Abstract
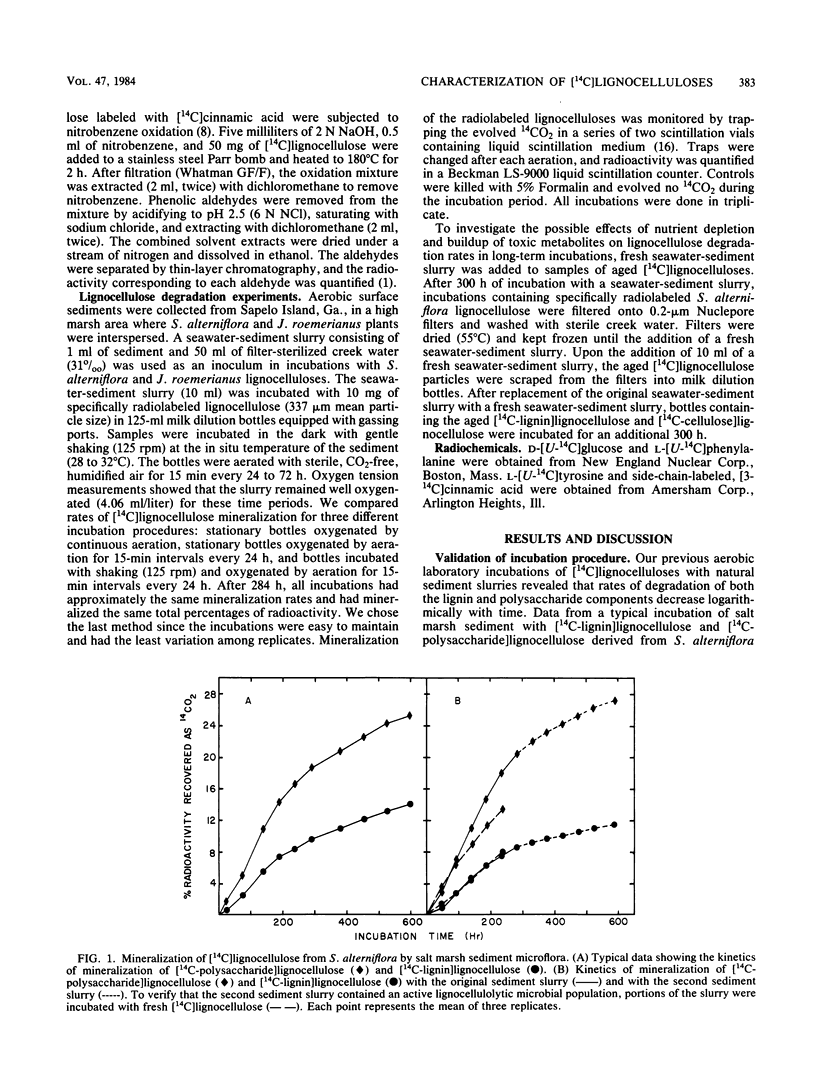
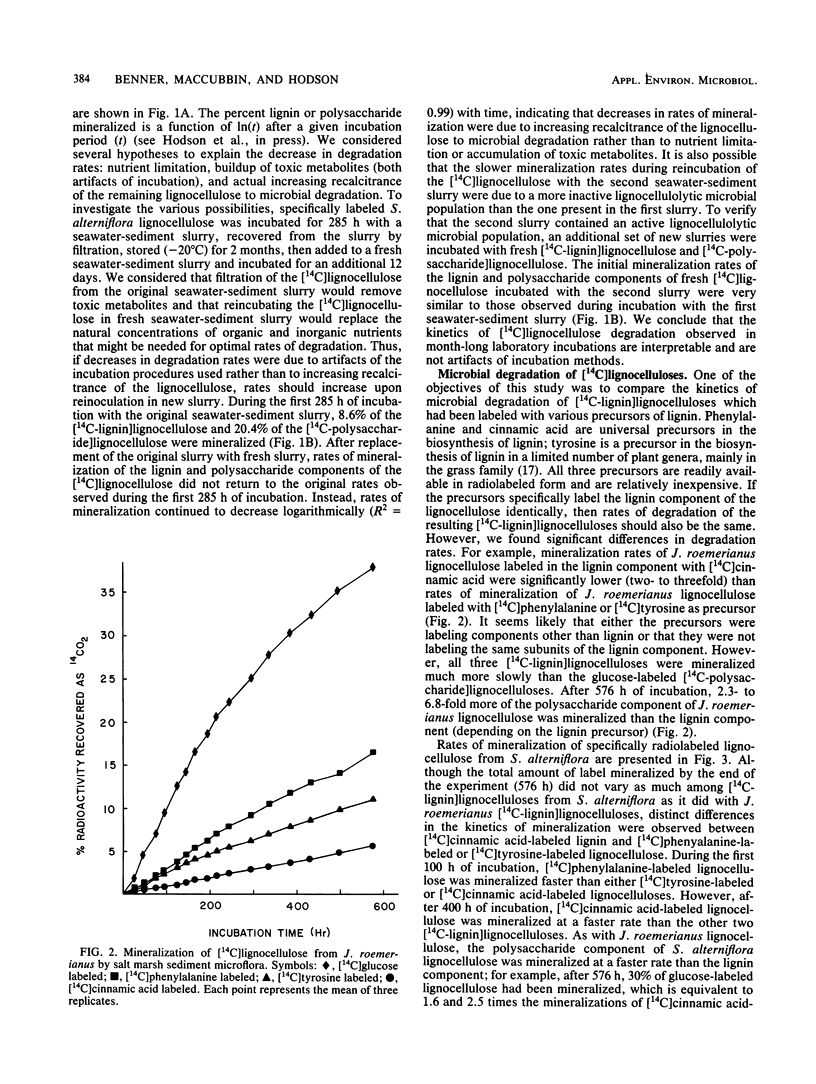
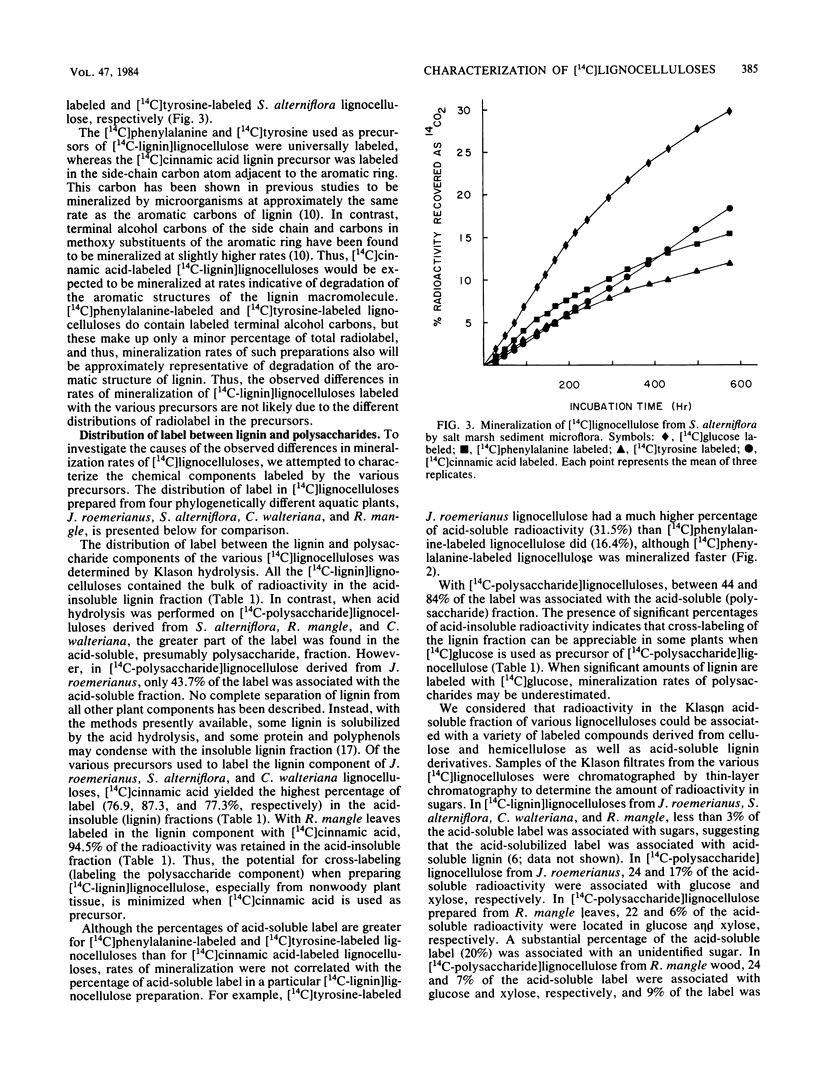
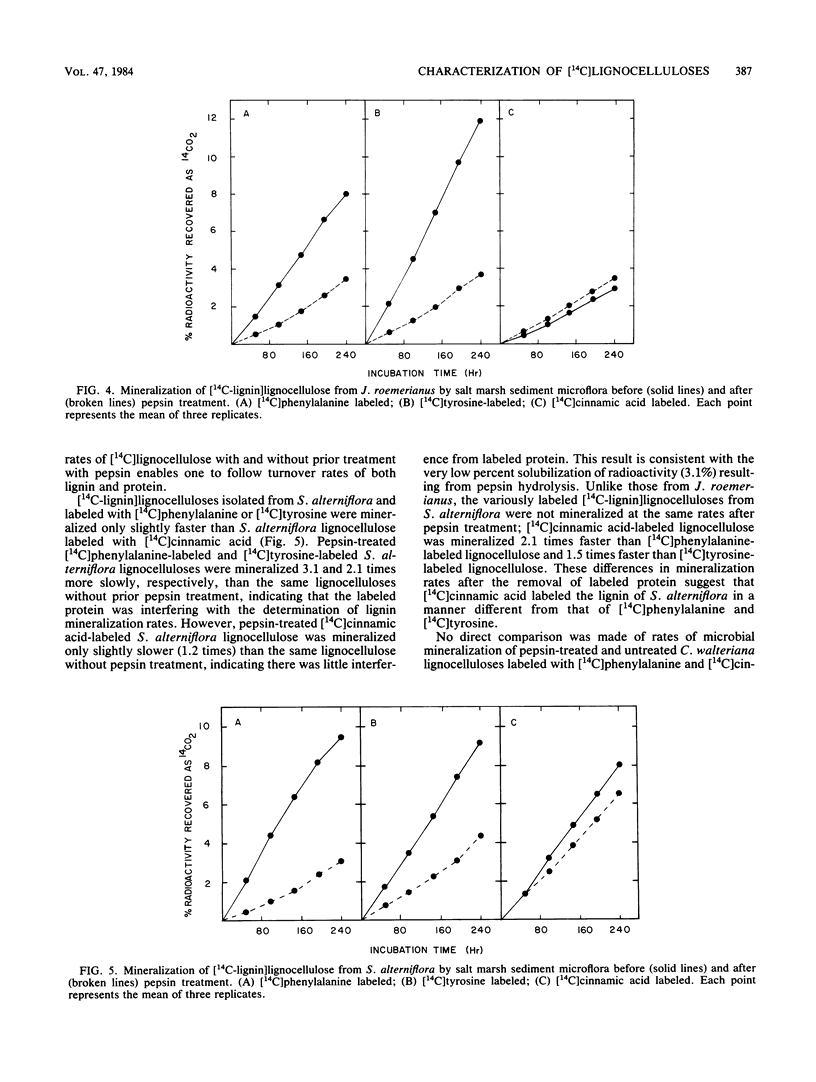
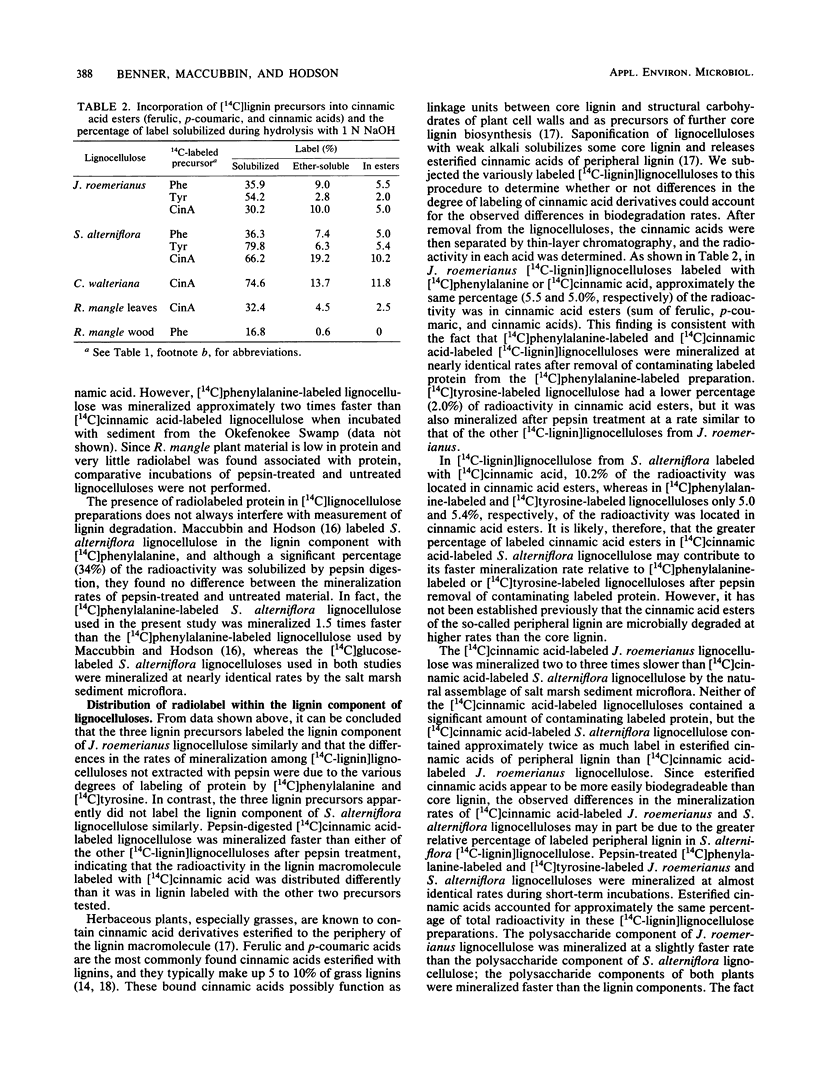
Specifically radiolabeled [14C-lignin]lignocelluloses were prepared from the aquatic macrophytes Spartina alterniflora, Juncus roemerianus, Rhizophora mangle, and Carex walteriana by using [14C]phenylalanine, [14C]tyrosine, and [14C]cinnamic acid as precursors. Specifically radiolabeled [14C-polysaccharide]lignocelluloses were prepared by using [14C]glucose as precursor. The rates of microbial degradation varied among [14C-lignin]lignocelluloses labeled with different lignin precursors within the same plant species. To determine the causes of these differential rates, [14C-lignin]lignocelluloses were thoroughly characterized for the distribution of radioactivity in nonlignin contaminants and within the lignin macromolecule. In herbaceous plants, significant amounts (8 to 24%) of radioactivity from [14C]phenylalanine and [14C]tyrosine were found associated with protein, although very little (3%) radioactivity from [14C]cinnamic acid was associated with protein. Microbial degradation of radiolabeled protein resulted in overestimation of lignin degradation rates in lignocelluloses derived from herbaceous aquatic plants. Other differences in degradation rates among [14C-lignin]lignocelluloses from the same plant species were attributable to differences in the amount of label being associated with ester-linked subunits of peripheral lignin. After acid hydrolysis of [14C-polysaccharide]lignocelluloses, radioactivity was detected in several sugars, although most of the radioactivity was distributed between glucose and xylose. After 576 h of incubation with salt marsh sediments, 38% of the polysaccharide component and between 6 and 16% of the lignin component (depending on the precursor) of J. roemerianus lignocellulose was mineralized to 14CO2; during the same incubation period, 30% of the polysaccharide component and between 12 and 18% of the lignin component of S. alterniflora lignocellulose was mineralized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand J. M. Studies on grass lignins. I. Separation and quantitative determination of p-hydroxybenzaldehyde, vanillin and syringaldehyde by thin-layer chromatography. J Chromatogr. 1966 Mar;21(3):424–429. doi: 10.1016/s0021-9673(01)91336-6. [DOI] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976 May;31(5):714–717. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L., Pometto A. L. Preparation of specifically labeled C-(lignin)- and C-(cellulose)-lignocelluloses and their decomposition by the microflora of soil. Appl Environ Microbiol. 1977 Jun;33(6):1247–1251. doi: 10.1128/aem.33.6.1247-1251.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L. Lignocellulose decomposition by selected streptomyces strains. Appl Environ Microbiol. 1978 Jun;35(6):1041–1045. doi: 10.1128/aem.35.6.1041-1045.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccubbin A. E., Hodson R. E. Mineralization of detrital lignocelluloses by salt marsh sediment microflora. Appl Environ Microbiol. 1980 Oct;40(4):735–740. doi: 10.1128/aem.40.4.735-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOMHOF D. W., TUCKER T. C. THE SEPARATION OF SIMPLE SUGARS BY CELLULOSE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1965 Feb;17:300–306. doi: 10.1016/s0021-9673(00)99872-8. [DOI] [PubMed] [Google Scholar]


