Abstract
Benzylisoquinoline alkaloids, such as the analgesic compounds morphine and codeine, and the antibacterial agents berberine, palmatine, and magnoflorine, are synthesized from tyrosine in the Papaveraceae, Berberidaceae, Ranunculaceae, Magnoliaceae, and many other plant families. It is difficult to produce alkaloids on a large scale under the strict control of secondary metabolism in plants, and they are too complex for cost-effective chemical synthesis. By using a system that combines microbial and plant enzymes to produce desired benzylisoquinoline alkaloids, we synthesized (S)-reticuline, the key intermediate in benzylisoquinoline alkaloid biosynthesis, from dopamine by crude enzymes from transgenic Escherichia coli. The final yield of (S)-reticuline was 55 mg/liter within 1 h. Furthermore, we synthesized an aporphine alkaloid, magnoflorine, or a protoberberine alkaloid, scoulerine, from dopamine via reticuline by using different combination cultures of transgenic E. coli and Saccharomyces cerevisiae cells. The final yields of magnoflorine and scoulerine were 7.2 and 8.3 mg/liter culture medium. These results indicate that microbial systems that incorporate plant genes cannot only enable the mass production of scarce benzylisoquinoline alkaloids but may also open up pathways for the production of novel benzylisoquinoline alkaloids.
Keywords: (S)-reticuline, magnoflorine, scoulerine
Higher plants produce divergent chemicals, such as alkaloids, terpenoids, and phenolic compounds, in secondary metabolism. Among these chemicals, alkaloids are very important in medicine because of their high biological activities. Alkaloids are low-molecular-weight, nitrogen-containing compounds that are found in ≈20% of plant species. Most alkaloids are derived from amines produced by the decarboxylation of amino acids, such as histidine, lysine, ornithine, tryptophan, and tyrosine. Benzylisoquinoline alkaloids are a large and diverse group of pharmaceutical alkaloids with ≈2,500 defined structures. In the benzylisoquinoline alkaloid pathway, aporphine-type alkaloids, such as magnoflorine and corydine, and protoberberine-type alkaloids, such as berberine and coptisine, are produced via (S)-reticuline from tyrosine. (S)-Reticuline is a branch-point intermediate in the biosynthesis of many types of benzylisoquinoline alkaloids and also a nonnarcotic alkaloid of pharmaceutical significance that is useful in the development of antimalarial and anticancer drugs (ref. 1 and references therein). Recent studies have also suggested that these alkaloids may be useful as novel medicines. For example, the aporphine-type alkaloid magnoflorine has been reported to protect HDL during oxidant stress to prevent the development of atherosclerotic disease and to inhibit human lymphoblastoid cell-killing by HIV-1 (2–4). A recent report stated that the antimicrobial agent berberine had cholesterol-lowering activity (5).
Because of the high interest in their potential for medicinal use, some benzylisoquinoline alkaloids have been chemically synthesized via total synthesis. For example, the total synthesis of the narcotic analgesic morphine has been reported by Gates and Tschudi (6). Although chemical synthesis has been applied to alkaloid production, an enzymatic synthesis would be desirable for environmentally friendly and highly efficient alkaloid production.
Plant metabolic engineering often has been tried to increase the amount of an alkaloid pathway end product, and selected plant cells can produce sufficient quantities of metabolites for industrial application (7). However, only a few successful examples of plant metabolic engineering have been reported, particularly for the accumulation of benzylisoquinoline alkaloid metabolites. To date, transgenic opium poppy plant with RNAi of codeinone reductase and transgenic California poppy cells with RNAi of berberine bridge enzyme (BBE) have been reported for the production of reticuline (1, 8). Although transgenic poppy is advantageous for the production of reticuline, the amount of product varies a great deal in plants and cultured cells, and plants and cultured cells take a long time to grow (9). Furthermore, these transgenic poppies accumulate some methylated derivatives of reticuline. Although a transgenic approach can be a very powerful tool for metabolic engineering, this technology, including RNAi, still needs to be further improved before it can be used for desired metabolite production.
Recently, some attempts to reconstruct entire plant biosynthetic processes have been examined in microbial systems (10, 11). Microbial systems may be able to improve not only the quantity but also the quality of secondary metabolites because they do not include other plant metabolites. Although microbial systems offer several advantages for the biotransformation of chemicals, they also have disadvantages, such as limited substrate availability, particularly for plant metabolites. The combination of microbial and plant-derived genes would be useful for the establishment of efficient systems for the production of various compounds. Here, we report the establishment of a microbial system for the production of reticuline, the key intermediate for benzylisoquinoline alkaloids, with the use of microbes with plant enzyme genes. Furthermore, we propose that a system that combines two different microbes with different biosynthetic potentials to produce more divergent chemicals may be a universal system. This advanced system is shown to produce two kinds of benzylisoquinoline alkaloids, magnoflorine and scoulerine.
Results and Discussion
Experimental Design for Benzylisoquinoline Alkaloid Production in Microbes.
Whereas our advanced system consisted of a two-step synthesis of alkaloids with Escherichia coli and Saccharomyces cerevisiae cells, we first tried to produce reticuline, which is an important intermediate in benzylisoquinoline alkaloid biosynthesis in E. coli cells. Various types of benzylisoquinoline alkaloids were then synthesized from reticuline by using S. cerevisiae cells because some plant enzymes are not necessarily expressed in bacteria in an active form. This combined system of E. coli and S. cerevisiae cells is particularly advantageous for the coexpression of plant enzymes, which are compartmentalized in cytosol and endoplasmic reticulum (ER), in an active form within the cell, and also for producing chemicals derived from different pathways.
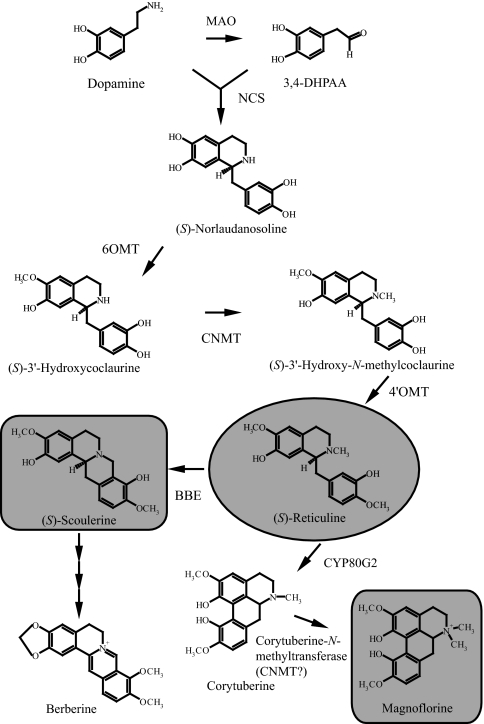
To produce benzylisoquinoline alkaloids in microbes, we first modified the benzylisoquinoline alkaloid pathway [Fig. 1 and supporting information (SI) Fig. S1]. Although benzylisoquinoline alkaloid biosynthesis begins with the conversion of tyrosine to dopamine and 4-hydroxyphenylacetaldehyde (4HPAA), which are condensed to (S)-norcoclaurine by norcoclaurine synthase (NCS) (12–18), these first steps are difficult to reconstruct for the efficient production of divergent benzylisoquinoline alkaloids. To simplify this situation, monoamine oxidase (MAO), NCS, norcoclaurine 6-O-methyltransferase (6OMT), coclaurine-N-methyltransferase (CNMT), and 3′-hydroxy-N-methylcoclaurine-4′-O-methyltransferase (4′OMT) were used to synthesize reticuline from dopamine in E. coli (18–21). The coupling of dopamine and 3,4-dihydroxyphenylacetaldehyde (3,4-DHPAA) enabled us to skip the step of the cytochrome P450 hydroxylase (CYP80B). Because MAO does not appear to play a role in the biosynthesis of alkaloids in opium poppy (22), microbial MAO was incorporated into reticuline biosynthesis to synthesize 3,4-DHPAA by the deamination of dopamine. In our previous study, two kinds of NCS, CjNCS1 and CjPR10A, have been isolated from C. japonica cells and characterized (18). CjPR10A is sufficiently expressed in an active form in E. coli cells, whereas CjNCS1 forms a larger complex in plant cells, and the recombinant enzyme expressed in E. coli cells has considerably lower activity than that of native enzyme. Because CjPR10A was more suitable than CjNCS1 in the microbial highly efficient production system, CjPR10A was used as NCS enzyme in reticuline biosynthesis.
Fig. 1.
Benzylisoquinoline alkaloid biosynthetic pathway reconstructed in microbes. MAO, MAO from Micrococcus luteus (GenBank accession no. AB010716); NCS, NCS from Coptis japonica (GenBank accession no. AB267399); 6OMT, 6OMT from Coptis japonica (GenBank accession no. D29811); CNMT, CNMT from Coptis japonica (GenBank accession no. AB061863); 4′OMT, 4′OMT from Coptis japonica (GenBank accession no. D29812); CYP80G2, CYP80G2 from Coptis japonica (GenBank accession no. AB288053); BBE, BBE from Coptis japonica.
In the second step of aporphine-type alkaloid biosynthesis, the recently identified P450 enzyme (CYP80G2), corytuberine synthase (23), was used in S. cerevisiae with C. japonica CNMT, which has a relatively broad substrate specificity and can N-methylate corytuberine to synthesize magnoflorine (Fig. 1). Similarly, BBE of C. japonica was expressed in S. cerevisiae to produce scoulerine from reticuline.
Reticuline Production in E. coli.
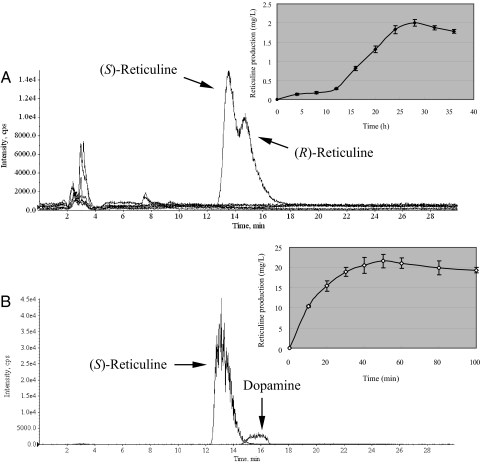
First, we examined high reticuline production as a key intermediate of benzylisoquinoline alkaloid in E. coli. For in vivo production, transgenic E. coli-expressing biosynthetic genes (i.e., MAO, NCS, 6OMT, CNMT, and 4′OMT) necessary to produce reticuline from dopamine were cultured with 2 mM dopamine in the medium. This culture produced mainly (R,S)-reticuline at a yield of 2.0 mg/liter medium within 28 h (Fig. 2A and Fig. S2). Dopamine and the resultant reticuline did not inhibit the growth of E. coli cells. An overall yield of reticuline from dopamine was 1.3%. The low overall yield was attributed to instability of dopamine. Dopamine is easily oxidized to be a melanin-like pigment without being introduced to E. coli cells (Fig. S3A). The light browning of the culture medium of transgenic E. coli-expressing reticuline biosynthetic genes compared with that of E. coli-containing empty vectors suggested that dopamine was converted into reticuline before oxidation and polymerization (Fig. S3). The improvement of utilization efficiency of dopamine will be needed for in vivo large-scale production. An advantage of this system is that (R,S)-reticuline was produced without the addition of a methyl group donor, S-adenosyl-l-methionine (SAM), because the regeneration of SAM in microbial cells is known to maintain in vivo methylation activity during bioconversion (24).
Fig. 2.
LC-MS analysis of reticuline produced in E. coli (A) or (S)-reticuline synthesized in vitro (B). The selected ion monitoring (SIM) parameters are as follows: m/z = 153 (3,4-DHPAA), m/z = 154 (dopamine), m/z = 288 (norlaudanosoline), m/z = 302 (3′-hydroxycoclaurine), m/z = 316 (3′-hydroxy-N-methylcoclaurine), m/z = 330 (reticuline). The inset shows the time course of reticuline production in E. coli (A) or (S)-reticuline synthesized in vitro (B). Error bars represent the SD of three independent measurements.
An increase in the amount of dopamine in the medium up to 5 mM further improved the yield of (R,S)-reticuline to a maximum of 11 mg/liter of culture (overall yield was 2.9%). Reticuline produced in E. coli was racemic (Fig. 2A), whereas NCS stereospecifically produced the (S)-form (18). We speculated that NCS could not function efficiently in E. coli cells and a spontaneous condensation reaction occurred to form norlaudanosoline, because E. coli cells expressing reticuline biosynthetic genes without NCS also produced racemic reticuline at the same level (data not shown). A reduction in the concentration of dopamine (≈100 μM) was not sufficient to synthesize (S)-reticuline (data not shown). In plant cells, dopamine is synthesized in the cytosol from 3,4-dihydroxphenylalanine (l-dopa) by dopa decarboxylase and then transported and accumulated within vacuoles at a concentration of 1 mg/ml (25, 26), suggesting that the compartmentalization of dopamine prevents the chemical coupling of amine and aldehyde. Because E. coli cells do not compartmentalize dopamine, the chemical coupling of dopamine and 3,4-DHPAA would dominate the NCS reaction for long periods of incubation. Optimized expression levels of reticuline synthetic genes, particularly MAO and NCS, were a critical factor for in vivo production of (S)-reticuline in E. coli cells.
To examine the dominant production of (S)-reticuline, crude enzymes from transgenic E. coli cells were prepared and reacted with dopamine and SAM. Unexpectedly, stereospecific (S)-reticuline was synthesized from dopamine with crude enzymes without fine-tuning of each enzyme level or purification (Fig. 2B). (S)-Reticuline was synthesized from 2 mM dopamine at a yield of 22 mg/liter (an overall yield was 14.4%) within 1 h (Fig. 2B), and no formation of (R)-reticuline was detected in this system. The enzyme reactions proceeded, and the pathway intermediates were not detected, suggesting that feedback regulation by the reaction product or co-factor limitations did not affect the synthesis of (S)-reticuline. This sufficiently high conversion rate and the lack of an intermediate would simplify purification of the reaction product, (S)-reticuline. An increase in the amount of dopamine added improved the yield of (S)-reticuline up to 55 mg/liter (an overall yield was 14.4%) within the same time scale. This in vitro biomanufacturing system can produce optically active (S)-reticuline much faster (i.e., 1 h) than the fermentation of cultured plant cells or transgenic plants (months to year).
Biosynthesis of Intermediates in a Benzylisoquinoline Alkaloid Pathway.
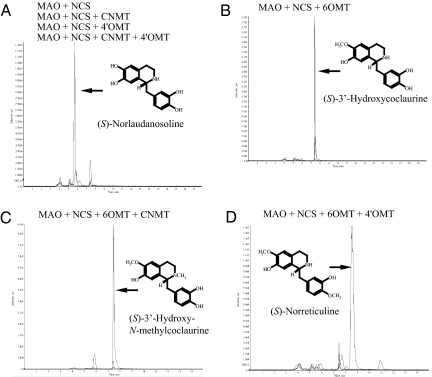
To characterize the biomanufacturing properties of our system, the biosynthesis of intermediates in a benzylisoquinoline alkaloid pathway from (S)-norlaudanosoline to (S)-norreticuline was also examined by using transgenic E. coli cells expressing various combinations of methyltransferase enzymes: 6OMT, CNMT, and 4′OMT. LC-MS analysis clearly showed that four kinds of benzylisoquinoline intermediates up to (S)-reticuline were synthesized as a dominant product in our modified biomanufacturing system (Fig. 3 and Fig. S4). The results in Fig. 3A indicated that the CNMT or 4′OMT reaction could hardly proceed without the 6OMT reaction, and 6OMT played a crucial role in benzylisoquinoline alkaloid biosynthesis. This is consistent with the nature of 6OMT as a rate-limiting step, as shown in California poppy cells (27). These results support the notion that the substrate specificities of biosynthetic enzymes regulate metabolite production, and we can control the production of various intermediates.
Fig. 3.
Production of intermediate alkaloids based on combinations of biosynthetic genes. Reactions were performed in vitro. Th gene combinations are as follows: MAO + NCS, MAO + NCS + CNMT, MAO + NCS + 4′OMT, MAO + NCS + CNMT + 4′OMT (A); MAO + NCS + 6OMT (B); MAO + NCS + 6OMT + CNMT (C); and MAO + NCS + 6OMT + 4′OMT (D). SIM parameters are as follows: m/z = 288 (norlaudanosoline), m/z = 302 (3′-hydroxycoclaurine), m/z = 316 (3′-hydroxy-N-methylcoclaurine or norreticuline), m/z = 330 (reticuline). The products were identified by comparison to authentic chemicals with regard to the fragmentation spectrum in LC-MS/MS (Fig. S4).
Because the production of benzylisoquinoline alkaloids from dopamine was so efficient in our system, the formation of a supercomplex of a metabolic channel was characterized in crude extract from transgenic E. coli cells. However, gel-filtration analysis showed that each recombinant NCS, 6OMT, CNMT, and 4′OMT, existed as a single polypeptide, and complex formation was not observed (data not shown). This and previous results in Figs. 2 and 3 indicated that the substrate specificities of the biosynthetic enzymes were critical factors for bioconversion and that 6OMT, CNMT, and 4′OMT reacted in succession, without metabolon formation. Our microbial system should provide insights into bioengineering of plant alkaloid production in microbes and the creation of novel biosynthetic pathways.
Benzylisoquinoline Alkaloid Production in Microbes.
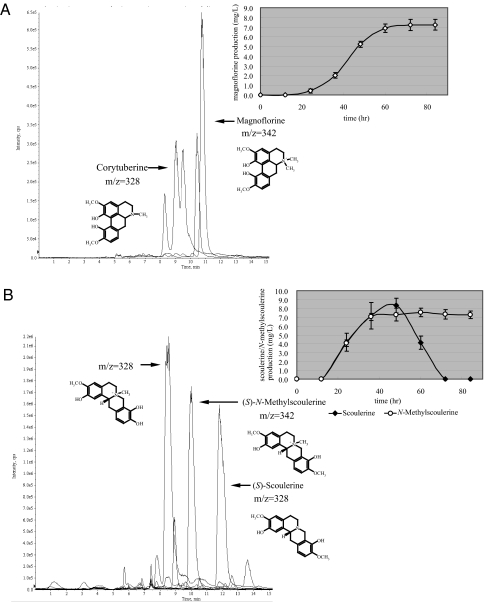
For benzylisoquinoline alkaloid production in microbes, we targeted magnoflorine for medicinal purposes. For magnoflorine production, transgenic E. coli cells expressing reticuline biosynthetic genes were cultured with 5 mM dopamine in the medium. After a certain period of culture, S. cerevisiae cells expressing CYP80G2 and CNMT were added to the culture medium, in which E. coli produced reticuline from dopamine. Liquid chromatography (LC)-MS analysis showed that magnoflorine and corytuberine were produced in the coculture medium of E. coli and S. cerevisiae (Fig. 4A and Fig. S5 A and B). In addition to corytuberine, uncharacterized byproducts (m/z 328) were also produced in the coculture medium. One of them should be dehydroreticuline, the oxidative product of reticuline by CYP80G2 (23). Magnoflorine was synthesized at a yield of 7.2 mg/liter culture within 72 h. In this system, magnoflorine was produced without the addition of a methyl group donor, SAM, along with reticuline.
Fig. 4.
LC-MS analysis of magnoflorine (A) or scoulerine (B) produced in mixed culture of microbes. SIM parameters are as follows: m/z = 153 (3,4-DHPAA), m/z = 154 (dopamine), m/z = 288 (norlaudanosoline), m/z = 302 (3′-hydroxycoclaurine), m/z = 316 (3′-hydroxy-N-methylcoclaurine), m/z = 328 (corytuberine or scoulerine), m/z = 330 (reticuline), m/z = 342 (magnoflorine or N-methylscoulerine). The inset shows the time course of magnoflorine or scoulerine/N-methylscoulerine production in microbes. E. coli culture, which produced reticuline, was mixed with S. cerevisiae with CYP80G2-CNMT (A) or BBE (B) and cocultured. Cultures were sampled at various intervals and production levels were quantified. The amount of N-methylscoulerine production was calculated by using scoulerine as a standard. The error bars represent the standard deviation of three independent measurements.
On the other hand, a protoberberine-type alkaloid, scoulerine, was produced when S. cerevisiae cells expressing BBE were used instead of those that expressed CYP80G2 and CNMT (Fig. 4B and Fig. S5 C and D). Scoulerine was synthesized at a yield of 8.3 mg/liter culture within 48 h. The overall yield of magnoflorine or scoulerine from dopamine was 1.9 or 2.2%, respectively. These results showed that the conversion efficiency of magnoflorine or scoulerine from reticuline in S. cerevisiae cells were 65.5% or 75.5%, respectively. It was indicated that a yield improvement of reticuline production in E. coli cells resulted in the efficient production of benzylisoquinoline alkaloids in microbes. In this system, N-methylscoulerine, a precursor of protopine-type and rhoeadine-type alkaloids, was also synthesized by the N-methylation of scoulerine. These results suggest that our combination system may be very useful for synthesizing diverse isoquinoline alkaloids, such as bisbenzylisoquinoline, benzophenanthridine, protoberberine, and morphinan alkaloids, by using S. cerevisiae cells that express the desired biosynthetic genes.
Conclusions
Our success in the reconstruction of a benzylisoquinoline alkaloid pathway in a microbial system may lead to ways for giving microbial cells the ability to produce plant alkaloids. Our system can provide a variety of isoquinoline alkaloid skeletons besides norlaudanosoline by the combination of other amines and aldehydes for substrates. The widespread application of our system may lead to further progress with microbial systems for use in the pharmaceutical industry, which needs a diverse chemical library to develop more advanced tools for chemical therapy, such as anticancer, antidiabetes, and antimalarial drugs (28, 29). Bioengineering by using microbial, plant, and other biological resources is a novel basic tool for manufacturing a broad range of plant-derived metabolites, particularly isoquinoline alkaloids.
Materials and Methods
Chemicals.
(S)-Reticuline was a gift from P. J. Facchini (University of Calgary, Calgary, BC, Canada). Magnoflorine was a gift from R. Nishida (Kyoto University, Kyoto, Japan). (R,S)-Reticuline, (R,S)-norreticuline, (R,S)-3′-hydroxycoclaurine, and (R,S)-scoulerine were gifts from Mitsui Chemicals, Inc. (R,S)-3′-hydroxy-N-methylcoclaurine was prepared as described previously (28). (R,S)-Norlaudanosoline was purchased from Acros Organics.
Construction of E. coli Expression Vectors for Reticuline Production.
Expression vectors for each enzyme were constructed as described previously (18–21). cDNAs were amplified by PCR with the respective primers (Table S1). Reticuline biosynthetic genes were expressed from two compatible vectors in E. coli (Fig. S6). MAO and NCS genes were ligated into pKK223–3 vector (Amersham Pharmacia), whereas 6OMT, CNMT, and 4′OMT genes were ligated into pACYC184 vector. The expression vector of MAO and NCS was constructed as described below. The PCR product of the MAO gene (EcoRI-HindIII fragment) was ligated into the pKK223–3 vector digested with EcoRI and HindIII by using a DNA Ligation kit (Takara Shuzo Co.). The PCR product of the NCS gene (NruI–BamHI fragment) was ligated into the pKK223–3 vector containing the MAO gene digested with NruI and BamHI to give the expression vector of MAO and NCS. The expression vector of 6OMT, CNMT, and 4′OMT was constructed as described below. The PCR products of the 6OMT (KpnI–BglII fragment), 4′OMT (BglII–BamHI fragment), and CNMT (BamHI–PstI fragment) genes were ligated into the pUC18 vector digested with KpnI and PstI. The PvuII fragment containing three genes was prepared from the resultant construct and then ligated into the pACYC184 vector digested with EcoRV and NruI to give an expression vector containing 6OMT, CNMT, and 4′OMT. MAO and other genes (NCS, 6OMT, CNMT, and 4′OMT) were under the control of the tac or T7 promoter, respectively. The vectors for the production of pathway intermediates other than reticuline were constructed by deleting unnecessary genes from the above plasmid.
Heterologous Expression of Recombinant Reticuline Synthetic Genes in E. coli.
The expression vectors for reticuline biosynthesis were introduced into E. coli BL21 (DE3). E. coli cells containing each plasmid were grown at 37°C in LB medium. After induction with 1 mM isopropyl β-d-thiogalactoside (IPTG), E. coli cells were incubated at 20°C for 24 h. Crude enzymes were extracted from the resultant E. coli cells with Tris buffer (50 mM Tris·HCl, pH 7.5, containing 10% glycerol and 5 mM 2-mercaptoethanol).
Production of Reticuline and Related Intermediates in E. coli.
For the in vivo production of reticuline, E. coli cells were incubated at 25°C for 28 h in LB medium supplemented with dopamine at IPTG induction. Culture medium was recovered, and the supernatant after protein precipitation with an equal volume of methanol was used to measure alkaloid production by LC-MS.
In vitro alkaloid production was conducted with crude enzymes prepared from transgenic E. coli cells in reaction buffer [50 mM Tris·HCl (pH 7.5), 10% glycerol, and 5 mM 2-mercaptoethanol] containing 5 mM dopamine and 1 mM SAM. After incubation at 37°C for 60 min and protein precipitation with an equal volume of methanol, alkaloid production was determined by LC-MS.
Construction of Expression Vectors for Magnoflorine/Scoulerine Production and Its Expression in S. cerevisiae.
The coexpression vector pGYR for P450 and yeast NADPH-P450 reductase was provided by Y. Yabusaki (Sumitomo Chemical Co., Ltd). This vector contained glyceraldehyde-3-phosphate dehydrogenase promoter and terminator (30). The cloning site of pGYR was further modified to contain an SpeI site to construct pGYR–SpeI. Full-length CYP80G2 cDNA was amplified by PCR by using single-stranded cDNAs synthesized from 1.3 μg of total RNA of cultured C. japonica cells with oligo(dT) primer and SuperScript III RNase H-reverse transcriptase (Invitrogen) and then ligated into the SpeI site of pGYR-SpeI to generate yeast expression vector, pGS-CYP80G2 (23). The expression vector of CNMT was constructed as described below. Full-length CNMT cDNA was amplified by PCR with KpnI–CNMT-F and CNMT–SalI-R primers (Table S1). The PCR product of CNMT gene was ligated into the KpnI and SalI site of pAUR123 vector (Takara Shuzo Co.) to generate the yeast expression vector pAUR123-CNMT. To construct the expression vector for BBE, full-length BBE cDNA was amplified by PCR in the same way as for CYP80G2 and then ligated into the HindIII and EcoRI sites of pYES2 vector (Invitrogen) to generate the yeast expression vector pYES2-BBE (N.I., T.T., E. Dubouzet, and F.S., unpublished construct).
For the in vivo production of magnoflorine or scoulerine, the expression plasmids for CYP80G2 and CNMT were introduced into yeast strain AH22 (31) and that for BBE was introduced into BJ5627 by the LiCl method (32), respectively. These recombinant yeast cells were cultivated in synthetic defined (SD) medium at 28°C, 180 rpm, as described elsewhere (33).
Magnoflorine or Scoulerine Production in Microbes.
For the in vivo production of magnoflorine, E. coli cells were incubated at 25°C for 12 h in LB medium supplemented with 5 mM dopamine at IPTG induction, and S. cerevisiae cells expressing CYP80G2 and CNMT were incubated at 28°C for 20 h in SD medium. S. cerevisiae cells and 2% glucose were added to E. coli culture medium, and incubation at 28°C was performed for an additional 72 h. For scoulerine production, S. cerevisiae cells expressing BBE and 2% galactose were added to E. coli culture medium, and incubation at 28°C was performed for an additional 48 h in the same way as for magnoflorine production. Culture medium was recovered, and the supernatant after protein precipitation with an equal volume of methanol was used to measure magnoflorine/scoulerine production by LC-MS.
LC-MS Analysis of Products.
Benzylisoquinoline alkaloid production was measured by LC-MS (API 3200, Applied Biosystems Japan Ltd.) with an Agilent HPLC system: column, ODS-80Ts (4.6 × 250 mm; Tosoh, Inc.); solvent system, 20% acetonitrile containing 0.1% acetic acid; and flow rate, 0.5 ml/min at 40°C. Products were identified by coelution with authentic chemicals and comparison with authentic chemicals with regard to the fragmentation spectrum in LC–tandem MS (LC-MS/MS) (Figs. S2, S4, and S5). An overall yield of reaction products was calculated as half the amount of dopamine in total because benzylisoquinoline alkaloids are the dimeric alkaloids of dopamine.
To distinguish between the (R) and (S) forms of reticuline, we used a chiral column (SUMICHIRAL-CBH, 4.0 × 100 mm; Sumika Chemical Analysis Service) with LC-MS. The solvent system was 5% acetonitrile containing 0.1% acetic acid, adjusted to pH 7.0 with NH4OH (flow rate, 0.4 ml/min at 25°C).
Supplementary Material
Acknowledgments.
This work was supported in part by Research for the Future Program of the Japan Society for the Promotion of Science Grant JSPS-RFTF00L01606 (to F. S.), by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Japan Foundation for Applied Enzymology (to F.S.), and by a postdoctoral fellowship from the Japan Society for the Promotion of Science (to H.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802981105/DCSupplemental.
References
- 1.Allen RS, et al. RNAi-mediated replacement of morphine with the nonnarcotic alkaloid reticuline in opium poppy. Nat Biotechnol. 2004;22:1559–1566. doi: 10.1038/nbt1033. [DOI] [PubMed] [Google Scholar]
- 2.Hung TM, et al. Magnoflorine from Coptidis rhizoma protects high density lipoprotein during oxidant stress. Biol Pharm Bull. 2007;30:1157–1160. doi: 10.1248/bpb.30.1157. [DOI] [PubMed] [Google Scholar]
- 3.Hung TM, et al. Protective effect of magnoflorine isolated from Coptidis rhizoma on Cu2+-induced oxidation of human low density lipoprotein. Planta Med. 2007;73:1281–1284. doi: 10.1055/s-2007-981615. [DOI] [PubMed] [Google Scholar]
- 4.Rashid MA, et al. Anti-HIV alkaloids from Toddalia asiatica. Nat Prod Res. 1995;6:153–156. [Google Scholar]
- 5.Kong W, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 6.Gates M, Tschudi G. The synthesis of morphine. J Am Chem Soc. 1952;74:1109–1110. [Google Scholar]
- 7.Sato F, Inui T, Takemura T. Metabolic engineering in isoquinoline alkaloid biosynthesis. Curr Pharm Biotechnol. 2007;8:211–218. doi: 10.2174/138920107781387438. [DOI] [PubMed] [Google Scholar]
- 8.Fujii N, Inui T, Iwasa K, Morishige T, Sato F. Knockdown of berberine bridge enzyme by RNAi accumulates (S)-reticuline and activates a silent pathway in cultured California poppy cells. Transgenic Res. 2007;16:363–375. doi: 10.1007/s11248-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 9.Sato F, Yamada Y. In: Bioengineering and Molecular Biology of Plant Pathways. Bohnert HJ, Nguyen HT, Lewis N, editors. Vol 1. Amsterdam: Elsevier; 2008. pp. 311–346. [Google Scholar]
- 10.Rathbone DA, Bruce NC. Microbial transformation of alkaloids. Curr Opin Microbiol. 2002;5:274–281. doi: 10.1016/s1369-5274(02)00317-x. [DOI] [PubMed] [Google Scholar]
- 11.Ro DK, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 12.Stadler R, et al. Revision of the early steps of reticuline biosynthesis. Tetrahedron Lett. 1987;28:1251–1254. [Google Scholar]
- 13.Stadler R, Kutchan TM, Zenk MH. (S)-norcoclaurine is the central intermediate in benzylisoquinoline alkaloid biosynthesis. Phytochemistry. 1989;28:1083–1086. [Google Scholar]
- 14.Samanani N, Facchini PJ. Purification and characterization of norcoclaurine synthase. The first committed enzyme in benzylisoquinoline alkaloid biosynthesis in plants. J Biol Chem. 2002;277:33878–33883. doi: 10.1074/jbc.M203051200. [DOI] [PubMed] [Google Scholar]
- 15.Samanani N, Liscombe DK, Facchini PJ. Molecular cloning and characterization of norcoclaurine synthase, an enzyme catalyzing the first committed step in benzylisoquinoline alkaloid biosynthesis. Plant J. 2004;40:302–313. doi: 10.1111/j.1365-313X.2004.02210.x. [DOI] [PubMed] [Google Scholar]
- 16.Luk LY, Bunn S, Liscombe DK, Facchini PJ, Tanner ME. Mechanistic studies on norcoclaurine synthase of benzylisoquinoline alkaloid biosynthesis: An enzymatic Pictet-Spengler reaction. Biochemistry. 2007;46:10153–10161. doi: 10.1021/bi700752n. [DOI] [PubMed] [Google Scholar]
- 17.Berkner H, et al. High-yield expression and purification of isotopically labeled norcoclaurine synthase, a Bet v 1-homologous enzyme, from Thalictrum flavum for NMR studies. Protein Expr Purif. 2007;56:197–204. doi: 10.1016/j.pep.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Minami H, Dubouzet E, Iwasa K, Sato F. Functional analysis of norcoclaurine synthase in Coptis japonica. J Biol Chem. 2007;282:6274–6282. doi: 10.1074/jbc.M608933200. [DOI] [PubMed] [Google Scholar]
- 19.Roh JH, et al. Purification, cloning, and three-dimensional structure prediction of Micrococcus luteus FAD-containing tyramine oxidase. Biochem Biophys Res Commun. 2000;268:293–297. doi: 10.1006/bbrc.2000.2113. [DOI] [PubMed] [Google Scholar]
- 20.Morishige T, Tsujita T, Yamada Y, Sato F. Molecular characterization of the S-adenosyl-L-methionine:3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase involved in isoquinoline alkaloid biosynthesis in Coptis japonica. J Biol Chem. 2000;275:23398–23405. doi: 10.1074/jbc.M002439200. [DOI] [PubMed] [Google Scholar]
- 21.Choi KB, Morishige T, Shitan N, Yazaki K, Sato F. Molecular cloning and characterization of coclaurine N-methyltransferase from cultured cells of Coptis japonica. J Biol Chem. 2002;277:830–835. doi: 10.1074/jbc.M106405200. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt J, Boettcher C, Kuhnt C, Kutchan TM, Zenk MH. Poppy alkaloid profiling by electrospray tandem mass spectrometry and electrospray FT-ICR mass spectrometry after [ring-13C6]-tyramine feeding. Phytochemistry. 2007;68:189–202. doi: 10.1016/j.phytochem.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Ikezawa N, Iwasa K, Sato F. Molecular cloning and characterization of CYP80G2, a cytochrome P450 which catalyzes an intramolecular C-C phenol coupling of (S)-reticuline in magnoflorine biosynthesis, from cultured Coptis japonica cells. J Biol Chem. 2008;283:8810–8821. doi: 10.1074/jbc.M705082200. [DOI] [PubMed] [Google Scholar]
- 24.Morishige T, Choi KB, Sato F. In vivo bioconversion of tetrahydroisoquinoline by recombinant coclaurine N-methyltransferase. Biosci Biotechnol Biochem. 2004;68:939–941. doi: 10.1271/bbb.68.939. [DOI] [PubMed] [Google Scholar]
- 25.Roberts MF, McCarthy D, Kutchan TM, Coscia CJ. Localization of enzymes and alkaloidal metabolites in Papaver latex. Arch Biochem Biophys. 1983;222:599–609. doi: 10.1016/0003-9861(83)90558-1. [DOI] [PubMed] [Google Scholar]
- 26.Kutchan TM, Rush M, Coscia CJ. Subcellular localization of alkaloids and dopamine in different vacuolar compartments of Papaver bracteatum. Plant Physiol. 1986;81:161–166. doi: 10.1104/pp.81.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inui T, Tamura K, Fujii N, Morishige T, Sato F. Overexpression of Coptis japonica norcoclaurine 6-O-methyltransferase overcomes the rate-limiting step in benzylisoquinoline alkaloid biosynthesis in cultured Eschscholzia californica. Plant Cell Physiol. 2007;48:252–262. doi: 10.1093/pcp/pcl062. [DOI] [PubMed] [Google Scholar]
- 28.Cui W, et al. Potential cancer chemopreventive activity of simple isoquinolines, 1-benzylisoquinolines, and protoberberines. Phytochemistry. 2006;67:70–79. doi: 10.1016/j.phytochem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Lee YS, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 30.Sakaki T, Akiyoshi-Shibata M, Yabusaki Y, Ohkawa H. Organella-targeted expression of rat liver cytochrome P450c27 in yeast. J Biol Chem. 1992;267:16497–16502. [PubMed] [Google Scholar]
- 31.Oeda K, Sakaki T, Ohkawa H. Expression of rat liver cytochrome P-450MC cDNA in Saccharomyces cerevisiae. DNA. 1985;4:203–210. doi: 10.1089/dna.1985.4.203. [DOI] [PubMed] [Google Scholar]
- 32.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikezawa N, et al. Molecular cloning and characterization of CYP719, a methylenedioxy bridge-forming enzyme that belongs to a novel P450 family, from cultured Coptis japonica cells. J Biol Chem. 2003;278:38557–38565. doi: 10.1074/jbc.M302470200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.