Abstract
The dramatic effects of the anti-IgE mAb omalizumab to lower free IgE levels and FcεRI levels on basophils contrast with more modest clinical effects. Accordingly, whether IgE modulates FcεRI levels and FcεRI-dependent mediator release in vitro on human skin mast cells (MCTC type) that had matured in vivo is of interest. IgE reversibly enhanced FcεRI levels on MCTC cells in a dose- and time-dependent manner (up-regulation t1/2 of 4–5 days with 1–3 μg/ml IgE), without affecting cell proliferation. A molar ratio of omalizumab to IgE of 0.9 at baseline prevented receptor up-regulation by 50%, whereas adding omalizumab to MCTC cells already with IgE-enhanced FcεRI levels at molar ratios of 5, 12.5, and 31 reduced FcεRI levels to baseline with respective t1/2 values of 8.7, 6.3, and 4.8 days. MCTC cells with IgE-enhanced FcεRI levels were more sensitive to stimulation with a low dose of anti-FcεRI mAb in terms of degranulation and production of PGD2, GM-CSF, IL-6, IL-13, and TNF-α. Reducing up-regulated FcεRI levels with omalizumab also reduced mediator release to a low dose of anti-FcεRI mAb to baseline by 3–4 wk. Thus, reducing free IgE should decrease the hypersensitivity of allergic individuals to low naturally occurring concentrations of allergens.
Mast cells and basophils of mice and humans are key effector cells of immediate hypersensitivity reactions that are initiated by aggregating the high-affinity receptor for IgE, FcεRI, on the surface of such cells. Cross-linking of FcεRI bound to IgE with multivalent Ag or IgG anti-IgE, or of FcεRI directly with IgG against the α-chain of FcεRI results in the immediate release of the preformed mediators stored in secretory granules (degranulation), including histamine, tryptase, β-hexosaminidase, and heparin, followed shortly thereafter by the de novo production and secretion of arachidonic acid metabolites such as PGD2 and leukotriene C4, and later the de novo production of cytokines, including GM-CSF, TNF-α, IL-6, and IL-13. The actions of these mediators lead to the clinical signs and symptoms associated with acute and chronic allergic reactions.
Interestingly, IgE itself regulates the level of FcεRI on the surface of mast cells and basophils. In mice, binding of monomeric IgE to FcεRI improves mast cell survival (1) and may directly enhance mediator release (2) within minutes after binding by spontaneously aggregating these receptors (3). Over days, monomeric IgE increases surface expression of FcεRI on basophils (4) and mast cells (5). In humans, too, developing or immature mast cells derived in vitro from progenitor cells in fetal liver (6), bone marrow, or cord blood (7) exposed to monomeric IgE in culture exhibit enhanced surface expression of FcεRI. Furthermore, such mast cells with IgE-enhanced FcεRI levels respond to lower concentrations of Ag or anti-IgE, and release greater maximal amounts of preformed histamine (human and mouse) and serotonin (mouse) as well as newly synthesized lipid mediators, and cytokines and chemokines when challenged with high concentrations of these stimulants. A similar effect has been observed for human basophils obtained from peripheral blood, where enhanced surface expression of FcεRI by IgE results in an enhanced degranulation response to FcεRI cross-linking (8, 9). Modulation of FcεRI levels by IgE has been attributed to a reduction in receptor loss rather than enhanced synthesis (10).
Although all human mast cells express histamine, heparin proteoglycan, and tryptase in their secretory granules, and Kit and FcεRI on their surface, mast cells have been divided into two types. The type of mast cell in normal skin is called the MCTC type, and is distinguished from the MCT type of mast cell by expression of chymase, carboxypeptidase, and cathepsin G in their secretory granules, and CD88 on their cell surface (11-14). MCTC cells predominate in skin, conjunctiva, bowel submucosa, perivascular tissue, and airway smooth muscle, and also reside in nasal mucosa, airway mucus glands, and mucosa of the conducting airways (15). In asthmatics, a marked increase in the MCTC type of mast cell in airway smooth muscle has been observed (16). Skin cells proliferate and retain their mature phenotype when MCTC cultured with stem cell factor (SCF)3 in serum-free medium (17). Such tissue-derived mast cells differ in some respects from in vitro-derived mast cells, e.g., skin MCTC cells express activating FcγRIIa and not inhibitory IIb, whereas cord blood-derived mast cells express FcγRIIb and not IIa (18).
In humans in vivo, serum IgE levels correlate with FcεRI levels on peripheral blood basophils (19). Moreover, treatment of allergic patients with the humanized anti-IgE mAb, omalizumab (which binds the Fc portion of free IgE and thereby prevents IgE binding to FcεRI), decreases free but not total IgE levels in serum. Basophils examined after omalizumab treatment exhibit diminished expression of surface FcεRI, the t1/2 for the decrease being ~3 days (20). However, because basophils are relatively short-lived cells (turning over every few days), those examined after treatment may represent mostly newly developed cells that matured in an environment of low levels of free IgE. Despite the marked reductions in free IgE levels in serum and FcεRI levels on basophils in response to anti-IgE therapy, the clinical response of atopic asthmatics and rhinitic patients, although significant, is far less dramatic (21-24). Consequently, the question arises as to whether mature, long-lived, tissue-derived mast cells respond as do peripheral blood basophils and developing mast cells to variations in IgE concentrations. Suggesting they do are the observations that omalizumab-treated atopic subjects show diminished responses to allergen skin tests and the mast cells in biopsies taken from such sites show diminished FcεRI levels on mast cells by immunocytochemistry (25).
The current study shows that MCTC cells from human skin undergo a dramatic up-regulation of surface FcεRI when exposed to elevated but physiological levels of IgE and become hyperresponsive to low levels of IgE cross-linking. This enhancement in receptor expression and consequent hypersensitive phenotype is shown to be preventable by omalizumab, and also reversible if free IgE is either removed by washing or neutralized by omalizumab. Within 3–4 wk after the onset of receptor down-regulation, both FcεRI levels and hypersensitivity return to baseline. This indicates that mast cells with IgE-enhanced FcεRI expression in vivo may be highly sensitive to low naturally occurring concentrations of allergens, and reducing free IgE levels should decrease FcεRI levels as well as this hypersensitive phenotype.
Materials and Methods
Reagents
The IgG anti-FcεRIα mAb (22E7) was generously provided by J. P. Kochan (Hoffman-LaRoche) (26); recombinant human SCF by Amgen and humanized anti-IgE mAb (omalizumab) by Genentech (South San Francisco, CA). Collagenase type 2 (Worthington Biochemical); hyaluronidase, type 1 DNase, amphotericin B, antibiotic/antimycotic solution, p-nitrophenyl N-acetyl-β-d-glucosaminide, soybean trypsin inhibitor (SBTI) (3), and 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (Sigma-Aldrich); X-VIVO 15 medium (Cambrex); rat anti-mouse IgG (A85-1), mouse IgG1 (X40) isotype control, mouse anti-human Kit (YB5.B8; IgG1), mouse anti-CD64 (10.1), mouse anti-CD32 (FLI8.26), and mouse anti-CD16 (3G8) (BD Biosciences); and chimeric IgE mAb (human Fcε and mouse Fab) (clone JW8/1; Serotec) and polyclonal human IgE (RDI Division of Fitzgerald Industries International) were purchased as indicated.
Purification and culture of mature human skin-derived mast cells
Fresh samples of human skin from breast reductions, mastectomies, or abdominoplasties were obtained from Department of Pathology at Virginia Commonwealth University, Cooperative Human Tissue Network of the National Cancer Institute, or the National Disease Research Interchange, as approved by the Human Studies Internal Review Board at Virginia Commonwealth University. Mast cells were dispersed from human skin, enriched, and placed into culture essentially as described (17). After removing s.c. fat by blunt dissection, residual tissue was cut into 1- to 2-mm3 fragments and digested with type 2 collagenase (1.5 mg/ml), hyaluronidase (0.7 mg/ml), and type 1 DNase (0.3 mg/ml) for 3 h at 37°C in HBSS buffer (1× HBSS, 0.04% NaHCO3, 1% FBS, 1.25 μg/ml Amphotericin B, and 1× antibiotic/antimycotic solution). The dispersed cells were collected by filtering the first digest through a no. 80 mesh stainless-steel sieve, and then through a 70-μm nylon cloth (BD Biosciences). The cells were washed, resuspended in HBSS buffer, layered over a Percoll cushion, and centrifuged at 1600 rpm at 15°C for 20 min. Nucleated cells at the buffer/Percoll interface were collected, washed, and resuspended at 5 × 105 cells/ml in serum-free X-VIVO 15 medium (Cambrex) containing 100 ng/ml recombinant human SCF. The cells were cultured in 24-well plates at 2 ml/well with weekly medium changes, and the cells were split when they reached a concentration of ~2 × 106 cells/ml. The percentages of mast cells were assessed cytochemically by metachromatic staining of cytospun cells with acidic toluidine blue. Typically, cultures of mature mast cells of 95–100% purity were obtained by 6 wk as assessed by cytochemistry or by flow cytometry with anti-Kit (YB5.B8) and anti-FcεRIα (22E7) mAbs, and 8- to 12-wk-old cultures were used in the experiments described below.
FcεRI expression
To study the surface expression of FcεRI, skin-derived mast cells were cultured in X-VIVO 15 medium containing 100 ng/ml SCF and varying amounts of polyclonal human IgE, typically 1 μg/ml IgE for 7 days unless stated otherwise. FcεRI surface expression also was examined after IgE was removed from the medium by washing the cells three times and placing them in culture with IgE-free X-VIVO 15 containing SCF or by addition of omalizumab to IgE-containing medium, or by removing IgE and adding omalizumab. Surface expression of FcεRI was assessed by flow cytometry after labeling with 22E7 and quantitated and expressed as mean fluorescence intensity (MFI).
Mast cell activation
Mature human skin mast cells (106 cells/ml) were activated with varying concentrations of 22E7 at 37°C. Activation of cells for cytokine production was conducted in X-VIVO 15 containing 100 ng/ml recombinant human SCF and 100 μg/ml SBTI (27) for 24 h. Activation to assess degranulation and PGD2 production was for 30 min in X-VIVO 15 medium without SCF or SBTI. Briefly, the cells were washed and suspended at 2 × 106 cells/ml in the appropriate medium and preincubated for 5 min at 37°C. Then, an equal volume of prewarmed medium containing varying amounts of 22E7 was added, and the cells were incubated for 30 min or 24 h at 37°C.
β-Hexosaminidase and PGD2 release and measurements
Human skin cells were activated for 30 min with varying concentrations of 22E7 at 37°C as described above. Following the activation period, the mast cells and supernatant were separated by centrifugation at 4000 rpm for 10 min at 4°C. The cell pellet was resuspended in equal volume PBS, sonicated (power 4, 50% pulse cycle times four pulses; Branson sonifier, model no. 350), and microfuged to remove debris. β-Hexosaminidase from both supernatant and cell lysate was assayed by measuring release of p-nitro-phenol from the substrate p-nitrophenyl N-acetyl-β-d-glucosaminide as described (28). Absorbance values were read at 405 nm. Degranulation was calculated as a net percentage release value using the following formula: net % release = ((stimulated releasate − spontaneous releasate)/(stimulated(releasate + retentate) − spontaneous releasate)) × 100.
PGD2 was measured from the same releasate used to measure β-hexosaminidase. The PGD2 enzyme immunoassay kit from Cayman Chemical was used, and the assay was conducted according to the manufacturer’s instructions. The lower limit of detection was 10 pg/ml.
Cytokine measurements
Human skin cells were activated for 24 h with the indicated concentrations of 22E7 at 37°C as described above. Following the activation period, mast cells were separated from the releasates by centrifugation (model 5810; Eppendorf AG) at 4000 rpm for 10 min at 4°C. Cytokines released upon activation and present in the supernatants were measured by ELISA in a 384-well format (27). Purified and biotinylated rat Abs (BD Biosciences) specific for IL-6, GM-CSF, TNF-α, and IL-13 and standard recombinant cytokines were as follows: IL-6 (rat MQ2-13A5/MQ2-39C3), GM-CSF (rat BVD2-23B6/BVD2-21C11), TNF-α (rat MAb1/MAb11), and IL-13 (rat JES10-5A2/B69-2). Wells were coated overnight at 4°C with capture mAbs, blocked with 1% BSA in PBS for 1 h at room temperature, washed with 0.05% Tween 20/PBS, and incubated overnight at 4°C with experimental samples or serially diluted recombinant human cytokines (IL-6, GM-CSF, and TNF-α were from BD Biosciences, and IL-13 from Pierce Endogen) of known concentration to generate standard titrations curves. The wells were washed and incubated with biotinylated detection Abs for 1 h at room temperature, washed, incubated with avidin-peroxidase for 30 min at room temperature, washed, and developed with the peroxidase substrate, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid. Absorbance values at 405 nm were measured using a SpectraMax 384 Plus UV-VIS plate reader (Molecular Devices). The lower limit of detections under these conditions was 31 pg/ml.
Mast cell proliferation
Cellular proliferation was assessed by labeling mature human skin mast cells with CFSE using the CellTrace CFSE Cell Proliferation kit (Molecular Probes). Human mast cells (106), prewarmed in 1 ml of PBS containing 0.1% BSA, were incubated with 1 μl of a 5 mM stock solution of CFSE in DMSO (final concentration of 5 μM) at 37°C for 10 min. The labeling was stopped by adding 5 ml of ice-cold culture medium and incubating for 5 min on ice. The cells were then washed three times in fresh culture medium, and suspended at 2 × 105 cell/ml in X-VIVO 15 medium containing SCF (100 ng/ml) with or without IgE (1 μg/ml) and placed in culture. Fluorescence intensity was analyzed daily for 8 days by flow cytometry. At the same time, surface expression of FcεRI was determined with 22E7 as described above.
Results
Monomeric IgE up-regulates FcεRI expression on mature human skin mast cells
To determine the effect of IgE mAb on the surface expression of FcεRI on human mature skin mast cells, the cells were cultured with 3 μg/ml chimeric human Fcε:mouse Fab IgE mAb for up to 27 days. Surface expression of FcεRI was measured at multiple time points by flow cytometry using the noncompetitive anti-FcεRI mAb, 22E7. During the time course of this experiment, SCF and IgE along with medium were replenished at weekly intervals. Fig. 1A shows that IgE mAb dramatically increased FcεRI surface expression on human skin mast cells. Under these conditions, the MFI levels of FcεRI increased ~10-fold over basal levels by 2 wk. A t1/2 of 3.6 days for FcεRI up-regulation by IgE mAb was calculated after fitting the data to an exponential equation. To determine the dose-response effect of IgE on surface FcεRI levels, human skin mast cells were cultured with 0–10 μg/ml IgE mAb for 2 wk, after which FcεRI expression levels were measured. As shown in Fig. 1B, the ED50 of chimeric IgE mAb at 2 wk was calculated to be 0.6 μg/ml (240 IU/ml). The receptor-enhancing effect of IgE was restricted to FcεRI, because no effect on expression levels of Kit, FcγRI, FcγRII(a,b,c), or FcγRIII was observed after 7 days of culture with IgE (Fig. 1C).
FIGURE 1.
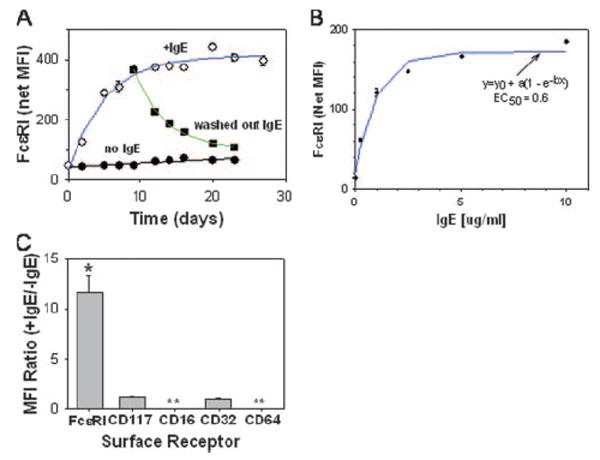
IgE enhances FcεRI expression on the surface of skin mast cells. A, Time course for up-regulation of FcεRI by chimeric human IgE mAb (3 μg/ml) and down-regulation by washing out IgE as measured by flow cytometry. ○, Up-regulation with IgE; ■, washout of IgE; ●,no IgE. The t1/2 for FcεRI up-regulation (3.6 days) was calculated after fitting the data to an exponential equation [y = yo+a(1 − e−bt)], where y is the MFI at t > 0 h, y0 is the MFI at 0 h, t is time (in hours), a is the maximal net increase in MFI, and b is a calculated constant. The t1/2 for FcεRI down-regulation (5.1 days) was calculated from the exponential decay equation [y = yo + ae−bt], where yo is the FcεRI-dependent MFI in cultures for which IgE had never been added, y is the MFI over time after IgE had been removed, a is the net increase in MFI above y0 at the time IgE was re moved, t is the time after IgE had been removed, and b is a calculated constant. B, Dose-response of FcεRI up-regulation by IgE after 2 wk. The ED50 (0.6 μg/ml) was calculated by fitting the data to the exponential equation y =y0 + a(1 – e−bx), where y is the MFI when the IgE concentration (in micrograms per milliliter) is >0, y0 is the MFI when IgE concentration is 0, x is the IgE concentration, a is the maximal net increase in MFI, and b is a calculated constant. C, FcεRI is selectively up-regulated on skin mast cells by IgE. IgE (1 μg/ml) was added to skin mast cell cultures for 7 days at which time the MFI ratios (+IgE/ −IgE cultures) for FcεRI, CD117, CD16, CD32, and CD64 were determined. *, p < 0.05 compared with unity, Student’s paired two-tailed t test. **, None detected.
The reversibility of IgE-enhanced expression of FcεRI was examined by removing IgE (washing three times) from cells that had been in culture with IgE mAb (3 μg/ml) for 10 days and reculturing them in IgE-free medium. Washing out the IgE resulted in the decline of FcεRI expression with a calculated t1/2 of 5.1 days after fitting the data to an exponential decay equation (Fig. 1A).
Anti-IgE mAb (omalizumab) down-regulates and prevents IgE-induced FcεRI augmentation
Whether omalizumab could prevent or reverse IgE-enhanced expression of FcεRI on human mature mast cells was examined in the current system. In one set of experiments, neutralizing doses were added simultaneously with IgE to examine prevention of IgE-enhanced FcεRI expression, and in a second set omalizumab was added after FcεRI had been up-regulated by IgE to examine down-regulation of this receptor. For these experiments, a commercial preparation of polyclonal human IgE (Biodesign International, Saco, ME) was used, in part because sales of the chimeric IgE mAb had been discontinued, and also because polyclonal IgE may better reflect natural conditions. As shown in Fig. 2A, IgE-enhanced FcεRI expression could be attenuated by coincubating increasing amounts of anti-IgE with IgE. Anti-IgE added to IgE at a weight ratio of 0.7 (molar ratio of 0.9) decreased the enhancement of the FcεRI MFI at 1 wk by 50%, whereas a weight ratio of 2.3 (molar ratio of 2.9) decreased IgE enhancement by 90%.
FIGURE 2.
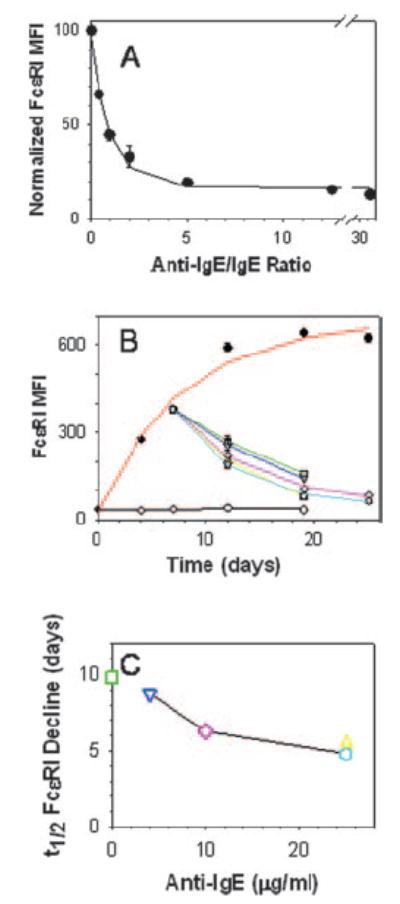
IgE-dependent enhancement of FcεRI on skin mast cells is both prevented and reversed by anti-IgE mAb. A, Anti-IgE attenuated IgE-mediated enhancement of FcεRI MFI. Polyclonal human IgE (1 μg/ml) in medium containing varying amounts of anti-IgE (omalizumab) was added to skin mast cell cultures for 7 days, at which time the FcεRI MFI was determined. The data were fit to an exponential decay equation (y = y0 + ae−bx), where y0 is the MFI at the time IgE with or without anti-IgE were added to the mast cell cultures, a is the maximal net increase in MFI above y0, b is a calculated constant, and x is the weight ratio of anti-IgE to IgE. Data from each of three separate cultures were first normalized to the maximal MFI achieved (set to 100), and then combined for analysis. Maximal MFI values were 292, 328, and 673. B, Anti-IgE down-regulates IgE-enhanced FcεRI expression. Skin mast cells were cultured without (○, black line) or with (●, red line) polyclonal human IgE (1 μg/ml) for 1 wk. At that time, the cells were washed, and a portion was resuspended with IgE-free medium (□, green line) and another portion with medium containing anti-IgE (25 μg/ml) (Δ, yellow line). Alternatively, IgE was left in the culture and anti-IgE was added at concentrations of 4 μg/ml (▽, blue line), 10 μg/ml (◊, purple line), and 25 μg/ml (
 , light blue line). Each data point is the mean of triplicate determinations. C, Relationship of the t1/2 for the decline in IgE-enhanced FcεRI MFI to the amount of anti-IgE. Data from B were used to calculate t1/2 values. Symbols are the same as in B, and symbol colors correspond to the line colors in B.
, light blue line). Each data point is the mean of triplicate determinations. C, Relationship of the t1/2 for the decline in IgE-enhanced FcεRI MFI to the amount of anti-IgE. Data from B were used to calculate t1/2 values. Symbols are the same as in B, and symbol colors correspond to the line colors in B.
Down-regulation of IgE-enhanced FcεRI is shown in Fig. 2B. First, addition of 1 μg/ml polyclonal IgE resulted in a >10-fold increase in FcεRI expression on mast cells by 7 days of culture (378 ± 12 MFI units) compared with mast cells cultured without IgE (35 ± 2 MFI units). The continued presence of IgE until day 25 further increased FcεRI MFI to 624 ± 11, and yielded a t1/2 for FcεRI up-regulation by polyclonal IgE of 4.9 days. Removal of IgE from the cultures by stringent washing alone at day 7 resulted in a 58% decrease in FcεRI MFI to 158 ± 1 by day 12 with a calculated t1/2 of 9.8 days. Of note, the t1/2 of FcεRI decline by washing out IgE in these experiments was somewhat longer than that observed in Fig. 1. This might reflect differences in the monoclonal and polyclonal IgE preparations used. Fig. 2B also shows the effect of different doses of omalizumab on FcεRI expression on mast cells that had been cultured for 7 days with 1 μg/ml IgE. A dose-dependent decline in FcεRI MFI was observed when omalizumab was added to the cultures at 4 μg/ml (5:1 anti-IgE to IgE molar ratio), 10 μg/ml (12.5:1), and 25 μg/ml (31:1). At these concentrations, respective FcεRI MFI levels decreased by 62, 73, and 79% 12 days after addition of omalizumab, and the respective t1/2 values for the declines in FcεRI MFIs were calculated to be 8.7, 6.3, and 4.8 days. Furthermore, when IgE was removed and anti-IgE (25 μg/ml) added, an 80% reduction in expression was observed by 12 days and the rate of decline yielded a t1/2 of 5.5 days, similar to the results observed at the highest concentrations of anti-IgE in the presence of IgE. Thus, at each of the concentrations of omalizumab used in the presence of IgE, FcεRI levels declined more rapidly than when IgE was simply removed from the medium (Fig. 2C). This suggests that excess omalizumab is more effective at reducing IgE-enhanced FcεRI levels than simply washing away free IgE, perhaps by trapping IgE as it spontaneously dissociates from FcεRI.
IgE has no effect on proliferation of human skin mast cells
Because monomeric IgE has been implicated in murine mast cell proliferation, the possibility that monomeric polyclonal IgE might also affect proliferation of human skin mast cells was considered. Cells were labeled with CFSE, an indicator of cellular proliferation, and cultured with and without 1 μg/ml IgE. Loss of CFSE fluorescence and FcεRI expression were measured at daily intervals by FACS analysis. Fig. 3 shows the patterns of mast cells cultured without (A and B) and with (C and D) IgE after 8 days in culture. As expected, mast cells cultured with IgE expressed significantly more FcεRI than cells cultured without IgE. However, the patterns of CFSE fluorescence were similar in both groups. In other words, the losses of CFSE fluorescence in the plus and minus IgE groups were comparable over the 8 days experiment (B and D). To better quantify receptor expression and CFSE fluorescence, data were extracted from each of the three distinct gates based on the FcεRI/CFSE profiles (Fig. 3A: R5, R6, and R7, and Fig. 3B: R2, R3, and R4). Table I shows the MFI data for FcεRI expression and CFSE labeling for the representative experiment in Fig. 3. As indicated in the two histograms, a stable MFI for CFSE labeling in the three gated groups of both cultures confirmed comparable distributions of proliferating cells. Interestingly, an inverse relationship between CFSE labeling and FcεRI expression on IgE-cultured mast cells was observed. Although FcεRI MFI was stable among all three gated groups in the cells cultured without IgE, it was 4.4-fold higher on IgE-cultured cells that had not proliferated (R2) compared with those that had undergone two rounds of division (R4). Thus, although basal FcεRI levels do not vary appreciably with mast cell proliferation, IgE-enhanced FcεRI levels appear to be higher on cells that had not proliferated.
FIGURE 3.
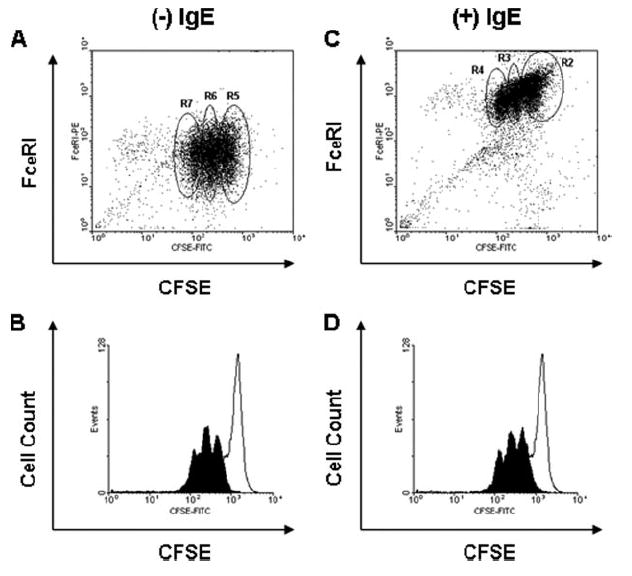
IgE-enhanced expression of FcεRI does not affect proliferation. Skin mast cells were labeled with CFSE and cultured without (A and B) and with (C and D) polyclonal human IgE (1 μg/ml) for 8 days, and analyzed for relationships between FcεRI expression and CFSE fluorescence (A and C) and between cell counts and CFSE fluorescence (B and D). The open histograms in B and D were produced within 24 h after CFSE labeling, whereas the closed histograms were produced 8 days after labeling. Mean data from the regions labeled in the A and C are presented in Table I from four independent experiments.
Table I.
FcεRI expression and CFSE labelinga
| Gate | CFSE | FcεRI |
|---|---|---|
| −IgE | ||
| R5 | 517 | 57 |
| R6 | 232 | 51 |
| R7 | 105 | 47 |
| +IgE | ||
| R2 | 520 | 1522 |
| R3 | 227 | 970 |
| R4 | 118 | 717 |
Expressed as MFI as described in Fig. 3.
Increased expression of FcεRI enhances degranulation and production of PGD2 and cytokines when FcεRI is aggregated at low concentrations of anti-FcεRI mAb
Because mast cell activation is initiated by FcεRI aggregation, the consequence of IgE-enhanced FcεRI expression on mediator release was of interest. After 7 days of culture in the presence or absence of IgE, human skin mast cells were challenged with suboptimal to optimal concentrations of 22E7 and analyzed for the effect of IgE-enhanced FcεRI to affect degranulation and release of PGD2 (Fig. 4), and production of GM-CSF, TNF-α, IL-6, and IL-13 (Fig. 5). Strikingly, activation with 0.001 μg/ml 22E7 resulted in significant enhancements in degranulation and PGD2 release from mast cells with IgE-enhanced FcεRI expression. Net percentage β-hexosaminidase release modestly increased from 0 to 0.1 4%, whereas PGD2 production markedly increased from 0.8 ± to 59 ± 6 ng/106 mast cells. In contrast, the maximal levels of degranulation and PGD2 release observed at higher 22E7 concentrations were not affected by up-regulating FcεRI expression. Also noteworthy is that spontaneous release values for β-hexosaminidase and PGD2 were not affected by the IgE incubation.
FIGURE 4.
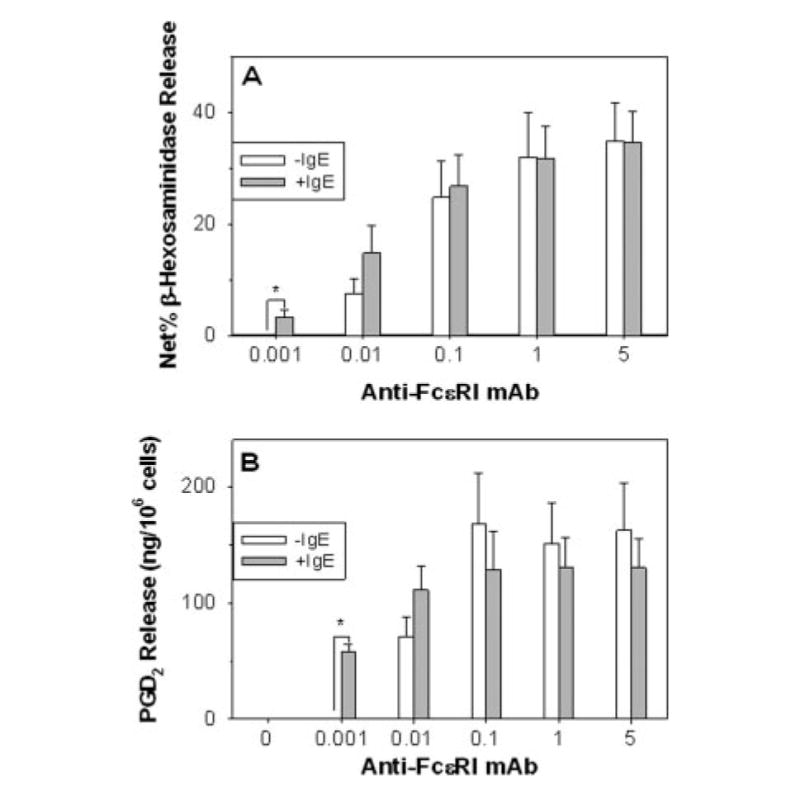
Skin mast cells with IgE-enhanced FcεRI expression are more sensitive to 22E7 in terms of degranulation and PGD2 secretion. Skin mast cells were cultured without or with polyclonal human IgE (1 μg/ml) for 1 wk, and then stimulated with varying concentrations of 22E7 for 30 min at 37°C. Degranulation in terms of β-hexosaminidase release (A) and PGD2 release (B) were then assessed. Mean ± SE data are shown for five (β-hexosaminidase) and four (PGD2) independent experiments. Spontaneous percentage release values (mean ± SD) of 5.3 ± 1.2 (−IgE) and 5.2 ± 1.4 (+IgE) were not significantly different. *, p < 0.05 between −IgE and +IgE.
FIGURE 5.
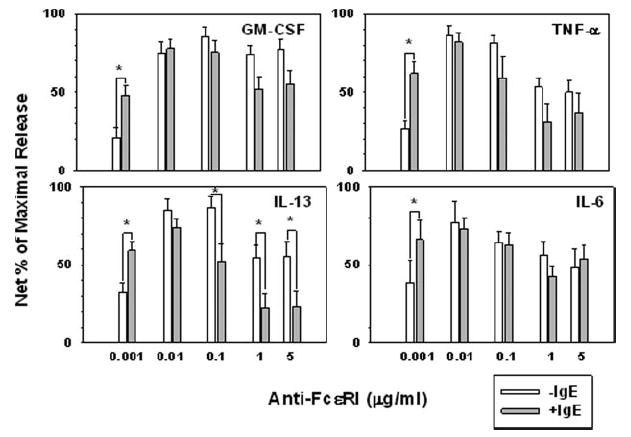
Skin mast cells with IgE-enhanced FcεRI expression are more sensitive to low-level FcεRIα aggregation-induced cytokine secretion. Skin mast cells were cultured without or with polyclonal human IgE (1 μg/ml) for 7 days, and then stimulated with varying concentrations of 22E7 for 24 h at 37°C in medium containing SCF and SBTI. The concentrations of cytokines in the cell-free supernatants were quantified by ELISA. The bars represent the mean ± SEM of 12 (GM-CSF), 10 (TNF-α), and 9 (IL-13 and IL-6) independent experiments with different mast cell preparations. Spontaneous release values (median) for minus vs plus IgE groups, respectively, did not differ significantly (Mann-Whitney rank sum test, p > 0.05) for GM-CSF (120 and 100 pg/ml), TNF-α (72 and 43 pg/ml), IL-13 (<32 and <32 pg/ml), and IL-6 (545 and 621 pg/ml). Maximal release values (net) varied between different mast cell preparations: GM-CSF (199–25,998 pg/106 cells), TNF-α (176–2,677 pg/106 cells), IL-13 (110–2,506 pg/106 cells), and IL-6 (590–4,997 pg/106 cells). *, p < 0.05 (Student’s paired two-tailed t test between −IgE and +IgE).
The effects of IgE-enhanced FcεRI on 22E7-stimulated cytokine production are shown in Fig. 5. Mast cells were activated for 24 h with 0–5 μg/ml 22E7 in culture medium containing SBTI to prevent cytokine degradation (27). A high degree of variability in the amount of cytokine produced among human mast cell cultures from different individuals was noted (data not shown). Consequently, to enable data from different experiments to be combined and analyzed, the data from each experiment were normalized to the maximal cytokine level and illustrated as a percentage of maximum release. The maximal release of IL-6, GM-CSF, TNF-α, and IL-13 from mast cells expressing basal FcεRI levels tended to occur at a higher concentration of 22E7, generally 0.1 μg/ml, than for mast cells expressing high FcεRI levels, 0.01 μg/ml. Furthermore, mast cells with enhanced compared with basal FcεRI expression released significantly and substantially more cytokine upon activation with 0.001 μg/ml 22E7. This included increases in mean normalized net production values (as a percentage of maximal) from 39 to 66% for IL-6, from 21 to 48% for GM-CSF, from 32 to 59% for IL-13, and from 26 to 62% for TNF-α. Interestingly, the amounts of IL-13 produced by mast cells that had been activated with 0.1, 1.0, and 5.0 μg/ml 22E7 were significantly lower from mast cells with IgE-enhanced FcεRI than from those with basal FcεRI levels. Spontaneous release values for these cytokines were not significantly affected by up-regulating FcεRI with IgE. Also, after FcεRI levels on skin mast cells had been enhanced with IgE, a detectable increase in MFI with 22E7 above an isotypematched control was only evident at 0.01 μg/ml 22E7 and above, even though mediator release was observed at 0.001 μg/ml 22E7. The key point is that mast cells with IgE-enhanced FcεRI expression became more sensitive to stimulation with a low level of FcεRI cross-linking in terms of degranulation, PGD2 release, and cytokine production.
Down-regulation of IgE-induced FcεRI expression to basal levels reduces sensitivity to FcεRI cross-linking to basal levels
We questioned whether the hypersensitive phenotype of mast cells with enhanced FcεRI expression would revert to baseline levels after reducing the number of receptors on those cells. To answer this question, human skin mast cells were cultured in the absence or presence of IgE (1 μg/ml) for 7 days and then with added omalizumab (25 μg/ml) over a 4-wk period to down-regulate receptor expression, similar to what was shown in Fig. 2. At weekly intervals, FcεRI expression was measured; samples of mast cells were activated with low (0.001 μg/ml) and high (1 μg/ml) concentrations of 22E7; and degranulation and production of PGD2 were assessed. In these experiments, omalizumab and IgE were replenished weekly. Fig. 6 shows clearly that down-regulating IgE-induced FcεRI surface expression with anti-IgE Ab over a 4-wk period (Fig. 6A) also reduces the level of degranulation (B) and the amount of PGD2 secretion (D) following activation with a low concentration (0.001 μg/ml) of 22E7. As expected, mast cells expressing basal and enhanced levels of FcεRI responded equally to activation with a high concentration (1.0 μg/ml) of 22E7, and this response was unaffected by anti-IgE Ab over the course of these experiments (Fig. 6, C and E). Surprisingly, a delay in the decreased release of β-hexosaminidase and PGD2 relative to the down-regulation of FcεRI was apparent. Although FcεRI expression had decreased 68% by 7 days after anti-IgE exposure, degranulation and PGD2 release were as high as or slightly higher than at day 0 (Fig. 6, B and D). However, both degranulation by 3 wk and PGD2 release by 4 wk had diminished to basal levels.
FIGURE 6.
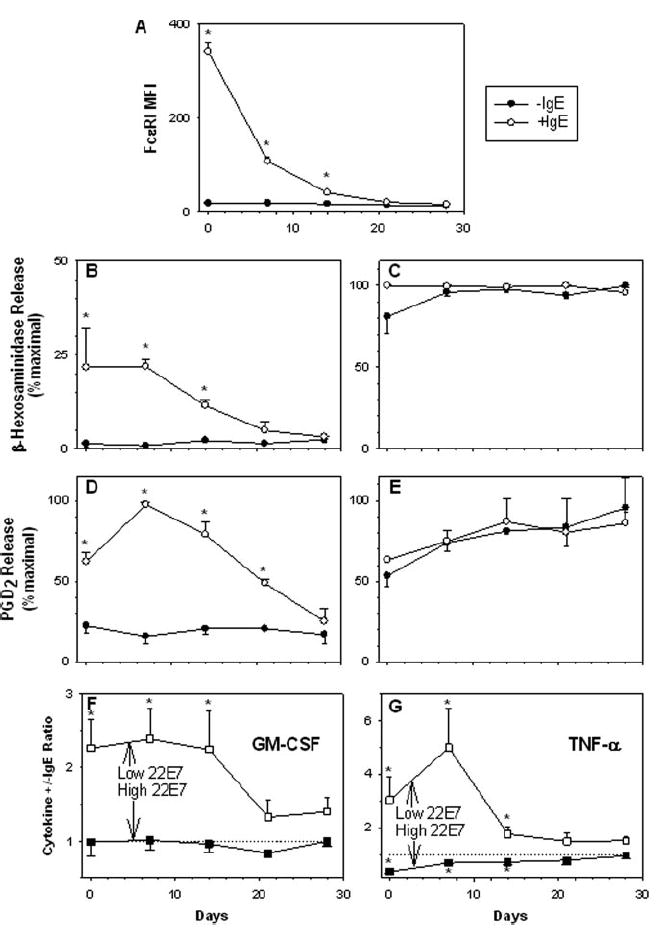
Down-regulation of IgE-enhanced FcεRI expression with omalizumab reverses augmented degranulation and production of PGD2, GM-CSF, and TNF-α to basal levels. Skin mast cells were cultured for 7 days without (●) or with (○) polyclonal human IgE (1 μg/ml) (A–E), and then the anti-IgE mAb omalizumab (25 μg/ml) was added over a 4-wk period. Omalizumab and IgE were replenished weekly. FcεRI levels were measured weekly (A) and samples of mast cells were activated with 0.001 μg/ml (B and D) or 1 μg/ml (C and E) 22E7. Degranulation (B and C) and PGD2 release (D and E) then were assessed. Mean ± SE are shown for three experiments with different mast cell preparations. Respective spontaneous release values for −IgE and +IgE groups did not significantly differ for β-hexosaminidase, 7.9 ± 1.2 and 8.1 ± 1.5% 0.11 and for PGD2, 0.38 ± 0.11 and 0.34 ± 0.18 ng/106 cells. GM-CSF (F) and TNF-α (G) release was determined by ELISA following activation with 22E7 for 24 h at 37°C in medium containing SCF and SBTI. To compare different mast cell cultures, the ratio of the percentage of net maximal release from the +IgE/ −IgE groups stimulated with 0.001 μg/ml (□) and 1 μg/ml (■) of 22E7 at each time point is plotted against days in culture with omalizumab. The dashed line (ratio, 1) indicates the point at which the percentage net maximal release of the +IgE and −IgE groups is equal. The data represent the mean ± SEM of four to five independent experiments with different mast cell preparations. *, p < 0.05 between −IgE and + IgE using a Student’s two-tailed t test.
In a second set of experiments, whether the increased production of GM-CSF and TNF-α from cells with up-regulated FcεRI expression could also be reduced to basal levels after down-regulating receptor expression with omalizumab was determined. The experimental protocol was similar to that described in Fig. 6, A–E, as was the decline of FcεRI (data not shown). To perform these comparisons with different mast cell preparations that gave variable maximal production levels of cytokines, the data were normalized within each experiment by determining a release ratio (plus or minus IgE) at each time point after stimulations with 0.001 and 1 μg/ml 22E7. Plotting this ratio against omalizumab incubation time (in days) displays the reversing effect of this mAb on up-regulated production of GM-CSF and TNF-α at 0.001 μg/ml 22E7 and the down-regulated TNF-α production at 1.0 μg/ml 22E7, as ratios in each case approach unity. At 0.001 μg/ml 22E7, GM-CSF and TNF-α production returned to baseline for mast cells with IgE-enhanced FcεRI expression by 3–4 wk of omalizumab treatment. At 1 μg/ml 22E7, GM-CSF ratios never varied significantly from unity, indicating IgE-enhanced FcεRI levels did not affect GM-CSF release at this high level of FcεRI aggregation. In contrast, TNF-α production from mast cells with increased FcεRI expression when activated with 1 μg/ml 22E7 was significantly lower than from mast cells with basal levels of receptor as indicated by a ratio of <1 at day 0. TNF-α showed this tendency in Fig. 5, although statistical significance was not achieved. Perhaps this inhibition of high-dose 22E7-initiated release of IL-13 shown in Fig. 5C and of TNF-α shown in Fig. 6G reflect a diminished size or number of receptor aggregates when high concentrations of 22E7 are used with mast cells having a high FcεRI surface density. However, degranulation and PGD2 release were not similarly affected. Importantly, omalizumab down-regulates IgE-enhanced FcεRI levels on skin MCTC cells and soon thereafter reverses the increased sensitization of these cells to low-level FcεRI aggregation in terms of degranulation and production of PGD2 and cytokines.
Discussion
The present study shows that monomeric IgE markedly and reversibly enhances the expression of the FcεRI on mature human skin mast cells of the MCTC type in a dose- and time-dependent manner. Mast cells incubated with 1 μg/ml IgE (400 IU/ml) for 7 days showed a >10-fold increase in the MFI of FcεRI compared with cells in culture medium alone. IgE-enhanced FcεRI expression was at least 90% prevented by coadministration of the anti-IgE mAb omalizumab (≥2.9 μg/ml), and was reversed either by washing out free IgE (t1/2, 9.8 days) or by addition of omalizumab (4, 10, and 25 μg/ml; t1/2, 8.7, 6.1, and 4.8 days, respectively) to IgE-containing cultures. A dramatic reduction in surface FcεRI levels on peripheral blood-derived basophils cultured in vitro also occurs with removal of IgE (9), but addition of the anti-IgE mAb, CGP51901, failed to accelerate this reduction (9). Thus, IgE-enhanced expression of FcεRI on human mature skin mast cells is preventable with coadministration of omalizumab, and reversible by removal or neutralization of free IgE.
The functional consequences of enhanced FcεRI expression on mediator release following aggregation of this receptor on skin MCTC cells were addressed. Compared with mast cells expressing basal levels of receptor, those with IgE-enhanced levels of FcεRI were significantly more sensitive to activation at a low concentration of 22E7 (0.001 μg/ml), exhibiting greater degranulation and greater, production of PGD2 and the cytokines IL-13, TNF-α IL-6, and GM-CSF. Furthermore, maximal release of these mediators at an optimal concentration of IgE (0.1–1 μg/ml) was not affected by up-regulating FcεRI levels, even though the absolute amounts of mediators released varied considerably among mast cells from different subjects. Basal levels of FcεRI levels on basophils, unlike for skin mast cells in culture, are suboptimal for achieving maximal mediator release. Peripheral blood basophils with IgE-enhanced levels of FcεRI, compared with those with basal FcεRI levels, also show increased sensitivity with respect to histamine and cytokine secretion when stimulated with Ag, but apparently not when stimulated with anti-IgE or anti-FcεRI (29).
Results of the current study differ somewhat from those using murine mast cells as well as those using human mast cells derived in vitro from umbilical cord blood progenitors. In the murine system (5, 30, 31), mast cells with IgE-enhanced FcεRI expression, when stimulated with Ag, exhibit increased sensitivity and maximal degranulation to FcεRI-dependent activation. The maximal release values of IL-6, IL-4, and vascular endothelial growth factor are also increased. Enhancement of maximal degranulation also occurred ex vivo with mouse peritoneal mast cells on an IgE−/− background after administration of IgE to these animals led to enhanced FcεRI expression in vivo. In the case of human cord blood-derived mast cells, exposure to IgE (5 μg/ml) for 4–6 days led to a 2- to 3-fold increase in surface FcεRI (7), which in turn was associated with increased maximal degranulation and production of leukotriene C4 and PGD2 (7), vascular endothelial growth factor (31), and MIP-1α (32), with little increase in sensitivity to anti-IgE stimulation. The current study differs from these observations on immature or developing human mast cells in that the hypersensitive phenotype of mature MCTC cells derived from human skin with IgE-enhanced FcεRI expression was observed only at a low (0.001 μg/ml 22E7) level of FcεRI cross-linking. At higher 22E7 concentrations, no significant effect on the maximal magnitude or sensitivity of the degranulation response or of PGD2 and cytokine production was observed between the −IgE and +IgE groups of mature human skin MCTC cells. These differences between mature in vivo-differentiated mast cells used here and presumably less mature in vitro-developed mast cells used in other studies might reflect disparities in maturation. For example, mast cells developing in vitro would not encounter the entire repertoire of growth-related factors or experience cognate interactions with other cell types likely to have occurred in vivo. This notion is supported by the observation that IgE enhances surface FcεRI expression on human mast cells derived in vitro from progenitor cells in fetal liver only after exposure to IL-4 within the first 4 wk of culture (6). This “priming” effect has presumably already occurred for the mature MCTC cells used in the current study under physiological conditions in vivo. In addition, the cytokines IL-3, -4, -5, and -6 are known to prime human cord blood-derived mast cells for survival and cytokine production (7, 33-37). Another possible explanation is that heterogeneity in the type of mast cell dispersed from skin should be minimal, in contrast to studies using in vitro-derived mast cells that might contain a mixture of MCT and MCTC types.
Our in vitro data show that omalizumab prevents and reverses IgE-enhanced FcεRI expression. Moreover, down-regulation of IgE-enhanced FcεRI expression to basal levels by 3–4 wk also reversed the hypersensitive phenotype that resulted from IgE-enhanced FcεRI expression. However, although sensitivity to receptor aggregation and FcεRI expression were restored to normal levels by 4 wk, there appeared to be a 1- to 2-wk delay in the down-regulation of sensitivity relative to the decrease in receptor expression (Fig. 6). For example, 1 wk after addition of omalizumab to FcεRI up-regulated mast cells, FcεRI expression measured by MFI had decreased by >50%, whereas degranulation, PGD2 secretion, and production of GM-CSF and TNF-α had not appreciably changed. Whether the apparent dissociation between the FcεRI level and mediator release after FcεRI cross-linking is due to an alteration in intracellular signaling or reflects a threshold under which FcεRI levels must drop before hypersensitivity diminishes remains to be explored. Consistent with this current in vitro study with skin mast cells was the observation that treatment of three allergic rhinitis subjects with omalizumab for 70 or 196 days, compared with untreated controls, resulted in a decreased staining intensity with anti-FcεRI mAb of mast cells in skin biopsies and a decreased response to allergen by intradermal skin testing (25). Whether this decreased response results from diminished sensitivity of those mast cells that had been present throughout the time course of treatment, or reflects the arrival of new mast cells with low sensitivity because they had developed under conditions of low IgE, is not known. The in vitro concentrations of omalizumab used in the current study (4–25 μg/ml) are comparable to the concentrations achieved in serum for subjects dosed at 300 mg s.c. every 4 wk (20–50 μg/ml) based on their weight and IgE level (38). This dose of omalizumab is designed to achieve a molar excess over baseline IgE levels of 15–20:1, and to reduce mean free IgE levels to ~25 ng/ml.
Overall, the current data suggest that IgE-induced augmentation of FcεRI on skin MCTC cells affects the release of preformed granule mediators as well as de novo lipid and cytokine mediators at low levels of FcεRI cross-linking. The typical dose of allergen to which mast cells are exposed in a natural setting also is likely to be at the low end of the dose-response range. Limited aggregation of FcεRI in vivo might also be predicted based on the presumption that these cells will be sensitized with polyclonal IgE molecules recognizing many Ags, but may only be exposed to a limited array of these Ags. Enhanced sensitivity of atopic patients to these natural exposures could be due to IgE-enhanced expression of FcεRI on the surfaces of mast cells and basophils. Down-regulation of IgE-enhanced FcεRI with anti-IgE on mature or developing MCTC cells may diminish clinical sensitivity to natural allergen exposures.
Footnotes
This work was supported in part by grants from the National Institutes of Health (R01-AI27517), Philip Morris USA, Philip Morris International, and Genentech (to L.B.S.).
Abbreviations used in this paper: SCF, stem cell factor; SBTI, soybean trypsin inhibitor; MFI, mean fluorescence intensity.
Disclosures Lawrence B. Schwartz receives royalties from Virginia Commonwealth University through licensing agreement with Phadia on tryptase immunoassay; advisory board honoraria from Genentec and Novartis; spouse receives honoraria for speaking engagements from Novartis and Genentec, Merck, AstraZeneca and Schering.
References
- 1.Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, Liu FT, Galli SJ, Kawakami T. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 3.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc Natl Acad Sci USA. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lantz CS, Yamaguchi M, Oettgen HC, Katona IM, Miyajima I, Kinet JP, Galli SJ. IgE regulates mouse basophil FcεRI expression in vivo. J Immunol. 1997;158:2517–2521. [PubMed] [Google Scholar]
- 5.Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, Kinet JP, Galli SJ. IgE enhances mouse mast cell FcεRI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med. 1997;185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia HZ, Du ZM, Craig S, Klisch G, Noben-Trauth N, Kochan JP, Huff TH, Irani AM, Schwartz LB. Effect of recombinant human IL-4 on tryptase, chymase, and Fcε receptor type I expression in recombinant human stem cell factor-dependent fetal liver-derived human mast cells. J Immunol. 1997;159:2911–2921. [PubMed] [Google Scholar]
- 7.Yamaguchi M, Sayama K, Yano K, Lantz CS, Noben-Trauth N, Ra C, Costa JJ, Galli SJ. IgE enhances Fcε receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fcε receptor I expression and mediator release. J Immunol. 1999;162:5455–5465. [PubMed] [Google Scholar]
- 8.Saini SS, MacGlashan DW, Jr, Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, Bochner BS. Down-regulation of human basophil IgE and FCεRIα surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- 9.MacGlashan D, Jr, McKenzie-White J, Chichester K, Bochner BS, Davis FM, Schroeder JT, Lichtenstein LM. In vitro regulation of FcεRIα expression on human basophils by IgE antibody. Blood. 1998;91:1633–1643. [PubMed] [Google Scholar]
- 10.MacGlashan D, Jr, Xia HZ, Schwartz LB, Gong J. IgE-regulated loss, not IgE-regulated synthesis, controls expression of FcεRI in human basophils. J Leukoc Biol. 2001;70:207–218. [PubMed] [Google Scholar]
- 11.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schechter NM, Irani A-MA, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145:2652–2661. [PubMed] [Google Scholar]
- 13.Irani A-MA, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase: selective localization to MCTC cells. J Immunol. 1991;147:247–253. [PubMed] [Google Scholar]
- 14.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz LB. Mast cells and basophils. In: Zweiman B, Schwartz LB, editors. Inflammatory Mechanisms in Allergic Diseases. Marcel Dekker; New York: 2002. pp. 3–42. [Google Scholar]
- 16.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 17.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97:2045–2052. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W, Kepley CL, Morel PA, Okumoto LM, Fukuoka Y, Schwartz LB. FcγRIIa, not FcγRIIb, is constitutively and functionally expressed on skin-derived human mast cells. J Immunol. 2006;177:694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malveaux FJ, Conroy MC, Adkinson NF, Jr, Lichtenstein LM. IgE receptors on human basophils: relationship to serum IgE concentration. J Clin Invest. 1978;62:176–181. doi: 10.1172/JCI109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, Sterbinsky SA, Hamilton RG, Lichtenstein LM. Down-regulation of FcεRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 21.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, Van As A, Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 22.Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, McAlary M, Fowler-Taylor A, Racine A, Gupta N, et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286:2956–2967. doi: 10.1001/jama.286.23.2956. [DOI] [PubMed] [Google Scholar]
- 23.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, Taylor AF, Rohane P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 24.Soler M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, Thirlwell J, Gupta N, Della CG. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 25.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J Allergy Clin Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Riske F, Hakim J, Mallamaci M, Griffin M, Pilson B, Tobkes N, Lin P, Danho W, Kochan J, Chizzonite R. High affinity human IgE receptor (FcεRI): analysis of functional domains of the β-subunit with monoclonal antibodies. J Biol Chem. 1991;266:11245–11251. [PubMed] [Google Scholar]
- 27.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175:2635–2642. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz LB, Lewis RA, Seldin D, Austen KF. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981;126:1290–1294. [PubMed] [Google Scholar]
- 29.MacGlashan D, Schroeder JT. Functional consequences of FcεRIα up-regulation by IgE in human basophils. J Leukoc Biol. 2000;68:479–486. [PubMed] [Google Scholar]
- 30.Hsu C, MacGlashan D., Jr IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol Lett. 1996;52:129–134. doi: 10.1016/0165-2478(96)02599-0. [DOI] [PubMed] [Google Scholar]
- 31.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fcε receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano K, Yamaguchi M, De Mora F, Lantz CS, Butterfield JH, Costa JJ, Galli SJ. Production of macrophage inflammatory protein-1α by human mast cells: increased anti-IgE-dependent secretion after IgE-dependent enhancement of mast cell IgE-binding ability. Lab Invest. 1997;77:185–193. [PubMed] [Google Scholar]
- 33.Yanagida M, Fukamachi H, Ohgami K, Kuwaki T, Ishii H, Uzumaki H, Amano K, Tokiwa T, Mitsui H, Saito H, et al. Effects of T-helper 2-type cytokines, interleukin-3 (IL- 3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood. 1995;86:3705–3714. [PubMed] [Google Scholar]
- 34.Toru H, Ra C, Nonoyama S, Suzuki K, Yata J, Nakahata T. Induction of the high-affinity IgE receptor (FcεRI) on human mast cells by IL-4. Int Immunol. 1996;8:1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C-4 synthase expression by interleukin 4. J Exp Med. 2001;193:123–133. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochi H, De Jesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and-5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc Natl Acad Sci USA. 2000;97:10509–10513. doi: 10.1073/pnas.180318697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oskeritzian CA, Wang Z, Kochan JP, Grimes M, Du Z, Chang HW, Grant S, Schwartz LB. Recombinant human (rh)IL-4-mediated apoptosis and recombinant human IL- 6-mediated protection of recombinant human stem cell factor-dependent human mast cells derived from cord blood mononuclear cell progenitors. J Immunol. 1999;163:5105–5115. [PubMed] [Google Scholar]
- 38.Hochhaus G, Brookman L, Fox H, Johnson C, Matthews J, Ren S, Deniz Y. Pharmacodynamics of omalizumab: implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin. 2003;19:491–498. doi: 10.1185/030079903125002171. [DOI] [PubMed] [Google Scholar]


