Abstract
Erythrocytosis can arise from deregulation of the erythropoietin (Epo) axis resulting from defects in the oxygen-sensing pathway. Epo synthesis is controlled by the hypoxia inducible factor (HIF) complex, composed of an α and a β subunit. There are 2 main α subunits, HIF-1α and HIF-2α. Recently, a HIF-2α Gly537Trp mutation was identified in a family with erythrocytosis. This raises the possibility of HIF2A mutations being associated with other cases of erythrocytosis. We now report a subsequent analysis of HIF2A in a cohort of 75 erythrocytosis patients and identify 4 additional patients with novel heterozygous Met535Val and Gly537Arg mutations. All patients presented at a young age with elevated serum Epo. Mutations at Gly-537 account for 4 of 5 HIF2A mutations associated with erythrocytosis. These findings support the importance of HIF-2α in human Epo regulation and warrant investigation of HIF2A in patients with unexplained erythrocytosis.
Introduction
An erythrocytosis is defined by a raised hemoglobin (Hb) level and increased red cell mass. It is commonly associated with variable serum erythropoietin (Epo) levels. EPO gene transcription is oxygen-sensitive. Decreased oxygen tension is sensed by the kidney, which results in increased Epo synthesis via the hypoxia inducible factor (HIF) transcription complex. This complex is composed of a β subunit and a variable α subunit, of which there are 2 main isoforms, HIF-1α and HIF-2α.1 Hydroxylation of the α subunit in a conserved LXXLAP motif2–4 (where underlining indicates the hydroxylated proline) by prolyl hydroxylase domain protein (PHD)5,6 allows recognition by the von Hippel-Lindau (VHL) tumor suppressor protein,7 thereby targeting this subunit for degradation. Aberrant function of the oxygen sensing pathway, which controls Epo production, appears to be a major cause of erythrocytosis, with mutations being described in VHL8–12 and PHD2.13–15
As with other inherited human diseases, erythrocytosis has been exemplary in aiding our understanding of fundamental physiologic pathways. A recent study of the HIF2A gene in 3 generations of one family with erythrocytosis uncovered a novel Gly537Trp mutation located near the hydroxylacceptor Pro-531.16 Functional studies indicate that the Gly537Trp HIF-2α mutant is less efficiently hydroxylated than wild-type protein.16 Consequently, HIF-2α is stabilized in normoxia, and thus it can be inferred that HIF-2α is a key regulator of EPO gene transcription in humans. Pursuing this further, screening of 75 additional patients has now revealed additional mutations in 4 patients.
Methods
Patients
A total of 75 patients were selected on the basis of having serum Epo in the normal range or above from a registry of idiopathic erythrocytosis patients.10,13 These patients form 46% and 26% of the total, respectively, whereas the frequency of erythrocytosis patients with suppressed serum Epo is 28%.17 All patients were negative for defects in the VHL8–12 and PHD213–15 genes and gave informed written consent on entering the study, which had been approved by the Queen's University, Belfast Research Ethics Committee, in accordance with the Declaration of Helsinki.
Case reports
Patient H2.
A 23-year-old woman from Scotland presented with raised Hb (191 g/L) and hematocrit (Hct; 0.57). Red cell mass was 186% of the predicted value, whereas other parameters were normal (Table 1). Two months after the initial presentation, her Epo level was 42.6 IU/L (normal range [NR], 5.3-16.5 IU/L, Hb, 158 g/L; Hct, 0.47). She had 3 venesections during the year of presentation and one additional venesection 2 years later. No further treatment has been required. Her last Epo measurement (August 2007) was 15.7 IU/L; (NR, 2.5-10.5 IU/L; Hb, 152 g/L; Hct, 0.45).
Table 1.
Hematologic indices for erythrocytosis patients positive for HIF-2α exon 12 mutations
| H1* | H2 | H3 | H4 | H5 | |
|---|---|---|---|---|---|
| HIF mutation | Gly537Trp | Met535Val | Gly537Arg | Gly537Arg | Gly537Arg |
| Age at presentation, y | 23 | 23 | 20 | 16 | 21 |
| Hemoglobin, g/L | 217 | 191 | 181 | 201 | 205 |
| Hematocrit | 0.64 | 0.57 | 0.52 | 0.62 | NA |
| WBC, ×109/L | 7.2 | 6.0 | normal | 6.1 | normal |
| Platelets, ×109/L | 226 | 223 | normal | 168 | normal |
| Epo, IU/L (NR) Hb, Hct† | 31.1 (2.5-10.5) 186, 0.55 | 42.6 (5.3-16.5) 158, 0.47 | 112 (10-21) 133, 0.44 | >200 (3-20) 124, NA | 66 (2.5-10.5) 173, 0.60 |
NA indicates not available.
Previously reported.16
Hb and Hct measurements at the time the Epo assay was performed.
Patient H3.
A woman from the south of England presented at 20 years with Hb of 181 g/L and Hct of 0.52 with normal white cell and platelet counts (Table 1). She experienced itch, but no splenomegaly was detected on examination. A bone marrow biopsy showed erythroid hyperplasia only. She has been managed by intermittent venesections over the past 30 years. Her Epo level has remained greatly elevated with a recent measurement of 112 IU/L (NR, 10-21 IU/L; Hb, 133 g/L; Hct, 0.44). Her 3 children all have normal indices.
Patient H4.
A 16-year-old Dutch girl presented with headache, dizziness, and fatigue. Investigations showed Hb 201 g/L and Hct 0.62 with normal white cell and platelet counts (Table 1). Maintenance venesections were performed 4 times a year. She then had a mesenteric thrombosis at 21 years, and the Hct was reduced to 0.40. She remained on heparin for 1 year, which was followed by coumarin treatment. She developed a palpable spleen at the age of 36 years. Recent investigations indicated an Epo level of more than 200 IU/L (NR, 3-20 IU/L). Her father, who died at the age of 41 years from a mesenteric infarction, was diagnosed with erythrocytosis and had regular venesections.
Patient H5.
A 21-year-old man of mixed Indian and Kenyan ancestry presented with Hb of 205 g/L while living in Uganda. His only symptom was intermittent itch. His platelet count and white cell count were not raised (Table 1). He was treated by venesection. When reinvestigated in 2007 at the age of 44 years, serum Epo was 66 IU/L (NR, 2.5-10.5 IU/L), spleen size was normal on ultrasound scan, and bone marrow showed marked erythroid hyperplasia. His 11-year-old daughter was diagnosed with an elevated Hb of 175 g/L but normal platelets and white cell counts. Her serum Epo was elevated at 12.4 IU/L (NR, 2.5-10.5 IU/L; Hb, 188 g/L; Hct, 0.56).
Mutation screening
Polymerase chain reaction (PCR)–direct sequencing was performed for exon 12 of HIF2A as described previously.16
Results and discussion
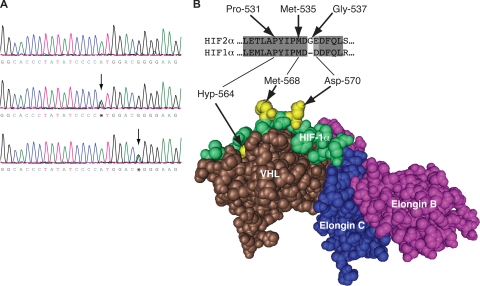
We reported the first case of HIF-2α–associated erythrocytosis in a family with raised serum Epo levels. The proband, H1, presented at age 23.16 Sequencing exon 12 of HIF2A revealed 4 patients, diagnosed in their early 20s or earlier, with 2 novel mutations (Figure 1A). By comparison, the mean age of patients on our erythrocytosis registry is 37 years.17 One patient (H2) harbored a heterozygous A to G at base 1603 (c.1603A>G) resulting in replacement of methionine with valine at amino acid 535 (p.Met535Val). Three patients (H3, H4, and H5) were heterozygous for a c.1609G>A mutation, which predicts replacement of glycine with arginine at amino acid 537 (p.Gly537Arg). Somewhat surprisingly, no mutation directly involved the LXXLAP motif. Nonetheless, HIF2A mutations are present at a significant frequency in this group of patients. Family studies revealed that all 3 children of H3 had normal hematologic indices and were negative for the c.1609G>A mutation, whereas the daughter of H5, who has erythrocytosis, possesses the same mutation as her father (p.Gly537Arg). No further HIF2A mutations were detected in the remaining patients included in this study.
Figure 1.
Identification of the c.1603A>G and c.1609G>A mutations in the HIF2A gene. (A) Detection of the c.1603A>G and c.1609G>A mutations by PCR-direct sequencing. PCR was performed on total peripheral blood DNA using a set of primers to specifically amplify exon 12 of the HIF2A gene. Sequencing detected a heterozygous A to G change at base 1603 in patient H2 (middle panel) and G to A at base 1609 in patient H5 (bottom panel) as indicated by arrows compared with wild-type sequence (top panel). Shown are nucleotides 1587 to 1615. Bases are as follows: G, black; A, green; T, red; C, blue. (B) Three-dimensional structure of the VHL:ElonginC:ElonginB complex bound to hydroxyproline-564 HIF-1α peptide (residues 556-575). The structure was generated using Cn3D from PDB coordinates (1LM8) deposited by Min et al.18 The positions of hydroxyproline-564 (Hyp-564), Met-568, and Asp-570 are shown (all in bright yellow). The sequences of HIF-2α and HIF-1α at the primary hydroxylation site are compared, with the corresponding HIF-2α residues indicated.
Both Met-535 and Gly-537 are conserved throughout evolution in HIF-2α.16 Furthermore, Met-535 is present in all 3 human HIF isoforms, whereas, in contrast, Gly-537 is unique to the HIF-2α isoform. Both residues are close in primary structure to the hydroxylacceptor Pro-531. Although the structure of either HIF-1α or HIF-2α bound to PHD2 has not been reported, the configuration of a HIF-1α peptide bound to VHL has been published.18,19 This structure reveals that both Met-535 and Gly-537 of HIF-2α reside in a region (residues 534-538) that separates the amino acids (residues 528-533 and residues 539-542) that are predicted to make essential contacts with VHL (Figure 1B).
The present erythrocytosis cases share a number of features in common with the previously reported kindred. First, all have elevated Epo. Second, they generally present at a relatively young age. The single exception is the grandmother of H1, who presented at age 54.16 Third, all have heterozygous mutations, suggesting that one mutant allele is sufficient to cause erythrocytosis. Fourth, none of the patients has thus far displayed evidence of a VHL-like syndrome, although long-term follow-up will be required to make a more definitive statement. It might also be noted that there is a clinical history of thrombosis in 2 of the patients described here as well as one of the patients previously reported16; a similar observation has been made with the VHL-associated Chuvash polycythemia. The increased thrombosis did not appear to correlate with increased Hct and was not affected by venesection in the Chuvash cases.20
In conclusion, these results support HIF2A mutations, all of which thus far reside in exon 12, as a cause of erythrocytosis and further substantiate an important role for HIF-2α in the regulation of Epo synthesis in humans.
Acknowledgment
This work was supported in part by National Institutes of Health grant R01 CA090261 (F.S.L.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.J.P., T.R.J.L., M.F.M., and F.S.L. designed research and analyzed data; M.J.P. performed research; P.A.B., G.C., A.W.D., A.R.G., D.O., M.G.R., R.v.W., M.W., and M.F.M. contributed and clinically assessed the patients; M.J.P., T.R.J.L., M.F.M., and F.S.L. wrote the paper; P.A.B., G.C., A.W.D., A.R.G., D.O., M.G.R., R.v.W., and M.W. read and approved the paper.
Conflict-of-interest disclosure: F.S.L. receives research grant support from GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Melanie J. Percy, Department of Haematology, Floor C, Tower Block, Belfast City Hospital, Lisburn Road, Belfast BT9 7AB, Northern Ireland; e-mail: melanie.percy@belfasttrust.hscni.net.
References
- 1.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 2.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 3.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 4.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 6.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 7.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 8.Ang SO, Chen H, Hirota K, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 9.Pastore YD, Jelinek J, Ang S, et al. Mutations in the VHL gene in sporadic apparently congenital polycythemia. Blood. 2003;101:1591–1595. doi: 10.1182/blood-2002-06-1843. [DOI] [PubMed] [Google Scholar]
- 10.Percy MJ, McMullin MF, Jowitt SN, et al. Chuvash-type congenital polycythemia in 4 families of Asian and Western European ancestry. Blood. 2003;102:1097–1099. doi: 10.1182/blood-2002-10-3246. [DOI] [PubMed] [Google Scholar]
- 11.Cario H, Schwarz K, Jorch N, et al. Mutations in the von Hippel-Lindau (VHL) tumor suppressor gene and VHL-haplotype analysis in patients with presumable congenital erythrocytosis. Haematologica. 2005;90:19–24. [PubMed] [Google Scholar]
- 12.Perrotta S, Nobili B, Ferraro M, et al. Von Hippel Lindau-dependent polycythemia is endemic on the island of Ischia: identification of a novel cluster. Blood. 2006;107:514–519. doi: 10.1182/blood-2005-06-2422. [DOI] [PubMed] [Google Scholar]
- 13.Percy MJ, Zhao Q, Flores A, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Percy MJ, Furlow PW, Beer PA, et al. A novel erythrocytosis-associated PHD2 mutation suggests the location of a HIF binding groove. Blood. 2007;110:2193–2196. doi: 10.1182/blood-2007-04-084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Sheikh M, Moradkhani K, Lopez M, Wajcman H, Préhu C. Disturbance in the HIF-1alpha pathway associated with erythrocytosis: further evidences brought by frameshift and nonsense mutations in the prolyl hydroxylase domain protein 2 (PHD2). Blood Cells Mol Dis. 2008;40:160–165. doi: 10.1016/j.bcmd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Percy MJ, Furlow PW, Lucas GS, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:52–58. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percy MJ, Furlow PW, Jones FGC, et al. Erythrocytosis caused by mutations in the PHD2 and VHL genes. Blood. 2007;110:1069A. [Google Scholar]
- 18.Min JH, Yang H, Ivan M, et al. Structure of an HIF-1alpha-pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 19.Hon WC, Wilson MI, Harlos K, et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 20.Gordeuk VR, Sergueeva AI, Miasnikova GY, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103:3924–3932. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]