Abstract
Despite the critical role that TGF-β plays in renal fibrosis, transgenic mice that overexpress human latent TGF-β1 in the skin exhibit normal renal histology and function even though circulating levels of latent TGF-β1 are an order of magnitude higher than wild-type animals. In fact, latent TGF-β1 seems to protect against renal inflammation in a model of ureteral obstruction. It is unknown, however, whether latent TGF-β1 also has this effect in immunologically mediated forms of renal disease such as anti-GBM crescentic glomerulonephritis. We induced anti-GBM disease in wild-type and transgenic mice overexpressing latent TGF-β1 in keratinocytes. After 14 days, wild-type mice developed progressive crescentic glomerulonephritis with severe renal inflammation and fibrosis. In transgenic mice, proteinuria was reduced by 50%, renal function was preserved, and the formation of glomerular crescents was suppressed by 70%. In addition, transgenic animals had reduced renal inflammation, evidenced by a 70% decrease in the accumulation of T cells and macrophages, and reduced expression of renal IL-1β, TNFα, and MCP-1 by 70 to 80%. Progressive renal fibrosis was also prevented in the transgenic mice, and these protective effects were associated with elevated levels of latent, but not active, TGF-β1 in plasma and renal tissue. Renal Smad7 was up-regulated and both NF-κB and TGF-β/Smad2/3 activation were suppressed. In conclusion, mice overexpressing latent TGF-β1 in the skin were protected against anti-GBM crescentic glomerulonephritis, possibly via Smad 7-mediated inhibition of NF-κB-dependent renal inflammation and TGF-β/Smad2/3-dependent fibrosis.
TGF-β1 exhibits a broad spectrum of biological functions in inflammation, immune response, and tissue repair/ fibrosis.1,2 Although TGF-β1 play a critical role in renal fibrosis,3 little attention has been paid to the potential role of this cytokine in inflammatory and immune-mediated kidney diseases. TGF-β is an anti-inflammatory cytokine and functions in both autocrine and paracrine manners to regulate cell proliferation, apoptosis, chemotaxis, immunity, and inflammatory response.1,2 This is illustrated by the finding that mice with targeted disruption of TGF-β1 develop a wasting syndrome associated with multifocal inflammation at about 3 wk after birth.4 In addition, systemic administration or overexpression of TGF-β produces an inhibitory effect on erosive arthritis,5 autoimmune encephalomyelitis,6 insulitis in nonobese diabetic mice,7 and systemic lupus erythematosus in the MRL/lpr/lpr mice.8 These findings provide strong evidence for the anti-inflammatory properties of TGF-β in the disease conditions.
It has been shown that mice with overexpression of active TGF-β1 in the liver develop severe renal damage with progressive renal fibrosis.9 In contrast, we have recently found that TGF-β1 Tg mice overexpressing a human Wt-TGF-β1 in the skin exhibit a normal renal histology and function without detectable kidney abnormalities, despite a ten-fold increase in circulating levels of latent TGF-β1.10 Moreover, we also found that mice with elevated levels of a latent form of TGF-β1 in plasma and renal tissues are able to prevent renal inflammation in a mouse model of obstructive kidney.10 These contradictory findings suggest a complex role of TGF-β1 in renal pathophysiological conditions.
Although it is known that mice overexpressing latent TGF-β1 can prevent renal inflammation in a model of obstructive kidney induced by ureteral ligation,10 it remains unclear whether latent TGF-β1 is able to protect against renal injury under the immunological condition. To address this question, we examined the functional role of TGF-β1 in an immunologically induced kidney disease induced in TGF-β1 Tg mice by administrating the sheep anti-mouse glomerular basement membrane (GBM) antibody. In this model, a severe and rapidly progressive crescentic GN was developed and the protective role of TGF-β1 in immune-mediated kidney disease was investigated.
RESULTS
Circulating and Renal Tissue TGF-β1 in Normal and Diseased Mice with Anti-GBM Crescentic GN
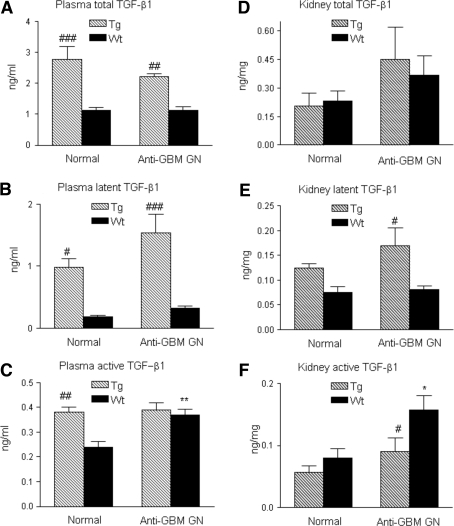
As reported previously,10 normal Tg mice showed a significant higher level of total TGF-β1 in plasma, but not in renal tissues (Figure 1, A and D). Of them, more than 50% were a latent form of TGF-β (Figure 1B). In the disease conditions, levels of plasma and renal latent TGF-β1 remained higher in Tg mice (>70%), but lower in Wt mice (<30%, Figure 1, B and E). Interestingly, active TGF-β1 in plasma was higher in normal Tg mice, accounting for <15% of total plasma TGF-β1, however, this was not altered during anti-GBM GN. In contrast, levels of active TGF-β1 were significantly increased in the diseased Wt mice compared with normal (Figure 1C), accounting for more than 30% of total plasma TGF-β1 in Wt mice. Importantly, active TGF-β1 was significantly increased in the diseased kidney in Wt mice (a two-fold increase), but it remained normal in Tg mice (Figure 1F).
Figure 1.
Circulating levels of TGF-β1 in plasma and renal tissues in normal and anti-glomerular basement membrane (GBM) GN animals. (A) Circulating levels of total TGFβ1; (B) circulating levels of latent TGF-β1; (C) circulating levels of active TGFβ1; (D) levels of total TGFβ1 in renal tissues; (E) levels of latent TGF-β1 in renal tissues; (F) levels of active TGFβ1 in renal tissues. Each bar represents the mean ± SEM for groups of six to eight mice. *P < 0.05, **P < 0.01 compared with the normal Wt mice; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the Wt mice.
Mice Overexpressing TGF-β 1 are Protected against Crescentic GN
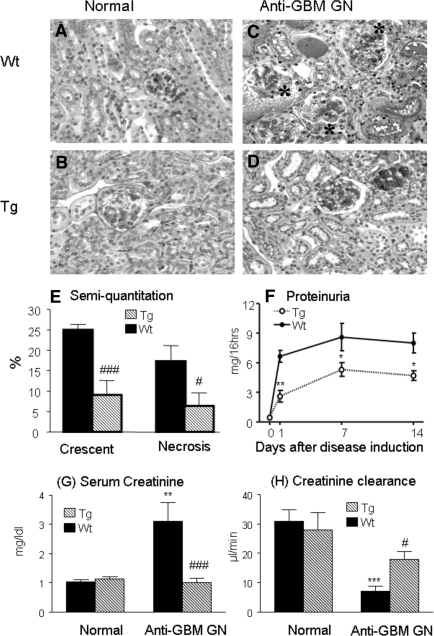
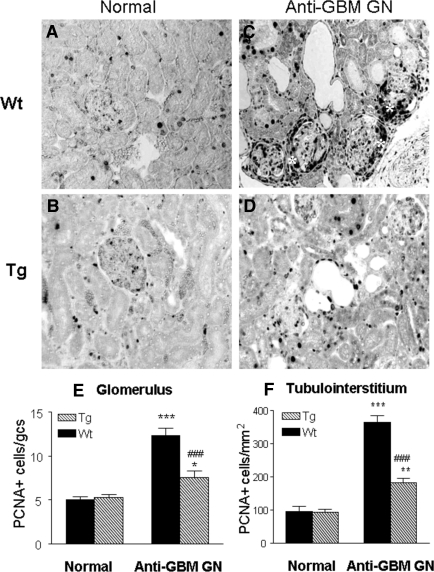
Histologically, both normal Wt and Tg mice exhibited a normal renal histology (Figure 2, A and B). However, Wt mice developed severe crescentic GN with progressive renal inflammation and fibrosis (Figure 2, C and E). This was accompanied by a marked increase in proteinuria (Figure 2F), a rise in serum creatinine, and a fall in creatinine clearance (Figure 2, G and H). All of these pathologic changes were substantially inhibited in Tg mice (Figure 2, D through H), resulting in 60% to 70% reduction of glomerular crescents and total urinary protein excretion, and prevention of a rise in serum creatinine and a fall of creatinine clearance. This was associated with a marked inhibition of cell proliferation as shown in Figure 3. Indeed, massive PCNA+ proliferating cells in glomerular crescents and in areas of tubulointerstitial damage were largely reduced in Tg mice compared with Wt mice (Figure 3, C through F).
Figure 2.
TGF-β1 Tg mice are protected against crescentic GN in a model of anti-GBM GN. (A, B) Kidneys from both K5.TGFβ1wt transgenic (Tg) and wild type (Wt) mice show normal renal histology. (C) A representative kidney from a Wt mouse with anti-GBM GN shows severe glomerular crescentic formation (*), segmental glomerular necrosis, and tubulointerstitial damage with numerous mononuclear cell infiltration at day 14 after induction of disease. (D) A representative kidney from a Tg mouse shows relatively normal histology at day 14 after induction of disease. (E) Semiquantitative analysis of histology. (F) Urinary protein concentrations. (G) Plasma creatinine. (H) Creatinine clearance. Each bar represents the mean ± SEM for groups of eight mice. *P < 0.05, **p <0.01, ***P < 0.001 compared with the normal mice; #P < 0.05, ###P < 0.001 compared with the Wt mice. Tissue sections are stained by periodic acid–Schiff methods. Magnification, ×200.
Figure 3.
Immunohistochemistry shows that massive kidney cell proliferation [proliferating cell nuclear antigen (PCNA)+] in Wt mice with anti-GBM crescentic GN at day 14 is blocked in TGF-β1 Tg mice. Proliferating cells are identified with the anti-PCNA antibody as the nuclear staining pattern. (A, B) PCNA+ cells in normal Wt (A) and Tg (B) mice; (C, D) PCNA+ cells in the diseased kidneys in Wt (C) and Tg (D) mice; (E, F) semiquantitative analysis of PCNA+ cells within the kidney. Note that massive cell proliferation is found in the diseased kidney in Wt mice (C), contributing significantly to severe glomerular crescentic formation (*), which is prevented in Tg mice (D). Each bar represents the mean ± SEM for a group of eight mice with anti-GBM GN and six normal mice. *P < 0.05, **P < 0.01, ***P < 0.001 compared with normal mice; ###P < 0.001 when compared with the Wt mice. Magnification, ×200.
Complement-Mediated Renal Injury is Inhibited in TGF-β1 Tg Mice with Anti-GBM Crescentic GN
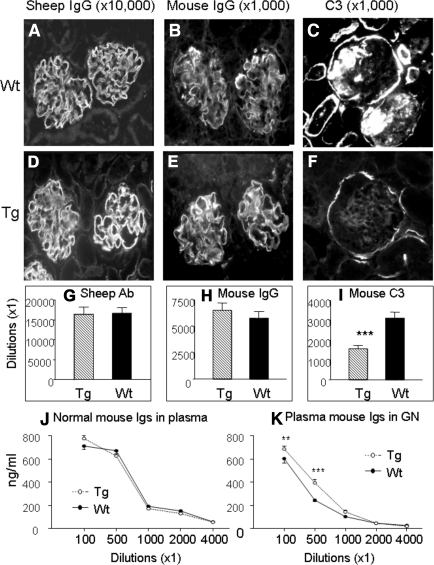
Immunofluorescence showed that there was no difference in immune deposition of the sheep anti-mouse GBM IgG (Figure 4, A, D, and G) and mouse IgG (Figure 4, B, E, and H) along the GBM in both Wt and Tg mice. However, strong complement C3 deposition noted in Wt mice was significantly inhibited in Tg mice (Figure 4, C, F, and I). Circulating levels of mouse immunoglobulins were comparable in both normal Wt and Tg mice, but significantly increased in the diseased Tg mice (Figure 4, J and K).
Figure 4.
Effect of overexpression of latent TGF-β1 in mice with anti-GBM GN (day 14) on antibody and complement deposition and mouse Ig production. (A, D) Representative immunofluorescence staining for deposition of mouse anti-sheep IgG in Wt (A) and Tg (D) animals; (B, E) representative immunofluorescence staining for deposition of mouse IgG in Wt (B) and Tg (E) animals; (C, F) representative immunofluorescence staining for deposition of C3 in Wt (C) and Tg (F) animals. (G, H, I) Semiquantitative analysis. Note that although there is no significant difference in both sheep anti-GBM and mouse IgG deposition along GBM, deposition of C3 within the glomerulus is significantly reduced (F, I). (J, K) ELISA shows that there is no difference in plasma concentrations of mouse immunoglobulins in Wt and Tg mice, however, an increase in mouse Ig production is found in Tg. Tg mice (hatched bars), Wt mice (solid bars). Each bar represents the mean ± SEM for groups of eight mice. ***P < 0.001 compared with the Wt mice. Tissue sections are stained with serial dilutions of the antibody and semiquantitated as described in Concise Methods. Magnification, ×200.
Cell-Mediated Renal Injury in Anti-GBM Crescentic GN is Inhibited in TGF-β Tg Mice
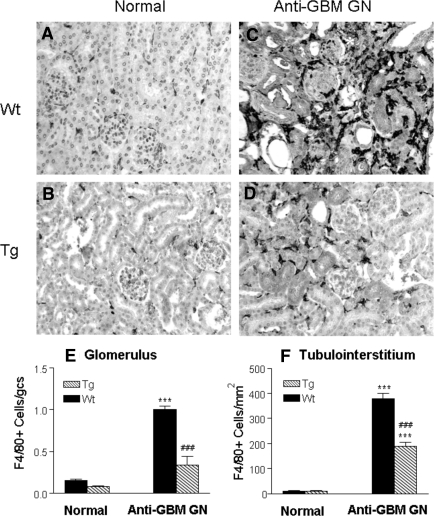
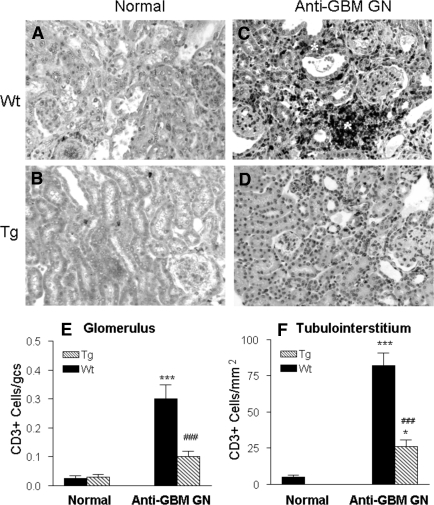
T cell and macrophage infiltration is a typical feature of anti-GBM crescentic GN. As shown in Figure 5 and Figure 6, although no difference in numbers of T cells and macrophages was found in the kidney in normal Wt and Tg mice (Figure 5A and Figure 6A), Wt mice developed a dense T cell and macrophage infiltration within the diseased kidney, contributing significantly to glomerular crescentic formation and severe tubulointerstitial damage (Figure 5C and Figure 6C), which was substantially inhibited in Tg mice as shown in Figure 5D and Figure 6D. Quantitative analysis confirmed these observations (Figure 5, E and F and Figure 6, E and F), respectively.
Figure 5.
Immunohistochemistry shows that macrophage infiltration within the kidney with anti-GBM crescentic GN at day 14 after disease induction is markedly inhibited in TGF-β1 Tg mice. (A, B) Normal Wt (A) and Tg (B) kidneys stained for F4/80+ T cells. (C, D) Diseased kidneys from Wt (C) and Tg (D) mice stained for F4/80+ cells. Note that extensive macrophage infiltration within the diseased kidney in Wt mice (C) contributes significantly to severe glomerular and tubulointerstitial damage, including glomerular crescentic formation (*). In contrast, macrophage infiltration within the diseased kidney is inhibited in TGF-β1 Tg mice (D). (E, F) Semiquantitative analysis. Each bar represents the mean ± SEM for a group of eight with anti-GBM GN and six normal mice. ***P < 0.001 compared with normal mice; ### P < 0.001 when compared with the Wt mice. Magnification, ×200.
Figure 6.
Immunohistochemistry shows that CD3+ T cell infiltration within the kidney with anti-GBM crescentic GN at day 14 is markedly inhibited in TGF-β1 Tg mice. (A, B) Normal Wt (A) and Tg (B) kidneys stained with the anti-CD3 antibody. (C, D) Diseased kidneys from Wt (C) and Tg (D) mice stained with the anti-CD3 antibody. Note that extensive CD3+ T cell infiltration within the diseased kidney in Wt mice (C) contributes significantly to severe glomerular and tubulointerstitial damage, resulting in focal tubulointerstitial damage (*). In contrast, CD3+ T cell infiltration within the diseased kidney in Tg mice is inhibited (D). (E, F) Semiquantitative analysis. Each bar represents the mean ± SEM for a group of eight mice with anti-GBM GN and six normal mice. *P < 0.05, ***P < 0.001 compared with normal mice; ### P < 0.001 when compared with the Wt mice. Magnification, ×200.
Upregulation of IL-1β, TNFα, and MCP-1 in Anti-GBM GN in Wt Mice is Prevented in TGF-β Tg Mice
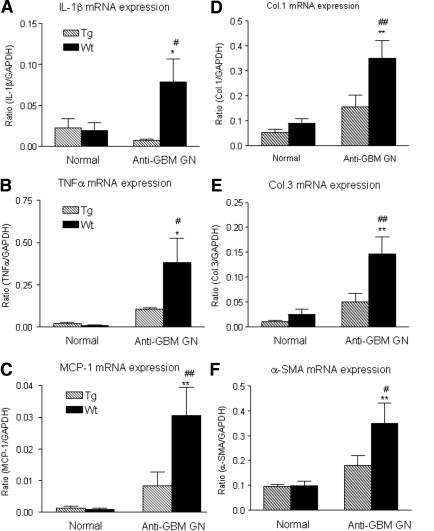
It has been well accepted that pro-inflammatory cytokines including IL-1 and TNFα as well as chemokines such as MCP-1 play an important role in the pathogenesis of anti-GBM GN. We next examined the expression of IL1β, TNFα, and MCP-1 by real-time PCR. As shown in Figure 7 (A through C), real-time PCR demonstrated that Wt mice exhibited a significant increase in the mRNA level of IL-1β, TNFα, and MCP-1 in the diseased kidney, which was prevented in the TGF-β1 Tg mice.
Figure 7.
Real time PCR shows renal inflammatory cytokines and chemokine (IL-1β, TNFα, and MCP-1) and extracellular matrix (collagen I, III, and α-SMA) mRNA expression in normal and anti-GBM GN (day 14) in both Wt and Tg mice. Total renal RNA extracted from normal and diseased kidney from Wt (solid bar) and Tg (hatched bar) mice were reverse transcribed and subjected to real-time PCR for IL-1β (A), TNFα (B), MCP-1 (C), collagen I (D), collagen III (E), and α-SMA (F). Each bar represents the mean ± SEM for a group of six mice. *P < 0.05, **P < 0.01 compared with the normal Wt mice. #P < 0.05, ##P < 0.01 when compared with the Wt.
Renal Fibrosis in Wt Mice with Anti-GBM GN is Prevented in TGF-β Tg Mice
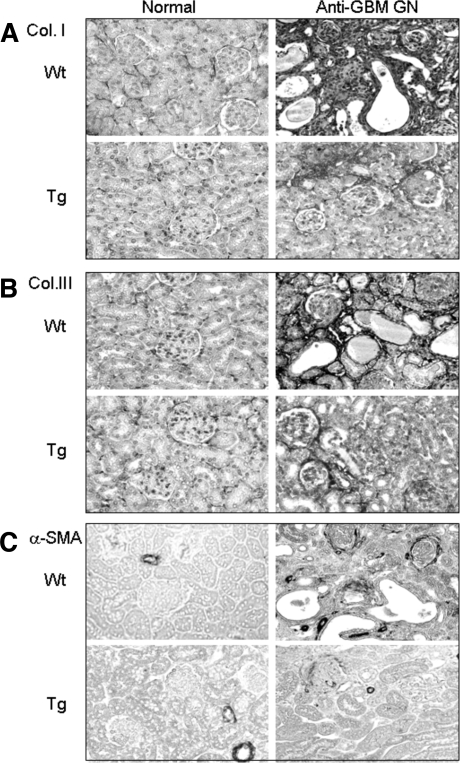
TGF-β is a key fibrotic growth factor in renal fibrosis. This is further demonstrated by the finding that mice overexpressing an active human TGF-β1 develop severe liver and kidney fibrosis.9 We next examined whether mice overexpressing a latent TGF-β1 develop renal fibrosis. Unexpectedly, real-time PCR showed that Tg mice with higher levels of latent TGF-β1 were protected from renal fibrosis as evident by a significant inhibition of increased renal collagen I, III, and α-SMA mRNA in Wt mice (Figure 7, D through F). This was further confirmed by immunohistochemical staining, showing that strong accumulation of collagen I, III, and α-SMA+ myofibroblasts in damaged areas of tubulointerstitium in Wt mice was inhibited in Tg mice (Figure 8).
Figure 8.
Immunohistochemistry shows extracellular matrix (collagen I, III, and α-SMA) accumulation within the kidney in normal and anti-GBM GN (day 14) in both Wt and Tg mice. (A) Collagen I; (B) collagen III; (C) α-SMA. Note that a strong accumulation of collagen I and III and α-SMA in the areas of tubulointerstitial damage is found in Wt mice with anti-GBM GN, which is substantially inhibited in Tg mice. Each picture represents an immunohistological change for a group of six normal and eight diseased mice. Magnification, ×200.
Upregulation of Smad7 and Inhibition of NF.κB/p65 and Smad2/3 Activation May be the Key Mechanisms by which TGF-β1 Tg Mice are Protected against Anti-GBM Crescentic GN.
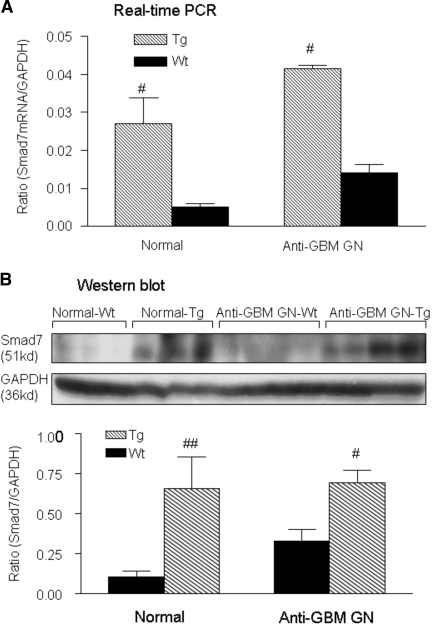
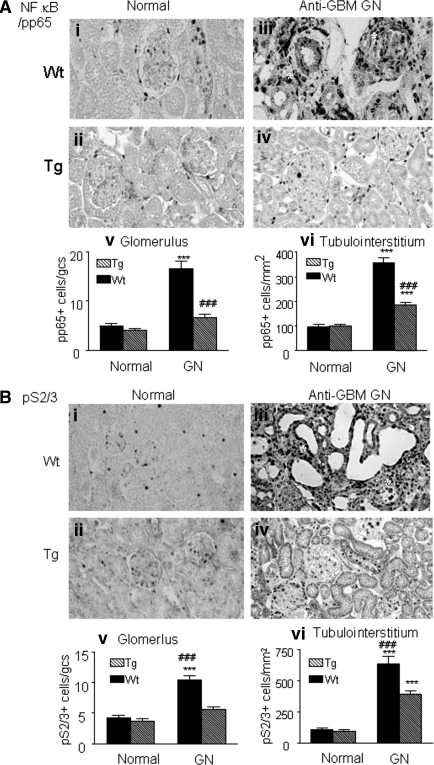
As shown in Figure 9, analyses by real-time PCR and Western blot revealed that, compared with wild-type mice, a marked increase in renal Smad7 mRNA and protein was found in both normal and diseased kidney in TGF-β1 Tg mice. This was associated with a marked inhibition of NF.κB/p65, but not p50 (data not shown), activation (Figure 10A). Indeed, compared with normal kidneys (Figure 10, Ai and Aii), marked activation of NF.κB/pp65 in glomerular crescents and areas of severe tubulointerstitial damage in Wt mice was abrogated in Tg mice (Figure 10, Aiii and Aiv), which was further demonstrated by quantitative analysis as shown in Figure 10 (Av and Avi). Similarly, upregulation of renal Smad7 in Tg mice also resulted in a marked inhibition of Smad2/3 activation (Figure 10B). Compared with normal kidneys where a few phosphorylated (p)-Smad2/3 were found within the glomerulus and tubulointerstitium (Figure 10, Bi and Bii), strong activation of p-Smad2/3 in areas of severe tubulointerstitial fibrosis was significantly inhibited in Tg mice (Figure 10, Biii and Biv), which was further confirmed by quantitative analysis (Figure 10, Bv and Bvi).
Figure 9.
Renal Smad7 is upregulated in both normal and anti-GBM disease mice that overexpress TGF-β1. (A) Real-time PCR, (B) Western blot analysis. Wt mice (solid bar), Tg mice (hatched bar), each bar represents the mean ± SEM for a group of six mice. #P < 0.05, ##P < 0.01 compared with Wt mice.
Figure 10.
Immunohistochemistry shows that NF.κB/p65 and Smad2/3 activation are blocked in TGF-β1 Tg mice with anti-GBM crescentic GN at day 14. Activation of NF.κB/p65 subunits and Smad2/3 are identified with the anti-phosphorylated p65 (pp65) or p-Smad2/3 antibody as the nuclear staining pattern. (Ai and Aii) NF.κB/pp65 nuclear location in the normal Wt and Tg mice; (Aiii and Aiv) activation of NF.κB/pp65 in the diseased kidney in Wt and Tg mice; (Av and Avi) semiquantitative analysis of NF.κB/pp65. (Bi and Bii) p-Smad2/3 nuclear location in the normal Wt and Tg mice; (Biii and Biv) activation of p-Smad2/3 in the diseased kidney in Wt and Tg mice; (Bv and Bvi) semiquantitative analysis of p-Smad2/3. Note that extensive activation of NF.κB/pp65 and p-Smad2/3 contributes significantly to severe glomerular and tubulointerstitial damage, resulting in glomerular crescent formation and focal tubulointerstitial damage (*). Each bar represents the mean ± SEM for a group of eight mice with anti-GBM GN and six normal mice. ***P < 0.001 compared with normal mice; ### P < 0.001 when compared with the Wt mice. Magnification, ×200.
DISCUSSION
We report here that mice overexpressing a latent form of TGF-β1 on the skin have a protective role in the development of crescentic GN. This was supported by the findings that Tg mice were able to prevent glomerular crescent formation, a rise in proteinuria and plasma creatinine, a fall of creatinine clearance, and the development of renal inflammation including T cell and macrophage accumulation, cell proliferation, expression of IL-1β, TNFα, and MCP-1, and complement C3 deposition. Unexpectedly, we also found that renal fibrosis was prevented in Tg mice as evident by suppression of collagen I, III, α-SMA mRNA expression and their matrix protein accumulation. Further study revealed that upregulation of renal Smad7, thereby preventing NF.κB/p65 and TGF-β/Smad2/3 from activation, may be a central mechanism by which TGF-β1 Tg mice are protected against renal inflammation and fibrosis in anti-GBM crescentic GN.
TGF-β1 is a pleiotropic cytokine with diverse roles in inflammation and fibrosis.1,2 TGF-β1 is produced and secreted as a latent complex, consisting of mature dimeric TGF-β1, a latency-associated peptide (LAP), and a latent TGF-β binding protein (LTBP). LAP binds to the N-terminal of TGF-β to prevent it from binding to its receptors, whereas LTBP-1 binds the LAP-TGF-β complex to prevent TGF-β from interacting with local matrix proteins. TGF-β must be released from LAP to become activated by factors including plasmin, thrombospondin-1, reactive oxygen species, and acid.11,12
There were several mechanisms whereby latent TGF-β1 may exert its anti-inflammatory properties in anti-GBM GN. First, blockade of IL-1, TNF-α, and MCP-1 and inhibition of T cell and macrophage infiltration could be the mechanism by which elevated levels of latent TGF-β1 in plasma and renal tissues in Tg mice inhibited anti-GBM crescentic GN. This was consistent with previous findings that TGF-β acts as an endogenous defender against IL-1β, TNFα, and MCP-1 production.13–17 Second, inhibition of complement C3 deposition within the inflamed kidney indicated that TGF-β1 may have a protective role in complement-mediated renal injury in anti-GBM GN. This may be associated with inhibition of complement C3 synthesis by TGF-β in response to inflammatory cytokines18,19 and may be also associated with suppression of complement activation locally by blocking renal inflammation in response to high levels of latent TGF-β1. Third, inhibition of cell proliferation could be another mechanism leading to suppression of crescentic GN in TGF-β1 Tg mice, because upregulation of Smad7 is able to block NF.κB-dependent cell proliferation and induce apoptosis.20,21 Finally, overexpression of latent TGF-β1 may produce an inhibitory effect specifically on T cell proliferation, activation, and Th1 differentiation, thereby blocking T cell-mediated crescentic GN. Administration or overexpression of TGF-β1 have been shown to suppress autoimmune diseases including arthritis,5 autoimmune encephalomyelitis,6 insulitis,7 and systemic lupus erythematosus.8 T cells engineered to specifically produce latent TGF-β1 reverse allergen-induced airway inflammation and suppress colitis,22,23 suggesting that latent TGF-β1 derived from immune effector cells plays a protective role in autoimmune diseases. In the studies presented here, we provided new information that latent TGF-β1 produced by skin keratinocytes also plays a protective role in immune-mediated crescentic GN via a paracrine manner. Indeed, TGF-β signaling is critical in maintaining peripheral T regulatory cells and inhibiting T cell activation and Th1 differentiation.24,25 Mice with T cell specific deletion of TGF-β1 gene develop lethal immune disease with massive T cell proliferation, activation, and Th1 differentiation further demonstrated an essential role for TGF-β1 in regulating T cell immunity.26 Thus, in addition to the inhibitory effect of latent TGF-β1 on NF.κB-dependent renal inflammation as described in these studies, it is likely that the protective role of latent TGF-β1 in crescentic GN may be attributable to the immunoregulatory role of TGF-β1 in T regulatory and Th1 differentiation, which will require further investigation.
What are signaling mechanisms of TGF-β1 leading to renal protection in mice overexpressing latent TGF-β1? It is known that TGF-β activates its downstream mediators, Smad2 and Smad3, to exert its biologic effects such as renal fibrosis.3,27 At the same time, TGF-β also induces Smad7 expression which, in turn, inhibits Smad2/3 signaling and fibrosis.3,27 In Wt mice, severe renal fibrosis was associated with over activation of Smad2/3 caused by an increase in renal active TGF-β1. This may also well explain the finding that mice overexpressing an active form of TGF-β1 in the liver result in a ten-fold increase in bioactive TGF-β1 in circulation and develop progressive liver and renal fibrosis.9 In contrast, we report here that mice overexpressing latent TGF-β1 were protected from renal fibrosis. This may be associated with a high level of renal Smad7, but a low level of activated Smad2/3 in response to a five-fold increase in plasma latent TGF-β1 associated with a less than one-fold increase in levels of plasma bioactive TGF-β1 and a two-fold increase in renal latent TGF-β1 with a normal level of bioactive TGF-β1 in the diseased kidney. These observations suggest that overexpression of Smad7 in response to higher levels of latent TGF-β1 with normal or marginally elevated levels of active TGF-β1 may be a critical mechanism of anti-renal fibrosis in Tg mice. This is consistent with previous reports that overexpression of renal Smad7 blocks TGF-β/Smad2/3-mediated renal fibrosis in vitro and in obstructive and remnant kidney diseases.28–30 Beyond the anti-fibrotic effect, Smad7 is capable of inhibition of renal inflammation. Overexpression of Smad7 in T cells has been shown to prevent a mouse model of anti-GBM GN.31 We have also shown that overexpression of renal Smad7 is capable of blocking renal inflammation by inhibiting NF.κB/p65 activation via induction of IκBα in vivo and in vitro.10,32 The study presented here added new information that overexpression of latent TGF-β1 resulted in upregulation of renal Smad7, which leads to inhibition of both NF.κB-dependent renal inflammation and Smad2/3-mediated renal fibrosis in anti-GBM GN. Thus, overexpression of Smad7 may be a central mechanism by which mice with overexpression of latent TGF-β1 were protected from renal fibrosis and inflammation.
An interesting observation in this study is that although Tg mice were protected against renal inflammation, they developed inflammatory skin lesions.33 The discrepancy between kidney and skin lesions may be associated with the local levels of latent and active TGF-β1, which has been discussed previously.10 However, it remains unclear as how increased latent TGF-β1 in circulation and kidney tissues upregulates renal Smad7. Smad7 is induced by TGF-β1 via a Smad2/3-dependent mechanism.27 However, we found that renal Smad7, which is high in the normal kidney, is reduced in the diseased kidney associated with over activation of TGF-β/Smad2/3 signaling and renal fibrosis.28,29 This suggests that TGF-β-induced Smad7 is regulated in a complex manner. It is possible that a persistent higher level of latent TGF-β1 in both plasma and kidney tissues as seen in Tg mice may allow maintenance of a low significant level of active TGF-β1 (a 0.6-fold increase compared with normal Wt mice), which may favor activation of Smad2/3 under the physiologic conditions, thereby inducing renal Smad7 expression without causing renal fibrosis. However, this was lost when Smad2/3 became overactivated in response to higher levels of active TGF-β1, as seen in Wt mice in this and other diseased models.9,28,29 It is also possible that upregulation of renal Smad7 may be post-transcriptionally regulated, as acetylation of Smad7 in response to TGF-β is able to protect it from Smurf-mediated ubiquitination and degradation.34,35 Nevertheless, although mechanisms whereby upregulation of renal Smad7 in mice with overexpression of latent TGF-β1 remain largely unknown, results from this study implicate that latent TGF-β1 may have therapeutic potential for renal inflammation and fibrosis.
CONCISE METHODS
Animal Model of Anti-GBM GN
K5.TGF-β1wt Tg mice were generated from ICR background mice as described previously.33 Accelerated anti-GBM GN was induced in both TGF-β1 Tg and Wt mice (20 g, aged 3 mo) following the established protocol.36,37 Groups of eight mice were euthanized on day 14. In addition, groups of six normal Wt and Tg mice were used as normal age-matched controls. Kidney tissues were collected for histology, immunohistochemistry, Western blot, and real-time PCR analyses. The Committee on the Use of Live Animals for Teaching and Research at the University of Hong Kong approved the experimental procedures.
Measurement of Proteinuria
All mice were housed in metabolic cages before and after induction of disease at days 0, 1, 7 and 14 for 16-h urinary protein excretion. Urinary protein excretion was determined using Coomasie Blue method. Plasma and urinary creatinine were determined by an ELISA kit as described previously.29,36
Measurement of TGF-β1 and Mouse Immunoglobulins in Plasma
TGF-β1 levels in plasma and renal tissues including the active form, the LAP, and total TGF-β1 were quantitatively analyzed by the human TGF-β1 DuoSet ELISA development system (DY240) that has been shown to recognize mouse TGF-β (R&D System Inc., Minneapolis, MN) as described previously.10,38 Circulating levels of mouse immunoglobulins were measured by ELISA following the established protocol.36
Western Blot Analysis
Protein from kidney tissues was extracted with RIPA lysis buffer and Western blot analysis was performed as described previously.10 After blocking nonspecific binding with 5% BSA, membranes were then incubated overnight at 4°C with the primary antibody against Smad7 (Santa Cruz Biotech Inc., Santa Cruz, CA), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Chemicon Inc., Temecula, CA), followed by a horseradish peroxidase-conjugated secondary antibody. The signals were visualized by an enhanced chemiluminescence system (Amersham, Piscataway, NJ).
Real-Time PCR
Total kidney RNA was isolated using the RNeasy kit, according to the manufacturer's instructions (Qiagen Inc., Valencia, CA). The cDNA was synthesized and real-time PCR was run with the Opticon2 real-time PCR machine (Bio-Rad, Hercules, CA) by using IQ SYBR green supermix reagent (Bio-Rad, Herculus, CA) as described previously.10 The primers used in this study, including mouse Smad7, IL-1β, TNF-α, collagen I and III, α-SMA, and GAPDH were described previously.10,39 The mouse MCP-1 primer used in this study was: forward 5′-CTTCTGGGCCTGCTGTTCA, reverse 5′- CCAGCCTACTCATTGGGATCA.
Histology and Immunohistochemistry
Changes in renal morphology were examined in methyl Carnoy's-fixed, paraffin-embedded tissue sections (4 μm) stained with hematoxylin and eosin or periodic acid–Schiff (PAS) methods. Immunostaining was performed in paraffin sections using a microwaved-based antigen retrieval technique.40 The antibodies used in this study included: rat anti-mouse monoclonal antibody to macrophages (F4/80) (Serotec, Ltd, Oxford, UK), rabbit polyclonal antibodies to CD3+ T cells (SP7) (Abcam, Cambridge, UK), PCNA, p-Smad2/3 (Santa Cruz Biotech Inc., Santa Cruz, CA), collagen I and III (Southern Tech, Birmingham, AL), α-SMA (Sigma, St. Louis, MO), and an activated NF.κB/pp65 (Ser276) subunit (Cell Signaling Tech. Beverly, MA).
Deposition of immune reactants within the kidney was assessed by direct immunofluorescence with FITC-conjugated polyclonal antibodies to sheep IgG, mouse IgG, and complement C3 as described previously.37 Semiquantitation of sheep IgG, mouse IgG, and C3 deposition was determined in tissue sections using an antibody titration method.35
Quantitative analyses of histology and immunostaining were carried on coded slides. Glomerular crescent formation and necrosis were scored by counting 50 glomeruli on PAS-stained section and expressed as percentage. The number of positive cells for CD3, F4/80, PCNA, p-Smad2/3 and NF.κB/pp65 was counted in 20 consecutive glomeruli and expressed as cells/glomerular cross-section (gcs), whereas positive cells in the tubulointerstitium were counted under high-power fields (×40) by means of a 0.0625-mm2 graticule fitted in the eyepiece of the microscope, and expressed as cells per mm2.
Statistical Analyses
Data obtained from this study are expressed as the mean ± SEM. Statistical analyses were performed using one-way ANOVA from GraphPad Prism 3.0 (GraphPad Software, Inc. San Diego, CA).
DISCLOSURES
None.
Acknowledgments
This study was supported by Grants from Research Grant Council of Hong Kong (RGCCERGHKU7592/06M and 7682/07M) to HYL and NIH/NCI (CA79998 and GM70966) to XJW.).
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Letterio JJ, Roberts AB: Regulation of immune responses by TGF-beta. Annu Rev Immunol 16: 137–161, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Roberts AB: Molecular and cell biology of TGF-beta. Miner Electrolyte Metab 24: 111–119, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Koka V, Lan HY: Transforming growth factor-beta and Smad signaling in kidney diseases. Nephrology (Carlton) 10: 48–56, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S: Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood 87: 1439–1445, 1996 [PubMed] [Google Scholar]
- 5.Chen W, Jin W, Cook M, Weiner HL, Wahl SM: Oral delivery of group A streptococcal cell walls augments circulating TGF-beta and suppresses streptococcal cell wall arthritis. J Immunol 161: 6297–6304, 1998 [PubMed] [Google Scholar]
- 6.Jin YX, Xu LY, Guo H, Ishikawa M, Link H, Xiao BG: TGF-beta1 inhibits protracted-relapsing experimental autoimmune encephalomyelitis by activating dendritic cells. J Autoimmun 14: 213–220, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Piccirillo CA, Chang Y, Prud'homme GJ: TGF-beta1 somatic gene therapy prevents autoimmune disease in nonobese diabetic mice. J Immunol 161: 3950–3956, 1998 [PubMed] [Google Scholar]
- 8.Raz E, Dudler J, Lotz M, Baird SM, Berry CC, Eisenberg RA, Carson DA: Modulation of disease activity in murine systemic lupus erythematosus by cytokine gene delivery. Lupus 4: 286–292, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Kopp JB, Factor VM, Mozes M, Nagy P, Sanderson N, Bottinger EP, Klotman PE, Thorgeirsson SS: Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest 74: 991–1003, 1996 [PubMed] [Google Scholar]
- 10.Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, Wang XJ, Lan HY: Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol 16: 1371–1383, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Lyons RM, Gentry LE, Purchio AF, Moses HL: Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol 110: 1361–1367, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D: The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Kitamura M, Suto T, Yokoo T, Shimizu F, Fine LG: Transforming growth factor-beta 1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. J Immunol 156: 2964–2971, 1996 [PubMed] [Google Scholar]
- 14.Kitamura M: Identification of an inhibitor targeting macrophage production of monocyte chemoattractant protein-1 as TGF-beta 1. J Immunol 159: 1404–1411, 1997 [PubMed] [Google Scholar]
- 15.Imai K, Takeshita A, Hanazawa S: Transforming growth factor-beta inhibits lipopolysaccharide-stimulated expression of inflammatory cytokines in mouse macrophages through downregulation of activation protein 1 and CD14 receptor expression. Infect Immun 68: 2418–2423, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suto TS, Fine LG, Shimizu F, Kitamura M. In vivo transfer of engineered macrophages into the glomerulus: endogenous TGF-beta-mediated defense against macrophage-induced glomerular cell activation. J Immunol 159: 2476–2483, 1997 [PubMed] [Google Scholar]
- 17.Kitamura M, Burton S, English J, Kawachi H, Fine LG: Transfer of a mutated gene encoding active transforming growth factor-beta 1 suppresses mitogenesis and IL-1 response in the glomerulus. Kidney Int 48: 1747–1757, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Barnum SR, Jones JL: Transforming growth factor-beta 1 inhibits inflammatory cytokine-induced C3 gene expression in astrocytes. J Immunol 152: 765–773, 1994 [PubMed] [Google Scholar]
- 19.Gerritsma JS, van Kooten C, Gerritsen AF, van Es LA, Daha MR: Transforming growth factor-beta 1 regulates chemokine and complement production by human proximal tubular epithelial cells. Kidney Int 53: 609–616, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Bottinger EP: Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest 108: 807–816, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lallemand F, Mazars A, Prunier C, Bertrand F, Kornprost M, Gallea S, Roman-Roman S, Cherqui G, Atfi A: Smad7 inhibits the survival nuclear factor kappaB and potentiates apoptosis in epithelial cells. Oncogene 20: 879–884, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, DeKruyff RH, Umetsu DT: CD4(+) T helper cells engineered to produce latent TGF-beta1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest 105: 61–70, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorbecke GJ, Umetsu DT, deKruyff RH, Hansen G, Chen LZ, Hochwald GM: When engineered to produce latent TGF-beta1, antigen specific T cells down regulate Th1 cell-mediated autoimmune and Th2 cell-mediated allergic inflammatory processes. Cytokine Growth Factor Rev 11: 89–96, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Li MO, Sanjabi S, Flavell RA: Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25: 455–471, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Marie JC, Liggitt D, Rudensky AY: Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25: 441–454, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Li MO, Wan YY, Flavell RA: T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26: 579–591, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Derynck R, Zhang YE: Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–84. 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ: Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535–1548, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hou CC, Wang W, Huang XR, Fu P, Chen TH, Sheikh-Hamad D, Lan HY: Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am J Pathol 166: 761–771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY: Smad7 inhibits fibrotic effect of TGF-Beta on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol 13: 1464–1472, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Kanamaru Y, Nakao A, Mamura M, Suzuki Y, Shirato I, Okumura K, Tomino Y, Ra C: Blockade of TGF-beta signaling in T cells prevents the development of experimental glomerulonephritis. J Immunol 166: 2818–2823, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Ng YY, Hou CC, Wang W, Huang XR, Lan HY: Blockade of NF.kB activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int Suppl 94: S83–S91, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Li AG, Wang D, Feng XH, Wang XJ: Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. Embo J 23: 1770–1781, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonsson M, Heldin CH, Ericsson J, Gronroos E: The balance between acetylation and deacetylation controls Smad7 stability. J Biol Chem 280: 21797–21803, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Jin YH, Jeon EJ, Li QL, Lee YH, Choi JK, Kim WJ, Lee KY, Bae SC: Transforming growth factor-beta stimulates p300-dependent RUNX3 acetylation, which inhibits ubiquitination-mediated degradation. J Biol Chem 279: 29409–29417, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Huang XR, Tipping PG, Shuo L, Holdsworth SR: Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int 51: 94–103, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Lan HY, Bacher M, Yang N, Mu W, Nikolic-Paterson DJ, Metz C, Meinhardt A, Bucala R, Atkins RC: The pathogenic role of macrophage migration inhibitory factor in immunologically induced kidney disease in the rat. J Exp Med 185: 1455–1465, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen SS, Cassone L, Lasco TM, McMurray DN: Effect of neutralizing transforming growth factor beta1 on the immune response against Mycobacterium tuberculosis in guinea pigs. Infect Immun 72: 1358–1363, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu P, Liu F, Su S, Wang W, Huang XR, Entman ML, Schwartz RJ, Wei L, Lan HY: Signaling mechanism of renal fibrosis in unilateral ureteral obstructive kidney disease in ROCK1 knockout mice. J Am Soc Nephrol 17: 3105–3114, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC: A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem 43: 97–102, 1995 [DOI] [PubMed] [Google Scholar]