Abstract
Leptin, an adipocyte-derived hormone, is known to regulate a variety of neuroendocrine functions. It inhibits the hypothalamo-pituitary-adrenal axis (HPA) in several animal models, however, the exact mechanism by which it does so is not known. Since norepinephrine (NE) is a key regulator of the HPA axis, we hypothesized that leptin could suppress HPA activity by decreasing NE levels. To study this, we implanted adult male Sprague Dawley rats with both a push-pull cannula in the paraventricular nucleus (PVN) and a catheter in the jugular vein. Animals were treated with either 0 or 100 μg or 500 μg of recombinant rat leptin (Lep). Push-pull perfusion was performed from 1000-1600 h. Perfusate samples were collected every 30 min and analyzed for NE levels using HPLC-EC. Blood samples were collected every 60 min and analyzed for coticosterone (CS) levels. To further understand the role of NE in this phenomenon animals were treated with either an α-1 adrenergic agonist, phenylephrine (PHE; 0.5 mg/KgBW), an α-2 adrenergic agonist, clonidine (CLON; 0.6 mg/Kg BW), or a β adrenergic agonist, isoproterenol (ISO; 0.2 mg/Kg BW) alone or in combination with 500 μg of Lep. Pretreatment and hourly posttreatment blood samples were collected, plasma was separated and analyzed for CS levels. Leptin administration decreased NE release in the PVN significantly by 30 min (p<0.05). It also significantly reduced plasma CS levels at 240 and 300 min (p<0.05). Administration of either PHE or CLON in combination with leptin prevented the leptin-induced decrease in CS. In contrast, administration of ISO along with leptin did not prevent the leptin-induced decrease in CS. These results indicate that leptin decreases hypothalamic NE and plasma corticosterone and that this effect is most probably mediated through alpha-adrenergic receptors.
Keywords: leptin, paraventricular nucleus, norepinephrine, Corticosterone, hypothalamus
1. INTRODUCTION
Leptin, an adipocyte-derived hormone, plays a major role in metabolic homeostasis by serving as a signaling molecule to the brain, providing information on the amount of body fat to the hypothalamus. Leptin has several central and neuroendocrine effects. It decreases food intake, increases energy expenditure, and affects the reproductive axis (Campfield et al., 1995; Cheung et al., 1997; Halaas et al., 1995; Pelleymounter et al., 1995). In addition, leptin has been shown to inhibit the hypothalamo-pituitary-adrenal (HPA) axis (Ahima et al., 2000).
A number of neurochemicals have been implicated in mediating leptin’s effects on HPA axis, however, the precise the mechanism by which leptin produces this effect is not clear. Regulation of the HPA axis is under the control of corticotrophin releasing hormone (CRH) neurons. These neurons are found in a number of areas in the brain including the hypothalamus (Chrousos, 1992; Nemeroff, 1992; Szafarczyk et al., 1987). In the hypothalamus, the paraventricular nucleus (PVN) contains a high concentration of CRH cell bodies. When stimulated, these neurons secrete CRH, which in turn causes the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary (Chrousos, 1992; Nemeroff, 1992; Szafarczyk et al., 1987). This then, acts on the adrenal cortex to cause an increase in corticosterone (CS) completing the activation of the stress axis. Norepinephrine (NE) plays a critical role in stimulating CRH secretion (Plotsky et al., 1989; Szafarczyk et al., 1987). The PVN receives rich noradrenergic innervation from brainstem noradrenergic nuclei and their terminals are known to synapse with CRH cell bodies in the PVN (Szafarczyk et al., 1987). Moreover, leptin receptors have been identified in the PVN and in brainstem noradrenergic nuclei (Grill and Kaplan, 2002; Hosoi et al., 2002). These findings suggest that the PVN and brainstem are probable sites of leptin’s actions.
In a recently published study, both systemic and central administration of leptin decreased NE concentrations in the PVN. This was accompanied by a reduction in serum CS (Clark et al., 2006). This study provided correlative evidence that a single injection of leptin could decrease NE levels in the PVN. The present study was done to investigate the dynamic changes in NE levels in the PVN after leptin administration. Push-pull perfusion in combination with HPLC was used to obtain NE release profiles along with simultaneous determination of changes in plasma corticosterone. To provide a mechanistic basis, we also investigated the role of adrenergic receptors in this phenomenon by using specific alpha and beta adrenergic agonists to reverse the effects of leptin on CS.
2. RESULTS
Histological examination of push-pull cannula location
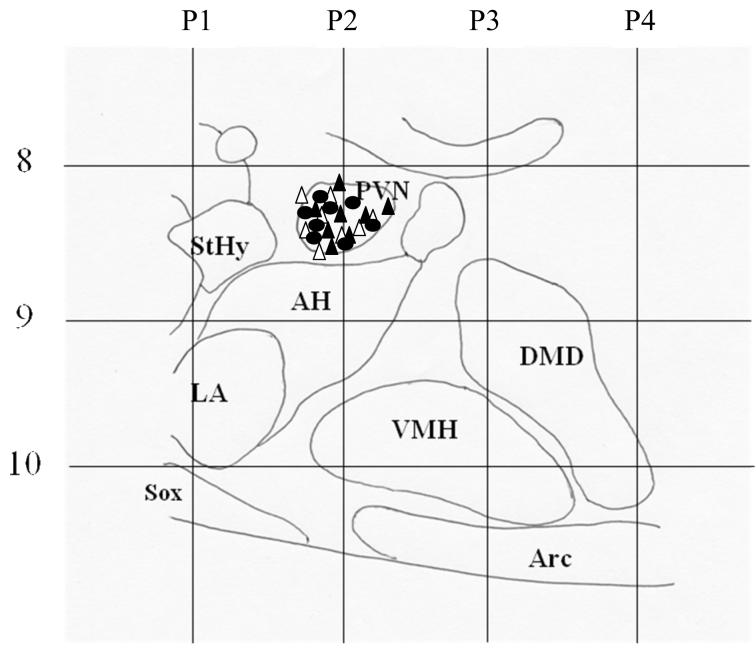
Push-pull cannula implantation sites in individual animals in control, 100 and 500 μg of leptin-treated groups are shown in a schematic diagram in Fig.1. All the cannulae were located in the vicinity of the PVN.
Fig 1.
A sagittal schematic diagram depicting the location of the push-pull cannulae in the control (△; n=8), 100 μg (▲; n=8) and 500 μg leptin (○; n=8) -treated groups. Cannula location was verified by histological examination. As indicated, all the cannulae were located in the PVN. The numbers P1-P4 indicate coronal plates extending from 1-4 mm posterior to Bregma. StHy: Striohypothalamic area; PVN: Paraventricular Nucleus; AH: Anterior hypothalamic area; LA: Lateroanterior hypothalamic nucleus; VMH: Ventromedial hypothalamus; DMD: Dorsomedial nucleus diffused; Arc: Arcuate Nucleus; Sox: Supraoptic decussation
Effects of systemic administration of leptin on NE release in the PVN and plasma CS
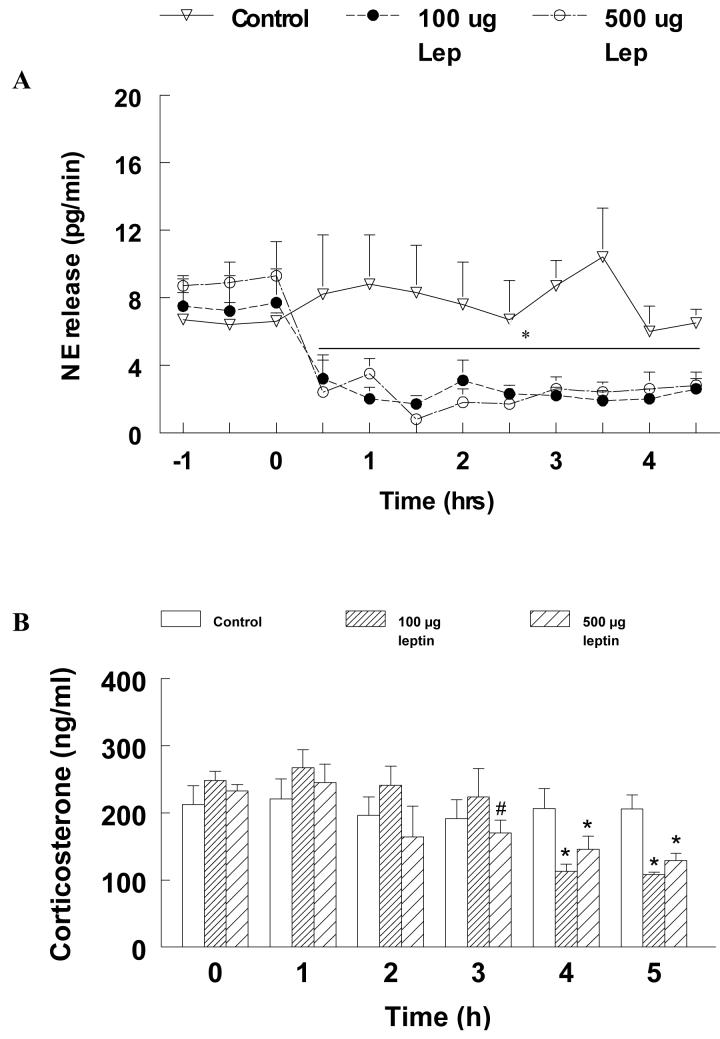
Profiles of NE release in the PVN in the different groups are shown in Fig.2A. The changes in NE levels were analyzed by two-way repeated measures ANOVA and the effect of treatment [F(2,132)=34.29], time [F(11,132)=10.13] as well as the interaction between treatment and time [F(22,132)=3.12] were found to be statistically significant (p<0.05). Pre-treatment NE levels were not significantly different between groups. Pre-treatment NE release (pg/min; mean ± SE) in the control group was 6.6 ± 0.5 and did not change signficantly for the duration of the experiment. In contrast, low dose leptin caused a 75% reduction in NE release from 7.7 ± 2 to 2.6 ± 0.6 within 30 min after which NE levels remained at that level for the rest of the observation period (p<0.05). Treatment with the higher dose of leptin produced a similar significant reduction in NE release from 9.3 ± 2 to 2.8 ± 0.8 within 60 min after which NE release remained free of any significant change (p < 0.05).
Fig. 2.
A. NE release (pg/min; mean±SE) in control and leptin-treated animals is shown. Leptin or its vehicle was administered at 0 h and perfusate samples from the PVN were collected at 30- min intervals. NE levels in the perfusates were measured using HPLC-EC. * indicates significant difference (p<0.05) compared to pretreatment levels and the control group.
B. Corticosterone (CS; ng/ml; mean±SE) measured at 1 h intervals in control and leptin treated animals. * indicates significant differences compared to pretreatment levels and the control group (p<0.05). # indicates significant difference from the pretreatment levels (p<0.05)
The effect of systemic administration of leptin on plasma CS is shown in Fig. 2B. Analysis of CS levels revealed a significant treatment [F(2,66)=68.1], time [F(5,66)=2.44] as well treatment vs. time interaction [F910,66)=6.689] (p<0.05). Pre-treatment plasma CS levels (ng/ml; mean ± SE) in the control group were 212.6 ± 28 and remained at that level for the duration of the experiment. Both high and low doses of leptin, however, caused a significant reduction in plasma CS compared to the control group and their respective pretreatment levels. Low dose leptin administration produced a significant decrease in plasma CS from 248.8 ± 13 during the pretreatment period to 155.5 ± 55.1 at 4 h. This decrease was also different from levels in the control group at that time point (p < 0.05). The higher dose of leptin also significantly reduced plasma CS from 256.3 ± 17.6 to 197 ± 20 (p<0.05). However, the decrease produced by the higher dose of leptin occurred much quickly (by 3 h rather than 4 h). This was also significantly lower than the levels observed in the control group at that time point. Moreover, the reduction in CS levels observed in both leptin-treated groups was similar. Plasma CS values remained low in both leptin-treated groups during the rest of the observation period.
Effects of adrenergic agonists on leptin-induced decrease in plasma CS
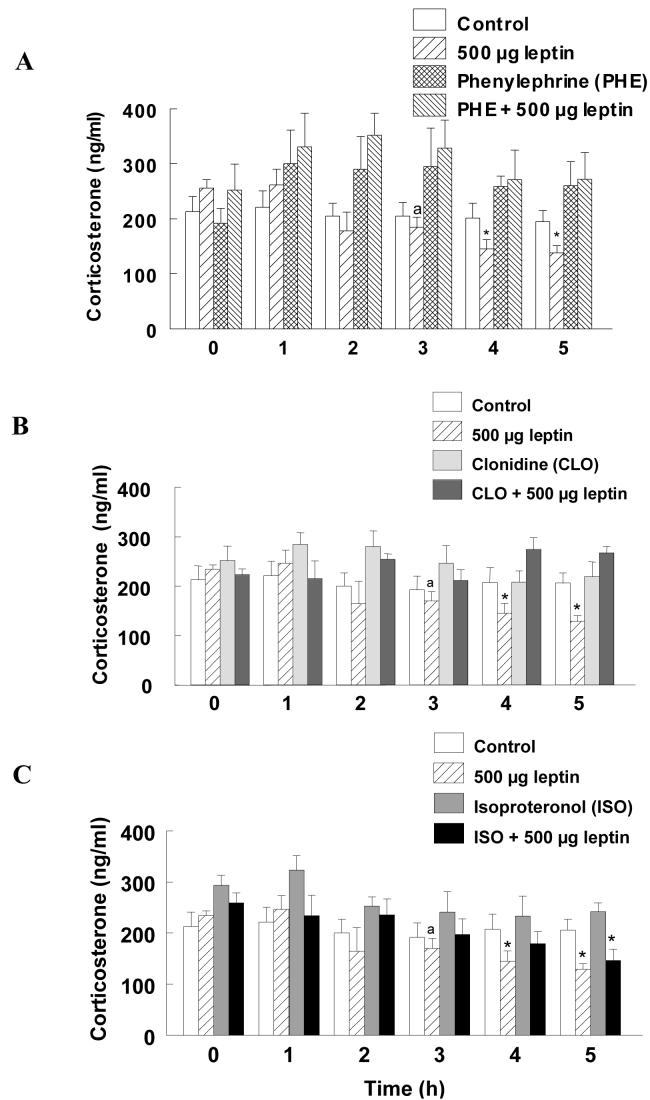
The effects of adrenergic agonists on leptin-induced decrease in CS are shown in Fig. 3. Statistical analysis of the results revealed that the effect of adrenergic agonists [F(5, 138)=3.049] and time [F(5, 138)=2.55] were significant (p<0.05). However, the interaction between treatment and time [F(25, 138)=0.98)] was not statistically significant. Pre-treatment CS levels were not different between groups. Plasma CS in the control group was 212.6 ± 28 and remained at that level for the duration of the experiment. Treatment with the α-1 agonist PHE, α-2 agonist CLON or the β agonist ISO did not appear to affect CS secretion when administered with the vehicle for leptin. In contrast, administration of PHE along with the 500 μg leptin completely prevented the leptin-induced decrease in plasma CS (Fig. 3A). CS levels in this group were 252.6±47 during the pretreatment period and remained at that level for the rest of the observation period. Similarly, administration of CLON along with the higher dose of leptin negated the leptin-induced decrease in plasma CS (Fig. 3B). In contrast to the α- adrenergic agonists, treatment with ISO failed to prevent the leptin-induced decrease in plasma CS. Although CS levels in this group at 3 and 4 h post treatment were not significantly different from pretreatment levels, it decreased significantly to 144.0±17 at 5 h (p<0.05) and was not different from the group that received 500 μg of leptin.
Fig. 3.
Effects of α (PHE and CLON) and β (ISO) adrenergic agonists on leptin-induced suppression of CS are shown in panels A, B and C, respectively. Leptin was administered at time 0 h and blood sampled through a venous catheter at hourly intervals for CS measurement. ‘a’ indicates significant differences compared to pretreatment levels. * indicates significant differences from pretreatment levels and the control group. CS levels in control and leptin treated groups are repeated in each panel for comparison purposes.
3. Discussion
Results from this study indicate for the first time that systemic administration of leptin can decrease NE release in the PVN while simultaneously reducing plasma CS. NE release in the PVN decreased 30 min following leptin administration and remained suppressed for the rest of the observation period. This was accompanied by decreased plasma CS levels at 4 and 5 h post-treatment. Although a dose-dependent effect was not apparent in NE levels, the higher dose of leptin advanced the decrease in CS by one hour compared to the low dose group. This effect was blocked by the α1 and α2 adrenergic agonists PHE and CLON, respectively. In contrast, the β-adrenergic agonist ISO did not have any effect on the leptin-induced decrease in plasma CS. These results indicate that leptin decreases hypothalamic NE and plasma CS and that this effect is most probably mediated via alpha-adrenergic receptors.
Several lines of evidence indicate that leptin inhibits the HPA axis or stress axis activity. Leptin deficient ob/ob mice and db/db mice that are incapable of sensing leptin, both have a hyperactive stress axis (Ahima et al., 1998; Campfield et al., 1995; Halaas et al., 1997; Halaas et al., 1995; Pelleymounter et al., 1995). Diabetic rats that have low leptin levels also have an activated stress axis and leptin administration normalizes CS levels in these animals (Barber et al., 2003). More direct evidence comes from recent studies in which chronic leptin infusion was able to decrease circulating CS levels (Nowak et al., 2002) and increase in endogenous leptin levels by feeding could decrease the hypercorticosolemia produced by fasting (Giovambattista et al., 2000). However, the mechanisms by which leptin decreases CS levels or HPA function is unclear.
A variety of neurochemicals are known to regulate the HPA axis. Among them, the catecholamines, specifically NE, is believed to have a stimulatory effect. The PVN of the hypothalamus has a high concentration of CRH cell bodies and receives rich noradrenergic innervation from the brain stem (Drolet and Rivest, 2001; Sawchenko and Swanson, 1982; Szafarczyk et al., 1988; Szafarczyk et al., 1987). Administration of NE into the PVN can stimulate CRH secretion, while a neurotoxic blockade of the ventral noradrenergic bundle, which carries noradrenergic fibers to the hypothalamus from the brain stem, markedly reduces NE levels and causes a significant reduction in CRH release (Szafarczyk et al., 1988; Szafarczyk et al., 1987). Taken together all these studies indicate that NE is stimulatory to HPA axis activity. Therefore, it is possible that the leptin-induced decrease in HPA function is mediated through a reduction in NE levels in the PVN. We recently demonstrated that both central and peripheral administration of leptin decreased NE concentrations in the PVN along with concurrent reductions in serum CS (Clark et al., 2006). The present study not only confirms these effects, but provides a time course for leptin’s action on NE levles in the PVN and plasma CS.
Leptin treatment decreased CS secretion significantly after 3 h in the high dose group and after 4 h in the low dose group. The higher levels of circulating leptin in the high dose group could have acted directly on the adrenal to suppress CS secretion. Both short and long forms of the leptin receptor are present in rat adrenocortical cells, and leptin has been shown to directly affect the growth of these cells as well as modulate glucocorticoid secretion (Malendowicz et al., 2004; Salzmann et al., 2004; Malendowicz et al., 2003). Also, there is evidence suggesting that leptin can directly inhibit glucocorticoid secretion from the adrenal gland (Szucs et al., 2001; Pralong et al., 1998). However, in the light of studies suggesting that both acute and chronic systemic administration of leptin can suppress CRH secretion from the PVN of the hypothalamus, ACTH from the anterior pituitary, and reduce glucocorticoid secretion from the adrenal gland, it is very possible that the suppression of glucocorticoid output by leptin is most likely mediated through central mechanisms.
The reduction in CS levels after leptin treatment was observed 2.5 hours after NE levels decreased in the PVN. Although the exact reason for this delay is not clear, it is possible that suppression of the HPA axis could take longer while stimulation of the HPA axis occurs more rapidly. In fact, it takes 1-2 days for glucocorticoids to suppress ACTH mRNA levels (Nakanishi et al., 1977).
Leptin has been shown to cross the blood-brain barrier (Banks et al., 1996) and leptin receptors have been identified in several areas of the brain including the PVN and the brainstem (Baskin et al., 1998; Hosoi et al., 2002; Meister and Hakansson, 2001; Mercer et al., 1998; Sawchenko, 1998). The decrease in NE levels in the PVN observed in this study could be brought about by a direct effect of leptin on either of these two areas. Leptin has been shown to decrease NE efflux from the hypothalamus in vitro, indicating that leptin is capable of directly affecting NE release from noradrenergic terminals in the hypothalamus (Francis et al., 2004). On the other hand, decreases in NE levels in vivo as observed in the present study can also be achieved by decreasing NE biosynthesis in brainstem NE neurons.
In the present study, leptin’s effects on plasma CS were reversed by the administration of alpha-adrenergic agonists, CLON and PHE, but not by the beta-adrenergic agonist ISO. A number of studies have investigated the role of adrenergic receptors in the regulation of CS. Systemic administration of CLON was able to reverse CS suppression after NE depletion (Daniels et al., 1993) and CLON by itself can also increase CS secretion (Daniels et al., 1993). Similarly, PHE, a selective α1 adrenergic agonist, stimulates CS secretion (Bugajski et al., 1995; Saphier and Feldman, 1989) which was blocked by an adrenergic antagonist prazosin (Gadek-Michalska et al., 1990; Saphier and Feldman, 1989). Systemic administration of the β adrenergic agonist, ISO, has also been shown to increase CS (Bugajski et al., 1995; Bugajski et al., 1991; Daniels et al., 1989) which was blocked by propranolol, a beta adrenergic antagonist. Administration of CLON or PHE alone did not increase plasma CS in the present study, however, treatment with both these agonists was able to completely reverse leptin-induced decrease in CS secretion. In contrast, ISO not only failed to increase CS, but it was unable to prevent the leptin-induced decrease in CS. Although it could be argued that a higher dose of ISO could have reversed these effects, the levels of CS in ISO-treated animals were comparable to the levels observed after CLON and PHE treatment. The reasons for the inability of all three adrenergic agonists to increase CS by themselves are unclear. This could be due to the fact that the doses of CLON, PHE and ISO that were used in the present study were lower compared to others that examined the effects of these compounds on CS levels (Bugajski et al., 1995; Bugajski et al., 1991; Daniels et al., 1993; Gadek-Michalska et al., 1990; Saphier and Feldman, 1989). However, results from the present study indicate that these doses were effective in dissecting the roles of adrenergic receptors in leptin-induced decrease in CS secretion.
These results indicate that leptin probably produces its inhibitory effects on CS by decreasing NE release in the PVN and that these effects are mediated through alpha adrenergic receptors and not through beta adrenergic receptors. It is also quite possible that the suppressive effects of leptin on serum CS could be due to direct effects on the adrenal gland. Leptin could also influence brain stem noradrenergic neurons to decrease NE levels in the PVN. We are currently investigating this possibility.
4. Experimental Procedure
Animals
Adult male Sprague-Dawley rats (3-4 months old) weighing approximately 350 g were obtained from Harlan Inc. (Indianapolis, IN). They were housed in light-controlled (lights on from 0700-1900 h), air-conditioned (23±2° C) animal quarters and were fed rat chow and water ad libitum. Animals were used in the experiments in accordance with the NIH guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at Michigan State University.
Cannula implantation and push-pull perfusion
The procedure for implanting a push-pull cannula in the PVN has been described before (MohanKumar and Quadri, 1993). Briefly, two groups of rats were anesthetized with sodium pentobarbital (50 mg/Kg BW, i.p.). They were implanted with a stainless steel cannula (22-G) in the PVN stereotaxically using the following coordinates with reference to the Bregma (Paxinos, 1987): 1.8 mm posterior, 0.2 mm lateral, 8.4 mm ventral as described before (Francis et al., 2000; MohanKumar and Quadri, 1993). After securing the cannula with dental cement, a stylet made of 30-G stainless steel tubing was used to plug the guide cannula to avoid blockage due to gliosis. After recovering from surgery, the animals were given at least a week of rest before experimentation.
The push-pull perfusion procedure has been described before (Francis et al., 2000; MohanKumar and Quadri, 1993). Briefly, the stylet was replaced by a cannula with the push and pull tubes connected to PE-20 tubings. These were attached to two channels of a peristaltic pump (P-3, Pharmacia, Sweden). Artificial cerebrospinal fluid was used as the perfusion medium (Francis et al., 2000; MohanKumar and Quadri, 1993). The perfusion medium was pushed through the push tube and collected from the pull tube. Samples were collected from 1000 h until 1600 h at 30-minute intervals and stored at -70 °C after the addition of 0.5 M HCLO4 at a ratio of 25:1 v/v. The perfusates were analyzed for NE concentrations using HPLC-EC.
Jugular Catheterization
The jugular catheterization procedure has been described before (Mohankumar et al., 1994). Briefly, a 0.75” long incision was made on the ventral surface of the neck under isoflurane anesthesia. The jugular vein was isolated by blunt dissection and punctured using a sterile 20G needle. A catheter made of silastic tubing (Dow Corning, Midland, MD) was inserted into the vein and held in position by two sutures. The free end of the catheter was passed under the skin and externalized at the base of the skull. The catheter was flushed with heparin saline (10 U/ml) and sealed. Blood samples were collected at hourly intervals, centrifuged at 800xg, plasma was separated and the cells were re-suspended in heparinized saline and re-introduced into the animal. Plasma samples were stored at -20° C until they were analyzed for CS by RIA.
Treatment
On the day of the experiment, animals were introduced into the perfusion cages at least two hours before treatment. After collecting two pretreatment push-pull perfusates and one blood sample, animals were injected i.p. with 0 (control, vehicle for leptin, n=8), 100 μg (low dose, n=8) or 500 μg (high dose, n=8) of rat recombinant leptin (R&D systems, Minneapolis, MN). Posttreatment perfusate samples were collected every 30 minutes and blood samples every 60 min from 1000 to 1600 h. At the end of the experiment, the location of the push-pull cannula was verified by histological examination and only those animals with a cannula in the PVN were included in the analysis.
In the second experiment, after collecting a pretreatment blood sample, animals were injected i.p. with either the α-1 adrenergic agonist, phenylephrine (PHE; 0.5 mg/Kg BW in sterile saline (n=5) followed 5 min later by 500 μl of sterile saline (vehicle for leptin); PHE followed 5 min later by 500 μg leptin (PHE+LEP, n=5); α-2 adrenergic agonist, clonidine (CLON; 0.6 mg/KgBW, n=8) followed by saline, or CLON followed by 500 μg leptin (CLON+LEP, n=9); a β adrenergic agonist, isoproterenol (ISO, 0.2 mg/KgBW, n=8) followed by saline, or ISO followed by 500 μg leptin (ISO+LEP, n=9). Posttreatment blood samples were collected every 60 min for 4 h. After separation of plasma, blood cells were reintroduced into the animal as described before.
HPLC
The HPLC-EC set up has been described previously (Francis et al., 2000; Mohankumar et al., 1994). Briefly, it consisted of a LC-4C amperometric detector (Bioanalytical Systems, West Lafayette, IN, USA), a phase II, 5 μm ODS reverse phase, C-18 column (Phenomenex, Torrance, CA, USA), a glassy carbon electrode, a CTO-10 AT/VP column oven, and an LC-10 AT VP pump (Shimadzu, Columbia, MD, USA). The composition of the mobile phase was as follows: monochloroacetic acid (14.14 g/L), sodium hydroxide (4.675 g/L), octanesulfonic acid disodium salt (0.3 g/L), ethylenediaminetetraacetic acid (0.25 g/L), acetonitrile (3.5%), and tetrahydrofuran (1.4%). The mobile phase was made in pyrogen-free water and then filtered and degassed through a Milli-Q purification system (Millipore, Bedford, MA, USA) and pumped at a flow rate of 1.8 ml/min. The sensitivity of the detector was 1 nA full scale, and the potential of the working electrode was 0.65 V. The column oven maintained the temperature of the column at 37 °C. Seventy five μl of the supernatant along with 25 μl of the internal standard (0.5 M dihydroxybenzylamine) was injected into the HPLC system. Class VP software (version 7.3, Shimadzu, Columbia, MD) was used to analyze the chromatograms. The sensitivity of the system was <1 pg.
Radioimmunoassay
Double antibody RIA was used to measure CS levels in the plasma. A Coat-A-Count kit for rat CS was obtained from Diagnostic Products Corp. (Los Angeles, CA, USA). The assays were performed in duplicate as per the manufacturer’s instructions.
Statistical Analysis
Changes in NE and CS levels were analyzed using two-way repeated measures ANOVA accounting for the treatment, time as well as the interaction effects between treatment × time combinations. The group wise comparisons were performed by Fisher’s LSD test.
Acknowledgement
This study was supported in part by NSF IBN 0236385 to SMJM and PSM and NIH AG 027697 to PSM and SMJM. Andrew Shin was supported by the Biomedical Health Research Initiative funds, MSU. We would like to thank Ms. Katrina Linning for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section Classification: Regulatory Systems
References
- Ahima RS, et al. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–7. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, et al. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Banks WA, et al. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Barber M, et al. Diabetes-induced neuroendocrine changes in rats: role of brain monoamines, insulin and leptin. Brain Res. 2003;964:128–35. doi: 10.1016/s0006-8993(02)04091-x. [DOI] [PubMed] [Google Scholar]
- Baskin DG, et al. Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes. 1998;47:538–43. doi: 10.2337/diabetes.47.4.538. [DOI] [PubMed] [Google Scholar]
- Bugajski J, et al. Adrenergic regulation of the hypothalamic-pituitary-adrenal axis under basal and social stress conditions. J Physiol Pharmacol. 1995;46:297–312. [PubMed] [Google Scholar]
- Bugajski J, et al. Catecholaminergic regulation of the hypothalamic-pituitary-adrenocortical activity. J Physiol Pharmacol. 1991;42:93–103. [PubMed] [Google Scholar]
- Campfield LA, et al. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Cheung CC, et al. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–8. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Regulation and dysregulation of the hypothalamic-pituitary-adrenal axis. The corticotropin-releasing hormone perspective. Endocrinol Metab Clin North Am. 1992;21:833–58. [PubMed] [Google Scholar]
- Clark KA, et al. Effects of central and systemic administration of leptin on neurotransmitter concentrations in specific areas of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R306–12. doi: 10.1152/ajpregu.00350.2005. [DOI] [PubMed] [Google Scholar]
- Daniels WM, et al. Alpha 2- and beta-adrenergic stimulation of corticosterone secretion in rats. Neurochem Res. 1993;18:159–64. doi: 10.1007/BF01474679. [DOI] [PubMed] [Google Scholar]
- Daniels WM, et al. The effect of partial noradrenergic denervation on corticosterone secretion in the rat. Neurochem Res. 1989;14:1187–90. doi: 10.1007/BF00965507. [DOI] [PubMed] [Google Scholar]
- Drolet G, Rivest S. Corticotropin-releasing hormone and its receptors; an evaluation at the transcription level in vivo. Peptides. 2001;22:761–7. doi: 10.1016/s0196-9781(01)00389-8. [DOI] [PubMed] [Google Scholar]
- Francis J, et al. Correlations of norepinephrine release in the paraventricular nucleus with plasma corticosterone and leptin after systemic lipopolysaccharide: blockade by soluble IL-1 receptor. Brain Res. 2000;867:180–7. doi: 10.1016/s0006-8993(00)02311-8. [DOI] [PubMed] [Google Scholar]
- Francis J, et al. Leptin inhibits norepinephrine efflux from the hypothalamus in vitro: role of gamma aminobutyric acid. Brain Res. 2004;1021:286–91. doi: 10.1016/j.brainres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A, et al. Effects of systemic and intracerebroventricular phenylephrine and clonidine on corticosterone secretion in rats. Endocrinol Exp. 1990;24:249–58. [PubMed] [Google Scholar]
- Giovambattista A, et al. Food intake-induced leptin secretion modulates hypothalamo-pituitary-adrenal axis response and hypothalamic Ob-Rb expression to insulin administration. Neuroendocrinology. 2000;72:341–9. doi: 10.1159/000054603. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- Halaas JL, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hosoi T, et al. Brain stem is a direct target for leptin’s action in the central nervous system. Endocrinology. 2002;143:3498–504. doi: 10.1210/en.2002-220077. [DOI] [PubMed] [Google Scholar]
- Meister B, Hakansson ML. Leptin receptors in hypothalamus and circumventricular organs. Clin Exp Pharmacol Physiol. 2001;28:610–7. doi: 10.1046/j.1440-1681.2001.03493.x. [DOI] [PubMed] [Google Scholar]
- Mercer JG, et al. Association of leptin receptor (OB-Rb), NPY and GLP-1 gene expression in the ovine and murine brainstem. Regul Pept. 1998;75-76:271–8. doi: 10.1016/s0167-0115(98)00078-0. [DOI] [PubMed] [Google Scholar]
- MohanKumar PS, Quadri SK. Systemic administration of interleukin-1 stimulates norepinephrine release in the paraventricular nucleus. Life Sci. 1993;52:1961–7. doi: 10.1016/0024-3205(93)90637-i. [DOI] [PubMed] [Google Scholar]
- Mohankumar PS, et al. Correlations of catecholamine release in the medial preoptic area with proestrous surges of luteinizing hormone and prolactin: effects of aging. Endocrinology. 1994;135:119–26. doi: 10.1210/endo.135.1.8013343. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, et al. Glucocorticoid effect on the level of corticotropin messenger RNA activity in rat pituitary. Proc Natl Acad Sci U S A. 1977;74:3283–6. doi: 10.1073/pnas.74.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. New vistas in neuropeptide research in neuropsychiatry: focus on corticotropin-releasing factor. Neuropsychopharmacology. 1992;6:69–75. [PubMed] [Google Scholar]
- Nowak KW, et al. Effects of prolonged leptin infusion on rat pituitary-adrenocortical function. Int J Mol Med. 2002;9:61–4. [PubMed] [Google Scholar]
- Paxinos GA, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1987. [Google Scholar]
- Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, et al. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989;10:437–58. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Saphier D, Feldman S. Adrenoceptor specificity in the central regulation of adrenocortical secretion. Neuropharmacology. 1989;28:1231–7. doi: 10.1016/0028-3908(89)90216-5. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol. 1998;402:435–41. [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, et al. Central catecholaminergic system stimulates secretion of CRH at different sites. Am J Physiol. 1988;255:E463–8. doi: 10.1152/ajpendo.1988.255.4.E463. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, et al. Further evidence for a central stimulatory action of catecholamines on adrenocorticotropin release in the rat. Endocrinology. 1987;121:883–92. doi: 10.1210/endo-121-3-883. [DOI] [PubMed] [Google Scholar]