Abstract
Thymic stromal lymphopoietin (TSLP) transgenic mice develop cryoglobulin-associated membranoproliferative glomerulonephritis, characterized by renal monocyte/macrophage infiltration, marked expansion of extracellular matrix, and variable intraluminal and mesangial deposits of cryoglobulins. A microarray approach was used to study global gene expression in glomerular RNA obtained from these mice, as well as from combined TSLP transgenic and Fcγ receptor IIb null mice (TSLP/FcIIb−/−), which develop aggravated membranoproliferative glomerulonephritis. Protease nexin-1 (PN-1) and tissue plasminogen activator (tPA), two potential regulators of fibrosis that are involved in the fibrinolytic and coagulation pathways, were dramatically upregulated in TSLP mice compared with wild-type controls. In situ hybridization revealed minimal expression of PN-1 mRNA in the glomeruli of wild-type mice, increased expression in TSLP mice, and the greatest expression in the mesangial cells of TSLP/FcIIb−/− mice. Immunohistochemistry demonstrated greater expression of PN-1, tPA, and PAI-1 in the mesangial cells of TSLP mice compared with wild-type and the greatest in TSLP/FcIIb−/− mice. In cultured mesangial cells, incubation with cryoglobulins induced an upregulation of PN-1 mRNA; increased expression of PN-1, tPA, and PAI-1 proteins; and stimulated secretion of TGF-β1. It is concluded that PN-1, tPA, PAI-1, and TGF-β1 are likely important mediators of murine cryoglobulinemic glomerulonephritis and that the cryoglobulins may directly upregulate their expression.
Accumulation of extracellular matrix (ECM) leading to glomerulosclerosis and interstitial fibrosis is a key feature of many chronic renal diseases. Activators and inhibitors of plasmin, a key enzyme involved with degradation of matrix and with fibrinolysis in coagulation pathways, have major roles in regulating renal fibrosing injury.1–3 Plasminogen activators (PA) are serine proteases that convert plasminogen into plasmin, which in turn degrades components of the ECM and activates growth factors that promote ECM accumulation. A variety of studies have reported decreased plasminogen activator (PA) activity and plasmin activity in glomeruli obtained from experimental glomerular injuries with mesangial matrix accumulation.1–5 Increased levels of the plasminogen activator inhibitor PAI-1, the best studied inhibitor of plasmin activity in renal injury, are observed in various forms of kidney diseases, leading to renal fibrosis and renal failure.1,4,6,7 As a result, plasminogen activator inhibitor-1 (PAI-1) is considered a major potential therapeutic target for amelioration of the process of matrix accumulation.8
PAI-1's activities are complex and involve inhibition of plasmin generation through its effects on both tissue type plasminogen activator (tPA) and urokinase type plasminogen activator (uPA). The activation of plasmin leads to degradation of ECM and activation of matrix metalloproteinases, both of which result in diminished fibrosis. In addition to these effects on matrix accumulation mediated by plasmin, PAI-1 has indirect and at times opposing effects because of its relationship with the profibrotic growth factor TGF-β. TGF-β directly increases expression of PAI-1, and, in turn, PAI-1 activity leads to diminished activation of TGF-β1 by plasmin, providing a physiologic feedback loop. Recently, it was demonstrated that PAI-1 may also exert profibrotic effects by directly recruiting monocytes/macrophages and myofibroblasts to sites of fibrosing renal injury, independent of its effects on plasmin.9
Protease nexin-1 (PN-1), like PAI-1, is a member of the serine protease inhibitor superfamily (serpins), which regulates matrix accumulation and coagulation under pathophysiologic conditions by inhibiting thrombin, plasmin, and both tPA, the predominant plasminogen activator in glomeruli,10 and uPA.11–14 PN-1, despite having activities comparable in many ways to PAI-1, has been little studied in the kidney; in particular, its role as a mediator of progressive or chronic kidney diseases has not been explored. Upregulation of PN-1 has been documented in the glomeruli of several experimental models of immune complex–mediated lupus-like glomerulonephritis15 as well as in atherosclerotic vessels,16 and there is evidence for a role for PN-1 in modulation of epithelial sodium channel activity in cortical collecting duct cells.17
Given the established effects of PAI-1 on fibrosing renal injury, we were intrigued by microarray analysis of glomeruli obtained from thymic stromal lymphopoietin (TSLP) transgenic mice, all of which develop cryoglobulinemic membranoproliferative glomerulonephritis (MPGN), which revealed marked upregulation of PN-1 in injured glomeruli compared with wild-type (WT) controls. To confirm the presence of PN-1 in this model of MPGN and explore the potential role of serpins and their substrates in glomerulonephritis, we performed studies to localize PN-1, PAI-1, and tPA by both immunohistochemistry (IHC) and in situ hybridization (ISH) in normal and nephritic mouse kidneys in vivo. These studies, supported by studies of mesangial cells in vitro, indicate coordinated upregulated expression of these key mediators of injury that may be directly induced by cryoglobulin deposition in cryoglobulinemic glomerulonephritis.
RESULTS
TSLP Transgenic Mice: A Model of Human Cryoglobulinemic Glomerulonephritis
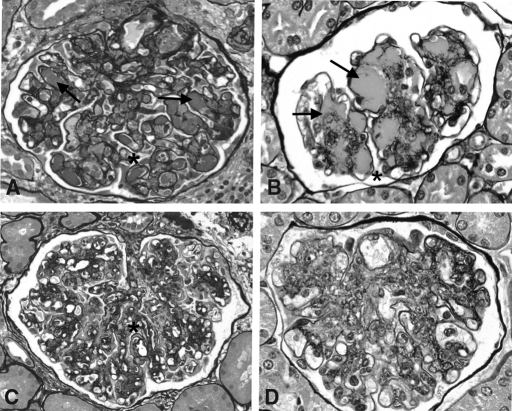
Renal involvement in TSLP transgenic mice is characterized by a large monocyte/macrophage infiltration, marked ECM expansion, increased glomerular cellularity, splitting of capillary basement membranes, and variable intraluminal as well as mesangial cryoglobulin deposits closely resembling the range of morphologic features of human cryoglobulinemic MPGN (Figure 1).
Figure 1.
Histology of TSLP transgenic mice and human cryoglobulinemic MPGN. Glomeruli from human cryoglobulinemic MPGN (A and C) and from TSLP transgenic mice (B and D) show a range of morphologic features, including intraluminal as well as mesangial cryoglobulin deposits (arrows, A and B), mesangial expansion and increased cellularity (A through D), and splitting of basement membranes (* in A, B, and C). In both humans and mice, the deposition of cryoglobulins can be histologically inapparent, and in these cases, the association of the MPGN with cryoglobulinemia is determined only by serologic evidence of cryoglobulins. Influx of monocytes, common but not invariably so in both humans and mice, is prominent in the capillaries and probably also in mesangial regions in these examples of human and murine MPGN (C and D, respectively) and confirmed by monocyte-specific immunohistochemical labeling.
RNA Microarray Analysis
RNA microarray analysis of glomerular RNA revealed a large number of differentially expressed transcripts in TSLP mice with MPGN when compared with WT littermate controls. Comparisons were made between female TSLP transgenic and WT mice at 50 d (64 total transcripts with more than two-fold change, P < 0.05), male TSLP transgenic and WT mice at 120 d (41 total transcripts with more than two-fold change, P < 0.05), male TSLP and TSLP/FcIIb−/− mice at 120 d (98 total transcripts with more than two-fold change, P < 0.05), and male TSLP and female TSLP transgenic mice at 50 d (222 total transcripts with more than two-fold change, P < 0.05). Among the genes with altered expression, two genes involved in the matrix regulatory and fibrinolytic pathways, PN-1 and tPA, were dramatically upregulated in TSLP mice.
The level of PN-1 in female TSLP transgenic mice was upregulated approximately three-fold compared with female control mice, but the expression level of PN-1 in male TSLP transgenic mice did not reach statistical significance compared with male WT mice; however, PN-1 expression in male TSLP/FcIIb−/− mice was upregulated >20-fold compared with male TSLP transgenic mice. In our model of MPGN, the female transgenic mice demonstrate more rapid onset of severe disease, and microarray analysis showed an eight-fold increase in the expression of PN-1 and approximately 7.5-fold higher levels of PAI-1 in females versus males at 50 d of age. tPA expression in male TSLP/FcIIb−/− mice was upregulated >30-fold compared with male TSLP transgenic mice but was not significantly upregulated in male or female TSLP mice compared with WT (Table 1).
Table 1.
Fold change of protease inhibitor and activator genes significantly increased in TSLP Tg and TSLPTg FcIIb−/− mice (more than two-fold change, P < 0.05)a
| Parameter | Gene Symbol | TSLP/WT
|
TSLP FcIIb−/−/TSLP, Males | |
|---|---|---|---|---|
| Females | Males | |||
| tPA | Plat | NS | NS | 30.64 |
| Serine protease inhibitor, clade E, PN-1 | SerpinE2 | 2.89 | NS | 23.07 |
| Cathepsin S | Ctss | 2.82 | NS | 6.01 |
| PAR-1 | F2r | NS | NS | 5.30 |
| Coagulation factor II (thrombin) receptor | F2r | NS | NS | 5.30 |
| Kallikrein 8 | Klk8 | NS | 4.44 | 3.53 |
PAR-1, proteinase activated receptor-1.
Upregulation of PN-1 mRNA in Mesangial Cells of TSLP Transgenic and TSLP/FcγIIb−/− Mice
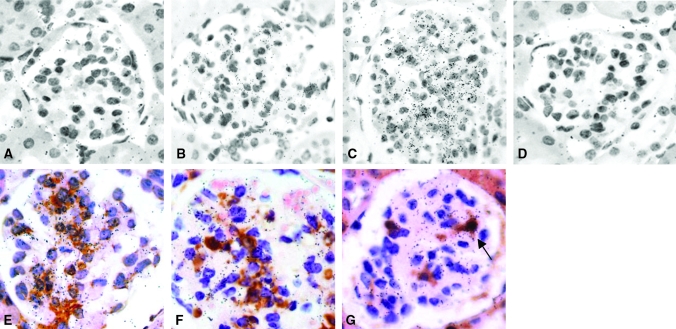
We confirmed the increased tissue expression of PN-1 mRNA by ISH on relevant tissue sections. WT mice demonstrated minimal expression of PN-1 mRNA in glomeruli (Figure 2A), with increased expression in TSLP transgenic mice (WT mice 0.3 ± 0.5 versus TSLP transgenic mice 1.4 ± 0.7, P < 0.001; Figure 2B), and greatest expression in glomeruli of TSLP/FcIIb−/− mice (2.8 ± 0.4, P < 0.001 [versus TSLP transgenic mice]; Figure 2C). To identify the source of PN-1 in glomeruli as either intrinsic renal cells (mesangial cells) or infiltrating monocyte/macrophages, we combined immunohistochemical labeling of α-smooth muscle actin (α-SMA), a marker of activated mesangial cells,18 and Mac-2, a marker for monocyte/macrophages, with ISH for PN-1 mRNA on renal tissues obtained from TSLP/FcγIIb −/− mice. Most cells that immunolabeled as expressors of α-SMA also expressed PN-1 mRNA (Figure 2D), whereas cells expressing both Mac-2 protein and PN-1 mRNA were a minor subset (<10%; Figure 2, E and F).
Figure 2.
Upregulation of PN-1 mRNA in mesangial cells of TSLP transgenic and TSLP/FcγIIb−/− mice. WT mice demonstrate little expression of PN-1 mRNA in glomeruli (A), with increased expression in TSLP transgenic mice (B), and greatest expression in glomeruli of TSLP/FcIIb−/− mice (C). Negative control hybridized with sense probe (D). In both TSLP mice (data not shown) and TSLP/FcIIb−/− mice (E through G), most of the cells immunolabeled with α-SMA also expressed PN-1 mRNA (E), whereas Mac-2+ monocyte/macrophages expressing PN-1 were a small subset of overall PN-1–expressing cells (F and G, arrow). (G) Negative control hybridized with the sense probe. Magnification, ×400.
Mesangial Expression of PN-1, tPA, and PAI-1 Proteins Is Increased in TSLP Transgenic and TSLP/FcγIIb−/− Mice
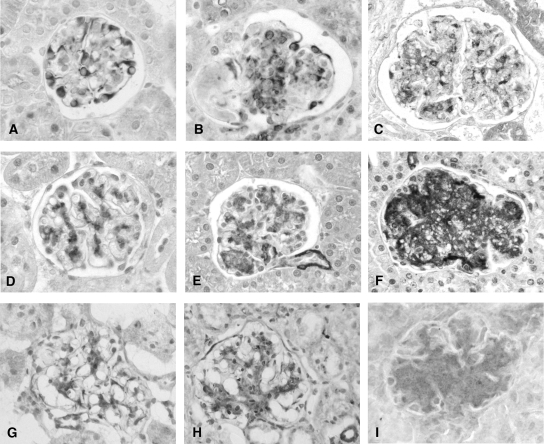
Localization of PN-1 and of tPA and its inhibitor PAI-1 was assessed by IHC on relevant tissue sections. PN-1 was localized to podocytes in WT mice (Figure 3A), with faint to absent staining of mesangial areas. In TSLP transgenic mice, additional de novo PN-1 expression was strong in mesangial areas and persisted in podocytes (Figure 3B). Mesangial expression of PN-1 was further increased in TSLP/FcIIb−/− mice (Figure 3C). Specificity of the PN-1 antibody was confirmed by immunoblotting of whole-kidney tissue lysates, yielding an expected band at approximately 45 kD, as well as antibody absorption with purified mouse PN-1. Mesangial expression of tPA protein was increased in TSLP transgenic mice (Figure 3D) compared with WT mice (Figure 3E), and the increased expression was more pronounced in TSLP/FcIIb−/− mice (Figure 3F). Focal expression of PAI-1 protein in WT mice was present in mesangial areas (Figure 3G) and was upregulated in TSLP transgenic mice Figure 3H) and more so in TSLP/FcIIb−/− mice (Figure 3I). Semiquantitative scoring of the immunohistochemistry results are presented in Table 2.
Figure 3.
Mesangial expression of PN-1, tPA, and PAI-1 proteins were increased in TSLP transgenic and TSLP/FcγIIb−/− mice. PN-1 is localized to podocytes in WT mice (A), and de novo mesangial expression is present in TSLP transgenic mice (B) and further upregulated in TSLP/FcIIb −/− mice (C). Mesangial expression of tPA protein was increased in TSLP transgenic mice (E) compared with WT mice (D) and was even more pronounced in TSLP/FcIIb−/− mice (F). Focal PAI-1 protein expression in WT mice was present in mesangial areas (G), which was upregulated in TSLP transgenic mice (H) and more so in TSLP/FcIIb−/− mice (I). Magnification, ×400.
Table 2.
IHC resultsa
| Parameter | PN-1 | tPA | PAI-1 |
|---|---|---|---|
| WT | 0.7 ± 0.2 | 0.9 ± 0.1 | 0.7 ± 0.2 |
| TSLP | 1.4 ± 0.4b | 2.1 ± 0.2b | 2.3 ± 0.4b |
| TSLP/FcIIb−/− | 2.3 ± 0.5c | 3.6 ± 0.2c | 3.2 ± 0.4c |
Semiquantitative scoring of glomerular expression of PN-1, tPA, and PAI-1.
P < 0.05 versus WT mice.
P < 0.05 versus TSLP mice.
Upregulation of PN-1 mRNA and of PN-1, tPA, and PAI-1 Proteins in Mesangial Cells In Vitro
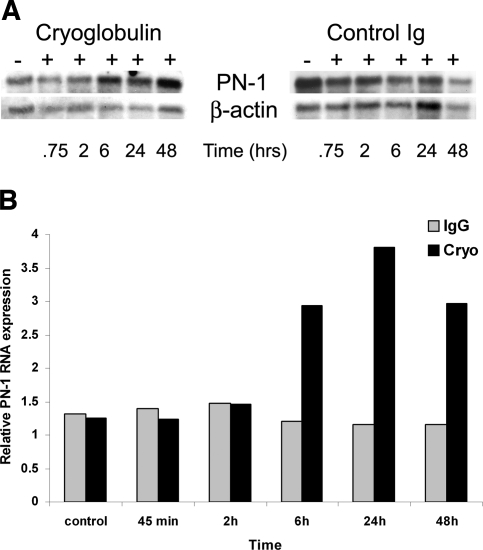
Mouse mesangial cells incubated with mouse IgG demonstrated no significant change of PN-1 mRNA expression by Northern analysis compared with mesangial cells without such exposure (Figure 4A). In contrast, there was upregulation of PN-1 in mesangial cells treated with cryoglobulin 6 h after exposure (2.4-fold higher than time-matched control), peaking at 24 h (3.3-fold higher than time-matched control; Figure 4B). The size of PN-1 was approximately 410 bp, consistent with data previously published.15
Figure 4.
Upregulation of PN-1 RNA in mesangial cells exposed to cryoglobulins. (A) Northern blot analysis. Mouse mesangial cells were incubated with cryoglobulin or mouse IgG (control Ig) for the indicated time. RNA extracted from each was analyzed by Northern blot (10 μg/lane) with a cDNA probe specific for PN-1. (B) PN-1 RNA expression. Mouse mesangial cells incubated with cryoglobulins demonstrate upregulated PN-1 RNA expression, increasing of 2.6-fold over control at 24 h. Upregulation became apparent at 6 h and peaked at 24 h. Mesangial cells incubated with control mouse IgG showed no significant change of PN-1 RNA expression. Values are normalized for differences in RNA loading.
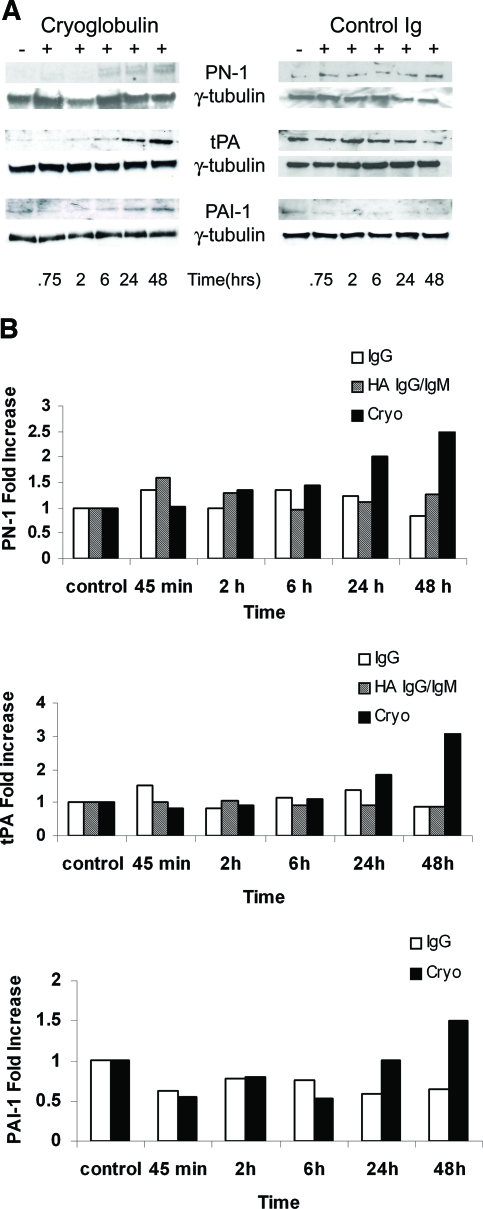
Upregulation of PN-1, tPA, and PAI-1 protein was demonstrated by Western blotting of lysates from mesangial cells treated with cryoglobulin (Figure 5A). Upregulation of each of these proteins became apparent at 6 to 24 h of the study and was maximal at 48 h, whereas no significant increase in these proteins was observed in mesangial cells incubated with mouse IgG (Figure 5B). Experiments performed with heat-aggregated (HA) IgG/IgM also demonstrated no significant change in expression of either PN-1 or tPA (Figure 5).
Figure 5.
Upregulation of PN-1, tPA, and PAI-1 protein by cryoglobulin in WT mesangial cells. (A) Western blot analysis. Mouse mesangial cells were incubated with cryoglobulins or HA IgG/IgM or mouse IgG (control Ig) for the indicated time. Cryoglobulin-induced upregulation of PN-1, tPA, and PAI-1 proteins became apparent at 24 h and maximal at 48 h. No significant increase in PN-1 or tPA was observed in mesangial cells incubated with HA-IgG/IgM (shown) or mouse IgG. Similarly, no increase was seen in PAI-1 expression after incubation with mouse IgG. γ-Tubulin was used as an internal control to ensure equal protein loading. (B) PN-1, tPA, and PAI-1 expression. Mesangial cells incubated with cryoglobulins demonstrated upregulated expression levels for PN-1 (2.8-fold), tPA (3.1-fold), and PAI-1 (1.5-fold) compared with control. Values were normalized for differences in protein loading.
Expression of TGF-β1 mRNA and Protein by Mesangial Cells Incubated with Cryoglobulin or Mouse Ig
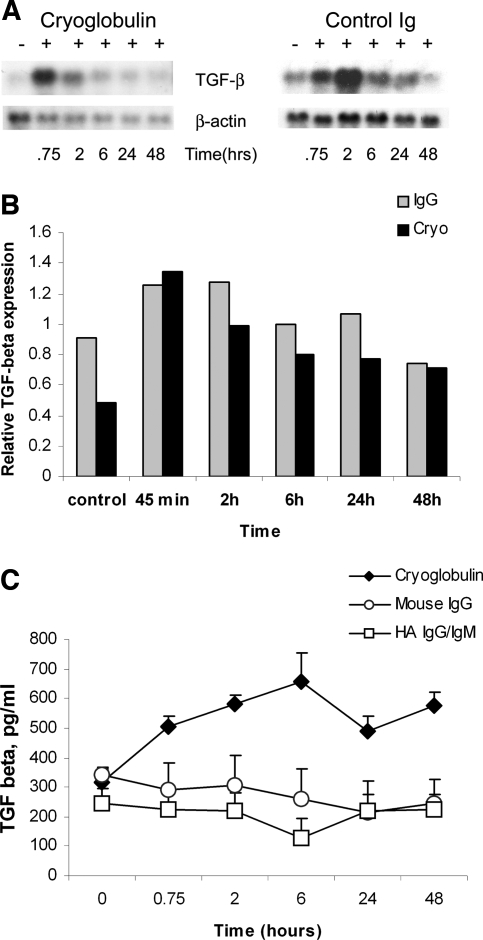
Incubation of cultured mouse mesangial cells with cryoglobulins caused a strong increase in TGF-β1 mRNA at 45 min, which gradually decreased during a 48-h period. Mesangial cells incubated with mouse IgG also showed upregulation of TGF-β1 mRNA at 45 min, which peaked at 2 h, and then decreased gradually (Figure 6, A and B).
Figure 6.
Expression of TGF-β1 mRNA and protein by mesangial cells incubated with cryoglobulin or mouse IgG. (A) Northern blot analysis. Mouse mesangial cells were incubated with cryoglobulin or control mouse IgG (control Ig) for the indicated time. Both cryoglobulin and mouse IgG induced a strong increase in TGF-β1 mRNA. Cryoglobulin induced a strong increase in TGF-β1 mRNA at 45 min, which gradually decreased up to 48 h. In contrast, mesangial cells incubated with mouse IgG showed upregulation of TGF-β1 mRNA at 45 min, which peaked at 2 h, and then decreased. (B) TGF-β1 RNA expression. There are no significant differences seen in expression levels of TGF-β1 resulting from exposure to cryoglobulins or IgG; values are normalized for equal RNA loading. (C) Analysis of conditioned medium derived from mouse mesangial cells incubated with cryoglobulin or HA IgG/IgM or mouse IgG by an ELISA specific for TGF-β1. Incubation with cryoglobulin induced increased release of TGF-β1 into culture medium as compared with control Ig. Values are the average of two experiments.
The level of TGF-β1 protein released by the mesangial cells was measured by TGF-β1–specific ELISA. Murine mesangial cells treated with cryoglobulins but not mouse IgG or HA IgG/IgM demonstrated a significant increase in secretion of TGF-β1 into the culture medium after 2 h of incubation (P < 0.05 versus baseline), peaking at 6 h (approximately two-fold; P < 0.01 versus baseline; Figure 6C). These results were averaged from two independent experiments for both cryoglobulin and HA IgG/IgM and mouse IgG treatment.
DISCUSSION
Three principal findings emerged from this study: (1) Markedly upregulated glomerular expression of the serpin PN-1 was identified by global microarray analysis and confirmed by Northern and Western blotting and by ISH and IHC in the TSLP model of cryoglobulinemic MPGN; (2) increased mesangial expression of tPA and PAI-1, also regulators of the proteases engaged in matrix proteolysis and fibrinolysis, were also demonstrated, with expression even more pronounced in combined TSLP/FcIIb−/− mice that have aggravated MPGN; and (3) upregulated production of these molecules and TGF-β1 was induced by cryoglobulins in murine mesangial cells in vitro.
PN-1 was identified previously as having a potential role in another model of murine cryoglobulinemic glomerulonephritis but has received no subsequent attention in studies of renal injury.15 We focused on PN-1 and related family members because of their marked upregulation in the TSLP mice as detected by gene array analysis.
We confirmed the increased tissue expression of PN-1 mRNA by ISH in diseased glomeruli. PN-1 mRNA expression was most striking in TSLP/FcIIb −/− mice, in which glomerular injury is accentuated, and the increased intensity of glomerular expression of PN-1 mRNA demonstrated by ISH corresponded well to the data obtained by microarray analysis. Using combined ISH and IHC techniques, we demonstrated that mesangial cells are the major source of PN-1 mRNA in this model of glomerulonephritis.
PAI-1 is the best characterized serine protease inhibitor, and its role in renal interstitial fibrogenesis has been established in models of PAI-1–deficient mice7 and in mice that overexpress PAI-1.6 Upregulation of PAI-1 in diseased glomeruli has also been well documented in experimental immune complex–mediated glomerulonephritis4,5,19,20 and in human glomerulonephritis21 and was observed in the mesangium of TSLP transgenic mice. PAI-1 can be induced by TGF-β1 in rodent kidneys and in cultured mesangial cells.22,23 TGF-β1 transgenic mice developed expanded ECM, which was accompanied by increased levels of PAI-1 in vivo.22 We previously reported upregulation of TGF-β1 mRNA in glomeruli by ISH that parallels the accumulation of ECM proteins in TSLP mice24 and now show that this correlates with upregulated PAI-1 expression. We suggest that PAI-1 plays an important role in the control of glomerular matrix accumulation in this murine model of MPGN as in other renal injuries and that the mechanisms of the PAI-1–related glomerular ECM production might be mediated through cytokines such as TGF-β1 released from intraglomerular macrophages and/or mesangial cells. PN-1 and PAI-1 have overlapping activities in this setting, and both likely contribute to the chronic mesangial injury of MPGN.
Microarray analysis and immunohistochemical studies of diseased kidneys also demonstrated upregulated tPA gene and protein expression in glomeruli of TSLP transgenic and TSLP/FcIIb −/− mice. tPA is inhibited by PAI-1 and PN-1, which leads to multiple events, including accumulation of fibrin, by inhibiting the generation of plasmin from plasminogen. It is something of a puzzle that tPA expression is upregulated in diseased glomeruli in TSLP transgenic mice, concurrent with its inhibitors PN-1 and PAI-1. It is possible that a balance of their net effects on plasmin activation helps to determine the extent of mesangial matrix accumulation in MPGN, but testing of this hypothesis awaits development of methods to measure the actual activities of these enzymes at the sites of glomerular injury.
The in vitro studies identified one mechanism for the mesangial upregulation of PN-1, tPA, and PAI-1 observed in vivo. Mouse mesangial cells incubated with cryoglobulins but not control Ig demonstrated upregulation of PN-1 mRNA and of PN-1, tPA, and PAI-1 proteins, correlating with the findings in vivo. Mouse mesangial cells incubated with cryoglobulins also demonstrated a significant increase in TGF-β1 gene expression, in accordance with our previous demonstrations of glomerular upregulation of TGF-β1 in TSLP transgenic and TSLP/FcIIb −/− mice.24,25 We also observed a significant increase in TGF-β1 mRNA in mesangial cells incubated with mouse IgG, a finding of unknown significance; however, further studies demonstrated that mesangial cells incubated with cryoglobulin secreted approximately 50% more TGF-β1 protein into the culture medium compared with the baseline, with no increased secretion of TGF-β1 protein by mesangial cells exposed to control mouse Ig.
In conclusion, we have shown that PN-1 is constitutively expressed by podocytes and identified a potential new role for PN-1 in MPGN by establishing its upregulation at mRNA and protein levels, localizing its increased expression largely to mesangial cells, and documenting its coordinated upregulation with another serpin family member, PAI-1, and other molecules that are important in the regulation of plasmin. The functional similarities of PN-1 to PAI-1 led to in vitro studies establishing that cryoglobulin deposition in the mesangium may be sufficient to initiate these patterns of protein expression, as well as to increase expression of TGF-β1, another key mediator of the matrix accumulation that characterizes MPGN. These studies provide the first mechanistic demonstration that these specific events in cryoglobulinemic glomerulonephritis may result directly from cryoglobulin deposition. We suggest that PN-1 is an underrecognized mediator of progressive glomerular injury and may, like PAI-1, be important in glomerulonephritides other than cryoglobulinemic MPGN.
CONCISE METHODS
Animals and RNA Extraction
The experimental protocol was reviewed and approved by the Animal Care Committee of the University of Washington in Seattle. The establishment of TSLP transgenic mice and combined TSLP transgenic FcγIIb receptor knockout mice (TSLP/FcγIIbR−/−) has been previously described.25,26
Three female and three male mice per experimental group (WT, TSLP transgenic, and TSLP/ FcγIIbR−/−) were killed at 50 d (females) and 120 d (males). These time points were chosen because female mice demonstrate faster progression of the disease compared with male mice and because renal pathology reaches a plateau at the times chosen for each gender with increasing mortality as mice age further.25,26 Although the rate at which disease evolves differs between genders, the fully developed disease is indistinguishable between males and females. At the end of the study, mice were anesthetized, kidneys were collected, and glomeruli were isolated from each mouse by a standard sieving method. The purity of the glomerular preparations was verified to be >85% by visual examination. Total glomerular RNA was isolated from each sample using the Qiagen RNeasy extraction protocol (Qiagen, Valencia, CA).
cDNA Microarrays and Data Analysis
Microarray assays were performed at the Center for Expression Array of the University of Washington (Seattle, WA). Initial quality control analysis (purity and integrity of RNA) was done using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Glomerular RNA from three mice per group were pooled and used for microarray analysis. Three micrograms of pooled total glomerular RNA was amplified, labeled, and fragmented following the Affymetrix eukaryotic target labeling protocol (Affymetrix, Santa Clara, CA). Samples were hybridized to an Affymetrix MG-U74Av2 GeneChip array containing 12,000 genes and processed according to standard Affymetrix procedures. Image processing and expression analysis were performed using Affymetrix MAS 5.0 software. The raw data were uploaded to the Rosetta Resolver System (Rosetta Biosoftware, Seattle, WA) Groups compared were WT versus TSLP transgenic and TSLP transgenic versus TSLP/FcγRIIb−/−, with statistical cuts of P < 0.05 considered indicative of differential expression. In accordance with proposed Minimum Information About a Microarray Experiment standards,27 raw data, including sample information, intensity measurements, error analysis, microarray content, and slide hybridization conditions, will be made available at the Expression Array Manager web site (http://expression.microslu.washington.edu).
IHC
Tissues from WT, TSLP transgenic, and TSLP/FcγRIIb−/− mice used in previous studies25,26 at identical time periods as those described were used for the immunohistochemical studies. Four-micrometer sections of frozen or methyl Carnoy–fixed, paraffin-embedded tissue were immunostained as described previously.24,28 The antibodies used were (1) anti-rat PAI-1 (American Diagnostica, Greenwich, CT); (2) anti-mouse tPA (American Diagnostica); (3) anti–Mac-2 (Cedarlane, Hornby, ON, Canada29); (4) anti–α-SMA, clone 1A4 (Sigma, St. Louis, MO); and (5) anti–PN-1 (R&D Systems, Minneapolis, MN). Negative controls for IHC included both substitution of the primary antibody with an irrelevant IgG or antisera from the same species and substitution with PBS. Specificity of the PN-1 antibody was established by antibody absorption using recombinant mouse PN-1 (R&D Systems) as previously published30 and by Western blots of whole-kidney lysates. Glomerular staining for tPA, PAI-1, and PN-1 was scored semiquantitatively from 0 to 4 as described previously and illustrated.24,28
ISH
Reverse transcription–PCR (RT-PCR) was performed using the Qiagen OneStep RT-PCR Kit, using the 3′ primer (5′-TAATGCCAAGGGCTTTCAGT-3′) and the 5′ primer (5′-CCTCTGCCTCTGAGTCCATC-3′), based on the published mouse PN-1 mRNA gene sequence (Genebank X70296)
The resulting PN-1 cDNA was cloned using the pGEM-T Vector System (Promega, Madison, WI), and the construct was used for ISH and Northern blotting as described previously.31 Negative controls included hybridization performed on replicate tissue sections using the sense riboprobe. The intensity of intraglomerular expression for each transcription was graded semiquantitatively as follows: 0, absent; 1, mild (<5 positive cells); 2, moderate (5 to 10 positive cells in a segmental distribution within the glomerulus); and 3, severe (>10 positive cells in a diffuse distribution) as described previously and illustrated.24
For identification of which cell types were the source of PN-1 mRNA in glomeruli, combined IHC and ISH was performed as described previously32 with IHC for markers specific for monocyte/macrophages (Mac-2) or for activated mesangial cells (α-SMA).18
Preparation of Murine Mesangial Cells and Experimental Protocols
Mesangial cells were derived from WT mice by a standard sieving method and characterized as described previously.33,34 A total of 2 × 104 cells were plated in six-well dishes and incubated in growth medium containing 20% FBS. Cell growth was arrested in medium containing 0.2% FBS for 48 h. Cryoglobulins were obtained from TSLP transgenic mice as described previously,26 washed, and diluted with cold sterilized PBS. These were kept at 4°C overnight, and the cryoprecipitates were confirmed on the next day. The precipitates were dissolved by warming to 37°C, and the concentration of solubilized protein was determined. HA IgG/IgM was prepared using a solution of 50% purified mouse IgG and 50% purified mouse IgM (both from Sigma), heating to 60°C for 10 min, and cooling on ice. WT mouse mesangial cells were incubated with 28 μg/ml cryoglobulins, HA IgG/IgM, or control purified mouse IgG in saline for varying time periods (45 min and 2, 6, 24, and 48 h). The concentration was chosen to approximate the circulating level of cryoglobulins in our model (unpublished observations) and in another mouse model of autoimmune glomerulonephritis with cryoglobulinemia.35
Measurement of TGF-β1 protein
Mesangial cells were treated with cryoglobulins, HA IgG/IgM, or mouse IgG; the medium was removed; and the amount of TGF-β1 protein secreted into the medium was measured using the Quantikine mouse/rat/porcine TGF-β1 Immunoassay Kit (R&D Systems). Subsequently, the cells were lysed, and total protein or total RNA was extracted and used in the Western and Northern blotting experiments.
Western Blot Analysis
Total cellular protein isolated from treated mesangial cells was used in Western blots performed as described previously34,36 using the same antibodies as used for IHC. Relative expression of each protein versus γ-tubulin expression was measured using NIH Image J software.
Northern Blotting
Total RNA was isolated using the Qiagen RNeasy Kit (Qiagen). Biotin-labeled probes to PN-1, TGF-β1,37 and β-actin (pTri-β-actin-mouse; Ambion, Austin, TX) were transcribed using the Detector RNA in vitro transcription Biotinylation kit (KPL, Gaithersburg, MD). After hybridization and high-stringency washes, the biotin-labeled probe was detected with streptavidin conjugated to alkaline phosphatase (KPL) and CDP star (KPL), and the membrane was exposed to x-ray film. Relative expression of each RNA transcript versus β-actin expression was measured using NIH Image J software.
Statistical Analyses
All values are expressed as means ± SD. Using SPSS 10.0 for Windows (SPSS, Chicago, IL), TGF-β1 concentration in medium was examined with one-way ANOVA after Tukey post hoc test. The microarray data were analyzed using the Rosetta Resolver System, with statistical significance of changes set at P < 0.05. For other analyses, the means were compared using the nonparametric Mann-Whitney U test between groups.
DISCLOSURES
None.
Acknowledgments
This work was supported by an unrestricted grant from Genzyme and grant DK66802 from the National Institutes of Health.
Portions of this work were presented at the 37th annual meeting of the American Society of Nephrology; October 27 through November 1, 2004; St. Louis MO; and at the 39th annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego CA.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Huang Y, Noble N: An unexpected role of plasminogen activator inhibitor-type 1 (PAI-1) in renal fibrosis. Kidney Int 67: 2502–2503, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Fogo AB: Renal fibrosis: Not just PAI-1 in the sky. J Clin Invest 112: 326–328, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eddy AA: Plasminogen activator inhibitor-1 and the kidney. Am J Physiol Renal Physiol 283: F209–F220, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Feng L, Tang WW, Loskutoff DJ, Wilson CB: Dysfunction of glomerular fibrinolysis in experimental antiglomerular basement membrane antibody glomerulonephritis. J Am Soc Nephrol 3: 1753–1764, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Malliaros J, Holdsworth SR, Wojta J, Erlich J, Tipping PG: Glomerular fibrinolytic activity in anti-GBM glomerulonephritis in rabbits. Kidney Int 44: 557–564, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA: Multifunctionality of PAI-1 in fibrogenesis: Evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67: 2221–2238, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, Eddy AA: PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60: 587–596, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Ingelfinger JR: Forestalling fibrosis. N Engl J Med 349: 2265–2266, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Eddy AA, Fogo AB: Plasminogen activator inhibitor-1 in chronic kidney disease: Evidence and mechanisms of action. J Am Soc Nephrol 17: 2999–3012, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Angles-Cano E, Rondeau E, Delarue F, Hagege J, Sultan Y, Sraer JD: Identification and cellular localization of plasminogen activators from human glomeruli. Thromb Haemost 54: 688–692, 1985 [PubMed] [Google Scholar]
- 11.Ferroni P, Guadagni F: Protease nexin-1: A regulator of vascular disease? J Thromb Haemost 4: 320–321, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Richard B, Pichon S, Arocas V, Venisse L, Berrou E, Bryckaert M, Jandrot-Perrus M, Bouton MC: The serpin protease nexin-1 regulates vascular smooth muscle cell adhesion, spreading, migration and response to thrombin. J Thromb Haemost 4: 322–328, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Scott RW, Bergman BL, Bajpai A, Hersh RT, Rodriguez H, Jones BN, Barreda C, Watts S, Baker JB: Protease nexin: Properties and a modified purification procedure. J Biol Chem 260: 7029–7034, 1985 [PubMed] [Google Scholar]
- 14.Baker JB, Low DA, Simmer RL, Cunningham DD: Protease-nexin: A cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell 21: 37–45, 1980 [DOI] [PubMed] [Google Scholar]
- 15.Moll S, Schaeren-Wiemers N, Wohlwend A, Pastore Y, Fulpius T, Monard D, Sappino AP, Schifferli JA, Vassalli JD, Izui S: Protease nexin 1 in the murine kidney: Glomerular localization and up-regulation in glomerulopathies. Kidney Int 50: 1936–1945, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Kanse SM, Chavakis T, Al-Fakhri N, Hersemeyer K, Monard D, Preissner KT: Reciprocal regulation of urokinase receptor (CD87)-mediated cell adhesion by plasminogen activator inhibitor-1 and protease nexin-1. J Cell Sci 117: 477–485, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Wakida N, Kitamura K, Tuyen DG, Maekawa A, Miyoshi T, Adachi M, Shiraishi N, Ko T, Ha V, Nonoguchi H, Tomita K: Inhibition of prostasin-induced ENaC activities by PN-1 and regulation of PN-1 expression by TGF-beta1 and aldosterone. Kidney Int 70: 1432–1438, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM: Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis: Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 87: 847–858, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moll S, Menoud PA, Fulpius T, Pastore Y, Takahashi S, Fossati L, Vassalli JD, Sappino AP, Schifferli JA, Izui S: Induction of plasminogen activator inhibitor type 1 in murine lupus-like glomerulonephritis. Kidney Int 48: 1459–1468, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Tang WW, Feng L, Loskutoff DJ, Wilson CB: Glomerular extracellular matrix accumulation in experimental anti-GBM Ab glomerulonephritis. Nephron 84: 40–48, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Hamano K, Iwano M, Akai Y, Sato H, Kubo A, Nishitani Y, Uyama H, Yoshida Y, Miyazaki M, Shiiki H, Kohno S, Dohi K: Expression of glomerular plasminogen activator inhibitor type 1 in glomerulonephritis. Am J Kidney Dis 39: 695–705, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Krag S, Osterby R, Chai Q, Nielsen CB, Hermans C, Wogensen L: TGF-beta1-induced glomerular disorder is associated with impaired concentrating ability mimicking primary glomerular disease with renal failure in man. Lab Invest 80: 1855–1868, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Gaedeke J, Noble NA, Border WA: Curcumin blocks multiple sites of the TGF-beta signaling cascade in renal cells. Kidney Int 66: 112–120, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Taneda S, Hudkins KL, Cui Y, Farr AG, Alpers CE, Segerer S: Growth factor expression in a murine model of cryoglobulinemia. Kidney Int 63: 576–590, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Muhlfeld AS, Segerer S, Hudkins K, Carling MD, Wen M, Farr AG, Ravetch JV, Alpers CE: Deletion of the fcgamma receptor IIb in thymic stromal lymphopoietin transgenic mice aggravates membranoproliferative glomerulonephritis. Am J Pathol 163: 1127–1136, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taneda S, Segerer S, Hudkins KL, Cui Y, Wen M, Segerer M, Wener MH, Khairallah CG, Farr AG, Alpers CE: Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am J Pathol 159: 2355–2369, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M: Minimum information about a microarray experiment (MIAME): Toward standards for microarray data. Nat Genet 29: 365–371, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Alpers CE, Hudkins KL, Gown AM, Johnson RJ: Enhanced expression of muscle-specific actin in glomerulonephritis. Kidney Int 41: 1134–1142, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg I, Cherayil BJ, Isselbacher KJ, Pillai S: Mac-2-binding glycoproteins: Putative ligands for a cytosolic beta-galactoside lectin. J Biol Chem 266: 18731–18736, 1991 [PubMed] [Google Scholar]
- 30.Alpers CE, Davis CL, Barr D, Marsh CL, Hudkins KL: Identification of platelet-derived growth factor A and B chains in human renal vascular rejection. Am J Pathol 148: 439–451, 1996 [PMC free article] [PubMed] [Google Scholar]
- 31.Alpers CE, Hudkins KL, Ferguson M, Johnson RJ, Rutledge JC: Platelet-derived growth factor A-chain expression in developing and mature human kidneys and in Wilms’ tumor. Kidney Int 48: 146–154, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Hudkins KL, Le QC, Segerer S, Johnson RJ, Davis CL, Giachelli CM, Alpers CE: Osteopontin expression in human cyclosporine toxicity. Kidney Int 60: 635–640, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Wolf G, Schroeder R, Thaiss F, Ziyadeh FN, Helmchen U, Stahl RA: Glomerular expression of p27Kip1 in diabetic db/db mouse: role of hyperglycemia. Kidney Int 53: 869–879, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Taneda S, Hudkins KL, Topouzis S, Gilbertson DG, Ophascharoensuk V, Truong L, Johnson RJ, Alpers CE: Obstructive uropathy in mice and humans: Potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol 14: 2544–2555, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Kikuchi S, Pastore Y, Fossati-Jimack L, Kuroki A, Yoshida H, Fulpius T, Araki K, Takahashi S, Lemoine R, Reininger L, Izui S: A transgenic mouse model of autoimmune glomerulonephritis and necrotizing arteritis associated with cryoglobulinemia. J Immunol 169: 4644–4650, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Banas MC, Parks WT, Hudkins KL, Banas B, Holdren M, Iyoda M, Wietecha TA, Kowalewska J, Liu G, Alpers CE: Localization of TGF-beta signaling intermediates Smad2, 3, 4, and 7 in developing and mature human and mouse kidney. J Histochem Cytochem 55: 275–285, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Pelton RW, Hogan BL, Miller DA, Moses HL: Differential expression of genes encoding TGFs beta 1, beta 2, and beta 3 during murine palate formation. Dev Biol 141: 456–460, 1990 [DOI] [PubMed] [Google Scholar]