Abstract
To increase our understanding of the molecular composition of the kidney glomerulus, we performed a meta-analysis of available glomerular transcriptional profiles made from mouse and man using five different methodologies. We generated a combined catalogue of glomerulus-enriched genes that emerged from these different sources and then used this to construct a predicted protein–protein interaction network in the glomerulus (GlomNet). The combined glomerulus-enriched gene catalogue provides the most comprehensive picture of the molecular composition of the glomerulus currently available, and GlomNet contributes an integrative systems biology approach to the understanding of glomerular signaling networks that operate during development, function, and disease.
Many kidney diseases and, importantly, approximately two thirds of all cases of ESRD originate with glomerular disease. Most cases of glomerular disease are caused by systemic disorders (e.g., diabetes, hypertension, lupus, obesity) for which the molecular pathogeneses of the glomerular complications are unknown. Our understanding of glomerular diseases is limited to a few monogenic disorders for which mutations have been identified in genes encoding glomerular proteins, such as components of the glomerular basement membrane or the podocyte filtration slits.1–4 These proteins are specifically, or particularly strongly, expressed in podocytes. Examples include nephrin, which was identified through its mutation in familiar nephrotic syndrome of the Finnish type,5 podocin, which is mutated in steroid-resistant nephrotic syndrome,6 and α-actinin-4, which is mutated in familial FSGS.7 In addition, mutations in the transcription factors WT1 and LMX1B, which are expressed selectively but not exclusively in podocytes, lead to glomerular disorders in the context of more complex syndromes referred to as nail-patella, Denys-Drash, or Frasier syndromes, respectively.8–10 Finally, glomerular basement membrane collagens and laminins have been shown to be mutated in Alport syndrome and Pierson congenital nephrotic syndromes, respectively.11,12 Genetic studies in mice have further revealed genes and proteins of importance for glomerulus development and function, such as podocalyxin,13 CD2AP,14 NEPH1,15 FAT1,16 forkhead box C2,17 transcription factor 21 (Pod1),18 protein tyrosine phosphatase receptor type O (GLEPP1) and synaptopodin.19,20
It is possible that some of these genes and proteins are targeted also in the common glomerular disorders triggered by systemic disease. It can be anticipated, however, that the identification of additional genes and proteins with glomerulus-specific expression will contribute further important information about glomerulus development, function, and disease. Importantly, the identification of larger pathways and networks of genes and proteins in the glomerulus would most likely facilitate the analysis of glomerular disease progression and help to unravel mechanisms operating in the common glomerular diseases of systemic origin. So far, a few efforts to map transcriptional profiles in glomeruli have been reported, each being successful in identifying limited subsets of glomerular markers but each also suffering from limitations in scale and technology.17,21–23 Our own two previous studies were based on large-scale sequencing of mouse glomerulus expressed sequence tag (EST) libraries. By comparing mouse glomerulus EST libraries with whole-kidney EST libraries, we found almost 500 glomerulus-enriched genes.21 We also used mouse glomerulus cDNA microarrays (GlomChip) to identify approximately 300 glomerulus-enriched genes.17 In two other large-scale transcriptional profiling studies, human glomeruli and other nephron segments were microdissected and analyzed by cDNA microarray technology,22 or serial analysis of gene expression (SAGE).23
As a result of the incomplete and only partially overlapping information derived from different species and different technical platforms, genome-scan and glomerulus disease gene-hunting projects suffer from the lack of a comprehensive source of basic information about the glomerular transcriptome. To facilitate the search for new causes of monogenic glomerular disorders and to help identify molecular pathways and networks involved in the pathogenesis of common glomerular diseases, we combined published and newly produced data in a meta-analysis of the glomerular transcriptome. On the basis of this analysis and with the aid of other available data and bioinformatics tools, we predicted a protein–protein interaction network in the glomerulus (GlomNet). We propose that the updated catalogue of glomerular transcripts together with GlomNet will facilitate the search for new genes/proteins and molecular pathways operating in glomerular assembly, physiology, and pathology.
RESULTS
Glomerulus-Enriched Gene Catalogue
We summarized and combined the results from five different expression-profiling platforms (Tables 1 and 2), four of which were previously published. These data sets include a mouse kidney EST library comparison,21 a mouse “GlomChip” cDNA microarray profiling,17 a human SAGE profiling,23 a human Stanford cDNA microarray profiling,22 and a newly added mouse Affymetrix Genome 430 2.0 Array profiling.
Table 1.
Summary of the characteristics of the five methodsa
| Method | Species | Total Features on the Platform | Glomerulus-Enriched Genes Reported | Selection Criteria |
|---|---|---|---|---|
| EST libraries comparison | Mouse | 13,368 EST | 497 | P < 0.05, more than three-fold difference with whole kidney tissue |
| GlomChip profiling | Mouse | 18,496 probes | 357 | P < 0.05, more than two-fold difference with nonglomerular kidney tissue |
| Affymetrix profiling | Mouse | 45,101 probes | 1013 | P < 0.05, more than two-fold difference with nonglomerular kidney tissue |
| SAGE profiling | Human | ∼50,000 tags | 153 | P < 0.01, seven-fold or more difference with at least three nephron libraries |
| Stanford cDNA microarray profiling | Human | 41,859 probes | 102 | Cluster analysis, genes predominantly expressed in glomeruli than others |
Species column shows the RNA sample source for the methods. For each method, total features on the platforms, number of genes identified (mouse/human), and the selection criteria for glomerulus-enriched genes are listed.
Table 2.
Summary of the statistics of the five methodsa
| Method | Glomerulus-Enriched Gene (Human) | Literature Confirmed (n [%]) | HPA Confirmed (n [%]) |
|---|---|---|---|
| EST libraries Comparison | 373 | 73 (19.6) | 17 (4.6) |
| GlomChip profiling | 326 | 63 (19.3) | 19 (5.8) |
| Affymetrix profiling | 914 | 84 (9.2) | 32 (3.5) |
| SAGE profiling | 153 | 37 (24.2) | 12 (7.8) |
| Stanford cDNA microarray profiling | 102 | 16 (15.7) | 3 (2.9) |
The numbers of glomerulus-enriched genes were counted as human Entrez genes or corresponding human homologous genes. Numbers of literature- and HPA-confirmed genes were also counted. The percentages in the parentheses show the percentage of the corresponding number among the total gene number.
Our own two previous studies compared gene expression profiles in glomerulus with whole kidney or nonglomerular kidney tissue.17,21 In the EST analysis,21 497 mouse UniGene clusters identified as glomerulus-enriched were mapped to 373 homologous human Entrez genes (NCIB Entrez Gene database, http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=search&db=gene). From the GlomChip profiling,17 357 mouse genes identified as glomerulus-enriched were mapped to 326 homologous human Entrez genes.
The SAGE profiling of human kidney identified 229 tags with higher levels in the glomerulus than in three other nephron portions.23 They were mapped to 153 human Entrez genes.
The Stanford cDNA microarray analysis of human kidney identified 196 cDNA clones that were predominantly expressed in the glomerulus compared with other parts of the nephron.22 They were mapped to 102 human Entrez genes.
We also compared the gene expression profile of mouse glomeruli with nonglomerulus kidney tissue on Affymetrix Mouse Genome 430 2.0 Arrays. Pure preparations of glomeruli were isolated from newborn mice as described previously.17 A total of 1013 mouse genes were identified as more than two-fold upregulated in the glomerulus, which were mapped to 914 homologous human Entrez genes.
In total, 1407 genes were identified as glomerulus-enriched in comparison with other parts of the kidney by at least one approach. The genes are listed in Supplementary Table S1 along with information on the approach by which they were identified.
Comparison and Evaluation of Different Approaches
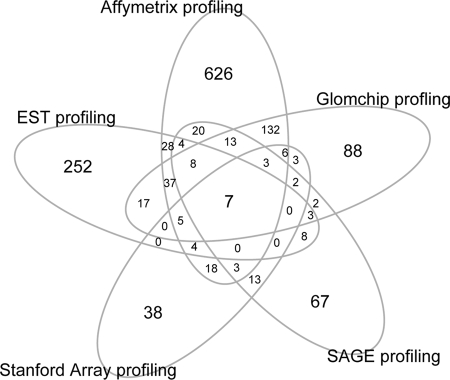
A comparison between the results of the five different approaches to identify glomerulus-enriched genes is illustrated in Figure 1. In total, 1407 genes were identified by at least one approach, and only seven genes (CDKN1C, ENG, EMCN, PTPRO, PLAT, IGFBP5, and PPAP2B) were identified in all studies. Of these, CDKN1C, PTPRO, and PLAT are expressed in podocytes, ENG and EMCN in endothelial cells, and IGFBP5 in mesangial cells. The cellular localization of PPAP2B in the glomerulus has not been reported. The five different approaches to identify glomerulus-enriched genes thus showed surprisingly limited overlap. Also, each approach identified a unique set of genes that were not identified by any of the other methods.
Figure 1.
Venn diagrams showing the overlap among the five glomerulus-enriched gene list identified by five different methods.
For assessment of the efficiency of each method in identifying glomerulus-enriched genes, the data sets were compared with published data on gene or protein expression in glomeruli by immunohistochemistry or in situ hybridization (see the “Literature-Confirmed” column in Supplementary Table S1). As shown in Table 2, the highest proportion of literature-confirmed glomerulus-expressed genes/proteins was found in the group of genes predicted by SAGE; 37 (24.2%) of 153 identified genes were confirmed. The EST data were 19.6%, GlomChip 19.3%, Stanford 15.7%, and Affymetrix 9.2% literature-confirmed, respectively.
The results of the different transcript profiling experiments were also compared with the public Human Protein Atlas (HPA) database, as described previously.21,24 The current release (version 2.0; http://www.proteinatlas.org) contains expression data for approximately 1500 antibodies in a variety of normal and cancerous human tissues. On the basis of the published stainings of kidney sections, the protein expression level in glomerular and tubular cells was assessed semiquantitatively. The result of protein expression comparison with the HPA database is shown in Table 2. The SAGE analysis showed the highest proportion of HPA-confirmed glomerulus-enriched proteins (12 [7.8%] of 153 genes confirmed). The results from GlomChip, EST, Affymetrix, and Stanford profilings were 5.8, 4.6, 3.5, and 2.9%, respectively (the identity of the HPA-confirmed genes is provided in Supplementary Table S1).
Obviously, the Affymetrix analysis, which assesses the expression of almost all genes, identified the largest number of glomerular markers validated by any of the other transcript profiling or by in situ RNA/protein detection (Figure 1, Table 2); however, Affymetrix profiling also identifies the largest total number of “unique” glomerulus-enriched transcripts. The proportion of unique genes (626 [approximately 68%] of 914) was also significantly higher in the Affymetrix analysis than in the other analyses except EST profiling (252 [approximately 67%] of 373). The proportion of genes identified by one method that was validated by at least one other method varied between 32% (Affymetrix) and 73% (GlomChip; Figure 1).
Construction of GlomNet
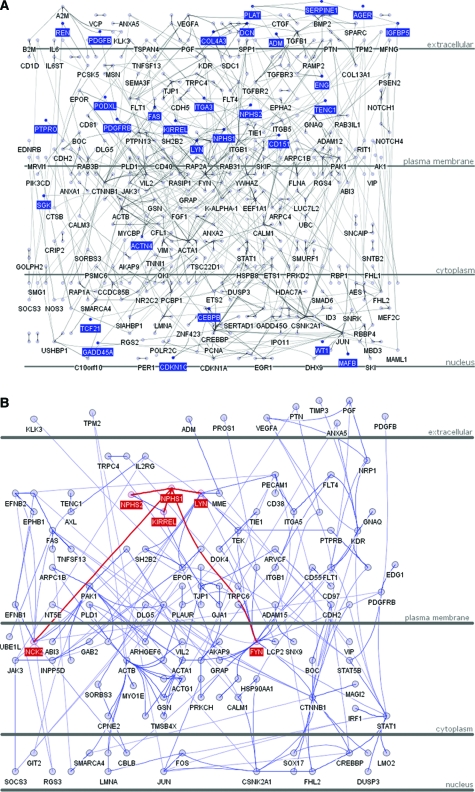
The Human Protein Reference Database (HPRD; http://www.hprd.org/) contains manually curated protein–protein interaction data from published literature. The data have been used to assemble the human protein “interactome.”25 Currently, 36,609 protein–protein interactions are depicted in HPRD (release 6). Among them, we found 772 direct interactions between 543 of the 1407 glomerulus-enriched gene products identified by any of the profiling methods (Supplementary Table S2). These include widely known glomerular protein interaction pairs, such as NPHS1–NPHS226 and NPHS1–FYN,27 as well as numerous other interaction pairs that are less well studied in the glomerulus. An assembly of the 772 interactions into a network—GlomNet—is illustrated in Figure 2A (a fully labeled network for zooming-in exploration is available as Supplementary Figure S1). Known genes whose mutation causes glomerular abnormality in humans or mice are highlighted within GlomNet in Figure 2A. This figure also illustrates that known glomerular disease proteins are located mainly in the extracellular, plasma membrane, and nuclear compartments.
Figure 2.
(A) Protein–protein interaction network for glomerulus-enriched genes (GlomNet). Proteins are represented with blue nodes, and interactions are represented with edges. The known glomerulus disease genes and the seven genes identified by all five methods are highlighted with deep blue color. (B) Protein–protein interaction network for Nephrin (NPHS1). The proteins directed interact with NPHS1, and the interactions are marked in red.
As an example of a putative signaling network, we depicted nephrin (NPHS1) and its first, second, and third neighbors in GlomNet (Figure 2B). By direct or indirect interactions with no more than two intermediate proteins, nephrin is predicted to contact 128 of the 543 proteins in GlomNet, present in extracellular, plasma membrane, cytoplasm, and nuclear compartments. It is interesting that at least four of these (PDGFB, PDGFRB, VEGFA, and KDR) have already been shown to be essential for glomerular development.28–30
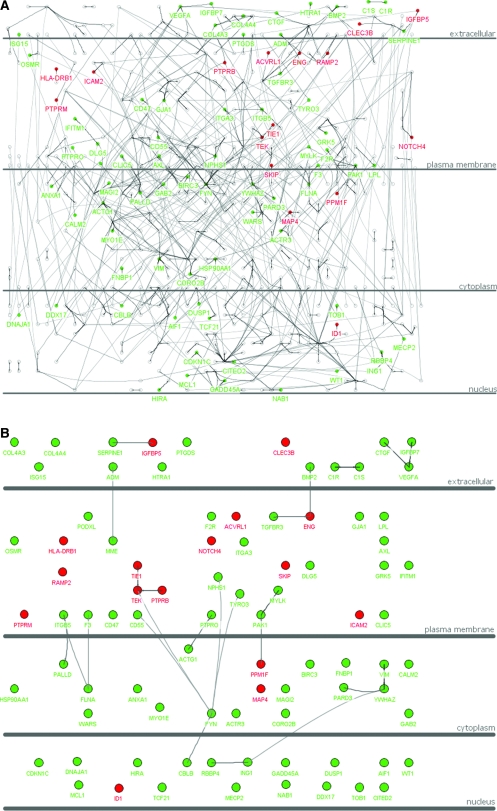
We also used the glomerulus-enriched gene catalogue and GlomNet to explore a published set of gene expression changes observed in human glomeruli isolated from patients with diabetic nephropathy (DN) in comparison with normal control subjects.31 This study identified 71 upregulated and 378 downregulated genes in the DN glomeruli. Twenty-five of the 71 upregulated genes and 129 of the 378 downregulated genes were found in the 1407 glomerulus-enriched gene catalogue (Supplementary Tables S3 and S4). Given that there are 25,000 to 30,000 genes in the mammalian genome, of which the DN-regulated genes (71 + 378 = 449) represent approximately 1.8% (449 of 25,000), the proportion of DN-regulated genes present in the 1407 glomerulus-enriched gene catalogue is significantly higher ([25 + 129]/1407 = 10.9%). An even higher proportion of the DN-regulated genes are represented in GlomNet (16 upregulated and 70 downregulated; [16 + 70]/543 = 15.8%; Figure 3A). The abundance of DN-affected genes in the 1407 catalogue and GlomNet lends support to our proposal that these tools may be of particular use in the search for glomerular pathomechanisms. It is interesting that several of the DN-affected genes link directly to each other in GlomNet, suggesting specific pathways that may be targeted by or be part of the pathogenic disease process (Figure 3B). It is interesting to note that many of the upregulated genes are expressed in endothelial cells (NOTCH4, TIE1, TEK [TIE2], ENG, PTPRB, ICAM2, and others), whereas many of the downregulated genes are expressed specifically in podocytes (NPHS1, PTPRO, PODXL, CDKN1C, TCF21, WT1, VEGFA, COL4A3, COL4A4, and others).
Figure 3.
(A) Gene expression pattern of DN-regulated genes over GlomNet. Red color indicates that the gene was upregulated in the DN glomeruli, and green color indicates that the gene was downregulated. (B) Illustration of DN-regulated genes over GlomNet. This is simplified from A, only showing the DN-regulated genes over GlomNet.
DISCUSSION
Several different techniques for high-throughput and genome-wide analyses of transcriptomes are now available. New technologies have also been developed for the isolation of cells and tissues and for the amplification of RNA from minute amounts of starting material such that complex transcriptional information can be obtained even from very limited numbers of cells. The kidney glomerulus is a structure from which transcriptome information might be particularly useful because this structure is severely afflicted by several common systemic disorders, such as diabetes and hypertension. Unfortunately, the molecular pathogeneses of such common glomerular diseases are unknown and treatment possibilities are limited. There is therefore an urgent need for new information and insights into the molecular composition and function of the glomerular cells and the identification of pathogenic pathways and, potentially, new glomerular drug targets.
A few studies have already been published on the glomerular transcriptome; however, these studies are difficult to compare and evaluate directly as a result of differences in technological platforms, species, and glomerulus isolation techniques. This study was done to facilitate comparison and to merge available glomerular transcriptome data into a single catalogue. By such meta-analysis of published and novel data from two different species—mouse and human—we produced the most comprehensive map of the glomerular transcriptome available.
EST sequencing and SAGE are direct ways to sample transcriptional information. Because significant differences in abundance require several EST/SAGE tags to be sequenced, the glomerulus-enriched transcripts identified by EST and SAGE approaches are likely also glomerulus-abundant transcripts. As such, they would also be predicted to be relatively easier to detect by other experiment methods, such as in situ hybridization or immunohistochemistry. This prediction partially agreed with the result from the literature and HPA, in which a higher proportion of SAGE and EST hits were confirmed than by the other methods (Table 2).
Affymetrix, GlomChip, and the Stanford cDNA all are different-sized microarray platforms that use customized methods to normalize and process the raw data, which can produce differences in the results. Affymetrix and Stanford arrays are near genome-wide, whereas GlomChip carries approximately 6000 genes isolated from glomerular cDNA libraries.17 The Affymetrix platform identified a higher number of glomerulus-enriched genes and also more literature- and HPA-confirmed genes than any of the other methods (Table 2); however, the proportion of the Affymetrix glomerulus-enriched genes that were confirmed by literature or HPA was lower than for the other methods. One explanation for this is that the Affymetrix array is more sensitive than the other methods and that many of the “unique” Affymetrix hits likely represent genes with expression levels undetectable by the other methods.
A comparison between the results from the two cDNA microarray approaches, GlomChip and Stanford, shows that GlomChip identified more glomerulus-enriched genes and both a higher number and proportion of genes confirmed by literature and HPA. This suggests that for the application to a specific tissue, a corresponding tissue-specific cDNA microarray might be preferable to a nonspecific cDNA microarray.
A comparison among the results of the five different transcription-profiling approaches (Figure 1) resulted in a surprisingly limited overlap, which may be caused by the different species (mouse and human) and developmental stages (adult and newborn) studied. The kidney glomerulus is likely also a target of immune-, stress-, and other environment-induced responses that may have varied between the studies. Conceivably, however, most of the transcriptional differences reflect the usage of different technical platforms and protocols. Raw data were also processed using different methods, and different criteria were used to categorize genes as glomerulus-enriched. Platform-specific features, however, would make it inappropriate to apply uniform criteria.
Conversely, the substantial number of “unique” genes, detected only by a single method, or genes detected by only two of five methods likely also reflects the high complexity of the glomerular transcriptome, confirmed, for example, by large-scale sequencing of glomerular cDNA libraries.21 We assume that genes contributing major functions in the glomerulus are evolutionary conserved between human and mouse and that each approach applied to date carries the potential for identifying the most significant genes in the glomerulus in relation to the respective scopes of the studies. From this assumption follows that the results of each approach are likely biologically meaningful and complementary to each other and that the pooled results, as presented here, provide a fuller and more useful picture of the glomerular transcriptome than any of the studies alone. Whereas emerging experimental data will undoubtedly modify this picture, we argue that the glomerulus-enriched gene catalogue presented here is of value to current nephrology research. With its defined method of construction, free access, and possibilities for updating, the glomerular gene catalogue should enhance our understanding of the molecular makeup of the kidney glomerulus and find use as a source of reference information for glomerulus disease research.
On the basis of the current version of the glomerular gene catalogue, we constructed GlomNet to facilitate identification of glomerular protein networks (Figure 1A). To our knowledge, this is the first attempt to describe the glomerulus by a systems biology approach. We predict that GlomNet will be particularly useful to predict links between previously uncharacterized glomerular proteins and known glomerular disease proteins. As an example of the current and future utility of GlomNet, we superimposed data from a recent glomerular transcription profiling study of DN.31 This analysis revealed interesting links between proteins affected in DN, pinpointing signaling pathways in both endothelial cells and podocytes that are differentially up- or downregulated. One of the downregulated transcripts in DN was Nephrin, which encodes a major component of the podocyte slit diaphragm and is mutated in congenital nephrotic syndrome.5 GlomNet illustrates that FYN, which directly interacts with Nephrin,27 together with three other proteins that interact with FYN, namely CD55, CBLB, and TYRO3, all are downregulated in DN, putatively pinpointing a pathway targeted in DN pathogenesis. Other transcripts that encode proteins reported to interact with Nephrin, such as Podocin (NPHS2) and KIRREL/NEPH1,32,33 were not found to be affected in this study of DN.
Microarray analyses have been applied to renal disease situations, and an increased focus on the glomerulus is expected along with methodologic improvements for glomerular isolation and comprehensive profiling of small biologic samples; however, deciphering the molecular mechanism from the large collections of transcriptome data is nevertheless challenging. GlomNet is a backbone and blueprint for protein networks in the glomerulus, which should facilitate the analysis of expression data from glomerular disease situations and help in identifying disease-related pathways and mechanisms.
CONCISE METHODS
Transcriptome Meta-analysis
EST Libraries Comparison.
By comparing glomerulus EST libraries with whole-kidney EST libraries as previously reported,21 497 mouse genes were identified as more than three-fold enriched in glomerulus. They were mapped to homologous human genes by NCBI HomoloGene assembly (http://www.ncbi.nlm.nih.gov/sites/entrez?db=homologene).
Glomerulus-Specific cDNA Microarray Profiling (GlomChip).
As described previously,17 357 mouse genes were identified as more than two-fold upregulated in the mouse glomerulus compared with rest of kidney. They were mapped to homologous human genes by NCBI HomoloGene assembly.
SAGE Profiling.
From the SAGE profiling,23 229 tags displayed significant higher expression level in the glomerulus than in other parts of the nephron. The threshold for statistical significance was set to P < 0.01 and seven-fold or more difference with at least three other nephron libraries. The unambiguously annotated tags were mapped to 153 human Entrez genes.
Stanford 41,859 cDNA Microarray Profiling.
Stringent criteria (described by Higgins et al.22) were used to produce a glomerular cluster with 196 clones whose IDs were searched in the SOURCE database (http://source.stanford.edu/cgi-bin/source/sourceSearch) and mapped to the corresponding Entrez Gene ID (Locuslink ID). A total of 162 clones that were unambiguously annotated were included in the analysis. They were mapped to 102 human Entrez genes.
Affymetrix Expression Array Profiling.
The RNA from mouse glomeruli and nonglomerular kidney tissue at P5 stage were isolated as described previously.17 Four glomerulus RNA samples and four nonglomerular kidney tissue sample were individually hybridized on eight Affymetrix Mouse Genome 430 2.0 arrays.
The array data were processed using Affy Package, and raw data were normalized using the gcrma package in the Bioconductor project (http://www.bioconductor.org). The t test was used to evaluate differential expression between glomerulus and nonglomerular kidney tissue sample. Multiple test correction of P values was done using the false discovery rate method.34 The fold change was calculated as the average expression difference between glomeruli and nonglomerular kidney tissue. The genes with more than two-fold upregulation in the glomeruli and corrected statistic P < 0.05 were identified as glomerulus-enriched genes. They were mapped to homologous human genes by NCBI HomoloGene assembly.
Construction of GlomNet
The protein–protein interaction pairs were downloaded from HPRD (release 6). The R program (http://www.r-project.org/) was used for the data processing. The interaction pairs formed by homophilic interaction of one protein were excluded. The interaction pairs formed by two glomerulus-enriched gene products were selected for further analysis.
The network visualization was done by using the Cytoscape program.35 Cerebral plug-in was used to layout using subcellular localization annotation.36 The protein cellular localization annotations were downloaded from HPRD. The proteins were classified to extracellular, plasma membrane, cytoplasm, and nucleus on the basis of their cellular compartment annotation and positioned in the corresponding layers in the graph. The proteins that are not annotated to these four categories were allowed to place in any layer optimized by the layout.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the Swedish Research Council and Novo Nordisk, Strategic Research, Söderberg, Hedlund and Wallenberg foundations (C.B. and K.T.); EU 6th frame work integrated project LYMPHANGIOGENOMICS, LSHG-CT-2004-503573; and the Inga-Britt and Arne Lundberg Foundation (C.B.).
Published online ahead of print. Publication date available at www.jasn.org.
M.T.'s current affiliation is Department of Clinical Cell Biology and Medicine, Chiba University, Chuo-ku, Japan.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Somlo S, Mundel P: Getting a foothold in nephrotic syndrome. Nat Genet 24: 333–335, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Khoshnoodi J, Tryggvason K: Unraveling the molecular make-up of the glomerular podocyte slit diaphragm. Exp Nephrol 9: 355–359, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Ly J, Alexander M, Quaggin SE: A podocentric view of nephrology. Curr Opin Nephrol Hypertens 13: 299–305, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL: Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet 19: 51–55, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Dreyer SD, Zhou G, Baldini A, Winterpacht A, Zabel B, Cole W, Johnson RL, Lee B: Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet 19: 47–50, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Klamt B, Koziell A, Poulat F, Wieacker P, Scambler P, Berta P, Gessler M: Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/-KTS splice isoforms. Hum Mol Genet 7: 709–714, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K: Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248: 1224–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schroder CH, Smeets HJ, et al.: Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 8: 77–81, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM: Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med 194: 13–27, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR: Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciani L, Patel A, Allen ND, ffrench-Constant C: Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol 23: 3575–3582, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C: Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25: 1160–1174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui S, Schwartz L, Quaggin SE: Pod1 is required in stromal cells for glomerulogenesis. Dev Dyn 226: 512–522, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Wharram BL, Goyal M, Gillespie PJ, Wiggins JE, Kershaw DB, Holzman LB, Dysko RC, Saunders TL, Samuelson LC, Wiggins RC: Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J Clin Invest 106: 1281–1290, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W: Synaptopodin: An actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139: 193–204, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L, Sun Y, Patrakka J, Mostad P, Norlin J, Xiao Z, Andrae J, Tryggvason K, Samuelsson T, Betsholtz C, Takemoto M: Glomerulus-specific mRNA transcripts and proteins identified through kidney expressed sequence tag database analysis. Kidney Int 71: 889–900, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Wang L, Kambham N, Montgomery K, Mason V, Vogelmann SU, Lemley KV, Brown PO, Brooks JD, van de Rijn M: Gene expression in the normal adult human kidney assessed by complementary DNA microarray. Mol Biol Cell 15: 649–656, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chabardes-Garonne D, Mejean A, Aude JC, Cheval L, Di Stefano A, Gaillard MC, Imbert-Teboul M, Wittner M, Balian C, Anthouard V, Robert C, Segurens B, Wincker P, Weissenbach J, Doucet A, Elalouf JM: A panoramic view of gene expression in the human kidney. Proc Natl Acad Sci U S A 100: 13710–13715, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, Andersson AC, Angelidou P, Asplund A, Asplund C, Berglund L, Bergstrom K, Brumer H, Cerjan D, Ekstrom M, Elobeid A, Eriksson C, Fagerberg L, Falk R, Fall J, Forsberg M, Bjorklund MG, Gumbel K, Halimi A, Hallin I, Hamsten C, Hansson M, Hedhammar M, Hercules G, Kampf C, Larsson K, Lindskog M, Lodewyckx W, Lund J, Lundeberg J, Magnusson K, Malm E, Nilsson P, Odling J, Oksvold P, Olsson I, Oster E, Ottosson J, Paavilainen L, Persson A, Rimini R, Rockberg J, Runeson M, Sivertsson A, Skollermo A, Steen J, Stenvall M, Sterky F, Stromberg S, Sundberg M, Tegel H, Tourle S, Wahlund E, Walden A, Wan J, Wernerus H, Westberg J, Wester K, Wrethagen U, Xu LL, Hober S, Ponten F: A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics 4: 1920–1932, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Gandhi TK, Zhong J, Mathivanan S, Karthick L, Chandrika KN, Mohan SS, Sharma S, Pinkert S, Nagaraju S, Periaswamy B, Mishra G, Nandakumar K, Shen B, Deshpande N, Nayak R, Sarker M, Boeke JD, Parmigiani G, Schultz J, Bader JS, Pandey A: Analysis of the human protein interactome and comparison with yeast, worm and fly interaction datasets. Nat Genet 38: 285–293, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T: Interaction with podocin facilitates nephrin signaling. J Biol Chem 276: 41543–41546, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB: Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C: Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Soriano P: Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8: 1888–1896, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Eremina V, Quaggin SE: The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens 13: 9–15, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Baelde HJ, Eikmans M, Doran PP, Lappin DW, de Heer E, Bruijn JA: Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis 43: 636–650, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS: Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest 112: 209–221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300, 1995 [Google Scholar]
- 35.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T: Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barsky A, Gardy JL, Hancock RE, Munzner T: Cerebral: A Cytoscape plugin for layout of and interaction with biological networks using subcellular localization annotation. Bioinformatics 23: 1040–1042, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.