Abstract
Recent studies indicated that hyperactivity of the hypothalamo-pituitary-adrenal system is a considerable risk factor for the precipitation of affective disorders, most notably of major depression. The mechanism by which this hyperactivity eventually leads to clinical symptoms of depression is unknown. In the present animal study, we tested one possible mechanism, i.e., that long-term exposure to high corticosterone levels alters functional responses to serotonin in the hippocampus, an important area in the etiology of depression. Rats were injected daily for 3 weeks with a high dose of corticosterone; electrophysiological responses to serotonin were recorded intracellularly from CA1 pyramidal neurons in vitro. We observed that daily injections with corticosterone gradually attenuate the membrane hyperpolarization and resistance decrease mediated by serotonin-1A receptors. We next used single-cell antisense RNA amplification from identified CA1 pyramidal neurons to resolve whether the functional deficits in serotonin responsiveness are accompanied by decreased expression levels of the serotonin-1A receptor. It appeared that expression of serotonin-1A receptors in CA1 pyramidal cells is not altered; this result was supported by in situ hybridization. Expression of corticosteroid receptors in the same cells, particularly of the high-affinity mineralocorticoid receptor, was significantly reduced after long-term corticosterone treatment. The present findings indicate that prolonged elevation of the corticosteroid concentration, a possible causal factor for major depression in humans, gradually attenuates responsiveness to serotonin without necessarily decreasing serotonin-1A receptor mRNA levels in pyramidal neurons. These functional changes may occur by a posttranscriptional mechanism or by transcriptional regulation of genes other than the serotonin-1A receptor gene itself.
Keywords: mineralocorticoid receptor, glucocorticoid receptor, intracellular recording, single-cell antisense RNA amplification, in situ hybridization
Corticosteroid hormones are secreted from the adrenal glands in a circadian pattern (1). In rats, corticosterone circulates in low amounts in the morning and at high concentrations in the evening. Temporary elevations in the level occur after stressful events. Corticosteroids can enter the brain and bind to two intracellular receptor subtypes (2): the mineralocorticoid receptor (MR; Kd ≈ 0.5 nM) and the glucocorticoid receptor (GR; Kd ≈ 5 nM). Because of the difference in affinity, changes in plasma corticosterone concentration alter the relative MR/GR occupation. Low levels of corticosterone mainly activate brain MRs, whereas high levels at the circadian peak or during stress activate GRs along with MRs (3–5). Electrophysiological studies in CA1 hippocampal neurons, which coexpress MR and GR, have shown that selective activation of corticosteroid receptors alters ion conductances and neurotransmitter responses within 1–2 hours (6). In particular, the membrane hyperpolarization mediated by serotonin-1A receptors (5-HT1A R) is sensitive to steroids. Predominant MR occupation suppresses 5-HT1A R-mediated responses, and this process requires protein synthesis (7–9). Additional GR occupation increases serotonin (5-HT) responses. Accordingly, 5-HT responses were large in acutely stressed animals compared with stressed animals pretreated with the GR-antagonist RU 38486 (10).
In addition to short-term increases in corticosteroid level after acute exposure to stress, chronic elevation of plasma corticosteroid levels also occurs, e.g., in association with affective disorders, most notably major depression (11, 12). In fact, hyperactivity of the hypothalamo-pituitary-adrenal system (yielding elevated corticosteroid levels) appears to exist before the manifestation of clinical symptoms of depression, suggesting that this hyperactivity forms a major risk factor for the precipitation of the disease (12, 13). The mechanism by which hyperactivity of the hypothalamo-pituitary-adrenal system eventually leads to clinical symptoms is largely unknown. Given the sensitivity of hippocampal 5-HT responses to corticosteroid levels, we hypothesize that chronic hypercorticism may alter the function of the hippocampal 5-HT system, which is important in the etiology of depression (14).
To test this hypothesis, we examined responses of CA1 hippocampal cells to 5-HT with intracellular recording in slices from rats that were injected daily with a high dose of corticosterone or vehicle for 3 weeks (15). To dissociate short-term from long-term effects of treatment, we also included a group that received a single corticosterone injection. Moreover, a 1-week corticosterone-treated group was incorporated to study the time course of changes in 5-HT responsiveness. In our electrophysiological recording, we focused on the maximal hyperpolarization and decrease in membrane resistance, which were shown to be indicators for 5-HT1A R activation in CA1 neurons (16). To resolve whether putative changes in the hippocampal 5-HT responses are caused by altered expression of the receptor responsible for these responses, we examined the transcript level of the 5-HT1A R in the entire CA1 area with in situ hybridization and in individual CA1 pyramidal neurons with the single-cell antisense RNA (aRNA) amplification technique (17). The advantage of the latter approach is that functional responses can be linked to transcriptional activity in identified CA1 pyramidal neurons. Moreover, the technique allows simultaneous examination of many different transcripts in single cells. Accordingly, we studied relative expression of several transcripts, including the MR and GR aRNA, in addition to the 5-HT1A R mRNA expression.
Materials and Methods
Animals.
Experimental conditions were similar to those described (e.g., refs. 7 and 18). In short, male Wistar rats (Harlan CPB, Horst, The Netherlands) were group-housed under standard conditions with alternating 12-h light/dark cycles (lights on at 8:00 a.m.). All experiments were approved by the local Animal Experiment Committee (DEC Project DED02).
Animals were injected subcutaneously with corticosterone (10 mg per rat per day, as described in ref. 15) before 9:30 a.m., either once or daily during 1 or 3 weeks. Corticosterone was dissolved each morning in ethanol (95%) and subsequently diluted in sunflower oil to a stock concentration of 10 mg corticosterone/200 μl. This dosage is assumed to saturate the GR for most of the day (3). Control animals were injected in a similar way with the vehicle solution. After each injection, the body weight was measured and the animal was returned to the home cage.
The age of the animals at the start of the treatment was selected such that animals were of comparable age at the day of the electrophysiological experiment (young adults; see Table 1). Because of a modest gain in body weight, animals receiving corticosterone for 3 weeks were subjected to a somewhat higher dose of corticosterone at the start of the experiment. This change in dosage is, however, small compared with the change seen when corticosterone is released for 3 weeks from a subcutaneous pellet (19); importantly, we did not observe any correlation between body weight at the start of the experiment and 5-HT response at the end of the treatment period.
Table 1.
Results of treatment with corticosterone
| Treatment | Body weight, g
|
Gland weight, mg/100 g
|
Plasma corticosterone, μg/dl | N | RMP, mV | Resistance, MΩ | Rectification, % | n | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Thymus | Adrenal | |||||||
| Acute | ||||||||||
| Vehicle | 212 ± 16 | 212 ± 16 | 302 ± 23 | 13.4 ± 1.3 | 9.6 ± 2.6 | 8 | −63.2 ± 0.8 | 49.7 ± 1.5 | 91.7 ± 1.2 | 15 |
| Corticosterone | 198 ± 22 | 198 ± 22 | 241 ± 36 | 13.4 ± 2.1 | 29.4 ± 12.8 | 6 | −65.8 ± 1.5 | 49.2 ± 1.5 | 93.0 ± 1.6 | 8 |
| 1 Week | ||||||||||
| Vehicle | 194 ± 15 | 223 ± 13 | 239 ± 16 | 15.1 ± 1.1 | 19.7 ± 6.2 | 8 | −61.5 ± 2.1 | 46.3 ± 2.8 | 91.4 ± 0.9 | 12 |
| Corticosterone | 208 ± 19 | 212 ± 18 | 66 ± 13*** | 9.7 ± 1.1** | 78.1 ± 6.2* | 7 | −62.2 ± 1.5 | 45.4 ± 3.2 | 91.1 ± 1.1 | 13 |
| 3 Weeks | ||||||||||
| Vehicle | 153 ± 15 | 276 ± 12 | 225 ± 8 | 13.5 ± 0.8 | 7.7 ± 4.4 | 7 | −64.7 ± 1.2 | 51.7 ± 2.7 | 91.7 ± 0.9 | 10 |
| Corticosterone | 145 ± 15 | 213 ± 9** | 122 ± 41* | 7.2 ± 0.7*** | 84.3 ± 23.7* | 8 | −63.5 ± 1.1 | 47.4 ± 1.9 | 93.4 ± 0.8 | 16 |
Long-term treatment with a high dose of corticosterone altered the body weight (in g), thymus and adrenal weight (in mg/100 g body weight), and the plasma corticosterone concentration (in μg/dl), as determined in N animals at the end of the treatment. Resting membrane potential (RMP; in mV), membrane resistance (in MΩ), and inward rectification (“sag”) in n cells were not significantly affected by either acute or long-term steroid treatment. All data represent mean ± SEM for N animals. For some parameters, animals were excluded from the analysis because no reliable data were obtained. No more than two animals per group were excluded on this basis. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Consistent with the high dose of corticosterone to which animals were subjected, plasma corticosterone levels were very much elevated, whereas gain in body weight and the weight of thymus and adrenals were attenuated, after both 1 and 3 weeks of corticosterone treatment (see Table 1 and ref. 20). Although vehicle-injected control animals displayed mildly elevated plasma corticosterone levels 90 min after injection, these elevations were probably temporary and did not cause, e.g., changes in adrenal weight.
Experimental Protocol.
On the day of the experiment, 60 min after the last injection, the animal was taken from the home cage and housed in a transportation cage for 30 min. The animal was then decapitated, and a blood sample was collected from the trunk to determine plasma corticosterone levels by a radioimmunoassay. Thymus and adrenal glands were routinely taken out and weighed.
After decapitation, the brain was removed from the skull. The right hemisphere was frozen instantly on powdered dry ice and stored at −80°C for in situ hybridization. For electrophysiology and single-cell RNA collection, the left hippocampus was removed from the animal. Dorsal transversal hippocampal slices (400 μm) were prepared with a tissue chopper. The slices were stored at room temperature in carbonated (95% O2/5% CO2) artificial cerebrospinal fluid consisting of 124 mM NaCl/3.5 mM KCl/1.25 mM NaH2PO4/1.5 mM MgSO4/25 mM NaHCO3/2 mM CaCl2/10 mM glucose. After at least 30 min, one slice at a time was transferred to the recording chamber and perfused with warm (33°C), carbonated artificial cerebrospinal fluid (pH = 7.4; flow rate of 2–3 ml⋅min−1). The slice was fixed between two nylon meshes and kept fully submerged.
Recording of 5-HT Responses.
Intracellular recordings were obtained from CA1 pyramidal neurons with conventional methods by using 4 M KOAc-filled glass microelectrodes (impedances: 80–130 MΩ). Voltage signals were passed to an NPI Instruments (Tann, Germany) amplifier. The membrane potential and current injections were continuously registered on a chart recorder. Hyperpolarizing currents of increasing magnitude were passed through the microelectrode to determine input resistance (e.g., ref. 7; see example in Fig. 1). Neurons included in this study displayed stable resting-membrane potentials between −50 and −70 mV, input resistances >30 MΩ, and spike amplitudes >80 mV.
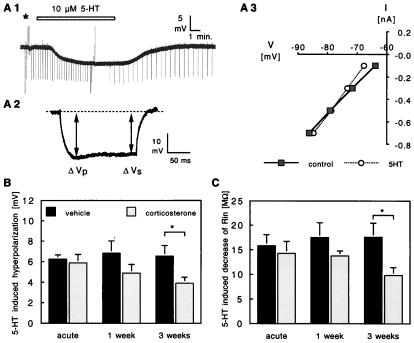
Figure 1.
(A) Typical response of a pyramidal CA1 neuron to bath-perfused 5-HT (A1, application period indicated by horizontal bar). In the presence of 5-HT, the membrane potential is hyperpolarized. This effect is reversible on washout of 5-HT. Before application of 5-HT, the cell was subjected to a fixed protocol (marked by ∗) during which inward rectification and membrane resistance were determined. Inward rectification (“sag”) was defined as the ratio × 100 of the sustained amplitude of the voltage deflection (ΔVs) and the peak voltage deflection (ΔVp), in response to a −0.5-nA current pulse of 180-ms duration (typical example shown in A2). Membrane resistance before and during 5-HT application was assessed from the inversed slope of the current–voltage relationship (A3), based on the ΔVp during negative and positive current pulses (−0.7 to 0.5 nA; 180-ms duration). Voltage deflections for the −0.7-nA and for the positive current pulses inducing an action potential were truncated in this chart recording. Changes in resistance were also continuously monitored, by applying −0.3-nA current pulses of 180-ms duration at a 15-s (before and during 5-HT) or 30-s (during washout) interval. (B) Changes in membrane potential during 5-HT application in hippocampal cells from vehicle-treated (black bars) and corticosterone-treated (open bars) animals. After 3 weeks of corticosterone treatment, the 5-HT-induced hyperpolarization was significantly smaller (P < 0.05) than in the vehicle-treated controls. The bars represent mean ± SEM, based on the same number of cells and rats as given in Table 1. (C) Mean (±SEM) change in membrane resistance during 5-HT application in hippocampal cells from vehicle- and corticosterone-treated animals. Similar to what was seen for the change in membrane potential, the 5-HT-induced reduction in resistance was significantly smaller (P < 0.05) in CA1 pyramidal neurons from 3-weeks corticosterone-treated animals than in the vehicle-treated controls.
Drugs were applied to the slices via the perfusion medium. 5-HT (creatinine sulfate complex; Sigma) was dissolved as 1 mM stock in artificial cerebrospinal fluid and stored at −20°C. Before application, the drug was diluted to the final concentration (10 μM). The concentration of 10 μM 5-HT was selected because previous studies have shown that this concentration is well above threshold and does not yield maximal responses (7, 16), thus allowing observation of both increased (10) and decreased (7) 5-HT responses after steroid treatment. Membrane potential and input resistance were recorded before, during, and after drug administration. The maximal hyperpolarization and decrease in resistance observed with this concentration is indicative for 5-HT1A R-mediated responses (16). After reversal of the 5-HT1A R-mediated effects, slowly activated but more persistent depolarizing responses, mediated by other 5-HT receptors, can still be discerned (16). Depolarizations and increases in resistance observed shortly after washout of 5-HT were therefore also routinely analyzed.
Single-Cell RNA Analysis.
In slices from the same animals that were recorded with microelectrodes for their 5-HT responsiveness, whole-cell K+ currents were measured under voltage-clamp conditions by using a Biologic (Grenoble, France) RK300 amplifier to establish the viability of randomly sampled, visually identified CA1 pyramidal cells in situ. After electrophysiological recording, cell contents were aspirated into the recording pipette (volume of patch pipette + cell contents was ≈10 μl) and transferred to a tube containing 20 units of RNase inhibitor. Subsequently, reagents, including avian myeloblastosis virus reverse transcriptase (RT, Seikagaku America) were added for first-strand cDNA synthesis (17, 21). The resulting single-stranded cDNA was linearly amplified to generate amplified aRNA. Purified first-round aRNA was further taken through a second round of amplification to generate 32P-labeled aRNA (17, 21). This aRNA population is antisense to the original poly(A)+ RNA and linearly amplified over the latter by greater than a million-fold (22). 32P-labeled aRNA was used to probe slot blots loaded with candidate cDNA clones (corresponding to neuronal mRNA sequences) as described (21). These included glial fibrillary acidic protein (courtesy of D. L. Feinstein, University of Illinois, Chicago, IL), MR (courtesy of P. D. Patel, Mental Health Research Institute, Ann Arbor, MI), GR (courtesy of K. R. Yamamoto, University of California, San Francisco, CA), 5-HT1A R (courtesy of E. Vreugdenhil, University of Leiden, Leiden, The Netherlands), neuronal 5-HT transporter (courtesy of B. J. Hoffman, National Institutes of Health, Bethesda, MD), and low molecular weight neurofilament (NF-L) and the voltage-gated potassium channel Kv1 (both courtesy of J. Eberwine, University of Pennsylvania, Philadelphia, PA).
Blots were analyzed on a PhosphorImager (Molecular Dynamics) by using imagequant software. For each gene, the intensity of bands corresponding to specific signal (above background) was expressed as a percentage of specific signal for the positive neuronal marker NF-L, as described (23). Use of this internal reference minimized variations between experiments arising from differences in specific activity of probe and absolute quantity of probe added. The signal for MR also was expressed as a percentage of the signal for the GR for each treatment group.
In Situ Hybridization.
Coronal cryosections (16 μm) were cut and thaw-mounted onto poly(l-lysine)-coated slides. From each animal, three consecutive sections from the dorsal hippocampus were mounted on the slide. After being dried at room temperature for 60 min, the sections were fixed with freshly prepared 4% paraformaldehyde in PBS for 5 min. They were then washed in PBS for 2 min, dehydrated in 70% ethanol for 5 min, and stored in 95% ethanol at 4°C until use.
PCR 1,000 plasmids containing a 350-bp insert coding for the third cytoplasmic loop of the rat 5-HT1A R (amino acids 220–345) in sense and antisense orientations were provided by Organon. These plasmids were linearized with EcoRI, and a [35S]thio-UTP-labeled riboprobe was made by in vitro translation with T7 RNA polymerase. For corticosteroid receptors, 35S-labeled cRNA antisense probes were used (24). The antisense MR probe was transcribed from a 513-bp rat-brain cDNA fragment, which encodes for the last 30 aa at the C terminus of the MR plus the adjacent highly specific 3′-untranslated region. A 500-bp fragment in the N-terminal region of the GR was subcloned from a 2.8-kb fragment of the rat-liver GR cDNA.
Hybridization was adapted from the method described by Meijer and de Kloet (25). In short, a hybridization mix was prepared containing 70% deionized formamide, 10% dextran sulfate, 0.06 M phosphate buffer, 3× SSC, 1× Denhardt’s solution, 10 mM DTT, 0.1 mg/ml yeast tRNA, and 0.1 mg/ml sheared salmon sperm DNA. Aliquots (1 ml) of this mix were added to 4 × 106 dpm of riboprobe and then 100 μl of the mix (containing 4 × 106 dpm of probe) was applied to every slide, each containing three tissue sections. Standard 24 × 50 mm microscopic coverslips were put on the slides, which were then placed in a moist chamber and hybridized overnight at 53°C. As a control, a few slides were hybridized with a sense probe. The next day, the coverslips were removed, and the slides were washed in 2× SSC at room temperature for 10 min, treated with RNase A at 37°C for 10 min (2 mg/100 ml in 0.5 M NaCl/0.1 M Tris, pH 7.5), and washed at 55°C in 2× SSC for 10 min, 1× SSC for 10 min, and 0.1× SSC for 60 min. The slides were then dried in an alcohol series and on the air and placed under Kodak X-OmatAR film for 2 weeks.
From each animal, three sections were analyzed. The hippocampal area within each autoradiogram was scanned and digitized. Background correction was performed, and the corrected image was stored on disk. Using a fixed sequence of gray level segmentation and erosion/dilation steps (26), we constructed a mask, leaving only the pyramidal and granule cell layers of the original image. The mean extinction value was determined for relevant hippocampal subfields, in each animal. The average extinction value (±SEM) was then calculated for the hippocampal subfields in all experimental groups.
Statistics.
For statistical analysis, data were analyzed for each animal. Data of vehicle- and corticosterone-treated groups were compared for each time point separately, i.e., for the acutely treated, the 1 week-treated, or the 3 week-treated groups. Data from vehicle and corticosterone treated groups were statistically analyzed with a two-tailed, unpaired Student’s t test, where P < 0.05 was considered to indicate significant difference.
Results
5-HT Responses.
In total, 74 CA1 neurons located in the pyramidal cell layer were intracellularly recorded, in hippocampal slices. The neurons were electrophysiologically identified as pyramidal neurons based on their resting membrane potential, input resistance, and inward rectification with hyperpolarizing pulses (e.g., ref. 7). Resting membrane potentials varied from −50 to −70 mV; the resistance, as determined from the slope of the current–voltage relationship (see Fig. 1), ranged from 30 to 74 MΩ. For all three treatment periods, the average membrane potential and resistance were not different between corticosterone- and vehicle-treated groups (Table 1). Inward rectification, which appeared as a “sag” of the membrane potential during a 0.5-nA hyperpolarizing pulse (Fig. 1), also showed no significant differences between groups.
In agreement with earlier reports (e.g., refs 7 and 16), 10 μM 5-HT hyperpolarized the membrane of nearly all CA1 pyramidal neurons and induced a decrease in membrane resistance (Fig. 1). In both the acute vehicle- and acute corticosterone-treated groups, the 5-HT-induced hyperpolarization amounted on average to ≈−6 mV, comparable to the 5-HT effects observed previously in animal groups with moderately elevated plasma corticosterone levels (10). Animals that were treated for 1 or 3 weeks daily with vehicle displayed similar responses to 5-HT. However, treatment for 3 weeks with a high dose of corticosterone yielded 5-HT-induced hyperpolarizations that were significantly smaller than observed in neurons from 3 weeks vehicle-injected animals (≈40% reduction; Fig. 1). Intermediate responses were observed in hippocampal cells from animals that had received corticosterone daily for 1 week.
The effect of steroid treatment on the 5-HT-induced change in resistance mimicked the effect seen for the hyperpolarization (Fig. 1). Three weeks of daily corticosterone injections attenuated the average 5-HT-induced change in resistance by ≈45% compared with the control group.
Single-Cell RNA Analysis.
In tissue from the same animals, we examined whether the attenuated 5-HT responses observed after prolonged corticosterone exposure were preceded or accompanied by a reduced 5-HT1A R mRNA expression. To this end, 5-HT1A R mRNA expression was determined in individual, visually identified pyramidal CA1 neurons in slices from the same animals in which 5-HT responses were examined (Fig. 2). Because the single-cell RNA amplification approach amplifies the total accessible cellular pool of poly(A)+ RNA, we could study expression of a number of mRNAs (in addition to the 5-HT1A R mRNA) from the same cell simultaneously, e.g., relative mRNA expression levels for MR and GR (see example in Fig. 2). We furthermore analyzed mRNA expression of (i) the neuronal 5-HT transporter, (ii) glial fibrillary acidic protein (GFAP), as a negative control for mRNA in neurons, and (iii) the potassium channel Kv1, as a negative control for steroid dependency (because there is presently no indication that the expression of this channel is regulated by corticosteroids). Unless stated otherwise, the hybridization to cDNAs corresponding to MR, GR, 5-HT1A R, GFAP, and Kv1 was expressed relative to the signal for NF-L (Fig. 2A).
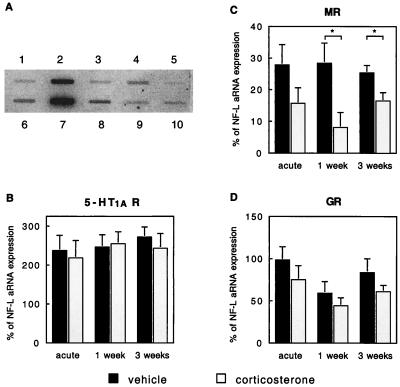
Figure 2.
(A) Expression profile of various candidate genes in an identified, CA1 hippocampal pyramidal neuron. The RNA from this neuron was obtained from a 1-week corticosterone-treated animal. One microgram of cDNA containing plasmid for GFAP (lane 1), NF-L (lane 2), MR (lane 3), GR (lane 4), neuronal 5-HT transporter (lane 5), CamKII (lane 6), 5-HT1A R (lane 7), Kv1 (lane 8), heat shock protein 90 (lane 9), and the plasmid pBluescript (nonspecific binding, lane 10) was linearized, heat-denatured, and gravity-applied to a membrane filter by using a slot blotter. Radiolabeled aRNA from the cell was hybridized for 2 days. After several wash steps, the filters were placed on a PhosphorImager screen for analysis. For each gene, the intensity of bands corresponding to specific signal (above background) was expressed as a percentage of specific signal for the positive neuronal marker NF-L. The signals for CamKII and heat shock protein 90 were not analyzed in the present study. (B) Expression of 5-HT1A R aRNA in identified CA1 pyramidal neurons relative to expression of NF-L was not significantly affected by either acute or long-term steroid treatment. The bars represent mean ± SEM, based on the following number of observations: acute vehicle, 10 cells; acute corticosterone, 15 cells; 1-week vehicle, 10 cells; 1-week corticosterone, 13 cells; 3-weeks vehicle, 15 cells; 3-weeks corticosterone, 15 cells. RNA was collected from 1–3 cells per animal. (C) In the same population of neurons, the expression of MR aRNA relative to expression of NF-L was significantly reduced after 1 or 3 weeks of corticosterone treatment. No significant effects were observed after acute corticosterone treatment. (D) Expression of GR aRNA in identified CA1 pyramidal neurons relative to expression of NF-L was not significantly affected by either acute or long-term steroid treatment.
All CA1 pyramidal neurons included in the present aRNA analysis study (n = 78) contained appreciable amounts of 5-HT1A R mRNA. As is evident from Fig. 2, however, 5-HT1A R mRNA expression (relative to NF-L expression) was not affected by either acute or chronic corticosterone treatment. Interestingly, in vehicle-treated controls (acute, 1-week, and 3-week groups pooled), the relative abundance of 5-HT1A R transcripts in each animal correlated quite well with the mean 5-HT-induced membrane hyperpolarization and change in resistance (ΔRin) within the same animal, as shown with a simple regression analysis (change in resting membrane potential, ΔRMP: r = 0.6, F = 5.39, P = 0.04; ΔRin: r = 0.6, F = 5.08, P = 0.04). This correlation was no longer observed when animals were treated with corticosterone (ΔRMP: r = 0.3, F = 1.13, P = 0.31).
In CA1 pyramidal neurons from all animals, MR mRNA was detectable, except for CA1 neurons from three animals treated for 1 week with corticosterone that had undetectable levels. Statistical analysis showed that markedly less MR mRNA was expressed in neurons from animals that were treated with corticosterone for 1 and 3 weeks (reduced by 72% and 36% compared with vehicle controls; Fig. 2).
GR mRNA was detected in every individual CA1 pyramidal neuron. Although GR mRNA expression tended to be lower after corticosterone treatment (Fig. 2), the differences did not attain statistical significance, possibly (partly) because of the degree of variation. When MR mRNA levels were expressed relative to GR mRNA levels, a significant decrease was observed in the 1-week corticosterone-treated group compared with the vehicle-treated group (0.17 ± 0.10 versus 0.52 ± 0.07, respectively; P < 0.01). No significant changes in MR/GR mRNA expression were found after either acute (P = 0.44) or 3-weeks corticosterone treatment (P = 0.16) in comparison to the control groups.
Relative abundance of GFAP was between 1% and 5% in the majority of the cells tested. In fact, in 43 of 78 cells, GFAP transcripts were not detected at all. The relative abundance of neuronal 5-HT transporter transcript in CA1 pyramidal neurons was also very low (3.5 ± 2.4% in the acute vehicle group). This agrees with immunocytochemical and in situ hybridization studies showing low neuronal 5-HT transporter protein levels in the CA1 hippocampal area (27, 28). Finally, mRNA levels for Kv1 were well above the background, but exhibited no steroid dependency (averages for the groups ranged between 20.6 ± 4.2 and 36.3 ± 11.0).
In Situ Hybridization.
To obtain an impression of the expression pattern for the 5-HT1A R, MR, and GR mRNA not only in the CA1 area but also in other hippocampal subfields, we used the in situ hybridization technique.
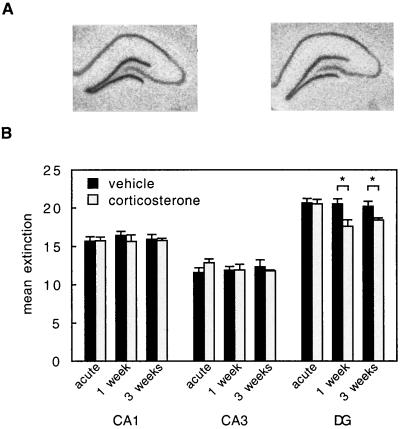
Fig. 3 shows that the expression level of the 5-HT1A R mRNA was high in the cell layer of the dentate gyrus relative to the CA1 and particularly the CA3 region. Analysis of the autoradiograms revealed that in the CA1 and CA3 area, acute or long-term elevation of corticosterone did not induce a change in the expression of 5-HT1A R mRNA compared with the vehicle-treated groups. However, in the dentate gyrus, 5-HT1A R mRNA expression was significantly reduced after 1-week or 3-week corticosterone treatment compared with the vehicle control groups.
Figure 3.
(A) Autoradiograms showing 5-HT1A R mRNA expression in the hippocampal lobes contralateral to those used for electrophysiological recording of 5-HT responses, in 3-weeks vehicle-treated (Left) and corticosterone-treated (Right) animals. (B) Mean extinction (±SEM; in arbitrary units) of mRNA expression for the 5-HT1A R in three hippocampal subfields. In the CA1 and CA3 area, corticosterone administered either acutely or long-term did not alter the 5-HT1A R mRNA expression. However, in the dentate gyrus, 1 or 3 weeks of corticosterone treatment significantly reduced the expression of the 5-HT1A R mRNA. For number of animals, see Tables 1 and 2.
Although the 5-HT1A R expression in the CA1 area was not affected by long-term corticosterone treatment, other transcripts showed steroid dependency. Thus, MR mRNA, which is abundantly expressed in all hippocampal subfields, was significantly reduced in the CA1 area as well as the dentate gyrus after 1 week or 3 weeks of corticosterone treatment (Table 2). No effects were observed after a single corticosterone injection. Similarly, GR mRNA expression levels in both the CA1 and dentate area were significantly reduced in animals receiving a 1-week or 3-weeks corticosterone treatment compared with the vehicle-treated control groups but not by acute exposure to corticosterone. The GR mRNA expression in the CA3 area was much lower and was not altered by corticosterone treatment.
Table 2.
Mean extinction (±SEM; in arbitrary units) of mRNA expression of the MR and GR in three hippocampal subfields
| Treatment | MR
|
GR
|
||||
|---|---|---|---|---|---|---|
| CA1 | CA3 | DG | CA1 | CA3 | DG | |
| Acute | ||||||
| Vehicle | 11.0 ± 0.2 | 9.2 ± 0.3 | 11.2 ± 0.3 | 19.1 ± 0.4 | 12.0 ± 0.3 | 18.9 ± 0.6 |
| Corticosterone | 10.7 ± 0.4 | 9.2 ± 0.3 | 11.1 ± 0.4 | 18.5 ± 0.4 | 12.4 ± 0.4 | 17.6 ± 0.4 |
| 1 Week | ||||||
| Vehicle | 12.2 ± 0.3 | 9.5 ± 0.4 | 11.4 ± 0.4 | 19.0 ± 0.4 | 11.3 ± 0.4 | 18.2 ± 0.4 |
| Corticosterone | 9.7 ± 0.9* | 8.6 ± 0.7 | 9.6 ± 0.7* | 15.2 ± 1.0* | 11.5 ± 1.0 | 15.4 ± 0.8* |
| 3 Weeks | ||||||
| Vehicle | 11.8 ± 0.3 | 9.2 ± 0.4 | 10.9 ± 0.2 | 19.2 ± 0.2 | 12.1 ± 0.5 | 18.9 ± 0.4 |
| Corticosterone | 9.6 ± 0.3* | 8.4 ± 0.4 | 9.0 ± 0.2* | 17.0 ± 0.3* | 11.8 ± 0.3 | 16.6 ± 0.4* |
The MR and GR mRNA expression is reduced after 1 and 3 weeks corticosterone treatment in both the dentate gyrus and the CA1 area, but not in the CA3 region. The number of animals used per group is given in Table 1. In two groups, however, extra animals were incorporated, one animal in the acute vehicle group (n = 9) and three animals in the acute corticosterone group (n = 9). *, P < 0.05.
Discussion
The objective of the present study was to investigate whether long-term exposure of animals to high corticosterone levels affects functional 5-HT responses of CA1 hippocampal cells. We found that daily injections during 3 weeks with a high dose of corticosterone—sufficient to occupy GRs for most of the day (3)—attenuates the 5-HT-induced membrane hyperpolarization and decrease in resistance, compared with responses in the vehicle group. This underscores that the timing of corticosterone exposure is important for the responsiveness to 5-HT (29). Interestingly, small 5-HT responses are also seen in nonstressed rats in the morning, but in these animals, 5-HT responses increase in amplitude when corticosteroid levels rise, thus occupying GRs in addition to MRs (7–10). The present data indicate that animals that are chronically exposed to high corticosterone levels display small 5-HT responses even when corticosteroid levels are high; in other words, they develop resistance to the GR-mediated enhancement of 5-HT responses.
The maximal hyperpolarization and decrease in input resistance are generally thought to be indicative of 5-HT1A R activation (16). Several observations argue against the possibility that steroid effects on other membrane properties contribute to our observations. First, steroid treatment did not affect passive membrane properties recorded before 5-HT administration. Second, the slowly induced depolarization and increased resistance caused by activation of non-5-HT1A receptors (16), as measured shortly after washout of 5-HT, were not significantly altered by either acute or long-term corticosteroid treatment (ref. 7, and data not shown). We tentatively conclude that the attenuated responses to 5-HT are probably caused by a diminished functional outcome of 5-HT1A R activation in pyramidal CA1 neurons.
Under our experimental conditions, the diminished functional outcome after long-term corticosterone treatment was not associated with a decreased 5-HT1A R mRNA expression in CA1 pyramidal neurons. This was found both with in situ hybridization and with single-cell RNA amplification, which allows study of 5-HT1A R expression in identified CA1 pyramidal neurons, i.e., the same type of neurons from which functional responses were obtained. This is partly in agreement with earlier in situ hybridization studies using various animals models with raised corticosterone levels, which in most (30–31), although not all (31), experimental conditions also found no change in 5-HT1A R mRNA expression in the CA1 area. Although mRNA levels are not necessarily linearly linked to transcriptional activity, the present data nevertheless suggest that high corticosteroid levels attenuate functional responses to 5-HT in the absence of transcriptional regulation of the 5-HT1A R gene.
The lack of transcriptional regulation of the 5-HT1A R gene when corticosterone levels are altered for a prolonged period of time does not apply to all genes. Earlier, we observed that the expression of some, but not all, calcium-channel subunits is altered with prolonged alterations of steroid level (21). In the present study, MR and GR mRNA expression were found to be reduced after 1 or 3 weeks of corticosterone treatment. This result agrees with studies showing reduced corticosterone binding after chronic exposure to very high corticosterone levels (32). In particular, a considerable reduction in MR mRNA expression was observed with chronic steroid treatment. Moreover, after 1 week of corticosterone treatment, the ratio of MR/GR mRNA expression was significantly reduced. The marked effects on MR mRNA expression, which is confined to pyramidal cells in the CA1 area, and the possibility of studying MR expression relative to GR mRNA expression in individual cells nicely illustrate the strength of using single-cell RNA amplification in addition to in situ hybridization. The present data suggests that with chronic hypercorticism, MR may be more sensitive to regulation by corticosterone than GR. This was also inferred from recent studies in which animals were subjected to unpredictable stress or corticosterone treatment for a considerable period (31, 33).
If transcriptional regulation of the 5-HT1A R gene does not take place with corticosterone treatment for 3 weeks, other mechanisms must underlie the observed functional deficit. One obvious explanation could be that translation of the 5-HT1A mRNA is less efficient, thus giving rise to less 5-HT1A R protein. However, binding studies performed thus far do not support this, because 5-HT1A R binding in the CA1 area was often (31, 34–37), although not in all cases (31, 38, 39), unaffected by chronic elevation of corticosterone levels. A second possibility is that very small changes in the amount of receptor transcript or protein have profound effects on 5-HT responsiveness. Yet, in vehicle-treated animals, we found that the relative 5-HT1A R mRNA expression in CA1 pyramidal neurons correlates quite well with both membrane hyperpolarization and resistance changes induced by 5-HT, indicating that changes in receptor expression may indeed be linked to functional responses. Interestingly, this correlation was no longer seen in corticosterone-treated rats. Finally, glucocorticoids may affect genes other than the 5-HT1A R gene, thus giving rise to intermediate proteins that modulate 5-HT1A R function (7, 40). The latter is supported by the fact that, so far, no glucocorticoid responsive element has been identified on the promoter sequence of the 5-HT1A R (Sequence Retrieval System, Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany). Possible intermediate proteins are the regulators of G protein signaling (RGS), which are known to alter 5-HT1A R-mediated K+ channels in heart cells (41). Interestingly, the expression of RGS4 in brain was recently found to be regulated by chronic stress (42).
Hyperactivity of the hypothalamo-pituitary-adrenal axis is a prominent feature in a considerable number of depressed patients (11, 12) and may in fact be causative to the disease (13). If so, the presently observed attenuation in 5-HT responsiveness with long-term elevated corticosteroid levels may play a role in the onset of functional deficits in the brain 5-HT system as found during depression (14). To further address this issue, future studies will need (i) to use models in which the rise in corticosterone level is from endogenous rather than exogenous sources and (ii) to study steroid/5-HT interactions in other brain areas (see reviews in refs. 43 and 44) in addition to the postsynaptic 5-HT effects in the hippocampus.
Acknowledgments
The critical discussions and reading of the manuscript by Drs. E. R. de Kloet and W. J. Wadman are gratefully acknowledged. This study was performed with support from grants Stichting Glaxo Research Netherlands no. 94-005, Netherlands Organization for Scientific Research no. 900-95-312, European Community no. 96-0179, and North Atlantic Treaty Organization no. 97-1033.
Abbreviations
- 5-HT
serotonin (5-hydroxytryptamine)
- 5-HT1A R
serotonin-1A receptor
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- NF-L
low molecular weight neurofilament
- GFAP
glial fibrillary acidic protein
- aRNA
antisense RNA
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dallman M F. Trends Endocrinol Metabol. 1993;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- 2.Reul J M, de Kloet E R. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 3.Reul J M, van den Bosch F R, de Kloet E R. J Endocrinol. 1987;115:459–467. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- 4.Reul J M, van den Bosch F R, de Kloet E R. Neuroendocrinology. 1987;45:407–412. doi: 10.1159/000124766. [DOI] [PubMed] [Google Scholar]
- 5.Spencer R L, Young E A, Chao P H, McEwen B S. Brain Res. 1990;514:37–48. doi: 10.1016/0006-8993(90)90433-c. [DOI] [PubMed] [Google Scholar]
- 6.Joëls M. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- 7.Joëls M, Hesen W, de Kloet E R. J Neurosci. 1991;11:2288–2294. doi: 10.1523/JNEUROSCI.11-08-02288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joëls M, de Kloet E R. Neuroendocrinology. 1992;55:344–350. doi: 10.1159/000126135. [DOI] [PubMed] [Google Scholar]
- 9.Karst H, Joëls M. Neurosci Lett. 1991;130:27–31. doi: 10.1016/0304-3940(91)90219-j. [DOI] [PubMed] [Google Scholar]
- 10.Hesen W, Joëls M. J Neuroendocrinol. 1996;8:433–438. doi: 10.1046/j.1365-2826.1996.04724.x. [DOI] [PubMed] [Google Scholar]
- 11.Gold P W, Wong M L, Chrousos G P, Licinio J. Mol Psychiatry. 1996;1:257–264. [PubMed] [Google Scholar]
- 12.Holsboer F, Barden N. Endocr Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- 13.Modell S, Lauer C J, Schreiber W, Huber J, Krieg J C, Holsboer F. Neuropsychopharmacology. 1998;18:253–262. doi: 10.1016/S0893-133X(97)00144-9. [DOI] [PubMed] [Google Scholar]
- 14.Mongeau R, Blier P, de Montigny C. Brain Res Rev. 1997;23:145–195. doi: 10.1016/s0165-0173(96)00017-3. [DOI] [PubMed] [Google Scholar]
- 15.Woolley C S, Gould E, McEwen B S. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 16.Andrade R, Nicoll R A. J Physiol (London) 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Proc Natl Acad Sci USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joëls M, de Kloet E R. J Neurosci. 1993;13:4082–4090. doi: 10.1523/JNEUROSCI.13-09-04082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karst H, de Kloet E R, Joëls M. Eur J Neurosci. 1999;11:889–898. doi: 10.1046/j.1460-9568.1999.00495.x. [DOI] [PubMed] [Google Scholar]
- 20.Akana S F, Scribner K A, Bradbury M J, Strack A M, Walker C D, Dallman M F. Endocrinology. 1992;131:585–594. doi: 10.1210/endo.131.2.1322275. [DOI] [PubMed] [Google Scholar]
- 21.Nair S M, Werkman T R, Craig J, Finnell R, Joëls M, Eberwine J H. J Neurosci. 1998;18:2685–2696. doi: 10.1523/JNEUROSCI.18-07-02685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madison R D, Robinson G A. BioTechniques. 1998;25:504–508. doi: 10.2144/98253rr06. [DOI] [PubMed] [Google Scholar]
- 23.Mackler S A, Brooks B P, Eberwine J H. Neuron. 1992;9:539–548. doi: 10.1016/0896-6273(92)90191-f. [DOI] [PubMed] [Google Scholar]
- 24.Van Eekelen J A, Jiang W, De Kloet E R, Bohn M C. J Neurosci Res. 1988;21:88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- 25.Meijer O C, de Kloet E R. Eur J Pharmacol. 1994;266:255–261. doi: 10.1016/0922-4106(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 26.Hendriksen H, Kamphuis W, Lopes da Silva F H. Mol Brain Res. 1997;50:257–266. doi: 10.1016/s0169-328x(97)00196-4. [DOI] [PubMed] [Google Scholar]
- 27.Lesch K P, Aulakh C S, Wolozin B L, Tolliver T J, Hill J L, Murphy D L. Mol Brain Res. 1993;17:31–35. doi: 10.1016/0169-328x(93)90069-2. [DOI] [PubMed] [Google Scholar]
- 28.Fujita M, Shimada S, Maeno H, Nishimura T, Tohyama M. Neurosci Lett. 1993;162:59–62. doi: 10.1016/0304-3940(93)90559-4. [DOI] [PubMed] [Google Scholar]
- 29.Beck S G, Choi K C, List T J, Okuhara D Y, Birnstiel S. Neuropsychopharmacology. 1996;14:27–33. doi: 10.1016/S0893-133X(96)80056-X. [DOI] [PubMed] [Google Scholar]
- 30.Holmes M C, French K L, Seckl J R. Mol Brain Res. 1995;28:186–192. doi: 10.1016/0169-328x(94)00207-u. [DOI] [PubMed] [Google Scholar]
- 31.Lopez J F, Chalmers D T, Little K Y, Watson S J. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky R M, Krey L C, McEwen B S. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman J P, Spencer R. J Neurosci. 1998;18:7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendelsohn S D, McEwen B S. Neuroendocrinology. 1991;54:454–461. doi: 10.1159/000125951. [DOI] [PubMed] [Google Scholar]
- 35.Mendelsohn S D, McEwen B S. Neuroendocrinology. 1992;56:881–887. doi: 10.1159/000126333. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe Y, Sakai R R, McEwen B S, Mendelsohn S D. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- 37.Pare W P, Tejani-Butt S M. Int Physiol Behav Sci. 1996;31:112–121. doi: 10.1007/BF02699783. [DOI] [PubMed] [Google Scholar]
- 38.McKittrick C R, Blanchard D C, Blanchard R J, McEwen B S, Sakai R R. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- 39.Flügge G. J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuhara D Y, Beck S G. J Pharmacol Exp Ther. 1998;284:1227–1233. [PubMed] [Google Scholar]
- 41.Doupnik C A, Davidson N, Lester H A, Kofuji P. Proc Natl Acad Sci USA. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni Y G, Gold S J, Iredale P A, Terwilliger R Z, Duman R S, Nestler E J. J Neurosci. 1999;19:3674–3680. doi: 10.1523/JNEUROSCI.19-10-03674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joëls M, de Kloet E R. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs B L, Azmitia E C. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]