Abstract
Bacterial acetyl-coenzyme A (acetyl-CoA) synthetase (AceCS), an evolutionarily conserved enzyme that converts acetate to acetyl-CoA, is activated by sirtuin-mediated deacetylation. Two recent studies show that this mechanism of regulation is also crucial for mammalian AceCS activity, indicating that control of metabolism at the step of converting acetate to acetyl-CoA is conserved. These findings highlight a metabolic regulatory network controlled by sirtuins that has implications for the mechanisms of calorie restriction and modulation of mammalian lifespan.
Introduction
The dietary regimen of calorie restriction extends lifespan in diverse organisms ranging from yeast to mammals. Genes encoding Silent information regulator 2 (Sir2) are essential for the effects of calorie restriction in many of the organisms studied [1], but how calorie restriction works is not yet known. It is thought, however, that calorie restriction invokes an ancient stress response that evolved to facilitate survival during times of adversity such as reduced food availability [2]. One of the changes associated with calorie restriction is a shift in the way in which energy is generated and used; that is, there is a shift from energy produced by glycolysis and the trichloroacetic acid (TCA) cycle towards energy produced by the breakdown of proteins and lipids and by increased gluconeogenesis to support glycolytic tissues such as the brain [3,4].
Acetyl-coenzyme A (acetyl-CoA) is a key molecule connecting several metabolic pathways that is generated from the breakdown of carbohydrates, lipids and amino acids, and from acetate [5]. Our knowledge of how mammals derive acetyl-CoA from acetate is surprisingly limited, possibly owing to the assumption that acetate metabolism is more relevant to bacteria than to mammals. But this view could change with the publication of two studies from the laboratories of Verdin [6] and Denu [7], which show that a mechanism of regulating acetate metabolism in bacteria also exists in mammals. These studies shed light on how we generate energy from the fuels that we consume and how we alter our metabolism when food supplies become dangerously low.
Conversion of acetate to acetyl-CoA
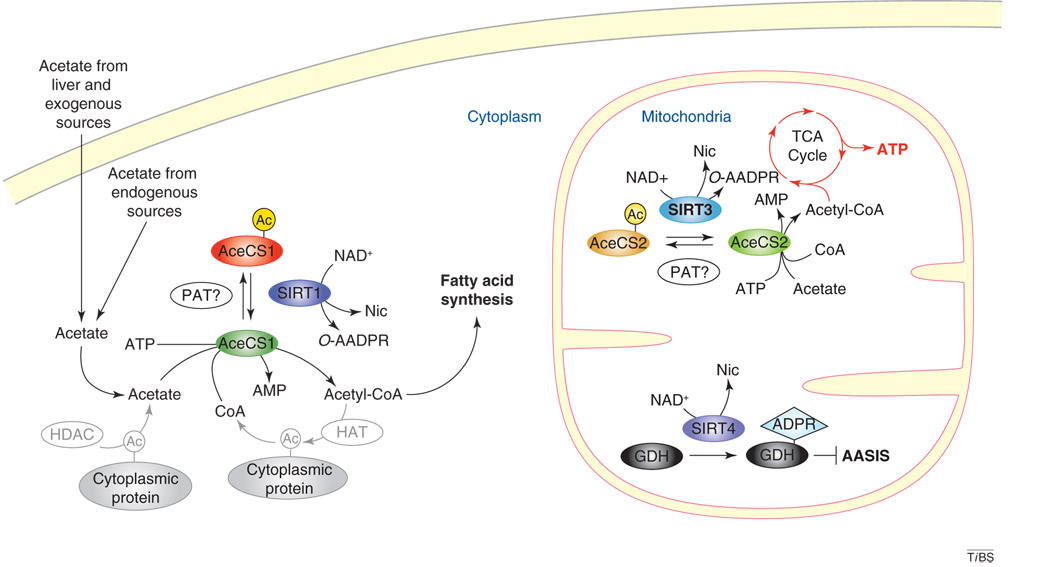
In mammals, acetate is derived from both exogenous sources (e.g. bacterial fermentation in the gut and dietary sources such as vinegar) and endogenous sources (e.g. ethanol metabolism, conversion of acetyl-CoA into acetate by acetyl-CoA hydrolase, hydrolysis of acetylcholine by acetylcholinesterases in the nervous system, and deacetylation of lysine residues by protein deacetylases) [5,8]. Acetate can be converted to acetyl-CoA either in the cytoplasm by AceCS1 or in the mitochondria by AceCS2 [9,10] (Figure 1). Acetyl-CoA is used in the synthesis of fatty acids, some amino acids and ketone bodies, and for entry of carbons into the TCA cycle.
Figure 1.
Subcellular locations for sirtuin regulation of AceCS activity. In the mitochondria, SIRT3 can deacetylate and activate AceCS2, leading to the conversion of acetate to acetyl-CoA, which in turn can be shunted into the TCA cycle, resulting in an increase in ATP production. In the cytoplasm, SIRT1 deacetylates AceCS1 to convert acetate into acetyl-CoA for use in fatty acid synthesis. In addition, AceCS1 might be involved in recycling acetate from deacetylation reactions, which could take place in the cytoplasm where numerous proteins subjected to reversible acetylation are localized. SIRT4, an ADP-ribosyltransferase, inhibits glutamate dehydrogenase (GDH) activity through ribosylation, leading to an inhibition of amino-acid-stimulated insulin secretion (AASIS) in β cells and the induction of gluconeogenesis in the liver. Abbreviations: PAT, protein acetyltransferase; Nic, nicotinamide; AMP, adenosine monophosphate; Ac, acetylated lysine residue; ADPR, ADP-ribosylation; HAT, histone acetyltransferase; HDAC, histone deacetylase.
Regulation of AceCS enzymatic activity by sirtuin proteins
Our knowledge about how AceCS proteins are regulated comes mostly from studies of bacteria by Escalante-Semerena and colleagues [11–13]. In Salmonella enterica, the catalytic activity of AceCS is regulated by acetylation. The bacterial protein acetyltransferase acetylates Lys609 in the catalytic pocket of AceCS to render it enzymatically inactive [11], and subsequent deacetylation by CobB reactivates the catalytic activity of AceCS [12]. CobB is a bacterial homolog of the yeast Sir2 deacetylase [13], the founding member of the so-called ‘sirtuin’ family of enzymes (the term sirtuins is derived from ‘sir-two-ins’ in reference to their homology to the yeast Sir2 protein).
The past 10 years have seen a marked increase in our understanding of sirtuins. They are found in all kingdoms of life and can carry out two posttranslational modifications of target proteins, deacetylation and mono-ADP ribosylation, both of which are coupled to the breakdown of NAD+. In yeast, worms and flies, overexpression of SIR2 increases lifespan, ostensibly by mimicking a calorie restriction diet. Mammals have seven Sir2 proteins, named SIRT1–SIRT7, which have key roles in regulating cell survival, fat metabolism and insulin secretion [14].
A conserved metabolic switch
Two recent papers demonstrate that the mode of AceCS regulation discovered in Salmonella is conserved in mammals [6,7]. Both studies show that AceCS2 is activated when it is deacetylated by SIRT3 (a mitochondrial sirtuin) [6,7]. Denu and colleagues [7] have further elucidated this conserved mechanism by demonstrating that SIRT1 activates cytoplasmic AceCS1 by deacetylation (Figure 1).
The diet known as calorie restriction induces numerous metabolic changes such as a shift away from glycolysis, a decrease in fatty acid biosynthesis in the liver while increasing it in the skeletal muscle, induction of gluconeogenesis, and improved insulin sensitivity [3,14]. How these changes occur is not well understood, but sirtuins might have an important role because the expression of mammalian SIRT1 and SIRT3 are induced under calorie restriction [15,16].
AceCS1 is present in all tissues, but is most abundant in kidneys, ovaries, testes and liver, where it is induced through transcriptional regulation by sterol-regulatory-element-binding proteins [9,17]. Activity of AceCS1 increases in concert with insulin in response to a carbohydrate-rich diet to induce fatty acid synthesis [18]. SIRT1 protein levels are increased during fasting and calorie restriction, which would lead to the deacetylation and activation of AceCS1, providing acetyl-CoA for fatty acid synthesis. Consistent with this hypothesis, overexpression of SIRT1 is sufficient to increase the synthesis of fatty acids from acetate [7]. However, expression of AceCS1 is reduced in the liver during fasting [18], which might explain why fatty acid synthesis is reduced under these conditions in the liver but not in other tissues such as skeletal muscle. It remains unclear under what conditions SIRT1, which is predominantly a nuclear protein [19], can function in the cytoplasm to deacetylate AceCS1. The subcellular distribution of SIRT1 might be tissue-specific because cytoplasmic SIRT1 has been observed in pancreatic β cells [20], suggesting that regulation of SIRT1 localization might be essential in its control of AceCS1 activity.
AceCS2 is present in the heart, kidney and skeletal muscle, but is noticeably absent from the liver. Induction of AceCS2 gene expression is regulated by the transcription factor KLF15, a member of the Sp1-like/Kruppel-like factor family [21]. It makes sense for AceCS2 expression to be suppressed in the liver, because the liver is one of the primary organs that generates acetate. AceCS2 activity in the liver would generate a ‘futile cycle’ in which acetate produced would be converted to acetyl-CoA, rather than excreted into the blood for use by extrahepatic tissues as an energy source.
Consistent with the notion that AceCS2 is involved in energy generation, increasing AceCS2 activity by overexpression leads to enhanced oxidation, as observed by CO2 generation [7,10]. Further studies are needed to determine whether activation of AceCS2 by increasing SIRT3 activity is sufficient to generate an effect on oxidation similar to that of AceCS2 overexpression. Under calorie restriction and ketogenic conditions, acetyl-CoA generated through the breakdown of fatty acids is used to produce ketone bodies that are excreted to extrahepatic tissues for use as an energy source. Expression of AceCS2 is induced in extrahepatic tissue under ketogenic conditions, suggesting that it might function in the metabolism of ketone bodies [10]. It will be interesting to determine whether AceCS2 and SIRT3 are coordinately regulated during calorie restriction and ketogenic conditions through the transcription factor KLF15.
A hallmark of calorie restriction is the truncation of the TCA cycle and the induction of gluconeogenesis [4]. Regulation of AceCS2 by SIRT3 provides further insight into how gluconeogenesis could be induced during calorie restriction. Pyruvate can be converted to carbohydrates through gluconeogenesis or to acetyl-CoA and can used for either the TCA cycle or fatty acid synthesis; thus, it is an intersection molecule in the metabolic network. A key regulatory step in the fate of pyruvate is carried out by the pyruvate dehydrogenase complex (PDH), which directs pyruvate to the TCA cycle (under low energy charge or low ATP:ADP ratio) or to fatty acid synthesis and gluconeogenesis (under high energy charge or high ATP:ADP ratio) [22]. Under conditions of high NADH to NAD+ and acetyl-CoA to CoA ratios (when the cellular energy charge is high), the PDH complex is inhibited and pyruvate is directed towards gluconeogenesis and fatty acid synthesis [22]. Increased SIRT3 levels during calorie restriction thus could have a dual effect on directing pyruvate into gluconeogenesis: first, by inhibiting PDH activity through increasing the NADH to NAD+ ratio (by converting NAD+ to nicotinamide through the breakdown of NAD+ during deacetylation reactions); and second, by increasing the acetyl-CoA to CoA ratio by boosting AceCS2 activity. Although AceCS2 is absent in the liver, the primary organ for gluconeogenesis, both AceCS2 and SIRT3 are expressed in the kidney, a secondary tissue that uses gluconeogenesis.
Sirtuins orchestrate metabolism in response to diet
Recent results have shown that sirtuins regulate metabolism at multiple points, consistent with mediating the physiology of calorie restriction (Figure 2). By deacetylating the transcriptional coactivator PGC1α, SIRT1 upregulates gluconeogenesis in the liver while repressing glycolysis and, by deacetylating AceCS1, it controls the flow of acetate into fatty acids [7,23,24]. SIRT1 also stimulates insulin secretion by repressing the uncoupling protein UCP2 [20,23]. SIRT3 regulates thermogenesis by increasing mitochondrial respiration [6,15]. Furthermore, by regulating AceCS2 activity, SIRT3 controls the levels of acetyl-CoA for use in metabolic pathways [6,15]. In addition, SIRT3 activity potentially could influence the switch to gluconeogenesis through regulation of the PDH complex during calorie restriction.
Figure 2.
Model of the coordinated regulation of metabolic alteration during calorie restriction. Calorie restriction induces the activity of SIRT1 and SIRT3 but reduces that of SIRT4. Regulation by sirtuins effects a metabolic shift into gluconeogenesis through SIRT1 regulation of the transcriptional coactivator PGC1α, SIRT3 regulation of AceCS2, and SIRT4 regulation of glutamate dehydrogenase (GDH). In addition, SIRT1 regulation of AceCS1, leading to changes in fatty acid synthesis, and SIRT3 regulation of AceCS2, resulting in changes in gluconeogenesis, are consistent with metabolic changes that take place during calorie restriction. PDH, pyruvate dehydrogenase; UCP2, uncoupling protein 2.
A recent paper from the laboratory of Guarente [25] shows that a third sirtuin, SIRT4, is also involved in metabolic regulation. SIRT4, which is localized to the mitochondria, is primarily an ADP-ribosyltransferase that downregulates glutamate dehydrogenase by targeting it for ribosylation [25]. During calorie restriction, glutamate dehydrogenase is upregulated by a reduction in ADP-ribosylation, which would induce gluconeogenesis in the liver and upregulate amino-acid-stimulated insulin secretion in pancreatic β cells.
Thus, these three sirtuins – SIRT1, SIRT3 and SIRT4 – each have a role in regulating the calorie-restriction-induced switch to gluconeogenesis, suggesting a coordinated sirtuin-regulated metabolic network operates during calorie restriction (Figure 2).
Concluding remarks
Although the ability of sirtuins to extend lifespan in simple organisms has been clearly established, we still have a great deal to understand about sirtuin biology in mammals. The two recent reports on the roles of SIRT1 and SIRT3 in regulating acetate metabolism, the TCA cycle and fatty acid metabolism provide an intriguing glimpse into how sirtuins might underlie the physiology and health benefits of calorie restriction. Future work on how the different sirtuin activities synergize to regulate global metabolic changes will further our understanding of the molecular mechanisms that modulate metabolism and longevity in response to calorie intake.
Acknowledgements
We thank Sean M. Armour for critical reading and comments on the manuscript. Our work is supported by funding from the National Institute on Aging, National Institutes of Health, the Beth Israel Deaconess Medical Center, and the Glenn Foundation for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guarente L, Picard F. Calorie restriction – the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Lamming DW, et al. Small molecules that regulate lifespan: evidence for xenohormesis. Mol. Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- 3.Dhahbi JM, et al. Calories and aging alter gene expression for gluconeogenic, glycolytic, and nitrogen-metabolizing enzymes. Am. J. Physiol. 1999;277:E352–E360. doi: 10.1152/ajpendo.1999.277.2.E352. [DOI] [PubMed] [Google Scholar]
- 4.Hagopian K, et al. Krebs cycle enzymes from livers of old mice are differentially regulated by caloric restriction. Exp. Gerontol. 2004;39:1145–1154. doi: 10.1016/j.exger.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe AJ. The acetate switch. Microbiol. Mol. Biol. Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwer B, et al. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallows WC, et al. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley BM, Williamson DH. Origins of blood acetate in the rat. Biochem. J. 1977;166:539–545. doi: 10.1042/bj1660539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luong A, et al. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 2000;275:26458–26466. doi: 10.1074/jbc.M004160200. [DOI] [PubMed] [Google Scholar]
- 10.Fujino T, et al. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 2001;276:11420–11426. doi: 10.1074/jbc.M008782200. [DOI] [PubMed] [Google Scholar]
- 11.Starai VJ, Escalante-Semerena JC. Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J. Mol. Biol. 2004;340:1005–1012. doi: 10.1016/j.jmb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Starai VJ, et al. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- 13.Tsang AW, Escalante-Semerena JC. CobB, a new member of the SIR2 family of eucaryotic regulatory proteins, is required to compensate for the lack of nicotinate mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase activity in cobT mutants during cobalamin biosynthesis in Salmonella typhimurium LT2. J. Biol. Chem. 1998;273:31788–31794. doi: 10.1074/jbc.273.48.31788. [DOI] [PubMed] [Google Scholar]
- 14.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 15.Shi T, et al. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 16.Cohen HY, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, et al. Transcriptional regulation of the murine acetyl-CoA synthetase 1 gene through multiple clustered binding sites for sterol regulatory element-binding proteins and a single neighboring site for Sp1. J. Biol. Chem. 2001;276:34259–34269. doi: 10.1074/jbc.M103848200. [DOI] [PubMed] [Google Scholar]
- 18.Sone H, et al. Acetyl-coenzyme A synthetase is a lipogenic enzyme controlled by SREBP-1 and energy status. Am. J. Physiol. Endocrinol. Metab. 2002;282:E222–E230. doi: 10.1152/ajpendo.00189.2001. [DOI] [PubMed] [Google Scholar]
- 19.Michishita E, et al. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moynihan KA, et al. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto J, et al. A Kruppel-like factor KLF15 contributes fasting-induced transcriptional activation of mitochondrial acetyl-CoA synthetase gene AceCS2. J. Biol. Chem. 2004;279:16954–16962. doi: 10.1074/jbc.M312079200. [DOI] [PubMed] [Google Scholar]
- 22.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem. Soc. Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 23.Bordone L, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 25.Haigis MC, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]