Abstract
You may have seen the bumper sticker “Eve was framed.” Thousands of years of being blamed for original sin and still many wonder, where's the evidence? Today, the tumor suppressor adenomatous polyposis coli (APC) may have the same complaint about accusations of a different type of CIN, chromosome instability. A series of recent papers, including three in this journal, propose that loss of APC function plays an important role in the CIN seen in many colon cancer cells. However, a closer look reveals a complex story that raises more questions than answers.
Does loss of APC promote CIN?
Adenomatous polyposis coli (APC) was first identified as a tumor suppressor gene mutated in familial colon cancer; it is also mutated in most sporadic cases (for review see Polakis, 2007). Its best-known role is as a negative regulator of Wnt signaling (for review see Clevers, 2006), but it also plays Wnt-independent roles in cytoskeletal regulation (for review see Näthke, 2004) through its ability to bind microtubules (MTs) and MT-associated proteins as well as associate with the actin cytoskeleton. APC has usually been implicated in chromosome instability (CIN) via its proposed cytoskeletal regulatory roles, but, as we see below, recent work also suggests possible roles for activated Wnt signaling in CIN.
When considering guilt or innocence, the first question is whether a crime actually occurred—does the loss of APC increase CIN? To answer this, we first must define CIN. In colon cancer, sequential mutations promote progression from polyp to adenoma to carcinoma. In colon and other cancers, advanced tumors exhibit CIN, with both aneuploidy (changes in chromosome number) and chromosome aberrations (translocations or other rearrangements) increasing as cancer progresses (Lengauer et al., 1998). Despite this strong correlation, it has remained uncertain whether CIN causes cancer or is a side effect of mutations in guardians of genome integrity; however, recent evidence supports a causal role (Weaver et al., 2007). Mutations in checkpoint mediators, DNA damage sensors, or kinetochore proteins help explain CIN but only account for ∼10% of aneuploid tumors (Cahill et al., 1998; Wang et al., 2004).
APC loss is the first step in colon carcinogenesis. If APC loss causes CIN, CIN should occur early in cancer progression. Data from a variety of laboratories suggest that many but not all APC mutant adenomas are aneuploid (e.g., 53% in Cardoso et al., 2006; also see Haigis et al., 2002; Sieber et al., 2002). Thus, although APC mutant adenomas often become aneuploid, some do not. Collectively, the data suggest that APC loss does not lead to wholesale failure in chromosome segregation in vivo but may trigger defects in the fidelity of chromosome segregation that promote cancer progression.
APC localizes to kinetochores, centrosomes, and astral MTs
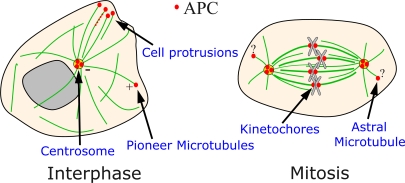
We next must ask where the crime occurred and whether APC was at the scene of the crime. APC's subcellular localization is complex and somewhat contentious (Fig. 1). There is agreement about APC localization during interphase: it localizes to puncta in cell protrusions near the ends of MTs (Näthke et al., 1996) and can surf on MT plus ends (Mimori-Kiyosue et al., 2000). Surprisingly, this does not require the +tip protein EB1 (Kita et al., 2006). +Tip localization is consistent with roles in stabilizing astral MTs, thus affecting spindle or contractile ring position during cytokinesis (Green et al., 2005; Caldwell et al., 2007). In mitosis, different groups report different localizations, each consistent with distinct roles in CIN. APC has been reported at kinetochores (Fodde et al., 2001; Kaplan et al., 2001) and at centrosomes (Banks and Heald, 2004; Louie et al., 2004). At kinetochores, it might regulate MT–kinetochore attachment or the spindle assembly checkpoint (SAC); at centrosomes, it could influence centrosome duplication or nucleation of spindle/astral MTs during mitosis. Both are consistent with roles in CIN. Truncated APC proteins like those found in tumors also have been localized to different sites, including puncta along spindle MTs (Green and Kaplan, 2003) or at centrosomes (Tighe et al., 2001). This diversity in reported sites of action raise questions about which is critical for CIN.
Figure 1.
APC localization. APC has been localized to several subcellular locations, some of which are highlighted here.
Evaluating a role in CIN
The first evidence linking APC and CIN came in 2001, when two groups reported that cultured embryonic stem (ES) cells homozygous mutant for APC (the Min or 1638T truncation mutations) become aneuploid in culture and accumulate rearranged chromosomes (Fodde et al., 2001; Kaplan et al., 2001). Kaplan et al. (2001) also reported lagging chromosomes, presumably precursors of CIN, whereas Fodde et al. (2001) found elevated numbers of tetraploid cells. Both groups found APC localized at kinetochores, suggesting a possible role in either kinetochore–MT attachment or in the SAC.
This was followed up by many groups who used three approaches to reduce APC function (see below). Some used colon cancer cell lines mutant for APC. Surprisingly, in tumors, there is no selection for homozygosity of null mutations; instead, one allele encodes a truncated protein retaining the N-terminal half of APC. It remains unclear whether these are dominant negative; recent work suggests that they are selected because they reduce but do not eliminate Wnt signaling (Albuquerque et al., 2002; McCartney et al., 2006). Others expressed similarly truncated APC proteins in wild-type cells, reasoning that they are dominant negative. However, caution must be used in interpreting these experiments. Because APC has many partners in both Wnt signaling and cytoskeletal regulation, high level overexpression of truncated APC may affect processes in which APC is not essential by sequestering binding partners that are essential for the process. In this paper, we focus on studies that used RNAi to reduce APC in otherwise wild-type cells, as this approach allows one to define the normal role of APC. We also consider some studies that express truncated APC, as dominant effects of these may well be relevant to tumorigenesis.
If APC is guilty of CIN, by what mechanisms does it act? Surprisingly, even simple loss-of-function experiments revealed diverse phenotypes and an equally diverse set of proposed mechanisms by which APC prevents CIN. In the following sections, we consider these models in turn, evaluating the evidence for and against each model by comparing and contrasting different studies.
The spindle assembly checkpoint: APC jumps into the SAC with BUB
The importance of correct chromosome segregation drove the evolution of a self-policing SAC that assures proper segregation of the duplicated genome (Musacchio and Salmon, 2007). The kinetochore protein complex mediates MT attachment to each chromatid. It is critical to ensure that the two sisters each attach to different spindle poles. Once this occurs, sister chromatids are pulled in opposite directions, generating tension between kinetochores. The SAC monitors kinetochore MT occupancy and tension across kinetochore pairs and is inactivated only when all chromosomes are correctly attached to both spindle poles, allowing anaphase onset and chromosome segregation. The SAC is regulated by MAD (mitotic arrest defective) and BUB (budding uninhibited by benzimidazoles) proteins, which localize to kinetochores. Defects in the SAC can lead to premature anaphase onset before both kinetochores of all chromosomes are properly attached and, thus, lead to defects in chromosome segregation (i.e., CIN).
One model for APC's role in CIN suggests that APC plays a key role in the SAC. To test this, one must assess whether a functional SAC is present in APC mutant cells. One way to do so is to disrupt kinetochore attachment or tension using MT poisons (nocodazole or taxol). This should result in SAC activation and arrest cells in mitosis, so an increased mitotic index suggests a functional SAC. In many wild-type cell types, nocodazole is quite effective at blocking mitotic exit, with 60–80% of the cells blocked in mitosis by 100 nm nocodazole (e.g., Draviam et al., 2006).
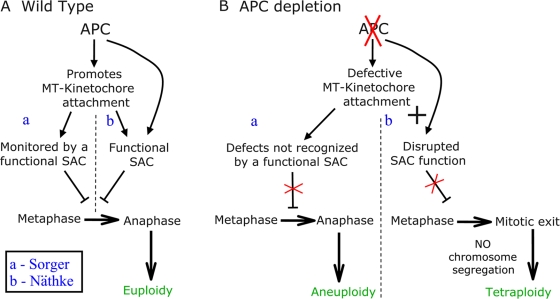
Näthke's laboratory suggests that APC is required for a functional SAC in U2OS cells (Dikovskaya et al., 2007). They report that APC-siRNA “substantially compromises the mitotic checkpoint” after nocodazole treatment (Dikovskaya et al., 2007) because APC-siRNA reduces mitotic arrest in response to nocodazole relative to control cells. However, it is important to note that the U2OS cells used had a modest response even to high levels of nocodazole relative to other cells (mitotic index decreased from 6% in control cells to 4% after APC-siRNA at 100 nM nocodazole, a standard dose, or from 22 to 12% at 5 μM nocodazole; some sublines of U2OS cells respond more robustly to 100 nM nocodazole, with a >70% mitotic index; Sihn et al., 2003). Another assay of SAC function is the proper localization of SAC proteins at kinetochores. APC depletion in U2OS cells reduced kinetochore Bub1 and BubR1 levels during prometaphase to ∼60% of normal (Dikovskaya et al., 2007). Together, these data suggest that in U2OS cells, APC loss compromises the SAC. In Näthke's model (Figs. 2 B and 3, B and C), this SAC defect leads to premature anaphase onset, which, in turn, triggers mitotic exit without cytokinesis, generating tetraploid cells.Finally, they suggest that APC loss inhibits apoptosis, which would normally be triggered by this sort of abnormal event.
Figure 2.
Models suggesting APC modulates the SAC or its response to attachment defects. (A) Proposed pathway in wild-type cells. APC promotes stable MT–kinetochore attachment through an unknown mechanism. The SAC monitors MT–kinetochore attachment and only allows mitotic exit once MT occupancy and tension are satisfactory. (a) In Sorger's model (Draviam et al., 2006), SAC function does not require APC. (b) In Näthke's model (Dikovskaya et al., 2007), APC plays a direct role in SAC function. (B) Proposed model accounting for chromosome segregation defects in the absence of APC. Disruption of APC seems to lead to defects in MT–kinetochore attachment, but models differ in what happens downstream. (a) In Sorger's model (Draviam et al., 2006), a functional SAC prolongs metaphase to attempt to correct attachment defects, but some defects remain undetected/uncorrected. This leads to mitotic exit of cells with lagging chromosomes, leading to aneuploidy. (b) In Näthke's model (Dikovskaya et al., 2007), defects in APC lead to compromised SAC function. This leads to mitotic exit without chromosome segregation, generating tetraploid cells.
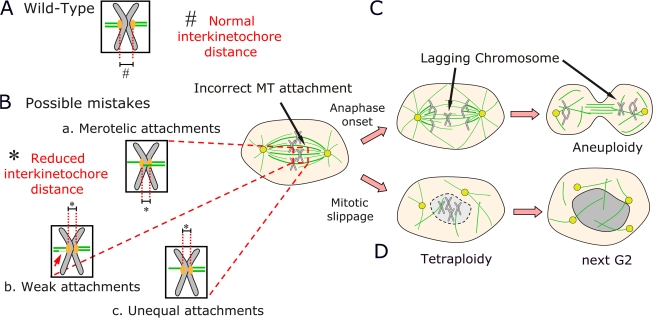
Figure 3.
Models suggesting roles for APC in MT–kinetochore attachment. (A) Wild-type MT attachment at the end of metaphase, when MT number is balanced and interkinetochore distance is normal. (B) In APC mutant cells, interkinetochore distance is reduced, which could be a result of several different defects in MT attachment. (C) A model in which these defects lead to lagging chromosomes and aneuploidy. (D) A model in which these defects and defects in the SAC lead to mitotic exit without cytokinesis and tetraploidy.
These interesting SAC defects may be cell type specific, however, rather than a general response to APC-siRNA. Sorger's laboratory found a functional SAC in APC-depleted HeLa cells (Draviam et al., 2006); nocodazole triggered >90% mitotic arrest in both wild-type and knockdown cells. Kaplan's laboratory found “a modest decrease in mitotic index” (60 to 45%) in APC-depleted 293 cells relative to controls and concluded that “the spindle checkpoint is functional” (Green et al., 2005). They also found a functional SAC in APCMin mutant ES cells or blastocysts (Kaplan et al., 2001). Furthermore, Mad2 and BubR1 are normally recruited to kinetochores in APC-depleted HeLa cells (Draviam et al., 2006) or Xenopus laevis egg extracts (Zhang et al., 2007).
Perhaps the most accurate assay of SAC function is to directly monitor mitosis by live cell imaging. Sorger's laboratory found that APC depletion delayed anaphase onset threefold; this delay was abolished by codepleting Mad2 (Draviam et al., 2006). This suggests that APC depletion leaves a functional SAC; they attributed subsequent CIN to the failure to fully detect/respond to defects in MT–kinetochore attachment caused by APC depletion. Cells are delayed in mitosis but ultimately proceed through it in an error-prone way. In contrast, Näthke's group saw the opposite: APC depletion reduced time to anaphase onset, suggesting a defective SAC (Dikovskaya et al., 2007). Thus, different groups using different cell lines find quite variable effects of APC loss on SAC precision. We feel this raises serious questions about whether defects in the SAC are a primary effect of APC loss in all cell types. Furthermore, all agree that there is not wholesale abrogation of this checkpoint in the absence of APC, as is seen in the absence of Mad2 (Dobles et al., 2000), suggesting that APC is not essential for the SAC.
APC and MTs: along for the ride or steering the ship?
APC may also play additional roles at kinetochores, helping to explain why APC and EB1 coimmunoprecipitate with Bub proteins and why Bub1/BubR1 phosphorylates APC in vitro (Kaplan et al., 2001; Zhang et al., 2007). In particular, given its role as a +tip protein, APC could help anchor MTs at kinetochores during mitosis. Virtually all researchers ascribe some role for defects in kinetochore–MT attachment in explaining APC's phenotype. Some, like Sorger (Draviam et al., 2006) and Mao (Zhang et al., 2007), suggest that this is the primary defect.
Mao's laboratory suggests that APC is a BubR1 target in a SAC-independent function (Zhang et al., 2007). They found that depleting either APC or EB1 from Xenopus egg extracts disrupts metaphase chromosome alignment, and kinase-dead BubR1 inhibits APC recruitment to kinetochores. Thus, they suggest that APC and EB1 act together or in parallel to promote stable kinetochore–MT interactions. This is an interesting model, but it will be important to extend these studies to intact cells.
One key to high-fidelity chromosome segregation is ensuring that the right number of MTs attach to each kinetochore (Fig. 3, A and B). If APC regulates this, one might expect to see decreased kinetochore–MT density in its absence. However, kinetochore–MT bundles were similar in fluorescent intensity in APC-depleted HeLa cells chilled to remove non-kinetochore–MTs, whereas CLIP170-depleted cells had a 70% reduction (Draviam et al., 2006). Kaplan's laboratory found slightly reduced kinetochore–MTs in APC− versus APC+ colorectal tumor cell lines, but this difference was lost by anaphase (Green and Kaplan, 2003). Thus, APC loss does not dramatically disrupt kinetochore–MT attachment.
Another way of assessing whether all is right at the kinetochore–MT interface is to examine distance between the kinetochores on sister chromatids, a measure of tension. Once both kinetochores are attached, opposing pulling forces pull them apart, with cohesion between sisters preventing segregation (Pinsky and Biggins, 2005). If APC regulates either MT–kinetochore attachment or kinetochore–MT dynamics, interkinetochore distance might be altered in APC mutant cells (Fig. 3 B). Strikingly, cells expressing APC truncations or that are APC depleted have reduced interkinetochore distance (Tighe et al., 2004; Green et al., 2005; Draviam et al., 2006; Dikovskaya et al., 2007). This is one of the few phenotypes consistent among all studies. Interestingly, EB1 siRNA has similar effects (Green et al., 2005; Draviam et al., 2006). This suggests that reduced interkinetochore distance is a fundamental effect of APC loss; thus, experiments exploring mechanisms by which it occurs are needed to explain APC's role in CIN.
Sorger, Mao, and Kaplan's laboratories further found that APC depletion disrupts metaphase chromosome alignment and chromosome segregation (Figs. 2 A and 3 B). Sorger's group found that chromosome congression occurred in APC- or EB1-depleted cells, suggesting bioriented kinetochore attachment, but metaphase plates were less compact, and most kinetochore pairs were misoriented relative to the spindle axis (Draviam et al., 2006). Kaplan's laboratory found that APC mutant tumor cells also have less compact metaphase plates; the expression of truncated APC1-1450 or APC or EB1 depletion also led to failure of some chromosomes to reach the metaphase plate (Green and Kaplan, 2003; Green et al., 2005). In Xenopus extracts, Mao's laboratory found even more dramatic defects in metaphase chromosome alignment after APC or EB1 depletion, leading to chronic SAC activation and mitotic arrest (Zhang et al., 2007). Once anaphase began, 30–65% of APC- or EB1-depleted cells (Green et al., 2005; Draviam et al., 2006) and >80% of cells overexpressing truncated APC1-1450 (Green and Kaplan, 2003) contained lagging chromatin strands. Thus, the studies from Mao and Sorger and the earlier paper from Kaplan are all consistent with defects in kinetochore–MT attachment leading to highly penetrant defects in the segregation of individual chromosomes, leading to aneuploidy and CIN.
APC and cytokinesis: does the road to aneuploidy pass through tetraploidy?
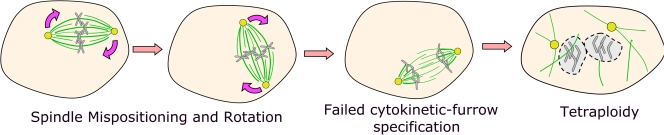
Another possible place for APC action is at plus ends of astral MTs. Kaplan's laboratory (Green et al., 2005) reported that one of the most striking effects of expressing truncated APC1-1450 was the reduction of astral MTs with consequent spindle mispositioning (Fig. 4). APC or EB1 depletion led to similar but less penetrant astral MT reduction and spindle mispositioning (Green et al., 2005). Sorger's laboratory also observed spindle mispositioning after APC depletion, including spindle rotation during metaphase (Draviam et al., 2006).
Figure 4.
Model suggesting APC regulates astral MT formation and thus cytokinesis. Cells depleted of APC sometimes have reduced astral MT arrays. This could trigger failed cytokinesis given the known role of astral MTs in defining and initiating formation of the cytokinetic furrow.
Caldwell et al. (2007) explored this further, finding that overexpression of the putative dominant-negative APC1-1450 leads to cytokinesis failure. They suggest that reduced MT contact with the cortex is the cause; consistent with this, they found a strong correlation between spindle rotation and failure to initiate a cytokinetic furrow (Fig. 4). The resulting tetraploidy could lead to aneuploidy after further divisions. One concern is that this occurred only after the overexpression of truncated APC1-1450 and was not reported in their earlier studies of APC knockdown (Green et al., 2005). Thus, it is possible that these effects are not strictly caused by APC loss of function. However, Kaplan's laboratory did find elevated aneuploidy and tetraploidy in intestines of APCmin/+ mice, even in crypts that are presumably heterozygous mutant (Caldwell et al., 2007). Thus, they concluded that defects in astral MTs and altered spindle positioning caused by APC mutations result in cytokinesis failure and that this is critical in CIN.
These data bring into focus one of the most substantial problems in comparing data from these different studies. It is difficult to reconcile the failure to properly segregate single chromosomes observed by Sorger or Kaplan after APC knockdown (Green et al., 2005; Draviam et al., 2006) and the outright cytokinesis failure leading to tetraploidy reported by Näthke (Dikovskaya et al., 2007) or the later Kaplan laboratory study (Caldwell et al., 2007). This must be resolved if we want to have a unified hypothesis for the role of APC in normal chromosome segregation and in CIN.
Does activated Wnt signaling cause CIN?
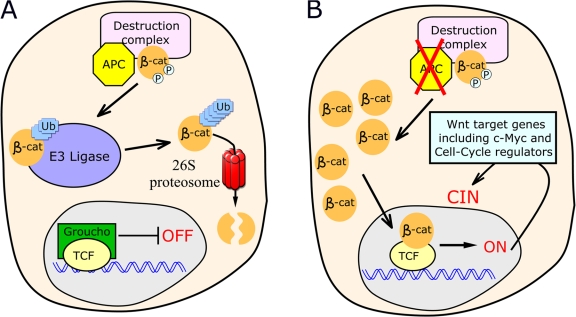
An additional critical issue with these models for APC function in CIN is that all assume that APC acts in CIN as a cytoskeletal regulator. However, APC's best-understood role is as a key negative regulator of Wnt signaling (Fig. 5; for review see Clevers, 2006). In APC's absence, Wnt signaling is inappropriately activated via stabilization of the key Wnt effector β-catenin and activation of downstream target genes by β-catenin–T cell factor (TCF) complexes. In the colon, where CIN caused by APC loss would have its greatest impact, Wnt signals regulate proliferation, maintaining stem cells (Reya and Clevers, 2005). Wnts stimulate proliferation by up-regulating the key transcription factor c-myc, which, in turn, down-regulates the cell cycle inhibitor p21. Thus, cells remain in cycle (van de Wetering et al., 2002). Strikingly, deletion of myc abrogates tumorigenic effects of the loss of APC (Sansom et al., 2007). APC loss activates Wnt signaling, locking cells in the stem cell fate and creating a colon polyp.
Figure 5.
The current model for APC function in Wnt signaling. (A) Functional APC assists in phosphorylating and targeting β-catenin for destruction. (B) Loss of APC leads to accumulation of β-catenin and, thus, transcription of Wnt-responsive genes.
Thus, it is possible, although not often appreciated, that APC loss contributes to CIN via activation of transcriptional targets of the Wnt pathway in its absence rather than through effects on cytoskeletal regulation. Cells lacking functional APC have levels of Wnt activation much higher than cells seeing endogenous Wnts; thus, their expression of cell cycle regulators may not match those of any normal cell, potentially altering cell cycle transitions.
Taketo's laboratory tested this hypothesis in cultured cells and the colon (Aoki et al., 2007). To assay CIN, they assessed the frequency of anaphase chromosome bridges (the anaphase bridge index [ABI]). In the normal colon, the ABI is ∼1%. In contrast, in polyps homozygous for truncated APC, this increased threefold, which is consistent with APC loss promoting CIN. They next examined whether activating the Wnt pathway downstream of APC using activated β-catenin affected CIN. Strikingly, this also elevated ABI five- to ninefold. Similar results were seen in ES cells (Aoki et al., 2007). To confirm that anaphase bridges accurately reflect CIN, they scored ES cell karyotypes after ∼10 doublings. In wild-type ES cells, 3–5% were abnormal, whereas in APC mutant cells or those expressing activated β-catenin, 15–22% were abnormal. Finally, blocking transcriptional effects of Wnt signaling with a dominant-negative TCF transcription factor reduced the ABI to levels near those in wild-type ES cells while expressing a β-catenin–independent version of TCF-induced CIN (as assessed by ABI). Thus, activating Wnt signaling downstream of APC led to CIN, and CIN caused by APC loss requires activation of Wnt target genes by TCF (Fig. 5). Interestingly, CIN frequency was much higher in culture than in vivo, even for wild-type cells; thus, cell culture may represent a sensitized environment. Taketo's laboratory also examined possible CIN mechanisms (Aoki et al., 2007). APC mutant or activated β-catenin–expressing ES cells both had a robust SAC, but prolonged MT depolymerization led to highly elevated numbers of 8N cells without apoptosis, suggesting mutant cells can escape the G2/M block without mitosis. This phenotype is strikingly similar to that reported by Näthke (Dikovskaya et al., 2007) and Kaplan (Caldwell et al., 2007), but in this case the proposed cause is activated Wnt signaling.
Behrens' laboratory provided further support for the hypothesis that Wnt signaling plays a role in CIN (Hadjihannas et al., 2006). In primary colon tumors, they found a very strong correlation between the level of Wnt signaling (assessed via a known Wnt target gene conductin) and the probability of CIN; 60% of CIN+ and only 7% of CIN− tumors showed a greater than fivefold activation of this Wnt target. Furthermore, they could mimic CIN by APC siRNA in cultured cells and could block this by simultaneously depleting β-catenin. Both Behrens' (Hadjihannas et al., 2006) and Näthke's (Dikovskaya et al., 2007) laboratories also examined whether removing APC increased CIN in colon cancer cells expressing activated β-catenin: if APC loss causes CIN in a cell in which Wnt signaling is already on, it would suggest that APC acts directly on chromosome segregation. In both cases, removal of APC increased CIN. However, it also increased activation of a Wnt reporter and an endogenous Wnt target, and, in the Behrens' laboratory study (Hadjihannas et al., 2006), codepletion of β-catenin abolished the effect of APC depletion. Together, all of these data suggest that APC loss may trigger CIN in part via activated Wnt signaling and downstream transcriptional effects; furthermore, the level of Wnt signaling elevation may be critical to whether CIN occurs.
How do we reconcile these disparate results?
Two key caveats may help explain discrepancies in the results of different APC-siRNA experiments. First, the efficiency of knockdown may vary between studies, and low levels of APC may rescue some but not all functions. For example, in kinetochore assembly, one must reduce CENP-A >10-fold to see an effect on CENP-I localization (Liu et al., 2006). Second, it is critical to remember that APC has a closely related paralogue, APC2. APC2 can regulate Wnt signaling, but its function in cytoskeletal regulation is unexplored. It is possible that, as in Drosophila melanogaster (Ahmed et al., 2002; Akong et al., 2002), the two APC family members play partially redundant roles in some tissues. This might explain different results of APC-siRNA if cells have different levels of APC2 expression.
Certain other data put limits on APC's roles in CIN. First, many of APC's cytoskeletal interactions are dispensable for its tumor suppressor function—APC1638T mutant mice lacking the C-terminal half of APC, including the MT- and EB1-binding sites, are viable and not tumor prone (Smits et al., 1999). Second, we think it is unlikely that truncated APC proteins seen in tumors have strong dominant effects on chromosome segregation. Null mutations in key kinetochore or SAC proteins are lethal to cells or organisms. Even less severe checkpoint defects seen in people carrying biallelic hypomorphic mutants in BubR1 result in massive aneuploidy, developmental defects, and cancer in many tissues (Hanks et al., 2004). None of this is characteristic of patients heterozygous for truncating APC mutations, whose tumors are largely restricted to the gastrointestinal tract. However, even weak dominant-negative effects of truncated APC causing modest reductions in segregation fidelity might promote tumor progression; slight reductions in SAC function can do so, as is demonstrated by the haploinsufficient cancer-prone phenotype of BubR1 or CENP-E heterozygous mice (Michel et al., 2001; Weaver et al., 2007).
It is also important to emphasize what does not go wrong when APC is depleted. Kinetochores still attach to MTs and align at least roughly at the metaphase plate, spindles are largely normal in structure (although with defects in position and astral MTs), and most, although not all, agree that there are no strong SAC defects. The downstream effects on chromosome segregation fall into two disparate categories: several laboratories report problems with alignment of individual chromosomes at the metaphase plate and subsequent loss of individual chromosomes, whereas other laboratories report cytokinesis failure.
Given the diversity of phenotypes and models, what can we conclude? First, we must seriously consider the possibility that activated Wnt signaling plays a role in CIN and that it may be the primary cause. Second, in evaluating possible cytoskeletal roles, we think it is important to focus on phenotypes seen in most cell types. All observe reduced interkinetochore distance. How might this happen? In principle, it could involve either defects in MT–kinetochore attachment or MT dynamics. Surprisingly, the effect seen is not predicted by the role of APC in interphase MT dynamics, where it promotes MT stability and growth (Kita et al., 2006). If loss of APC reduced MT stability without decreasing kinetochore attachment, in the simplest model, this would increase interkinetochore tension. However, the consequences of changes in MT–kinetochore attachment are difficult to predict given current data, depending on whether they involve global reduction in MT–kinetochore attachment or unbalanced attachment to the two kinetochores. One interesting avenue that should be carefully addressed is APC's possible role in regulating mitotic centromere-associated kinesin (MCAK), an MT-depolymerizing kinesin. MCAK is thought to monitor incorrect MT–kinetochore attachments, and defects in MCAK are known to lead to merotelic attachment and lagging chromosomes (Kline-Smith et al., 2004). These data, in combination with a known APC–MCAK interaction (Banks and Heald, 2004), suggest that APC may positively regulate MCAK, possibly by preventing aurora B's ability to phosphorylate and inactivate it.
We suspect that APC plays a modulatory but not essential role in several different processes. For example, imagine that in its absence, there are small changes in MT dynamics, in the strength of the MT–kinetochore attachment, in accuracy of the SAC, and in astral MTs, and, on top of that, the loss of APC triggers changes in the cell cycle and apoptosis via its role in Wnt signaling. Each defect might have little or no effect in isolation; however, in combination, they may destabilize the accuracy of chromosome segregation and, in extreme cases, lead to cytokinesis failure. However, we must be cautious in extrapolating work from cultured cells into the animal, as cultured cells may not be as “happy” as those in vivo and thus may be more susceptible to these effects.
In our view, the role of APC in CIN and the mechanisms by which it acts remain unclear. It is critical to continue analyzing the effects of APC loss both in cultured cells and in animal models, testing the reigning hypotheses and critically examining mechanisms.
Acknowledgments
We thank T. Maresca, B. McCartney, D. Roberts, E. Rogers, S. Rogers, G. Shemer, and the anonymous reviewers for helpful suggestions.
N.M. Rusan is supported by American Cancer Society grant PF-06-108-CCG. Our work on APC is supported by National Institutes of Health grant GM67236. We apologize to any authors whose work was not cited because of length constraints.
Abbreviations used in this paper: ABI, anaphase bridge index; APC, adenomatous polyposis coli; CIN, chromosome instability; ES, embryonic stem; MCAK, mitotic centromere-associated kinesin; MT, microtubule; SAC, spindle assembly checkpoint; TCF, T cell factor.
References
- Ahmed, Y., A. Nouri, and E. Wieschaus. 2002. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development. 129:1751–1762. [DOI] [PubMed] [Google Scholar]
- Akong, K., E. Grevengoed, M. Price, B. McCartney, M. Hayden, J. DeNofrio, and M. Peifer. 2002. Drosophila APC2 and APC1 play overlapping roles in Wingless signaling in the embryo and imaginal discs. Dev. Biol. 250:91–100. [DOI] [PubMed] [Google Scholar]
- Albuquerque, C., C. Breukel, R. van der Luijt, P. Fidalgo, P. Lage, F.J. Slors, C.N. Leitao, R. Fodde, and R. Smits. 2002. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum. Mol. Genet. 11:1549–1560. [DOI] [PubMed] [Google Scholar]
- Aoki, K., M. Aoki, M. Sugai, N. Harada, H. Miyoshi, T. Tsukamoto, T. Mizoshita, M. Tatematsu, H. Seno, T. Chiba, et al. 2007. Chromosomal instability by beta-catenin/TCF transcription in APC or beta-catenin mutant cells. Oncogene. 26:3511–3520. [DOI] [PubMed] [Google Scholar]
- Banks, J.D., and R. Heald. 2004. Adenomatous polyposis coli associates with the microtubule-destabilizing protein XMCAK. Curr. Biol. 14:2033–2038. [DOI] [PubMed] [Google Scholar]
- Cahill, D.P., C. Lengauer, J. Yu, G.J. Riggins, J.K. Willson, S.D. Markowitz, K.W. Kinzler, and B. Vogelstein. 1998. Mutations of mitotic checkpoint genes in human cancers. Nature. 392:300–303. [DOI] [PubMed] [Google Scholar]
- Caldwell, C.M., R.A. Green, and K.B. Kaplan. 2007. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J. Cell Biol. 178:1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, J., L. Molenaar, R.X. de Menezes, M. van Leerdam, C. Rosenberg, G. Moslein, J. Sampson, H. Morreau, J.M. Boer, and R. Fodde. 2006. Chromosomal instability in MYH- and APC-mutant adenomatous polyps. Cancer Res. 66:2514–2519. [DOI] [PubMed] [Google Scholar]
- Clevers, H. 2006. Wnt/beta-catenin signaling in development and disease. Cell. 127:469–480. [DOI] [PubMed] [Google Scholar]
- Dikovskaya, D., D. Schiffmann, I.P. Newton, A. Oakley, K. Kroboth, O. Sansom, T.J. Jamieson, V. Meniel, A. Clarke, and I.S. Näthke. 2007. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J. Cell Biol. 176:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobles, M., V. Liberal, M.L. Scott, R. Benezra, and P.K. Sorger. 2000. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell. 101:635–645. [DOI] [PubMed] [Google Scholar]
- Draviam, V.M., I. Shapiro, B. Aldridge, and P.K. Sorger. 2006. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 25:2814–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde, R., J. Kuipers, C. Rosenberg, R. Smits, M. Kielman, C. Gaspar, J.H. van Es, C. Breukel, J. Wiegant, R.H. Giles, and H. Clevers. 2001. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 3:433–438. [DOI] [PubMed] [Google Scholar]
- Green, R.A., and K.B. Kaplan. 2003. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 163:949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, R.A., R. Wollman, and K.B. Kaplan. 2005. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol. Biol. Cell. 16:4609–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjihannas, M.V., M. Bruckner, B. Jerchow, W. Birchmeier, W. Dietmaier, and J. Behrens. 2006. Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proc. Natl. Acad. Sci. USA. 103:10747–10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis, K.M., J.G. Caya, M. Reichelderfer, and W.F. Dove. 2002. Intestinal adenomas can develop with a stable karyotype and stable microsatellites. Proc. Natl. Acad. Sci. USA. 99:8927–8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks, S., K. Coleman, S. Reid, A. Plaja, H. Firth, D. Fitzpatrick, A. Kidd, K. Mehes, R. Nash, N. Robin, et al. 2004. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36:1159–1161. [DOI] [PubMed] [Google Scholar]
- Kaplan, K.B., A.A. Burds, J.R. Swedlow, S.S. Bekir, P.K. Sorger, and I.S. Nathke. 2001. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat. Cell Biol. 3:429–432. [DOI] [PubMed] [Google Scholar]
- Kita, K., T. Wittmann, I.S. Näthke, and C.M. Waterman-Storer. 2006. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol. Biol. Cell. 17:2331–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith, S.L., A. Khodjakov, P. Hergert, and C.E. Walczak. 2004. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell. 15:1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer, C., K.W. Kinzler, and B. Vogelstein. 1998. Genetic instabilities in human cancers. Nature. 396:643–649. [DOI] [PubMed] [Google Scholar]
- Liu, S.T., J.B. Rattner, S.A. Jablonski, and T.J. Yen. 2006. Mapping the assembly pathways that specify formation of the trilaminar kinetochore plates in human cells. J. Cell Biol. 175:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie, R.K., S. Bahmanyar, K.A. Siemers, V. Votin, P. Chang, T. Stearns, W.J. Nelson, and A.I. Barth. 2004. Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. J. Cell Sci. 117:1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney, B.M., M.H. Price, R.L. Webb, M.A. Hayden, L.M. Holot, M. Zhou, A. Bejsovec, and M. Peifer. 2006. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development. 133:2407–2418. [DOI] [PubMed] [Google Scholar]
- Michel, L.S., V. Liberal, A. Chatterjee, R. Kirchwegger, B. Pasche, W. Gerald, M. Dobles, P.K. Sorger, V.V. Murty, and R. Benezra. 2001. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 409:355–359. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue, Y., N. Shiina, and S. Tsukita. 2000. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J. Cell Biol. 148:505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio, A., and E.D. Salmon. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379–393. [DOI] [PubMed] [Google Scholar]
- Näthke, I.S. 2004. The adenomatous polyposis coli protein: the Achilles heel of the gut epithelium. Annu. Rev. Cell Dev. Biol. 20:337–366. [DOI] [PubMed] [Google Scholar]
- Näthke, I.S., C.L. Adams, P. Polakis, J.H. Sellin, and W.J. Nelson. 1996. The Adenomatous Polyposis Coli (APC) tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 134:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky, B.A., and S. Biggins. 2005. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 15:486–493. [DOI] [PubMed] [Google Scholar]
- Polakis, P. 2007. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 17:45–51. [DOI] [PubMed] [Google Scholar]
- Reya, T., and H. Clevers. 2005. Wnt signalling in stem cells and cancer. Nature. 434:843–850. [DOI] [PubMed] [Google Scholar]
- Sansom, O.J., V.S. Meniel, V. Muncan, T.J. Phesse, J.A. Wilkins, K.R. Reed, J.K. Vass, D. Athineos, H. Clevers, and A.R. Clarke. 2007. Myc deletion rescues Apc deficiency in the small intestine. Nature. 446:676–679. [DOI] [PubMed] [Google Scholar]
- Sieber, O.M., K. Heinimann, P. Gorman, H. Lamlum, M. Crabtree, C.A. Simpson, D. Davies, K. Neale, S.V. Hodgson, R.R. Roylance, et al. 2002. Analysis of chromosomal instability in human colorectal adenomas with two mutational hits at APC. Proc. Natl. Acad. Sci. USA. 99:16910–16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihn, C.R., E.J. Suh, K.H. Lee, T.Y. Kim, and S.H. Kim. 2003. p55CDC/hCDC20 mutant induces mitotic catastrophe by inhibiting the MAD2-dependent spindle checkpoint activity in tumor cells. Cancer Lett. 201:203–210. [DOI] [PubMed] [Google Scholar]
- Smits, R., M.F. Kielman, C. Breukel, C. Zurcher, K. Neufeld, S. Jagmohan-Changur, N. Hofland, J. van Dijk, R. White, W. Edelmann, et al. 1999. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 13:1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe, A., V.L. Johnson, M. Albertella, and S.S. Taylor. 2001. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe, A., V.L. Johnson, and S.S. Taylor. 2004. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J. Cell Sci. 117:6339–6353. [DOI] [PubMed] [Google Scholar]
- van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A.P. Haramis, et al. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 111:241–250. [DOI] [PubMed] [Google Scholar]
- Wang, Z., J.M. Cummins, D. Shen, D.P. Cahill, P.V. Jallepalli, T.L. Wang, D.W. Parsons, G. Traverso, M. Awad, N. Silliman, et al. 2004. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res. 64:2998–3001. [DOI] [PubMed] [Google Scholar]
- Weaver, B.A., A.D. Silk, C. Montagna, P. Verdier-Pinard, and D.W. Cleveland. 2007. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 11:25–36. [DOI] [PubMed] [Google Scholar]
- Zhang, J., S. Ahmad, and Y. Mao. 2007. BubR1 and APC/EB1 cooperate to maintain metaphase chromosome alignment. J. Cell Biol. 178:773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]