Abstract
Poverty is associated with increased risk of ESRD, but its contribution to observed racial differences in disease incidence is not well-defined. To explore the contribution of neighborhood poverty to racial disparity in ESRD incidence, we analyzed a combination of US Census and ESRD Network 6 data comprising 34,767 patients that initiated dialysis in Georgia, North Carolina, or South Carolina between January 1998 and December 2002. Census tracts were used as the geographic units of analysis, and the proportion of the census tract population living below the poverty level was our measure of neighborhood poverty. Incident ESRD rates were modeled using two-level Poisson regression, where race, age and gender were individual covariates (level 1), and census tract poverty was a neighborhood covariate (level 2). Neighborhood poverty was strongly associated with higher ESRD incidence for both blacks and whites. Increasing poverty was associated with a greater disparity in ESRD rates between blacks and whites, with the former at greater risk. This raises the possibility that blacks may suffer more from lower socioeconomic conditions than whites. The disparity persisted across all poverty levels. The reasons for increasingly higher ESRD incidence among US blacks as neighborhood poverty increases remain to be explained.
The higher incidence of ESRD among ethnic minorities in the United States was first recognized almost three decades ago.1 In 2004, incidence rates in blacks were almost 1000 per million compared with about 260 per million for whites.2 The possibility that the higher ESRD burden among black Americans can be attributed to their disproportionate representation at lower socioeconomic levels has been the subject of a number of investigations3–11 and reviews.12,13 Researchers have found that socioeconomic factors are associated with higher ESRD risk,14–16 perhaps because of barriers to health care (including delayed referral and presentation for ESRD care), lack of availability of healthy foods, exposure to environmental nephrotoxins, or other as yet unspecified factors in impoverished neighborhoods.12
The strength of the contribution of such community socioeconomic factors to the observed racial disparities in ESRD incidence remains inconclusive (Table 1). Contrary to expectations, adjustment for community socioeconomic status (SES) generally resulted in only a modest reduction in black-to-white relative risk for ESRD.3,4,8–11 Studies generally used large-area poverty measures (e.g., average per capita income in the county) to investigate the role of SES in racial disparities. Furthermore, few studies have assessed whether the relationship between community SES and ESRD risk differs for blacks and whites. The purpose of this study was to investigate the role of neighborhood poverty in observed racial differences in ESRD incidence in the southeastern United States using a small-area poverty measure, namely the percentage of the CT population living below poverty level.
Table 1.
Socioeconomic factors in studies of racial differences in ESRD
| Author, Year | Data Source, Population | Type of SES Measures | SES Measures | Findings | Limitations |
|---|---|---|---|---|---|
| Whittle et al., 19913 | Maryland regional ESRD registry, 534 cases of HTN-ESRD | area-based, 13 black and 13 white populations formed by grouped zip codes (20,495 to 420,489 persons) | % completing high school; % with household income of at least $10,000 | ||
| SES was associated with incidence, but did not reduce B:W RR significantly | SES measures from very large geographic areas lack of age-gender adjustment ecological analysis (26 populations) | ||||
| Brancati et al., 19924 | Maryland regional ESRD registry, 442 cases of DM-ESRD | % lacking regular source of health care, insurance, and college education; % with household income ≤$10,000 | |||
| Byrne et al., 19945 | New York Medicare program, 9,390 ESRD cases | area-based, zip code | median family income | ESRD incidence tended to decrease with increasing SES for whites, but not for blacks | crude ESRD rates calculated for each of SES levels |
| Young et al., 19946 | USRDS, 80,172 ESRD cases | area-based, county | average per capita income | B:W RR was decreasing with increasing income (race-income interaction) | SES measures from very large geographic areas |
| Perneger et al., 19957 | Maryland, Virginia, West Virginia, 716 cases and 361 controls | individual | household annual income, health insurance, number of missing teeth | crude B:W OR was 8.1 and decreased to 5.5 after adjusting for income | limited generalizability potential for selection bias |
| Klag et al., 19978 | MRFIT, 332,544 men | area-based, zip code | median family income | similar ESRD-SES associations were present in both races. B:W RRs were 1.59 (NS), 2.47 (S), and 1.62 (NS) for <15,000, 15,000 to 20,000, and ≥20,000 categories | limited generalizability assumed median family income was stable over 17 yr |
| Karter et al., 20029 | Kaiser Permanente, 62,432 diabetics | individual; area-based, census block | education (individual) average income (census block) | age-gender–adjusted estimates of ethnic differences did not differ substantially from those additionally adjusted for SES | limited generalizability (fully insured DM patients) |
| Tarver-Carr et al., 200210 | NHANES, 9,082 adults, 37 ESRD cases | individual | poverty status, educational attainment, marital status | age-gender–adjusted B:W RR was 2.7 and was reduced for just 12% after adjusting for SES (RR = 2.5) | limited power (only 37 cases) |
| Li et al., 200411 | Medicare 5% database, 1,055,236 beneficiaries | area-based, county | median family income | age-gender–adjusted B:W RR reduced from 3.5 to 2.9 after adjustment for SES and comorbidities, and to 2.1 in full model | SES contribution to reduced incidence is hard to assess limited generalizability (population 65 and older) |
MRFIT, Multiple Risk Factors Intervention Trial; HTN, hypertension; DM, diabetes mellitus; NS, not significant; S, statistically significant.
RESULTS
Between January 1, 1998, and December 31, 2002, 36,982 patients initiated dialysis therapy in ESRD Network 6 treatment facilities. Of those, 34,767 (94%) patients were >20 yr of age, had their race indicated on CMS-2728 form as “black” or “white,” and resided in Georgia, North Carolina, or South Carolina. The mean age of the patients was 61 yr and the numbers of males and females were almost equal. Almost 57% of incident ESRD cases were black, compared with the adult population of the three states, which had about 25% blacks.
The unadjusted black-to-white rate ratio (B:W RR) for all-cause ESRD was 3.9 (95% CI 3.8 to 4.0). As expected, age was strongly associated with ESRD incidence; i.e., individuals >60 yr of age were >10 times more likely to develop ESRD compared with those <40 yr of age (Table 2). Females were slightly less likely than males to have ESRD in the unadjusted analysis.
Table 2.
Association between ESRD incidence and individual and neighborhood level factors, univariate and stratified by race
| Predictor | Overall
|
White
|
Black
|
|||||
|---|---|---|---|---|---|---|---|---|
| Population Estimate | Number of ESRD Cases | Incidence Rate per 1,000,000 | Rate Ratio (95% CI) | Incidence Rate per 1,000,000 | Rate Ratio (95% CI) | Incidence Rate per 1,000,000 | Rate Ratio (95% CI) | |
| Individual level | ||||||||
| race | ||||||||
| white | 51,612,985 | 15,020 | 291 | REF | — | — | — | — |
| black | 17,257,135 | 19,747 | 1144 | 3.93 (3.85 to 4.02) | — | — | — | — |
| age | ||||||||
| 20 to 39 | 28,604,015 | 3,596 | 126 | REF | 53 | REF | 307 | REF |
| 40 to 49 | 14,577,885 | 4,664 | 320 | 2.54 (2.44 to 2.66) | 134 | 2.56 (2.37 to 2.77) | 831 | 2.71 (2.57 to 2.85) |
| 50 to 59 | 10,887,730 | 7,052 | 648 | 5.15 (4.95 to 5.36) | 302 | 5.78 (5.38 to 6.21) | 1924 | 6.26 (5.96 to 6.58) |
| 60 to 69 | 7,058,965 | 8,395 | 1189 | 9.46 (9.10 to 9.84) | 663 | 12.69 (11.86 to 13.59) | 3365 | 10.95 (10.44 to 11.50) |
| ≥70 | 7,741,525 | 11,060 | 1429 | 11.36 (10.94 to 11.80) | 976 | 18.69 (17.51 to 19.94) | 3430 | 11.17 (10.64 to 11.71) |
| gender | ||||||||
| male | 32,853,345 | 17,362 | 528 | REF | 336 | REF | 1148 | REF |
| female | 36,016,775 | 17,405 | 483 | 0.91 (0.90 to 0.93) | 249 | 0.74 (0.72 to 0.76) | 1141 | 0.99 (0.97 to 1.02) |
| Neighborhood level | ||||||||
| % below poverty | ||||||||
| <5 | 11,977,395 | 2908 | 243 | REF | 185 | REF | 730 | REF |
| 5 to 9.9 | 19,399,610 | 7182 | 370 | 1.52 (1.46 to 1.59) | 251 | 1.36 (1.29 to 1.43) | 1001 | 1.37 (1.27 to 1.48) |
| 10 to 14.9 | 15,726,865 | 6854 | 436 | 1.79 (1.7 to 1.87) | 295 | 1.60 (1.51 to 1.69) | 958 | 1.31 (1.22 to 1.41) |
| 15 to 19.9 | 9,703,270 | 6063 | 625 | 2.57 (2.46 to 2.69) | 373 | 2.02 (1.90 to 2.14) | 1144 | 1.57 (1.46 to 1.69) |
| 20 to 24.9 | 5,655,325 | 4736 | 837 | 3.45 (3.29 to 3.61) | 473 | 2.56 (2.39 to 2.74) | 1300 | 1.78 (1.66 to 1.92) |
| ≥25 | 6,407,655 | 7024 | 1096 | 4.51 (4.32 to 4.71) | 540 | 2.92 (2.72 to 3.13) | 1453 | 1.99 (1.86 to 2.13) |
REF, reference.
More than 50% of the white population in Georgia, North Carolina, and South Carolina resided in CTs with <10% of persons living below poverty level. In contrast, the corresponding number for blacks was 26%. Almost 40% of blacks lived in CTs with ≥20% of the population living below poverty level, compared with just 11% for whites (Table 3).
Table 3.
Black and white adult population of Georgia, North Carolina, and South Carolina by residential area poverty
| Population of the Census Tracts with % below Poverty Level
|
||||||
|---|---|---|---|---|---|---|
| <5 | 5 to 9.9 | 10 to 14.9 | 15 to 19.9 | 20 to 24.9 | ≥25 | |
| Black | 8% | 18% | 19% | 18% | 14% | 23% |
| White | 21% | 31% | 24% | 13% | 6% | 5% |
Neighborhood poverty was associated with higher all-cause ESRD rates in the crude analysis. Compared with the CTs with <5% of the population below poverty level (“richest”), those with 5% to 9.9% of the population below poverty had 1.5 times higher incidence rate (RR = 1.52, 95% CI 1.6 to 1.59), those with 10% to 14.9% had 1.8 times higher incidence rate (RR = 1.79, 95% CI 1.72 to 1.87), 15% to 19.9% had 2.6 times higher incidence rate (RR = 2.57, 95% CI 2.46 to 2.69), and those with >20% of the population below poverty (federal poverty areas) had >3 times higher incidence rates of all-cause ESRD (Table 2).
After adjustment for individual age and gender (model 1), the estimated B:W RR for all-cause ESRD increased to 5.0 (95% CI 4.8 to 5.1) compared with 3.9 in the crude model. After accounting for between-CT variation in ESRD incidence (model 2), the B:W RR was estimated at 4.4 (95% CI 4.3 to 4.5). The between-CT variance was important (statistically significant), suggesting that the geographic variation in overall ESRD rates can not be fully accounted for by differences in area age, race, and gender composition.
Next, we determined the degree to which the geographic variation in ESRD rates was comparable for blacks and whites (model 3). The estimated B:W RR was 4.3 (95% CI 4.0 to 4.6). The random effect parameter for race by CT was also statistically significant, indicating that the black-to-white disparity in ESRD incidence varied by CT after accounting for age and gender.
Next, we assessed the association of neighborhood poverty and race-poverty with ESRD incidence (model 4). The B:W RR varied across the neighborhood poverty strata; i.e., there was a statistically significant race–poverty interaction on the multiplicative scale (Table 4). For example, in the CTs with <5% population below poverty, B:W RR for ESRD was the largest at 4.7 (95% CI 4.1 to 5.5) and then declined with increasing neighborhood poverty to 3.2 (95% CI 2.8 to 3.6) in CTs with >25% of the population below the poverty level.
Table 4.
Multivariate analyses of the association between race and all-cause ESRD incidence (model 4)
| Fixed effects | Estimate | SEM | P Value |
|---|---|---|---|
| Intercept | −7.5664 | 0.0919 | <0.0001 |
| Race | 1.1548 | 0.0626 | <0.0001 |
| Age, yr | |||
| 20 to 40 | −2.6159 | 0.0198 | <0.0001 |
| 40 to 50 | −1.6367 | 0.0179 | <0.0001 |
| 50 to 60 | −0.8188 | 0.0156 | <0.0001 |
| 60 to 70 | −0.1893 | 0.0147 | <0.0001 |
| Gender | −0.2968 | 0.0108 | <0.0001 |
| Neighborhood poverty, % below poverty level | |||
| <5 | −0.8893 | 0.1241 | <0.0001 |
| 5 to 9.9 | −0.6920 | 0.1102 | <0.0001 |
| 10 to 14.9 | −0.4698 | 0.1115 | <0.0001 |
| 15 to 19.9 | −0.3574 | 0.1201 | 0.0029 |
| 20 to 24.9 | −0.0957 | 0.1326 | 0.4702 |
| Race-poverty product term | |||
| <5 | 0.3995 | 0.0883 | <0.0001 |
| 5 to 9.9 | 0.3915 | 0.0725 | <0.0001 |
| 10 to 14.9 | 0.3059 | 0.0715 | <0.0001 |
| 15 to 19.9 | 0.1609 | 0.0747 | 0.0312 |
| 20 to 24.9 | 0.1880 | 0.0809 | 0.0203 |
| Random Effects | Estimate | SEM | P Value |
|---|---|---|---|
| Random intercept variance (census tract random effect) | 3.0569 | 0.1197 | <0.0001 |
| Random slope variance (race random effect) | 0.4198 | 0.0266 | <0.0001 |
| Covariance of random slope and intercept | −0.0628 | 0.0579 | 0.2784 |
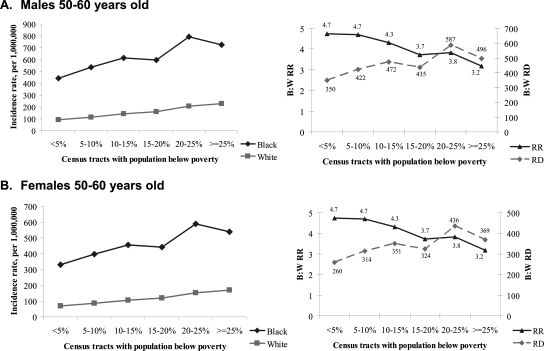
The model-derived estimates of ESRD incidence by race and CT poverty level for males and females aged 50 to 60 yr (typical age of ESRD onset) are shown in Figure 1. The rate patterns show that higher neighborhood poverty is associated with progressively higher ESRD incidence for both blacks and whites. However, for blacks greater increases in ESRD rates are observed as neighborhood SES declines compared with whites. For example, among black males aged 50 to 60 yr, residence in neighborhoods with >25% of the population below poverty compared with neighborhoods with <5% of residents below poverty was associated with 280 excess ESRD cases per million. In contrast, for white males of the same age, the excess ESRD risk was 134 per million (Figure 1A).
Figure 1.
Model-predicted ESRD incidence rates by neighborhood poverty and by race. RR, risk ratio; RD, risk difference.
DISCUSSION
Our main observation is that the association between neighborhood poverty and ESRD incidence differs for blacks and whites. Among blacks, neighborhood poverty was associated with a greater excess ESRD incidence than for whites. These observations suggest that if factors associated with neighborhood poverty were causal, the elimination of these factors would benefit black population more than white population with respect to ESRD incidence reduction, and the absolute difference in ESRD rates between blacks and whites would be reduced. For example, if individuals living in neighborhoods with >25% of the population below the poverty level had in fact lived in neighborhoods with <5% of the population below poverty, the estimated difference in ESRD rates between blacks and whites would be reduced from 496 to 350 per million in males aged 50 to 60 yr and from 369 to 260 per million in females aged 50 to 60 yr (Figure 1, A and B).
Of note, although under such conditions the absolute difference in rates between blacks and whites would be decreasing, the black-to-white rate ratio would, by contrast, be increasing slightly as community poverty decreases (Figure 1, A and B, right). Finally, our study suggests that even though the absolute difference in ESRD rates between blacks and whites decreases with decreasing poverty, the racial disparity persists even in the least impoverished neighborhoods.
Our finding that neighborhood poverty is associated with increased ESRD incidence is supported by a number of earlier studies.3,4,6 However, the studies that examined this association by race came to varying conclusions. In the study by Byrne, Netelman, and Luke,5 an association of ESRD incidence with SES, as measured by median income at the zip code of residence, was demonstrated for whites but not for blacks. These results are contrary to our observation of a strong association between ESRD rates and economic deprivation of the area of residence among both racial groups. In contrast, Young et al.6 found increasing absolute difference in ESRD incidence between blacks and whites with worsening economic conditions in the county of residence. Important limitations to both of these studies included using large heterogeneous geographic areas (counties and zip codes) to define neighborhood poverty and failure to account for clustering of subjects within these geographic areas. Also contrary to our findings was an investigation of the Atherosclerosis Risk in Communities (ARIC) study population that reported that the association of neighborhood SES with chronic kidney disease was weaker and less consistent among blacks compared with whites.17
A number of potential explanations may be offered for the patterns observed in our study. The persistence in black-to-white differences in ESRD incidence within the same level of community poverty may reflect the residual effects of income differences. For example, even within the same CT, blacks may be more economically disadvantaged than whites (i.e., may have lower income and lower wealth). We were unable to adjust for individual SES in our models, and the association of neighborhood poverty with ESRD rates and the residual racial disparities may have been diminished if we had done so.
We do not think, however, that the additional adjustment for current personal income would fully explain the persisting racial disparity in ESRD incidence within communities of similar degrees of poverty. Kidney failure develops over some period of time and socioeconomic effects likely accumulate throughout life.18 Thus, adjusting for earlier life socioeconomic position in addition to current SES might lend further insight into the role of SES in racial disparities in ESRD incidence.
It is possible that there are individual factors other than age, gender, and individual economic status that might confound the associations of both race and neighborhood poverty with ESRD incidence, such as a range of health-seeking and risky behaviors (smoking, drug use, etc.). Without information on these factors, a potential for bias caused by uncontrolled confounding can not be eliminated.
Moreover, even with comparable individual SES, access to health care, and comparable health care–seeking behaviors for blacks and whites, racial differences in quality of delivered care may exist within communities. For example, there are documented racial differences in the detection, treatment, and control of hypertension19 and diabetes20,21 as well as secondary prevention of cardiovascular disease,22 all conditions associated with increased risk of ESRD. Variations in the control of these conditions across communities would also not be unexpected, as rural/urban differences have been reported,23 and substantial differences in patterns of health care across communities have been extensively documented.24,25 The role of these factors in racial difference in ESRD incidence remains to be described.
The differences in prevention in addition to lifestyle differences would result in differential prevalence of hypertension and diabetes in blacks and whites, which is recognized in the literature. For example, NHANES I to III found that age-adjusted prevalence of hypertension in blacks was significantly higher than in whites (23% versus 13% in NHANES III).26 The gender- and age-adjusted prevalence of physician-diagnosed diabetes in adults >20 yr old was 8.2% for blacks and 4.8% for whites in NHANES III.27 The combination of diabetes and hypertension is about three times more common in blacks than in whites. This differential prevalence of hypertension and diabetes certainly suggests the higher risk of ESRD development in blacks. We were unable to control for individual hypertension and diabetes history because these data were not available to us. This is certainly a limitation of the study. However, a number of earlier studies investigating whether higher prevalence of these conditions in blacks was the major factor accounting for their higher ESRD risk came to a similar conclusion: Racial differences in ESRD incidence can not entirely be explained by differences in the prevalence of diabetes and hypertension.3,4,28,29
In addition to the potential for uncontrolled confounding by individual SES, diabetes, and hypertension, and possibly other individual level factors as described above, this study may also be limited by the fact that administrative units (CTs) may be not an ideal proxy for residential neighborhood. Furthermore, the study design does not allow us to address the issue of residential mobility or residential selection.
Another limitation of our study is that analysis was performed on the data from three southeastern states, and thus the results should be generalized with caution. We were not able to perform analysis using the nation-wide data because we only had access to the geocoded Network 6 ESRD surveillance data set. Geocoding all ESRD surveillance data would prove useful for future health disparities research.
The strength of our study is that we used population-based surveillance data from the three southern states, and thus the population was large and minorities were well represented. We used CTs as geographic units of analysis as opposed to larger areas, such as counties or zip codes. CTs are more homogeneous on social factors likely to confound the relationship between ESRD incidence and race (i.e., possibility for residual confounding is diminished) and are more appropriate proxies for social context and environmental exposures. The multilevel modeling approach used in this study incorporated information from subjects and CTs into a single analysis, providing the estimates that are adjusted for clustering of individuals within neighborhoods.
In conclusion, we found that neighborhood poverty was strongly associated with ESRD incidence in both blacks and whites. Furthermore, racial disparity in ESRD incidence (absolute difference in ESRD rates between blacks and whites) was more pronounced in poorer neighborhoods. Finally, racial disparity in disease incidence persisted across all levels of neighborhood poverty, even in the least impoverished neighborhoods. Further research is needed to investigate the pathways by which socioeconomic factors may affect ESRD risk in blacks and whites and how this effect may be ameliorated. Identifying potentially modifiable neighborhood factors affecting ESRD risk in blacks and whites is appealing because it might result in developing and targeting prevention strategies that are more practical and easier to implement than individual level interventions.
CONCISE METHODS
Selection of Geographic Unit and Socioeconomic Measure for Analysis
We selected CTs as the geographic unit of analysis. CTs are small (4000 inhabitants on average), relatively permanent statistical subdivisions of counties that are “designed to be homogeneous with respect to population characteristics, economic status, and living conditions”30 and are used by local and federal governments as administrative units for program planning and resource allocation. Consequently, CTs have been used as the unit of analysis in a number of studies of neighborhood effects on health.31–33
The measure of CT economic deprivation selected for this analysis was percentage of its population living below the poverty level. Previous research showed that this measure meaningfully summarized important aspects of the neighborhood socioeconomic conditions and consistently detected socioeconomic gradients across a wide range of health outcomes.32–35 To determine the poverty level, the Census Bureau uses a set of money income thresholds that vary by family size and composition. For example, the poverty level for a family of two adults and two children was $16,895 in 1999. Of note, the Census Bureau defines poverty areas as CTs or block numbering areas where at least 20% of residents live below the poverty level.
Data
Deidentified data on all incident patients who started renal replacement therapy in Georgia, North Carolina, and South Carolina dialysis facilities between January 1998 and December 2002 were obtained from ESRD Network 6. Network 6 is a part of a national system of ESRD networks maintaining the universal population-based ESRD registry for monitoring quality of the Medicare ESRD Program.
We used demographic data from the Centers for Medicare & Medicaid Services (CMS) Medical Evidence Report form (CMS-2728)36 completed by dialysis facilities within the network on all incident dialysis patients. Data included gender, race, age, and residential address, which was geocoded (converted to latitude and longitude) so that each patient could be placed in the appropriate CT. Data on race could be either self-reported by the patient or selected by the staff completing CMS-2728 form. The incident patient counts cross-tabulated by CT, age, race, and gender constituted the numerator data for rates calculation.
Age-race-gender-specific population estimates for each CT in Georgia, North Carolina, and South Carolina for the year 2000 were obtained from the US Census Bureau Summary File 1.30 We used these estimates to derive the denominators for rates calculation. Of note, race is self-reported in the US Census. Finally, for each CT we derived the proportion of population living below the poverty level in 1999 using the data from the US Census Bureau Summary File 3.30
Exclusions
We excluded patients <20 yr of age and those whose race was neither black nor white because of the relatively small number of patients in these age and race categories. The ArcView Geographic Information System (Environmental Systems Research Institute, Inc., Redlands, CA) was used to allocate patients to CTs using their geocoded residential addresses. We excluded patients who began dialysis in Network 6 facilities but who resided in CTs outside of Georgia, North Carolina, or South Carolina.
Statistical Analyses
Eligible incident ESRD patients were grouped into 80,960 strata corresponding to five age groups (20 to 39 yr, 40 to 49 yr, 50 to 59 yr, 60 to 69 yr, and ≥70 yr), two gender groups, and two race groups within each of the 4048 CTs in three states. The population of the states in 2000 was also stratified by these same factors. The CT-age-race-gender strata with zero population counts (i.e., no residents) in 2000 were excluded, resulting in a total of 75,363 cells. Each cell had the number of incident ESRD cases in 1998–2002 in the numerator and 2000 US Census population estimates multiplied by 5 yr of observation in the denominator.
The main outcome of interest was all-cause ESRD incidence, and the exposure of interest was race. Rates were modeled using the two-level Poisson regression. Age and gender were individual covariates (level 1), and percentage of the CT population living below poverty level was a neighborhood covariate (level 2). For the purposes of analysis, six categories for neighborhood poverty were created: CTs with <5%, 5% to 9.9%, 10% to 14.9%, 15% to 19.9%, 20% to 24.9%, and ≥25% of the population living below the poverty level (the last two categories meet the federal definition of poverty areas).
We fitted a series of models to meet the study objective. Model 1 included fixed parameters for patient race, age, and gender and was used to obtain the average B:W RR for ESRD conditional on individual age and gender. In addition to the parameters in model 1, model 2 included a random effect for CT, which allowed evaluation of the racial disparity in ESRD taking into account clustering of individuals within CTs. Model 3 added a random effect for patient race by CT to see whether racial differences in ESRD incidence varied across neighborhoods. Finally, model 4 was fitted to see whether there was a differential association of neighborhood poverty with ESRD risk among blacks and whites. Model 4 was constructed by adding to the model 3 CT poverty categories and including a cross-level interaction term between race and neighborhood poverty. Model 4 was then used to examine the pattern of the association between ESRD incidence and neighborhood poverty in blacks and whites, adjusting for demographics and allowing for correlations. Model-predicted ESRD incidence rates across six levels of neighborhood poverty by race were calculated and plotted for selected age and gender categories.
The statistical significance of the random parameters in models 2, 3, and 4 was tested using the approximate likelihood ratio test.37 All analyses were performed using SAS package version 9.0 (SAS Institute, Inc., Cary, NC). The two-level Poisson models were fitted using the NLMIXED procedure. The study was approved by the Emory University Institutional Review Board on December 30, 2004.
DISCLOSURES
None.
Acknowledgments
The analyses upon which this publication is based were performed under Contract Number 500 to 00-NW06 entitled End Stage Renal Disease Network Organization for the State of North Carolina, sponsored by the Centers for Medicare and Medicaid, Department of Health and Human Services. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, organizations imply endorsement by the U.S. Government.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Easterling RE. Racial factors in the incidence and causation of end-stage renal disease (ESRD). Trans Am Soc Artif Intern Organs 23: 28–33, 1977 [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System. USRDS 2003 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, 2003
- 3.Whittle JC, Whelton PK, Seidler AJ, Klag MJ: Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Arch Intern Med 151: 1359–1364, 1991 [PubMed] [Google Scholar]
- 4.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ: The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. JAMA 268: 3079–3084, 1992 [PubMed] [Google Scholar]
- 5.Byrne C, Nedelman J, Luke RG: Race, socioeconomic status, and the development of end-stage renal disease. Am J Kidney Dis 23: 16–22, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Young EW, Mauger EA, Jiang KH, Port FK, Wolfe RA. Socioeconomic status and end-stage renal disease in the United States. Kidney Int 45: 907–911, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Perneger TV, Whelton PK, Klag MJ: Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch Intern Med 155: 1201–1208, 1995 [PubMed] [Google Scholar]
- 8.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men: 16-year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 9.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV: Ethnic disparities in diabetic complications in an insured population. JAMA 287: 2519–2527, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Li S, McAlpine DD, Liu J, Collins AJ: Differences between blacks and whites in the incidence of end-stage renal disease and associated risk factors. Adv Ren Replace Ther 11: 5–13, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Powe NR: To have and have not: Health and health care disparities in chronic kidney disease. Kidney Int 64: 763–772, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Nzerue CM, Demissochew H, Tucker JK. Race and kidney disease: Role of social and environmental factors. J Natl Med Assoc 94[Suppl]: 28S–38S, 2002 [PMC free article] [PubMed] [Google Scholar]
- 14.Fored CM, Ejerblad E, Fryzek JP, Lambe M, Lindblad P, Nyren O, Elinder CG: Socio-economic status and chronic renal failure: A population-based case-control study in Sweden. Nephrol Dial Transplant 18: 82–88, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Cass A, Cunningham J, Snelling P, Wang Z, Hoy W: End-stage renal disease in indigenous Australians: A disease of disadvantage. Ethn Dis 12: 373–378, 2002 [PubMed] [Google Scholar]
- 16.Cass A, Cunningham J, Wang Z, Hoy W: Social disadvantage and variation in the incidence of end-stage renal disease in Australian capital cities. Aust N Z J Public Health 25: 322–326, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Merkin SS, Coresh J, Roux AV, Taylor HA, Powe NR: Area socioeconomic status and progressive CKD: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 46: 203–213, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Shoham DA, Vupputuri S, Kshirsagar AV: Chronic kidney disease and life course socioeconomic status: A review. Adv Chronic Kidney Dis 12: 56–63, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hertz RP, Unger AN, Cornell JA, Saunders E: Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 165: 2098–2104, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA: Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care 41: 1221–1232, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS: Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care 22: 403–408, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Qureshi AI, Suri MF, Guterman LR, Hopkins LN: Ineffective secondary prevention in survivors of cardiovascular events in the US population: Report from the Third National Health and Nutrition Examination Survey. Arch Intern Med 161: 1621–1628, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Mainous AG 3rd, King DE, Garr DR, Pearson WS: Race, rural residence, and control of diabetes and hypertension. Ann Fam Med 2: 563–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMott K: Healthcare practices vary widely from town to town: Regional Dartmouth Atlas. Health Syst Lead 4: 2–3, 1997 [PubMed] [Google Scholar]
- 25.Wennberg JE: Unwarranted variations in healthcare delivery: Implications for academic medical centres. BMJ 325: 961–964, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Brown C, Roccella EJ: Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the Health Examination surveys, 1960 to 1991. Hypertension 26: 60–69, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD: Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21: 518–524, 1998 [DOI] [PubMed] [Google Scholar]
- 28.McClellan W, Tuttle E, Issa A: Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis 12: 285–290, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM: Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med 321: 1074–1079, 1989 [DOI] [PubMed] [Google Scholar]
- 30.US Census Bureau. Available online at http://www.census.gov/. Accessed October 26, 2004.
- 31.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N: Racial disparities in context: A multilevel analysis of neighborhood variations in poverty and excess mortality among black populations in Massachusetts. Am J Public Health 95: 260–265, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R: Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US). J Epidemiol Community Health 57: 186–199, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieger N, Waterman PD, Chen JT, Soobader MJ, Subramanian SV: Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: Geocoding and choice of area-based socioeconomic measures: The Public Health Disparities Geocoding Project (US). Public Health Rep 118: 240–260, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R: Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: Does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol 156: 471–482, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV: Race/ethnicity, gender, and monitoring socioeconomic gradients in health: A comparison of area-based socioeconomic measures. The Public Health Disparities Geocoding Project. Am J Public Health 93: 1655–1671, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services: ESRD Network Organizations Manual. Available online at http://cms.hhs.gov/esrd/2.asp. Accessed February 12, 2004
- 37.Snijders TAB, Bosker, RJ: Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: SAGE Publications Ltd., 1999