Abstract
Mutations in the gene encoding podocin (NPHS2) cause autosomal recessive steroid-resistant nephrotic syndrome (SRNS). For addressing the possibility of a genotype–phenotype correlation between podocin mutations and age of onset, a worldwide cohort of 430 patients from 404 different families with SRNS were screened by direct sequencing. Recessive podocin mutations were present in 18.1% (73 of 404) of families with SRNS, and 69.9% of these mutations were nonsense, frameshift, or homozygous R138Q. Patients with these mutations manifested symptoms at a significantly earlier age (mean onset <1.75 years) than any other patient group, with or without podocin mutations, in this study (mean onset >4.17 yr). All but one patient affected by truncating or homozygous R138Q mutations developed SRNS before 6 yr of age. Patient groups with other recessive podocin mutations, with single heterozygous podocin mutations, with sequence variants, and with no podocin changes could not be distinguished from each other on the basis of age of onset. In conclusion, nephrotic syndrome in children with truncating or homozygous R138Q mutations manifests predominantly before 6 yr of life, and the onset of disease is significantly earlier than for any other podocin mutations. Because the age of onset can vary by several years among those with identical mutations, additional factors may modify the phenotype.
Nephrotic syndrome (NS) is a common childhood kidney disease caused by impaired glomerular function, which results in proteinuria, hypoalbuminemia, edema, and hyperlipidemia. Although underlying causes of NS are numerous, standard steroid treatment regimens are used to classify idiopathic forms of NS clinically. On the basis of the patients’ response to steroid treatment, NS is descriptively classified as steroid-sensitive NS (SSNS) or steroid-resistant NS (SRNS).
Positional cloning of various genes as mutated in patients with SRNS in recent years has led to the surprising finding that up to 28% of sporadic cases of SRNS are caused by recessive mutations of NPHS2.1 Mutations causing recessive NS in children were found in nephrin (NPHS1),2 podocin (NPHS2),3 laminin-β-2 (LAMB2), and phospholipase C-ɛ (PLCE1).4–6 Dominant mutations of exons 8 and 9 of the Wilms’ tumor 1 gene (WT1) also cause childhood NS.7 In adult patients with SRNS, dominant disease causing mutations in ACTN4 and TRPC6 were found.8–10 Conjointly, these findings have shown that podocyte dysfunction affecting the glomerular slit diaphragm is a key feature of SRNS.11
Among these SRNS genes identified so far, mutations in NPHS2 encoding podocin represent the most frequent cause.1,12 Patients with NPHS2 mutations show histology of FSGS in approximately 80% of cases and progress to ESRD. Extensive studies have documented that the detection of two NPHS2 mutations is clinically relevant, because affected patients do not respond to standard steroid treatment.1 Thus, adverse effects of such treatment attempts can be avoided in children with recessive NPHS2 mutations. In addition, patients with NPHS2 mutations have a reduced risk (8 versus 33%) for recurrence of FSGS in a kidney transplant.1,13
NPHS2 mutations have been found in patients presenting at a wide range of ages, in contrast to NPHS1 (nephrin) mutations, which lead to congenital NS with onset within the first 3 mo of life. The reason for this phenotypic variability of patients with NPHS2 mutations remains elusive. In our recent study on NS manifesting in the first year of life (NSFL), we identified NPHS2 mutations as the most frequent cause of both congenital (0 to 3 mo) and infantile (3 to 12 mo) NS among 89 European children with NSFL.14 In 94.1% of patients with NSFL caused by NPHS2 mutations, we found nonsense, frameshift, or homozygous R138Q mutations. These findings and the suggestive data by Weber et al.13 led to the question of whether specific NPHS2 mutations determine the age of initial onset of SRNS.
To evaluate this genotype–phenotype correlation among patients with NPHS2 mutations, we examined 430 patients from 404 families from a worldwide cohort who presented with SRNS between 0.0 and 21.0 yr of age for mutations in NPHS2 by direct sequencing of all eight exons. In this study, we demonstrate that nonsense, frameshift, or homozygous R138Q mutations are frequent recessive mutations of NPHS2. We show that these NPHS2 mutations are found in patients with a significantly earlier onset than in patients with other recessive mutations, single mutations, or sequence variants of NPHS2. We observe that identical mutations lead to onset of SRNS within a range of several years and speculate that additional factors modify the NPHS2 phenotype. These findings will be important for the prognostic evaluation and management of renal replacement therapy in children with SRNS.
Results
Frequency of NPHS2 Mutations
A total of 430 patients with SRNS from 404 families were analyzed for mutations of NPHS2 by direct DNA sequencing. Mutations in NPHS2 were present in 18.1% (73 of 404) of all families and affected 82 patients (Table 1). In families with more than one affected member, mutations were present in 39.1% (nine of 23 families). In families with one affected child, mutations were present in 16.8% (64 of 381 families; data not shown). The R138Q mutation that has been described as a European founder mutation3 was found in 57.5% (42 of 73) of families with the presence of two disease-causing mutations (Table 1, groups A through D; Supplementary Table 1). In 5.1% (21 of 404) of all families, only one single heterozygous mutation was detected (Table 1, groups E and F; Supplementary Table 1).
Table 1.
A total of 430 patients from 404 families with SRNS analyzed for mutations and sequence changes in NPHS2a
| Group | NPHS2 Mutation | Affected Individuals
|
Affected Families
|
Mean Onset (yr) | Onset Range (yr) | ||
|---|---|---|---|---|---|---|---|
| Total | Onset known | Total | Onset known | ||||
| A | Truncating × anyb | 32 | 31 | 29 | 28 | 1.75c | 0.0 to 9.1 |
| B | R138Q × R138Q | 27 | 26 | 22 | 21 | 1.77c | 0.0 to 5.4 |
| C | R138Q × missense | 10 | 9 | 9 | 8 | 5.95 | 0.0 to 14.3 |
| D | Missense × missense | 13 | 12 | 13 | 12 | 4.17 | 0.0 to 16.6 |
| 82 | 78 | 73 | 69 | 2.61 | 0.0 to 16.6 | ||
| E | Single × R229Q | 14 | 13 | 13 | 12 | 6.74 | 0.8 to 16.3 |
| F | Single × ? | 9 | 9 | 8 | 8 | 8.12 | 1.6 to 14.7 |
| G | R229Q × R229Q | 2 | 2 | 2 | 2 | 6.48 | 0.0 to 13.0 |
| R229Q × ? | 24 | 23 | 24 | 23 | |||
| 49 | 47 | 47 | 45 | 6.87 | 0.0 to 16.3 | ||
| H | No mutation | 299 | 267 | 284 | 253 | 6.4 | 0.0 to 21.0 |
| Total | 430 | 392 | 404 | 367 | 5.71 | 0.0 to 21.0 | |
A total of 430 patients from 404 families with SRNS were analyzed by direct exon sequencing for sequence changes in NPHS2. Clinical data on 392 patients from 367 families allowed for correlation on NPHS2 sequence variants with age of onset. Patients were categorized into patients with two disease-causing NPHS2 mutations (A through D), patients with a single NPHS2 mutations and/or sequence variant R229Q (E through G), and patients without NPHS2 variants (H).
Group A includes 14 individuals from 11 families with a single R138Q mutation accompanying the truncating mutation. The total number of families/individuals with R138Q mutations is 42/5.
Age of onset for both groups A and B was significantly lower than for all other groups (P < 0.01).
The distribution of the four groups with two recessive mutations (A through D) among 73 families with NPHS2 mutations and clinical data were as follows (Table 1): A, 39.7% (29 of 73) with one truncating mutation (nonsense or frameshift) in combination with any other mutation; B, 30.1% (22 of 72) with a homozygous R138Q mutation; C, 12.3% (nine of 73) with one R138Q mutation in combination with one missense mutation; and D, 17.9% (13 of 73) with two missense mutations other then R138Q (Table 1). Six previously unpublished NPHS2 mutations were found (Supplementary Table 1).
In 47 families, we detected only one single NPHS2 mutation or the sequence variant R229Q of unknown significance (Table 1, groups E through G). In these 47 families, one heterozygous mutation in combination with the R229Q sequence variant of unknown significance was present in 27.7% (13 of 47; E). In 17.0% (eight of 47) of families, one heterozygous NPHS2 mutation only was present (F), and in 55.3% (26 of 47) of families, the R229Q sequence variant only was present heterozygously (24 of 47) or homozygously (G; Table 1). The frequency of R229Q variants among all 404 families with SRNS in this study was 9.2% (37 of 404) in the heterozygous state and 0.5% (two of 404) in the homozygous state.
Correlation of NPHS2 Mutations and Age of Onset
Age at onset of SRNS was documented for 392 patients from 367 families and ranged from 0.0 to 21.0 yr. Two disease-causing NPHS2 mutations were found in 19.9% (78 of 392) of these patients. The relative frequency with which any two disease-causing NPHS2 mutations were detected declined with increasing age at manifestation.
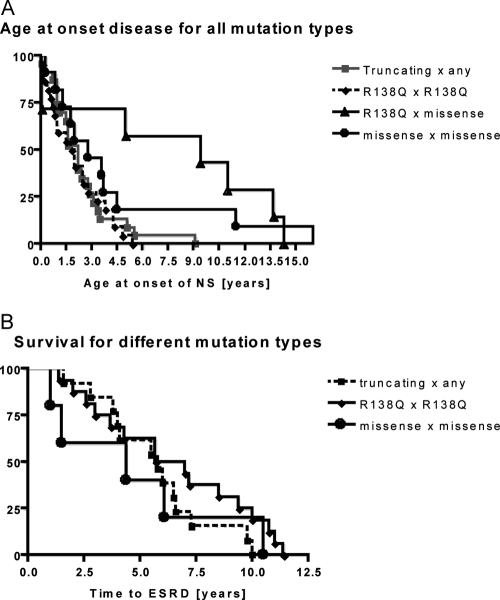
We then evaluated genotype–phenotype correlations between type of mutation and age of onset (Table 1). This correlation showed significantly earlier onset of SRNS in children with two disease-causing NPHS2 mutations (A through D; mean 2.61 yr; range 0.0 to 16.6 yr) than in children with single heterozygous NPHS2 mutations and sequence variant R229Q (E through G; mean 6.87 yr; range 0.0 to 16.3 yr; P < 0.0001). Children with two recessive NPHS2 mutations (A through D) also manifested significantly earlier when compared with children without NPHS2 sequence variants (H; mean 6.4 yr; range 0.0 to 21.0 yr; P < 0.0001). In particular, the presence of two truncating mutations (nonsense or frameshift) in NPHS2 (A) was associated with much earlier onset (mean 1.75 yr; range 0.0 to 9.1 yr). Likewise, the homozygous presence of the NPHS2 founder mutation R138Q (B) was associated with early onset (1.77 yr; range 0.0 to 5.4 yr). These two groups (A and B) were responsible for the earlier onset of patients with the presence of two NPHS2 mutations (groups A through D; Table 1). Figure 1A illustrates the significant difference in age at onset of disease between mutation groups A and C (P = 0.006). Age of onset for patients with the presence of two other disease-causing NPHS2 mutations was 5.95 yr (range 0.0 to 14.3 yr) for children with one R138Q and one missense mutation (C). It was 4.17 yr (range 0.0 to 16.6 yr) for children with two missense mutations other than R138Q (D). Both groups (C and D) showed no significant difference compared with patients without disease-causing mutations (E through G; Table 1). The mean age at onset of SRNS was 6.87 yr (range 0.0 to 16.3 yr) for patients with no disease-causing NPHS2 mutation (E through G) and 6.40 yr (range 0.0 to 21.0 yr) for children free of NPHS2 changes (H; Table 1). Patients with at least one truncating mutation (nonsense or frameshift) or with homozygous R138Q mutations therefore showed a significantly earlier onset of SRNS than any other group of NPHS2 changes. Survival analysis shows that there is no significant difference in the time interval from onset of disease to development of ESRD between different mutation types (Figure 1B).
Figure 1.
Kaplan Meier survival analysis of time intervals between NPHS2 mutation groups A (truncating × any), group B (R138Q × R138Q), group C (R138Q × missense), and group D (missense × missense). (A) Age at onset of disease for all four groups. Each mutation group was compared with group A (earliest age of onset). The age at onset of disease between groups A and C was significantly different (P = 0.006). (B) The time interval from onset of disease to development of ESRD. Data on development of ESRD were present in one of nine patients in group C; therefore, this group was excluded from the calculation. Each mutation group was compared with group D (shortest time interval). The time interval was not significantly different between the groups.
The three patient groups with a single mutation or the sequence variant R229Q of unknown significance manifested later; however, there was no significant difference of age of onset neither among groups E, F, or G nor when they were compared with 267 individuals without NPHS2 changes (H). Later onset of SRNS in children with single NPHS2 mutations could thus not be observed in this cohort of patients manifesting between 0.0 and 21.0 yr of age. The mean age at manifestation for individuals in these groups was as follows (Table 1): Group E, 6.74 yr (range 0.8 to 16.3 yr) for individuals with single mutation and sequence variant R229Q; group F, 8.12 yr (range 1.6 to 14.7 yr) for patients with single mutations only; and group G, 6.48 yr (range 0.0 to 13.0 yr) for children with one single heterozygous or a homozygous R229Q sequence variant (Table 1).
Seventy-one (91.0%) of 78 patients with two NPHS2 mutations and clinical data manifested before their ninth birthday. Six of the seven children who had two mutations and were older than 9 yr at diagnosis carried two missense mutations other then R138Q only.
Discussion
We report here an NPHS2 genotype–phenotype correlation for age of onset of SRNS in the largest cohort of children who had SRNS and were examined for NPHS2 mutations published to date. Of 404 families with SRNS included in this study, 73 (18.1%) of 404 carried two disease-causing NPHS2 mutations, and 5.1% (21 of 404) carried only one single heterozygous mutation. The R229Q sequence variant of unknown significance was present in 9.2% (37 of 404) of families heterozygously and in 0.5% (two of 404) of families homozygously.
The R138Q mutation was found as the most frequent mutation in 42 (57.5%) of 73 families with two disease-causing mutations. This confirms in our large cohort the role of R138Q as a founder mutation as had been stated by Boute et al.3 in the initial description of NPHS2. The proportion of children who had SRNS and carried two recessive NPHS2 mutations was particularly high among children who were younger than 6 yr. Notably, the presence of at least one truncation mutation or of a homozygous R138Q mutation led to strikingly earlier onset (<1.77 yr) compared with all other groups of NPHS2 mutations (<4.17 yr). These findings will be important for the counseling of affected families and the prognostic evaluation and management of renal replacement therapy in children with SRNS.
Our analysis shows a genotype–phenotype correlation between specific NPHS2 mutations and age at onset. Nonsense, frameshift (“truncating mutations”), or homozygous R138Q resulted in significantly earlier presentation of SRNS than all other NPHS2 changes. In patients with these severe mutations, SRNS had started before 6 yr of age in 98.2% of cases. These findings are consistent with a study by Weber et al.13 Our study further shows that children with one truncating mutation (nonsense or frameshift) and one additional R138Q mutation manifest particularly early (mean 1.0 yr; range 0.0 to 5.5 yr; Supplementary Table 1). This finding further supports the idea that R138Q acts as a severe mutation leading to early onset of SRNS. Functional data have shown that podocin with the R138Q mutation is retained in the endoplasmic reticulum and that this condition results in disrupted targeting of nephrin (NPHS1) to lipid raft microdomains.15–17
Patients with only one R138Q mutation in combination with one missense mutation (group C) and patients with two missense mutations other than R138Q (group D) manifested significantly later than patients with two severe mutations (groups A and B; Table 1). Only seven children with two NPHS2 mutations in this study manifested at >9 yr of age. Six of these seven children carried the missense mutation V290M or V180M.12 The latter had been reported in association with late onset of SRNS by Weber et al.13 Therefore recessive NPHS2 mutations as a rule do predominantly result in childhood SRNS before the age of 9 yr.
Our data also show that identical mutations may result in onset of SRNS over a spectrum of several years. This was seen best in patients who had homozygous R138Q mutations and presented between 0.0 and 5.4 yr of age. We therefore conclude that specific mutations have predictive value for age at onset of SRNS, but additional modifying factors are involved. One could speculate that single heterozygous mutations in NPHS1 could act as a modifier of the NS phenotype in children, but this has been described only extremely rarely (three cases,18 one case,13 and no cases14); therefore, we did not examine patients for NPHS1 mutations. We investigated the influence of gender on age of presentation in all 73 patients with disease-causing mutations. The mean age at onset of disease in male patients was 2.74 yr; the mean age at onset of disease in female patients was 2.42 yr. These differences were not statistically significant (Supplementary Table 2).
To determine whether ethnic background had an influence on the age of presentation in patients with disease-causing mutations, we calculated mean age at onset of disease for four different ethnic groups: European patients, Central Slavic patients, Turkish patients, and others. The differences in ages among the groups are small, and statistical analysis was not done because most groups were too small to reach significant conclusions.
Given that NPHS2 is a recessive disease, only the presence of two disease-causing mutations explains the phenotype. It is not unusual, however, that the second mutation may not be detected in mutational analysis in recessive diseases. The clinical significance of single mutations and the NPHS2 sequence variant R229Q have been discussed in various studies.18–22 We therefore included single mutations and the sequence variant of unknown significance R229Q in our analysis as separate groups. The mean age at presentation for patients in whom one single NPHS2 mutation or R229Q was detected (groups E and F) was significantly different neither from patients with two missense mutations other then R138Q (group D) nor from patients without NPHS2 changes (group H). Our data therefore neither support the idea that single heterozygous mutations result in late onset SRNS nor eliminate this option.
Specifically, the allele frequency of the R229Q sequence variant of unknown significance has been reported as high as 3.9% in healthy individuals.22 We found this change in 9.2% (37 of 404) of our families in the heterozygous state and in only 0.5% (two of 404) of all families in the homozygous state. R229Q has been discussed as a modifier of SRNS18–20; however, its involvement in the development of FSGS seemed inconclusive to other authors.22 Our study does not allow a conclusion that R229Q should be considered a disease-causing mutation.
Ardiles et al.21 suggested a correlation between NPHS2 mutations and age of onset of SRNS. Our data put their suggestion on a statistically solid ground with some modification. We conclude that not homozygosity versus heterozygosity of NPHS2 mutations but rather the allelic type of mutations determines the phenotype by onset of NS. We think that, in addition to NPHS2 mutations, modifying factors would explain the finding that identical mutations lead to presentation at different ages.
Conclusions
The presence of two recessive NPHS2 mutations caused SRNS in 18.1% (73 of 404) families in this study. We demonstrate for the first time statistical significance for early onset of SRNS in children with truncating or homozygous R138Q mutations of NPHS2 (<1.77 yr) versus children with all other sequence variants (>4.17 yr), including recessive NPHS2 mutations, with single detected NHPS2 mutations, with isolated R229Q sequence variants, or without NPHS2 mutations. This finding will be important for the prognostic evaluation and management of renal replacement therapy in children with SRNS. The vast majority (56 [98.2%] of 57) of children with two truncating or homozygous R138Q mutations presented at <6 yr of age. Truncating or homozygous R138Q mutations are frequent pathogenic NPHS2 mutations. In this study, they were detected in 71.9% (59 of 82) of all families. Our correlation for age of onset in SRNS does not allow a conclusion on a pathogenic significance of the frequent NPHS2 sequence variant R229Q.
Concise Methods
Patient and Data Recruitment
Human subject research was approved by the University of Michigan institutional review board and the ethics commission of the University of Freiburg, Germany. The diagnosis of NS was made by pediatric nephrologists in specialized centers on the basis of published criteria.23 Patient recruitment for this study was worldwide with predominance of Central European individuals. After informed consent, clinical data were provided by enrolling specialists using a standardized questionnaire (http://www.renalgenes.org), which we have described previously.1 Blood samples of patients with NS were acquired and used for DNA extraction by standard methods.
To evaluate NPHS2 genotype–phenotype correlations, we analyzed children affected by SRNS only. SRNS was defined according to published criteria by Arbeitsgemeinschaft für Pädiatrische Nephrologie and the International Study of Kidney Disease in Children (ISKDC).23,24 Families in which affected individuals had shown any response to standard steroid treatment were excluded from this study. All patients were examined for mutations in WT1 by direct sequencing of exons 8 and 9.
Of 446 families with SRNS, we excluded from our analysis 42 families in which we identified mutations in NPHS1, LAMB2, or WT1. The cohort analyzed for mutations in NPHS2 thus included 404 families with 430 patients with SRNS (Table 1).
To compare age of onset between different NPHS2 sequence changes, we defined onset of SRNS as time of first detection of nephrotic-range proteinuria (>40 mg/m2 per h) or diagnosis of persistent low-grade proteinuria (>4 mg/m2 per h). Age at onset of SRNS was documented for 392 patients from 367 families and ranged from 0 to 21.0 yr.
When evaluating frequency of mutations, we considered numbers of families, because siblings have identical mutations. When evaluating clinical data, we considered numbers of patients, because siblings may differ in their clinical characteristics (see Table 1). A subgroup of 202 patients (181 families) in this study had been included in the analysis by Ruf et al.,1 in which it was studied under the aspect of steroid responsiveness among children with NPHS2 mutations, and 89 patients (80 families) have also been studied in an analysis of genetic causes of NSFL.14
Categories of NPHS2 Mutations
Patients with two recessive mutations (groups A through D) were classified on the basis of the suggestive data by Weber et al.13 as patients with at least one nonsense or frameshift mutation in combination with any second mutation (A), patients with the homozygous founder mutation R138Q (B),patients with a single heterozygous R138Q mutation in combination with one different missense mutation (C), and patients with two missense mutations other than R138Q (D; Table 1).
The phenotypic relevance of single NPHS2 mutations and sequence variants of unknown significance in general and the frequent sequence variant R229Q in particular have been discussed by several authors as being involved in the pathogenesis of SRNS.18–22 Because the pathogenic relevance of these findings has not yet been demonstrated conclusively, we also included single detected mutations and the R229Q sequence variant of unknown significance as separate categories (E through G). We subclassified patients with one heterozygous NPHS2 mutation in combination with sequence variant R229Q (E), patients with one heterozygous NPHS2 mutation only (F), and patients with one heterozygous or a homozygous sequence variant R229Q only (G; Table 1). Patients without NPHS2 mutations or sequence variant R229Q were defined as group H.
Mutation Analysis of NPHS2 by Direct Sequencing
Genomic DNA was isolated from blood samples using the Puregene DNA purification kit (Gentra, Minneapolis, MN) following the manufacturer's guidelines. Mutation analysis was performed by exon-flanking direct sequencing of all eight exons of NPHS2.1 Exon primers are available from the authors. For sequence analysis, the software SEQUENCHER (Gene Codes, Ann Arbor, MI) was used. For all detected mutations and other sequence variants, sequencing of both strands was performed. Segregation of these changes was confirmed by direct sequencing of parental samples when available. The absence of previously unpublished mutations was shown in 160 control chromosomes from healthy individuals of matched ethnic origin.
Statistical Analysis
We tested for differences of mean values of age of onset using a t test and considered P < 0.05 significant. Range of age of onset is given in years.
Disclosures
None.
Supplementary Material
Acknowledgments
F.H. is supported by grants from the National Institutes of Health (P50-DK039255, R01-DK076683), the Smokler Foundation, and the Thrasher Research Foundation.
Following are the members of the Study Group of the Arbeitsgemeinschaft für Pädiatrische Nephrologie (APN): A. Noyan (Adana, Turkey); A. Bakkaloglu (Ankara, Turkey); S. Spranger (Bremen, Germany); S. Briese, D. Müller, U. Querfeld (Berlin, Germany); G. Reusz (Budapest, Hungary); R. Bogdanovic (Belgrad, Serbia); B. Beck, B. Hoppe, M.T.F. Wolf (Cologne, Germany); K. Dittrich, J. Dötsch, C. Plank, E.-M. Rüth, W. Rascher (Erlangen, Germany); P. Hoyer (Essen, Germany); M. Schröder (Frankfurt, Germany); M. Brandis, A. Fuchshuber, M. Pohl, C. von Schnakenburg (Freiburg, Germany); C. Mache (Graz, Austria); F. Schäfer, T. Knüppel, O. Mehls, B. Tönshoff, D. Wenning (Heidelberg, Germany); M. Kemper, D.E. Müller-Wiefel (Hamburg, Germany); J.H.H. Ehrich, G. Offner (Hannover, Germany); M. Barenbrock (Hamm, Germany); T. Jungraithmayr, B. Zimmerhackl (Innsbruck, Austria); J. Misselwitz (Jena, Germany); S. Wygoda (Leipzig, Germany); D. Böckenhauer (London, UK); M. Schuhmacher (Luebeck, Germany); M. Benz, M. Griebel, J. Höfele, L. Weber (Munich, Germany); H. Fehrenbach (Memmingen, Germany); M. Bulla, E. Kuwertz-Bröcking, A. Schulze Everding (Muenster, Germany); M. Shenoy (Newcastle, UK); L. Patzer (Halle, Germany); T. Seeman (Prague, Czech Republic); A. Gianviti, G. Rizzoni (Rome, Italy); O. Amon (Tübingen, Germany); C. Licht (Toronto, Ontario, Canada); J. Mühleder (Wels, Austria); G. Laube, T. Neuhaus (Zürich, Switzerland); T. Stuckert (Zwickau, Germany).
We thank the patients and their parents for participation in this study. F.H. is the Frederick G.L. Huetwell Professor and Doris Duke Distinguished Clinical Scientist.
Published online ahead of print. Publication date available online at www.jasn.org.
See related editorial, “Podocyte-Specific Gene Mutations Are Coming of Age,” on pages 190–191.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt APN: Patients with mutations in NPHS2 (Podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15: 722–732, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 4: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Hasselbacher K, Wiggins RC, Matejas V, Hinkes B, Mucha B, Hoskins BE, Ozaltin, Nürnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Bröcking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nürnberg P, Zenker M, Hildebrandt F: Recessive missense mutations in LAMB2 as a cause of isolated and non-Pierson type congenital nephrotic syndrome. Kidney Int 70: 1008–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nurnberg G, Garg P, Verma R, Chaib H, Hoskins BE, Ashraf S, Becker C, Hennies HC, Goyal M, Wharram BL, Schachter AD, Mudumana S, Drummond I, Kerjaschki D, Waldherr R, Dietrich A, Ozaltin F, Bakkaloglu A, Cleper R, Basel-Vanagaite L, Pohl M, Griebel M, Tsygin AN, Soylu A, Muller D, Sorli CS, Bunney TD, Katan M, Liu J, Attanasio M, O'toole JF, Hasselbacher K, Mucha B, Otto EA, Airik R, Kispert A, Kelley GG, Smrcka AV, Gudermann T, Holzman LB, Nurnberg P, Hildebrandt F: Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet 38: 1397–1405, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Mucha B, Ozaltin F, Hinkes BG, Hasselbacher K, Ruf RG, Schultheiss M, Hangan D, Hoskins BE, Everding AS, Bogdanovic R, Seeman T, Hoppe B, Hildebrandt F, Members of the APN Study Group: Mutations in the Wilms’ tumor 1 gene cause isolated steroid resistant nephrotic syndrome and occur in exons 8 and 9. Pediatr Res 59: 325–331, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB: A mutation in the TRPC6 cation channel causes focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Reiser J, Polu KR, Moller CC, Kelan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somlo S, Mundel P: Getting a foothold in nephrotic syndrome. Nat Genet 24: 333–335, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Karle SM, Uetz B, Ronner V, Glaeser L, Hildebrandt F, Fuchshuber A: Novel mutations in NPHS2 detected in both familial and sporadic steroid-resistant nephrotic syndrome. J Am Soc Nephrol 13: 577–579, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Weber S, Gribouval O, Esquivel EL, Moriniere V, Tete MJ, Legendre C, Niaudet P, Antignac C: NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int 66: 571–579, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Hinkes BG, Mucha B, Vlangos CH, Gbadegesin R, Liu J, Hoskins B, Hasselbacher K, Hangan D, Ozaltin F, Bakkaloglu A, Zenker M, Hildebrandt F, Members of the APN Study Group: Nephrotic syndrome manifesting in the first year of life: two-thirds are caused by mutations in four genes (NPHS1, NPHS2, WT1, or LAMB2). Pediatrics 119: e907–e919, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Huber TB, Simons M, Hartleben B, Semetz L, Schmidts M, Grundlach E, Saleem MA, Walz G, Benzing T: Molecular basis of the functional podocin-nephrin complex: Mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet 12: 3397–3405, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ohashi T, Uchida K, Uchida S, Sasaki S, Nihei H: Intracellular mislocalization of mutant podocin and correction by chemical chaperones. Histochem Cell Biol 119: 257–264, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Nishibori Y, Liu L, Hosoyamada M, Endou H, Kudo A, Takenaka H, Higashihara E, Bessho F, Takahashi S, Kershaw D, Ruotsalainen V, Tryggvason K, Khoshnoodi J, Yan K: Disease-causing missense mutations in NPHS2 gene alter normal nephrin trafficking to the plasma membrane. Kidney Int 66: 1755–1765, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Koziell A, Grech V, Hussain S, Lee G, Lenkkeri U, Tryggvason K, Scambler P: Genotype/phenotype correlations of NPHS1 and NPHS2 mutations in nephrotic syndrome advocate a functional inter-relationship in glomerular filtration. Hum Mol Genet 11: 379–388, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Tsukaguchi H, Sudhakar A, Le TC, Nguyen T, Yao J, Schwimmer JA, Schachter AD, Poch E, Abreu PF, Appel GB, Pereira AB, Kalluri R, Pollak MR: NPHS2 mutations in late-onset focal segmental glomerulosclerosis: R229Q is a common disease-associated allele. J Clin Invest 110: 1659–1666, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira AC, Pereira AB, Mota GF, Cunha RS, Herkenhoff FL, Pollak MR, Mill JG, Krieger JE: NPHS2 R229Q functional variant is associated with microalbuminuria in the general population. Kidney Int 65: 1026–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Ardiles LG, Carrasco AE, Carpio JD, Mezzano SA: Late onset of familial nephrotic syndrome associated with a compound heterozygous mutation of the podocin-encoding gene. Nephrology 10: 553–556, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Franceschini N, North KE, Kopp JB, McKenzie L, Winkler C: NPHS2 gene, nephrotic syndrome and focal segmental glomerulosclerosis: A HuGE review. Genet Med 8: 63–75, 2006 [DOI] [PubMed] [Google Scholar]
- 23.APN: Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet 1: 380–383, 1988 [PubMed] [Google Scholar]
- 24.ISKDC: Primary nephrotic syndrome in children: Clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity—A Report of the International Study of Kidney Disease in Children. Kidney Int 20: 765–771, 1981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.