Abstract
Although the impact of tumor immunology on the clinical management of most cancers is still negligible, there is increasing evidence that anticancer immune responses may contribute to the control of cancer after conventional chemotherapy. Thus, radiotherapy and some chemotherapeutic agents, in particular anthracyclines, can induce specific immune responses that result either in immunogenic cancer cell death or in immunostimulatory side effects. This anticancer immune response then helps to eliminate residual cancer cells (those that fail to be killed by chemotherapy) or maintains micrometastases in a stage of dormancy. Based on these premises, in this Review we address the question, How may it be possible to ameliorate conventional therapies by stimulating the anticancer immune response? Moreover, we discuss the rationale of clinical trials to evaluate and eventually increase the contribution of antitumor immune responses to the therapeutic management of neoplasia.
A daunting diversity of distinct molecular etiologies gives rise to one class of life-threatening diseases — cancer (1) — which affects half of the inhabitants of developed countries during their lifetime and kills one-third of them. The changes in the cell biology of tumor cells are conditioned by epigenetic and genetic reprogramming, genomic instability being an essential feature of both oncogenesis and tumor progression. This epigenetic and genetic modification of cancer cells is often accompanied by the emission of “danger signals,” as well as the expression of ectopic or mutated proteins. Thus, the antigenic characteristics of tumor cells can be perceived by the innate and cognate immune systems.
As defined primarily by Hanahan and Weinberg, the tumorigenic process stems from six hallmark criteria: i.e., growth signal self-sufficiency, resistance to growth-inhibitory signals, resistance to apoptosis, limitless growth potential, sustained angiogenesis, and metastasizing potential (1). A seventh potential hallmark of cancer, avoidance of immunosurveillance (2), allowing tumor cells to escape anticancer immune responses or to actively suppress them (2, 3), has come under close scrutiny. The question as to whether and to what extent immunosurveillance controls and shapes the development of human cancers has been examined in several recent reviews (4–6). There is increasing evidence that tumors develop more frequently in immunodeficient patients, for instance, in transplant recipients (7). This strongly suggests that at least part of the vast evidence in favor of an important role for immunosurveillance in oncogenesis and tumor progression, as obtained in mouse models, can be extrapolated to the human system.
Our ever-expanding understanding of the molecular and cellular etiology of cancer has not yet been accompanied by a parallel improvement in therapeutic outcome. The purpose of this review is to raise a series of related questions: Is the success (or the lack thereof) of anticancer chemotherapy or radiotherapy conditioned by contribution of the immune system? And, if so, is it possible to enhance conventional therapies by stimulating the anticancer immune response? How could we best design clinical trials to interrogate (and eventually increase) the contribution of antitumor immune responses to the therapeutic management of neoplastic disease?
Relationship between cancer and the immune system: a quick guide
As previously discussed in a recent review (4), the immune system may prevent tumor outgrowth. Thus, occult cancer becomes manifest in mice after ablation of T cells and/or injection of anti–IFN-γ antibodies, indicating that the adaptive immune response can keep cancer in check during the equilibrium state (6). However, cancer cells escape the innate and adaptive immune responses (immunosurveillance) by immunoselection (selection of nonimmunogenic tumor cell variants, also known as immunoediting) or immunosubversion (active suppression of the immune response).
Although the concept of immunoediting has been developed in mice, there is evidence that this idea may also apply to humans. Unstable microsatellite tumors in humans (which can be expected to carry more neoantigens than tumors with chromosomal instability) are prominently infiltrated by CTLs and are associated with a favorable prognosis (8–10). Tumor infiltration by T, NK, and NKT cells is a sign of improved prognosis in multiple human neoplasias, including melanoma (11), colon (12), and ovarian cancers (13). Spontaneous tumor regression coupled to massive lymphocyte infiltration has been noted in individual patients with basal cell carcinoma (14), Merkel cell carcinoma (15), and lung carcinoma (16). High levels of antibodies reactive against the tumor suppressor protein p53 have a positive prognostic value in ovarian (17) and gastric cancer (18). In patients with early breast cancer, survival is favorably influenced by a natural humoral immune response to mucin. Indeed, mucin MUC1, a heterodimeric transmembrane glycoprotein aberrantly overexpressed by most human carcinomas, is a tumor antigen recognized by T cells and is shared by different tumors such as breast, colon, pancreas, ovary, and lung carcinoma and may be tumor specific due to its differential glycosylation in normal versus tumor cells (19).
One important question concerns the impact of conventional anticancer chemotherapy on the relationship between the tumor and the immune system. Therapy that is applied during the tumor escape phase not only affects the tumor but also modulates the relationship between the tumor and the immune system. Thus, chemotherapy can, by simply reducing the tumor mass (debulking), reduce its immunosuppressive properties. As a proof of principle, the mere surgical removal of the primary tumor (mechanical debulking) can reverse tumor-induced immune tolerance, restoring the antibody- and cell-mediated immune responses, even in animals bearing metastatic breast cancer (20). By enforcing the selection of chemotherapy-resistant tumor cells and by inducing additional mutations (chemotherapy often involves mutagenic agents), therapy can induce the expression of new tumor antigens. Chemotherapy can cause immunogenic cancer cell stress or death and hence mediate a sort of cancer vaccination effect (as discussed below). Furthermore, chemotherapy can stimulate the immune system, either via a direct effect on immune effectors or regulatory mechanisms or indirectly, by causing lymphopenia followed by homeostatic proliferation of immune effectors that may be particularly active in the anticancer response. The combination of these effects may “reset” the relationship between the tumor and the immune system from the latest stage (escape) to a preceding state (elimination or equilibrium).
Immunostimulatory side effects of anticancer drugs
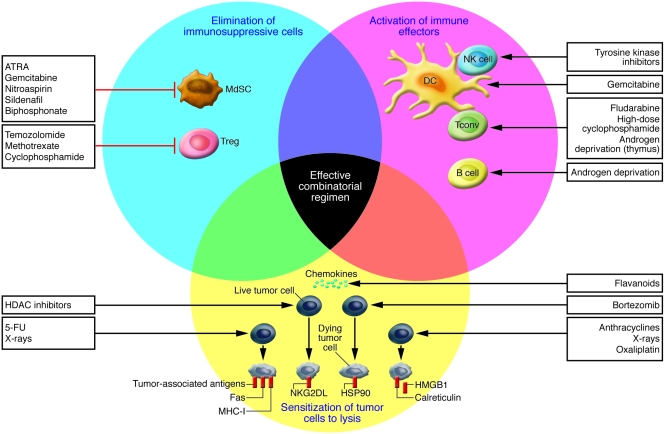
Although cytotoxic anticancer drugs as well as so-called targeted agents act mostly through direct effects on cancer cells, many among these agents have additional effects on the immune system that may contribute to their therapeutic efficacy (Figure 1).
Figure 1. Mechanisms of the impact of conventional anticancer therapies on immune responses.
Anticancer therapeutics can inhibit suppressive mechanisms of tumor-induced immune tolerance (blue circle), boost T and/or B cell responses (pink circle), or stress tumor cells in such a way that tumor cells become immunogenic and sensitive to lysis (yellow circle). The main drugs driving these effects are also shown. Cyclophosphamide at low doses, gemcitabine, and all-trans-retinoic acid (ATRA) act on immunosuppressive cells such as Tregs or myeloid suppressor cells (MdSC) to facilitate tumor attack by conventional effectors (Tconv). Pharmacological inhibition of MdSCs can also be achieved by nitroaspirin (96), sildenafil (97), and biphosphonate (98). Androgen deprivation boosts T and B cell responses. Strategies leading to lymphodepletion allow the establishment of memory effector T cells efficient in long-term protection against tumor cells. Tyrosine kinase inhibitors boost DC/NK cell crosstalk. The proteasome inhibitor bortezomib induces myeloma cell–surface expression of the molecular chaperone protein HSP90, which leads to DC uptake, antigen processing, and DC maturation. Anthracyclines, oxaliplatin, and irradiation promote tumor membrane expression of CRT and release of HMGB1 by tumor cells, which are required events for DC-mediated phagocytosis of dying tumors and cross-presentation of tumor antigens to T cells, respectively. Inhibitors of histone deacetylases (HDACs) promote the expression of NKG2D ligands (NKG2DL), sensitizing the tumor cell to NK cell–mediated lysis. Tumor cells exposed to x-rays express increased numbers of MHC class I molecules, tumor antigens, and Fas, favoring CTL attack. Flavanoid-mediated production of chemokines favors attraction of immune effectors into tumor beds. Ideally, an appropriate combination of chemotherapeutic agents could achieve all of these three types of beneficial effects.
Transient lymphopenia (abnormally low levels of blood lymphocytes) may activate homeostatic mechanisms that finally stimulate tumor-specific effector T cells. Thus, the therapeutic induction of lymphopenia has raised considerable interest in the context of adoptive cell transfer (ACT) therapies (which involve ex vivo generation of tumor-reactive lymphocytes from endogenous tumor-infiltrating lymphocytes and their activation and expansion before reinfusion into the tumor-bearing host) and vaccination against melanoma (21). Animal studies demonstrated that lymphoablation enhances the effectiveness of adoptively transferred tumor-specific CD8+ T cells (22). These experimental data have been tested in a clinical trial on 35 patients with metastatic melanoma refractory to conventional treatments. A highly lymphodepleting conditioning regimen was followed by ACT with tumor-infiltrating lymphocytes (TILs), resulting in 51% objective response rates associated with a persistent clonal lymphocytosis and/or antimelanocyte autoimmunity (23). In mice, lymphodepletion can be combined with vaccination strategies to promote the differentiation of central memory T cells specific for tumor antigens (24–27). The clinical relevance of these findings has been validated in a randomized clinical trial in myeloma (28). The in vivo–primed T cells of patients who were immunized with the 7-valent pneumococcal conjugate vaccine (PCV; Prevnar) were first collected following vaccination. Patients who then received high-dose chemotherapy and autologous stem cell transplantation as well as an infusion of these in vivo–primed and in vitro–expanded T cells, followed by subsequent booster immunizations, had a robust reconstitution of clinically relevant antimicrobial immunity within a month after transplantation (28). Defeating T cell fatigue after ACT against cancer or HIV has also been achieved with IL-15 (29), anti-CD40 (30), or programmed cell death 1 (PD-1) blockade (31). These findings suggest that, after some additional refinement, chemotherapy or irradiation-induced lymphodepletion, combined with additional manipulations such as ACT and immunopharmacological interventions, might achieve antitumor immune responses.
Hormonotherapy, the use of hormonal manipulation, is part of the clinical armamentarium for the management of breast and prostate cancer. The influence of androgens on lymphocyte development and activation has been reviewed (32). Experimental data indicate that androgen deprivation increases the number of naive T cells exported from the thymus and may therefore contribute to broadening the repertoire of T cell immunity, leading to effective antitumor immune responses and breaking of tumor-induced tolerance (33, 34). Moreover, androgen deprivation enhances the production of newly generated IgM+ naive B cells from bone marrow (35). Androgen ablation in prostate cancer patients may activate immune responses to some prostate tumor antigens by increasing the pool of naive T cells (as demonstrated by TCR rearrangement excision circle [TREC] analyses; TRECs are stable DNA circles excised from T cell germline DNA to allow for TCR formation during early T cell development and exist in high concentrations in thymic emigrants), decreasing and/or diluting the population of Tregs, and favoring the concomitant infiltration of T cells into tumor beds (36, 37). One study performed on 73 men bearing nonmetastatic prostate cancer and 50 controls showed that neoadjuvant hormonal and radiation therapy (but not radical prostatectomy) can elicit a tumor-specific autoantibody response (38). Humoral responses arose early and were durable in most cases, prompting the manipulation of B cell responses by immunotherapy.
Some agents (in particular cyclophosphamide but also fludarabine, gemcitabine, oxaliplatin, and 5-fluorouracil [5-FU]) can at least partially deplete or transiently inactivate tumor-protective Tregs (39–41). Several clinical trials have combined low doses of cyclophosphamide and antitumor vaccination for the treatment of metastatic melanoma, without any clear results, perhaps due to the lack of statistical power (42–46). In a small, randomized study performed on 42 metastatic breast cancer patients, cyclophosphamide was combined with a vaccine composed of a synthetic sialyl-Tn epitope linked to keyhole limpet hemocyanin (KLH) and an adjuvant. KLH is a natural protein isolated from the marine mollusk keyhole limpet and used as a carrier protein together with weak antigens to boost immune responses to haptens, self antigens, and idiotype proteins. A statistically significant increase in median survival from 12 months to 20 months was reported (47). A similar trend was observed in renal cell carcinoma patients immunized with allogeneic, mature DCs pulsed with tumor lysates and KLH and also receiving 300 mg/m2 i.v. cyclophosphamide 4 and 3 days before each monthly vaccination (48). In yet another study, 10 end-stage cancer patients received 1 daily dose of 100 mg cyclophosphamide, every other week, for 4 weeks (49). This “metronomic” (i.e., administered orally daily) cyclophosphamide treatment suppressed Treg inhibitory functions and restored the proliferative capacity of effector T cells as well as the cytotoxicity of NK cells (49, 50).
Many other anticancer agents also have immunostimulatory effects that have been demonstrated at the clinical level. For example, gemcitabine has been reported to enhance the frequency of IFN-γ–producing T cells and/or CD69+ activated cells in pancreatic cancer patients (51). In a phase I trial, gemcitabine elicited cellular immune responses in non–small cell lung cancers (52). One clinical trial examined the combined effects of gemcitabine and cytokines (GM-CSF and low doses of IL-2) in 42 patients presenting with advanced colorectal cancers. The objective responses rates and time to progression were encouraging and associated with increased tumor-specific CTL precursor frequencies in responders (53), prompting the initiation of a phase III trial. Another example is provided by a study of imatinib mesylate (IM; also known as STI571 and Gleevec), an inhibitor of the tyrosine kinases BCR-ABL, PDGFR, and KIT. Some patients with gastrointestinal stromal tumors (GISTs) that have no c-KIT mutation (and hence lack the IM target) responded to IM (54), suggesting that IM can exert indirect antitumor effects. Indeed, in a fraction of patients with GIST, IM causes an increase in NK activity. The activation of NK cells induced by IM constitutes a positive prognostic parameter (54), indicating that immunostimulation may contribute to the therapeutic effect of IM in patients, as has been suggested by animal experiments (55).
Immunogenic cancer cell stress and death
Transforming cells have to overcome both intrinsic (cell-autonomous) and extrinsic (immune-mediated) barriers to tumorigenesis. One important intrinsic barrier against transformation includes the activation of a DNA damage response after oncogenic stress, resulting in the activation of ataxia telangiectasia mutated (ATM), checkpoint kinase–1 (CHK1), and finally, p53-dependent apoptosis (56) or senescence (57). As a result, preneoplastic lesions and in situ carcinomas often manifest the activation of ATM, CHK1, and p53, while advanced cancers suppress or lose this DNA damage response. An extrinsic barrier against tumor growth consists of the elimination of transforming cells by cells from the innate and cognate immune systems. Intriguingly, these barriers may be linked in molecular terms (3, 58). Thus, the DNA damage response induces expression of NK cell group 2D (NKG2D) ligands on tumor cells in an ATM- and CHK1-dependent (but p53-independent) fashion (59). NKG2D is an activating receptor involved in tumor immunosurveillance that is expressed on NK, NKT, and γδT cells and resting (in mice) and/or activated (in humans) CD8+ T cells. The expression of NKG2D ligands on the surface of transforming cells thus can be expected to play a major role in tumor surveillance (Figure 1).
Although p53 is not required for the expression of NKG2D ligands in cells undergoing DNA damage (59), a recent study highlights an important cooperation between p53-induced tumor cell senescence and the innate immune system (57). Restoration of p53 function in established liver cancers led to tumor regression, but only when the mice carried an intact immune system. Thus, the reactivation of p53 led to the remission of liver cancer, an effect that was lost after ablation of NK cells and macrophages (57). These examples illustrate how molecules that are activated during early tumorigenesis (e.g., ATM, CHK1, p53) can alert the immune system to mediate an antitumor response. As chemotherapy with DNA-damaging agents often activates ATM, CHK1, and p53, it appears plausible, yet remains to be proven, that such agents also elicit ATM-, CHK1-, and p53-dependent immune effects.
Chemotherapeutic agents and radiation can increase the immunogenic properties of tumor cells by enhancing MHC class I expression (60), thereby increasing their vulnerability to CTLs. Similarly, some chemotherapeutic agents (such as genotoxic agents and histone deacetylase inhibitors) increase the expression of NKG2D ligands (59, 61), thus facilitating tumor cell lysis by NKG2D-expressing lymphocytes (including NK cells, NKT cells, and CTLs). Yet another frequent effect of DNA damage inflicted by radiotherapy or chemotherapy is the increase in the expression of death receptors (in particular Fas/CD95 and TNF-related apoptosis-inducing ligand [TRAIL] receptors) (62), enabling lysis of the tumor cells by Fas/CD95 ligand and TRAIL-positive immune effectors (Figure 1).
Tumor cell demise often occurs through apoptosis or necrosis. Although there are teleological arguments to suggest that apoptosis must be nonimmunogenic or even tolerogenic (because physiological cell death occurs through apoptosis but does not lead to autoimmunity), and although necrosis (which is often pathological) has been considered as being proinflammatory, the theoretical equations in which apoptosis equals nonimmunogenicity and necrosis equals immunogenicity do not withstand experimental verification. Instead, it seems that apoptosis is nonuniform in biochemical terms, meaning that various pathways can lead to cell death and induce stimulus-specific changes. Anthracyclines, oxaliplatin, and ionizing irradiation have the exceptional capacity to induce immunogenic cell death, while the vast majority of chemotherapeutic agents induce nonimmunogenic cell death (63–66). Anthracyclines, oxaliplatin, and ionizing irradiation also have the particular ability to induce the early, preapoptotic translocation of the chaperone calreticulin (CRT) from the lumen of the endoplasmic reticulum (endo-CRT) to the plasma membrane (ecto-CRT) (64, 65, 67). Ecto-CRT then facilitates the engulfment of the tumor cell by DCs yet does not induce DC maturation. Neutralization or knockdown of CRT abolishes the immunogenicity of tumor cell death, while exogenous supply of recombinant CRT can restore the immunogenicity per se of nonimmunogenic cell death (64, 65, 67) (Figure 1).
Another chaperone, HSP90, has recently been reported to be involved in the immunogenicity of human myeloma cell death elicited by the proteasome inhibitor bortezomib (68). In this in vitro model, HSP90 is exposed on the plasma membrane surface of myeloma cells following exposure to bortezomib and induces the phenotypic maturation of DCs (Figure 1). Yet another factor that acts on DCs is high mobility group box 1 protein (HMGB1), a chromatin-binding protein that is released from cells during the late stages of cell death induced by anthracyclines, oxaliplatin, and ionizing irradiation. HMGB1 then interacts with TLR4 on the surface of DCs and affects the intracellular fate of engulfed antigen from dying tumor cells. Ligation of TLR4 by HMGB1 inhibits the fusion of phagosomes (which contain antigenic cargo) with lysosomes, thereby allowing the antigen to traffic toward the antigen-presenting compartment (66). Neutralization or knockdown of HMGB1 abolishes the capacity of dying tumor cells to elicit anticancer immune responses, underscoring its contribution to tumor immunogenicity (66). The aforementioned examples illustrate that, depending on the cell death inducer, tumor cells can expose or release factors that affect their uptake by DCs (e.g., CRT), the maturation of DCs (e.g., HSP90), or antigen presentation by DCs (e.g., HMGB1).
Molecular and/or epidemiological evidence for an immune response during therapy
Pioneering work has highlighted a contribution of T cells to the antitumor effects mediated by cytotoxic agents (Tables 1 and 2) (69). An EL4 thymoma cell clone that was relatively resistant to the anthracycline doxorubicin in vitro gave rise to doxorubicin-sensitive tumors when implanted into immunocompetent C57BL/6 mice. This unexpected therapeutic effect, however, appeared to depend on splenocytes, suggesting that immunity could be indispensable to the antitumor effects mediated by anthracyclines (69). While this contention was not always supported by experimental data (Table 1), similar results have been obtained by other investigators in several other cancers (Table 2), provided that immunogenic chemotherapies (for example, anthracyclines or oxaliplatin) were administered. Thus, EL4 thymoma, Glasgow osteosarcomas, and CT26 colon cancer treated with oxaliplatin, as well as CT26 colon cancers and MCA205 fibrosarcomas treated with anthracyclines, exhibit much better therapeutic responses when the host is immunocompetent than when it is immunodeficient (e.g., athymic nu/nu mice) (64, 66, 67, 70). Similarly, the depletion of CD4+ or CD8+ T cells by injection of specific monoclonal antibodies compromises the therapeutic efficacy of doxorubicin on CT26 tumors (64). TS/A, a cell line derived from a spontaneous mammary adenocarcinoma in BALB/c mice, also responded much better to local radiotherapy in immunocompetent as compared with athymic hosts (66). Together, these results demonstrate that the efficacy of chemotherapy and radiotherapy is influenced by the immune system.
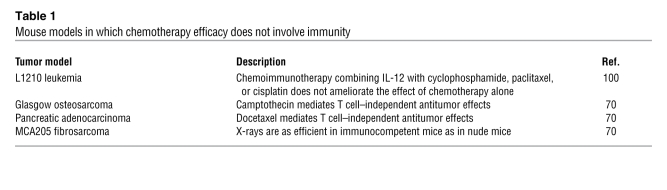
Table 1 .
Mouse models in which chemotherapy efficacy does not involve immunity
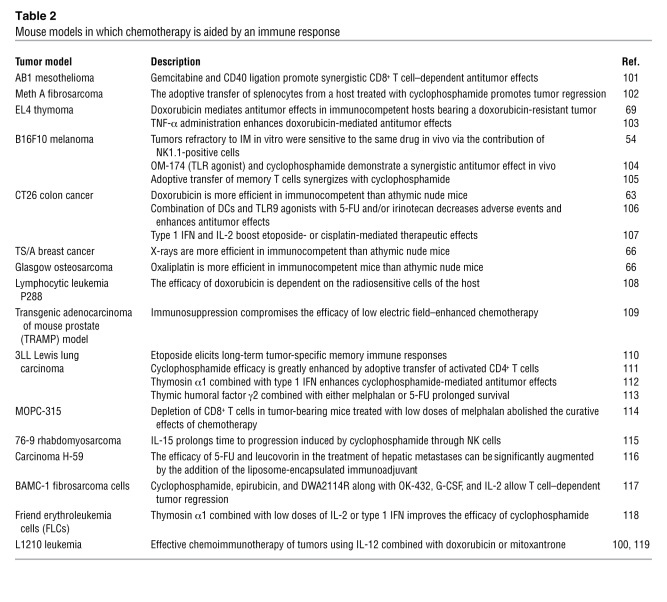
Table 2 .
Mouse models in which chemotherapy is aided by an immune response
The aforementioned results suggest that induction of immunogenic tumor cell death by anthracyclines, oxaliplatin, or ionizing radiation stimulates an immune response that helps keep the tumor in check. If this speculation were true, one would expect that tumor-derived proteins such as CRT and HMGB1 (which dictate the immunogenicity of tumor cell death) and the presence of their receptors on antigen-presenting cells would be required for full therapeutic success. Accordingly, the knockout of TLR4 and that of its downstream effector MyD88 (but not that of TIR domain–containing adapter-inducing IFN-β [TRIF], an adapter responding to activation of TLRs that mediates a signaling cascade distinct from that mediated by MyD88) reduced the therapeutic efficacy of immunogenic chemotherapies in mice bearing CT26 colon cancers, TS/A mammary cancers, EL4 thymomas, or Glasgow osteosarcomas (66).
In individuals of mixed European descent, there is a sequence polymorphism in TLR4 (896A→G, Asp299Gly, RefSNP identification number rs4986790) with an allelic frequency of approximately 6% (71, 72) that prevents the binding of HMGB1 to TLR4 in a dominant negative fashion (66, 70). A clinical study indicated that breast cancer patients that carry the Asp299Gly TLR4 allele do not differ from patients displaying the normal TLR4 allele for any of the classical prognostic factors. However, the Kaplan-Meier estimate of metastasis-free survival after local radiotherapy and systemic anthracycline chemotherapy indicated that the Asp299Gly TLR4 allele (but not the TLR4 mutation, RefSNP identification number rs1927911) is an independent predictive factor of early relapse (66, 70). In metastatic renal cancer, a polymorphism in the IL-4 promoter strongly influences prognosis (73).
Many investigators use the term immunochemotherapy to describe the therapeutic instillation of monoclonal antibodies that recognize tumor antigens. Of note, the efficacy of immunochemotherapy in rituximab (anti-CD20)–treated follicular lymphoma is, in part, dictated by the efficacy of antibody-dependent cellular cytotoxicity, as determined by the Fc receptor IIIA genotype (74, 75) and the content of tumor-associated macrophages (76).
For technical reasons, there are very few studies that address the frequency of tumor-specific effector or memory T cells or antibody titers before and after chemotherapy. In infants with acute myeloid leukemia (AML), the emergence of antileukemia precursors after chemotherapy correlates with the maintenance of hematologic remission (77). Recently, a dynamic immunomonitoring study reported the presence of leukemia-specific, TNF-α–producing CD4+ T cells in patients bearing chronic myelogenous leukemia and successfully treated with IM (78). Spontaneous and induced T cell reactivities were associated with hematological and cytogenetic responses in a majority of patients. Interestingly, antibody titers against antiphospholipids were associated with prolonged survival after retinoid acid treatment in promyelocytic leukemia (79, 80). Galanis et al. studied the prognostic value of neuronal autoantibodies in small cell lung carcinoma patients. Although the titers of the paraneoplastic autoantibodies were associated with less-extensive disease at presentation, the cisplatin-based chemotherapy caused patients to become immunocompromised, and time to progression could not be predicted according to a rise in the antibody titers (81).
Future investigation should address the question, In which cancers does the induction of specific antitumor immune responses by immunogenic chemotherapies or radiotherapy have a prognostic impact?
Immunochemotherapy
Based on the aforementioned premises, oncologists and immunotherapy experts might discuss the opportunity to optimize their current anticancer approaches. Such an optimization might stem from the sequential strategy depicted in Figure 2, associating: (a) suppressors of tolerogenic pathways; (b) priming of naive T and B cells with vaccines; (c) induction of cell death with cytotoxic drugs; (d) agents rendering nonimmunogenic cell death immunogenic; (e) chloroquine (to combat host defects in TLR4 or block autophagy and multidrug resistance); (f) and sustaining immune effectors with NK, NKT, or DC adjuvants (e.g., TLR agonists, glycolipids, cytokines, chemokines).
Figure 2. Steps required for successful cancer immunotherapy.
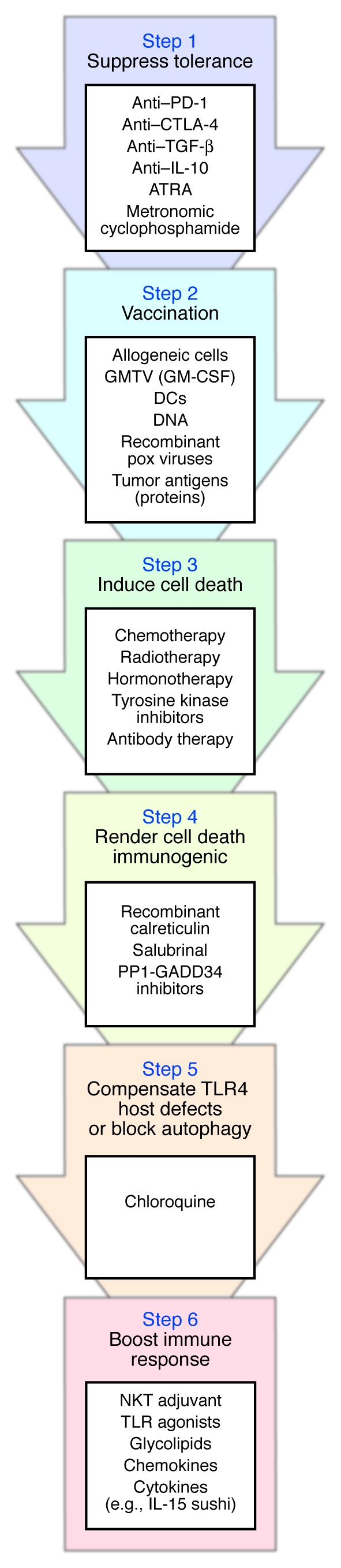
The immune system of cancer-bearing individuals suffers from tumor-induced tolerance, which should be alleviated (Step 1) before induction of an active immune response with tumor vaccines (Step 2). Some evidence suggests that prior vaccination (Step 2) favors the antitumor effects of chemotherapeutic agents (Step 3). Cell death triggered by chemotherapy or radiotherapy (Step 3) should then be rendered immunogenic via addition of compounds that enhance calreticulin expression at the tumor cell membrane (Step 4). To overcome putative TLR4 host defects, which can compromise the developing immune response, administration of chloroquine is indicated (Step 5). Finally, immune adjuvants should be given to sustain and enhance the ensuing antitumor immune response (Step 6). Potential mediators at each step are listed. GMTV, genetically modified tumor vaccines; PP1-GADD34, protein phosphatase 1 complexed to GADD34; IL-15 sushi, sushi domain of soluble IL-15 receptor α (99).
Future studies should focus on combination therapies and determine the optimal schedule of chemoimmunotherapy, since the kinetics of the host and tumor response are likely critical determinants of the therapeutic response. Moreover, such combinations, while potentially effective, might also be fraught with significant toxicity (i.e., infectious diseases or autoimmunity, as have already been observed with the use of lymphodepletion or anti-CTLA4 antibodies). Phase I trials of immunochemotherapy may help define the toxicity and the dosage at which an immune response is achieved, but sizeable randomized studies will be needed to evaluate the optimal schedule of such therapeutic combinations.
To date, very few clinical studies have combined conventional chemotherapies with anticancer vaccination and/or immunostimulatory regimens (Table 3). In particular, there is no systematic or randomized assessment of the order in which cytotoxic therapies and tumor vaccines should be administered. In one trial, 29 end-stage non–small cell lung cancer patients who were resistant to first-line platin-based therapy were enrolled in a study employing a vaccination protocol involving autologous DCs infected with adenoviral vectors encoding p53 (82). Since the tumor progressed in 23 of 29 patients, salvage chemotherapy by paclitaxel or carboplatin was proposed as a second line of chemotherapy. The response rate to this second-line chemotherapy was 61.5%, and up to 38% survived at one year following vaccination. The historical controls were known to exhibit a 6%–16% objective response rate to a second-line chemotherapeutic, and less than 20% were alive beyond one year. A direct positive correlation between immune response to p53 and clinical outcome could be observed. Only 30% of patients who did not develop a p53-specific response to vaccination responded to second-line chemotherapy, whereas 75% of p53 cellular immune responders had objective clinical responses to chemotherapy after vaccination (P = 0.08) (82).
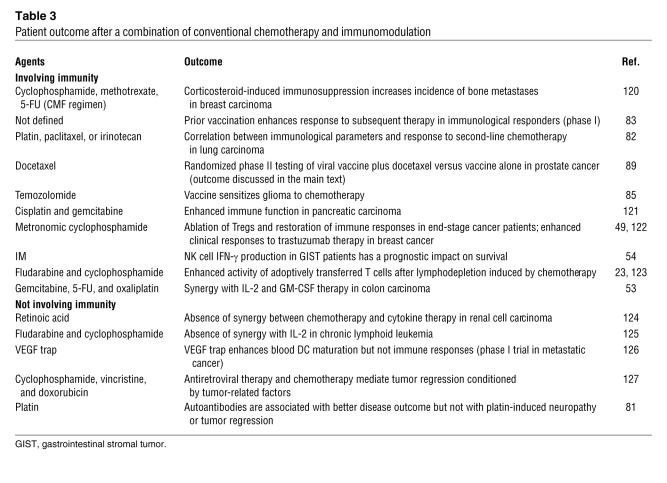
Table 3 .
Patient outcome after a combination of conventional chemotherapy and immunomodulation
In another study, 17 patients bearing a diverse range of advanced tumors received an encapsulated DNA vaccine encoding the carcinogen activator cytochrome P450 1B1 expressed by almost all human tumors. While 10 of 11 who did not develop anti-CYP1B1–specific T cells failed to respond to salvage therapy, 5 of 6 who elicited immunity against CYP1B1 exhibited marked response to the salvage regimen for more than one year (83).
A similar conclusion was reached in a retrospective examination of the impact of vaccination based on peptide- or lysate-loaded DCs on the efficacy of conventional therapy (craniotomy, stereotaxic radiosurgery, radiotherapy, treatment with temozolomide alone or in combination with nitrosourea) against glioblastoma (84, 85). Progression rates and overall survival were compared among 12 tyrosinase-related protein 2–based (TRP-2–based) vaccine–treated patients, 13 chemotherapy-treated patients, and 13 glioblastoma patients treated with a combination of the TRP-2–based vaccine and chemotherapy. The results suggested that chemotherapy synergized with previous therapeutic vaccination to slow glioblastoma progression and significantly extend patient survival relative to individual therapies. In some cases, where the authors could establish glioblastoma cell lines before and after vaccination, the immunoselected tumor cells exhibited increased sensitivity to temozolomide and carboplatin compared with primary tumor cells. These data suggest that immunological targeting of TRP-2 increases sensitivity to cell death inducers (86).
Given the evidence of a role for testosterone in modulating B and T cell immune responses, there is a strong rationale for combining androgen deprivation with vaccine therapy in prostate cancer, and this is further supported by some experimental data (87). As reviewed by Aragon-Ching et al. (32), phase I and phase II trials indicated that androgen ablation may boost immune responses elicited by DCs, allogeneic prostate cancer cell lysate, or GM-CSF–secreting tumor cell–based vaccines (88). Arlen et al. (89) were the first to conduct a randomized study evaluating the use of vaccine alone versus concomitant vaccine plus weekly administration of docetaxel and steroids, with the end points of monitoring immune responses in 28 patients bearing a metastatic, androgen-resistant prostate cancer. First, they showed that a vaccine (admixed recombinant vaccinia virus expressing prostate-specific antigen [PSA]) or B7.1 followed by sequential boosts with fowlpox virus-PSA can be administered with weekly docetaxel and dexamethasone comedication without compromising the ability of the patient to mount tumor-associated antigen–specific T cell responses as compared with vaccine alone. They also evaluated clinical responses to the combination therapy, vaccine alone, and chemotherapy after progression following vaccination. Whereas progression-free survival in the combination arm was similar to that of the historical controls (median, 3.2 versus 3.7 months), there was an increase in progression-free survival in patients who received docetaxel following disease progression on vaccine alone (6.1 versus 3.7 months). Patients who crossed over to docetaxel following vaccination continued to maintain PSA-specific T cell responses that corresponded to declining serum levels of PSA. A similar trend was observed in a report by Noguchi et al., in which low-dose estramustine (a nitrogen mustard) was combined with HLA-A24–restricted peptide vaccination for prostate cancer. Combination therapy was associated with augmented CTL responses, peptide-specific IgG responses, and decreased serum levels of PSA in 13 patients (90).
The feasibility and safety of combining 5-FU–based chemotherapy with carcinoembryonic antigen–derived (CEA-derived) peptides in adjuvants was evaluated in a study of 17 patients bearing colorectal cancer (91). Eight of 17 patients developed CEA-specific CTL responses, while 6 of 17 exhibited an objective response, a rate similar to that of historical controls treated with chemotherapy alone (91). A phase II trial enrolled 32 patients treated with anti-idiotype antibody mimicking the CEA antigen, with 14 of them additionally placed on a 5-FU–based regimen. All patients developed CEA-specific humoral and cellular immune responses (92). The 5-FU–based regimen, which is the standard of care for patients with localized colon cancer with nodal involvement, did not affect the immune response. These data warrant a phase III trial for patients with resected colon cancer.
Open questions in cancer immunology
Many clinical oncologists doubt the general impact of immune parameters on the practical management of neoplasia. How can clinical studies be designed in order to provide more ample evidence of the importance of cancer immunology?
It would be important to perform large-scale epidemiological studies that link the incidence and course of cancer — including therapeutic failure — to genotypic and phenotypic markers of immunodeficiency. As mentioned above, polymorphisms in the genes encoding TLR4 and cytokines may correlate with therapeutic outcome, but larger and more systematic studies (such as genome-wide SNP correlations) will be important in this area. It would be interesting as well to correlate the susceptibility to intracellular infectious agents (including viruses, listeria, tuberculosis, mycoplasma, etc.) with poor cancer chemotherapeutic responses. Moreover, an exhaustive meta-analysis should be performed on melanoma vaccination studies. Such a meta-analysis should address the putative positive impact of vaccination on later chemotherapeutic responses.
The prognostic impact of tumor-specific gene expression or proteomic profiles should be reinterpreted by studying markers of preexisting immune responses. The molecular profiling of follicular lymphoma at diagnosis indicated that the two gene-expression signatures that predicted survival comprised genes expressed by nonmalignant tumor-infiltrating cells, most likely myeloid and T cells. The nature of the infiltrating immune cells was the predominant feature of the cancer that predicted survival (93). It would be important to perform prospective trials in which serial tumor biopsies, performed before and after chemotherapy, are evaluated for signs of local immune responses as putative prognostic factors. If anticancer immune responses dictate long-term therapeutic success, then local signs of antigen-priming (i.e., the presence of DCs in a pseudolymphoid architecture and contexture) or T and NK cell responses (with the presence of effector memory T cells, preferentially of the Th1 type, that should outnumber Tregs) would correlate with favorable responses. This working hypothesis is supported by our recent data showing a correlation between pathological complete response to neoadjuvant chemotherapy and therapy-induced high CD8+ effector T lymphocyte and low Treg infiltrate levels in a series of 56 patients bearing locally advanced breast cancer (94).
The immunosuppressive side effects of massive chemotherapy should also be reevaluated. Although one cycle of lymphodepletion may constitute a therapeutic advantage due to homeostatic T cell repletion, repeated cycles of lymphodepletion may destroy the expanding population of tumor-specific immune effectors. For instance, in breast cancer, treatment with weak, repeated doses of anthracyclines (every week, ideally without glucocorticoids) should be compared with the usual therapeutic scheme in which intense doses are administered every three weeks (and usually accompanied by high doses of glucocorticoids to reduce the chemotherapy-associated discomfort) with simultaneous monitoring of tumor size (by PET/CT) and antitumor immune responses.
Along the same lines, it may be important to readdress the therapeutic management of different cancers, given the idea that chemotherapy should elicit an immune response. Hence, neoadjuvant chemotherapy (as opposed to adjuvant chemotherapy) of small cancers (such as early breast cancers discovered by routine radiographic screening) would offer the advantage that more tumor antigen would become available for the priming of T cells, while high-dose tolerance should not constitute a problem. Similarly, it could be important to preserve the sentinel lymph node rather than remove it systematically for disease staging purposes. Indeed, the sentinel lymph node constitutes the privileged site of antigen priming, in which the presence of activated DCs (expressing DC-LAMP — a protein only expressed in mature DCs) constitutes a positive prognostic marker, at least in melanoma (95).
Future studies might also include clinical trials to attempt to correct the defective chemotherapeutic response of breast cancer patients with the Asp299Gly TLR4 allele by administering chloroquine. Oral chloroquine is a widely used antimalaria agent that acts at an acceptable level of toxicity. In mice lacking TLR4, chloroquine restores the chemotherapeutic response to oxaliplatin, presumably because it ameliorates deficient antigen presentation by DCs. In TLR4-negative DCs, phagocytic cargo containing tumor antigen is rapidly destroyed by lysosomes, and lysosomal inhibition by chloroquine restores antigen presentation. Similarly, chloroquine reestablishes the defective presentation of dying tumor cell antigens by DCs from patients carrying the Asp299Gly TLR4 allele in vitro (66).
From a biotechnological point of view, drug-screening approaches could be used to investigate the capacity of compounds to promote the translocation of CRT to the cell surface of a responding cell line (selected on its response to anthracyclines for CRT exposure). This screening could select druggable compounds based on their capacity to induce immunogenic cell death (Figure 1).
In conclusion, we anticipate that clinical trials will corroborate the potential of assessing and eliciting anticancer immune responses for prognostic and therapeutic purposes, provided that issues regarding intellectual property, licensing, and liability do not represent significant obstacles for industrial partners to accept the sponsoring and/or launching of combination therapies. It is our collective hope that an expanding immunostimulatory pharmacopeia, together with ever-more-sophisticated anticancer vaccination techniques, eventually will allow us to trigger tumoricidal immune responses at will.
Acknowledgments
The authors are supported by grants from the Ligue Nationale contre le Cancer (to L. Zitvogel, L. Apetoh, and G. Kroemer), the Fondation pour la Recherche Médicale (to L. Apetoh and A. Tesniere), the European Union (grants “ALLOSTEM” and “DC-THERA” to L. Zitvogel; grants “Active p53,” “Apo-Sys,” “ChemoRes,” “Death Train,” “RIGHT,” and “Trans-Death” to G. Kroemer), Cancéropôle Ile-de-France, Institut National du Cancer, and Agence Nationale de la Recherche (to G. Kroemer).
Footnotes
Nonstandard abbreviations used: ACT, adoptive cell transfer; ATM, ataxia telangiectasia mutated; CEA, carcinoembryonic antigen; CHK1, checkpoint kinase–1; CRT, calreticulin; 5-FU, 5-fluorouracil; HMGB1, high mobility group box 1 protein; IM, imatinib mesylate; KLH, keyhole limpet hemocyanin; NKG2D, NK cell group 2D; PSA, prostate-specific antigen; TRP-2, tyrosinase-related protein 2.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1991–2001 (2008). doi:10.1172/JCI35180.
References
- 1.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Zitvogel L., Tesniere A., Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 4.Swann J.B., Smyth M.J. Immune surveillance of tumors. J. Clin. Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn G.P., Koebel C.M., Schreiber R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 6.Koebel C.M., et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 7.Buell J.F., Gross T.G., Woodle E.S. Malignancy after transplantation. Transplantation. 2005;80:S254–S264. doi: 10.1097/01.tp.0000186382.81130.ba. [DOI] [PubMed] [Google Scholar]
- 8.Naito Y., et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 9.Lu B.J., Lai M., Cheng L., Xu J.Y., Huang Q. Gastric medullary carcinoma, a distinct entity associated with microsatellite instability-H, prominent intraepithelial lymphocytes and improved prognosis. Histopathology. 2004;45:485–492. doi: 10.1111/j.1365-2559.2004.01998.x. [DOI] [PubMed] [Google Scholar]
- 10.Galon J., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 11.Haanen J.B., et al. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol. Immunother. 2006;55:451–458. doi: 10.1007/s00262-005-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pages F., et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. . N. Engl. J. Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. . N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 14.Halliday G.M., Patel A., Hunt M.J., Tefany F.J., Barnetson R.S. Spontaneous regression of human melanoma/nonmelanoma skin cancer: association with infiltrating CD4+ T cells. World J. Surg. 1995;19:352–358. doi: 10.1007/BF00299157. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T., Yoneda K., Manabe M., Demitsu T. Spontaneous regression of merkel cell carcinoma: a comparative study of TUNEL index and tumor-infiltrating lymphocytes between spontaneous regression and non-regression group. J. Dermatol. Sci. 2000;24:203–211. doi: 10.1016/S0923-1811(00)00103-1. [DOI] [PubMed] [Google Scholar]
- 16.Kerr K.M., et al. Partial regression in primary carcinoma of the lung: does it occur? Histopathology. 1998;33:55–63. [PubMed] [Google Scholar]
- 17.Goodell V., et al. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J. Clin. Oncol. 2006;24:762–768. doi: 10.1200/JCO.2005.03.2813. [DOI] [PubMed] [Google Scholar]
- 18.Mattioni M., et al. Serum anti-p53 antibodies as a useful marker for prognosis of gastric carcinoma. Int. J. Biol. Markers. 2007;22:302–306. doi: 10.1177/172460080702200410. [DOI] [PubMed] [Google Scholar]
- 19.Finn O.J., et al. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol. Rev. 1995;145:61–89. doi: 10.1111/j.1600-065X.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 20.Danna E.A., et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.CAN-03-2646. [DOI] [PubMed] [Google Scholar]
- 21.Muranski P., et al. Increased intensity lymphodepletion and adoptive immunotherapy — how far can we go? Nat. Clin. Pract. Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L., et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudley M.E., et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. . J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H.M., Poehlein C.H., Urba W.J., Fox B.A. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 25.Ge Q., Hu H., Eisen H.N., Chen J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2989–2994. doi: 10.1073/pnas.052714099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lou Y., et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–6790. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badovinac V.P., Messingham K.A., Jabbari A., Haring J.S., Harty J.T. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 28.Rapoport A.P., et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat. Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 29.Teague R.M., et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat. Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 30.Tuma R.A., Giannino R., Guirnalda P., Leiner I., Pamer E.G. Rescue of CD8 T cell-mediated antimicrobial immunity with a nonspecific inflammatory stimulus. J. Clin. Invest. 2002;110:1493–1501. doi: 10.1172/JCI16356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrell A. Defeating T-cell fatigue in HIV. Nat. Med. 2006;12:1124–1125. doi: 10.1038/nm1006-1124. [DOI] [PubMed] [Google Scholar]
- 32.Aragon-Ching J.B., Williams K.M., Gulley J.L. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front. Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 33.Drake C.G., et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roden A.C., et al. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 2004;173:6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 35.Viselli S.M., Stanziale S., Shults K., Kovacs W.J., Olsen N.J. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84:337–342. [PMC free article] [PubMed] [Google Scholar]
- 36.Mercader M., et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page S.T., et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am. J. Physiol. Endocrinol. Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 38.Nesslinger N.J., et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin. Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 39.Ghiringhelli F., et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 40.Lutsiak M.E., et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 41.Correale P., et al. 5-fluorouracil-based chemotherapy enhances the antitumor activity of a thymidylate synthase-directed polyepitopic peptide vaccine. J. Natl. Cancer Inst. 2005;97:1437–1445. doi: 10.1093/jnci/dji188. [DOI] [PubMed] [Google Scholar]
- 42.Berd D., Maguire H.C., Jr., McCue P., Mastrangelo M.J. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J. Clin. Oncol. 1990;8:1858–1867. doi: 10.1200/JCO.1990.8.11.1858. [DOI] [PubMed] [Google Scholar]
- 43.Miller K., et al. Improved survival of patients with melanoma with an antibody response to immunization to a polyvalent melanoma vaccine. Cancer. 1995;75:495–502. doi: 10.1002/1097-0142(19950115)75:2<495::AID-CNCR2820750212>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 44.Livingston P.O., et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J. Clin. Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 45.Jones R.C., et al. Immune response to polyvalent melanoma cell vaccine in AJCC stage III melanoma: an immunologic survival model. Ann. Surg. Oncol. 1996;3:437–445. doi: 10.1007/BF02305761. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell M.S., Von Eschen K.B. Phase III trial of Melacine melanoma theraccine versus combination chemotherapy in the treatment of stage IV melanoma [abstract]. Proc. Am. Soc. Clin. Oncol. 1997;16:494a. [Google Scholar]
- 47.MacLean G.D., Miles D.W., Rubens R.D., Reddish M.A., Longenecker B.M. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J. Immunother. Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Holtl L., et al. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol. Immunother. 2005;54:663–670. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghiringhelli F., et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghiringhelli F., et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plate J.M., Plate A.E., Shott S., Bograd S., Harris J.E. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol. Immunother. 2005;54:915–925. doi: 10.1007/s00262-004-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levitt M.L., et al. Phase I study of gemcitabine given weekly as a short infusion for non-small cell lung cancer: results and possible immune system-related mechanisms. Lung Cancer. 2004;43:335–344. doi: 10.1016/j.lungcan.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Correale P., et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J. Clin. Oncol. 2005;23:8950–8958. doi: 10.1200/JCO.2005.12.147. [DOI] [PubMed] [Google Scholar]
- 54.Borg C., et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J. Clin. Invest. 2004;114:379–388. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taieb J., et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 56.Bartkova J., et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 57.Xue W., et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gasser S., Raulet D. The DNA damage response, immunity and cancer. Semin. Cancer Biol. 2006;16:344–347. doi: 10.1016/j.semcancer.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Gasser S., Orsulic S., Brown E.J., Raulet D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reits E.A., et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armeanu S., et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 62.Chakraborty M., et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 63.Casares N., et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Obeid M., et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 65.Obeid M., et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 66.Apetoh L., et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 67.Obeid M., et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol. Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 68.Spisek R., et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maccubbin D.L., et al. Adriamycin-induced modulation of host defenses in tumor-bearing mice. Cancer Res. 1992;52:3572–3576. [PubMed] [Google Scholar]
- 70.Apetoh L., et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol. Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 71.Arbour N.C., et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 72.Kiechl S., et al. Toll-like receptor 4 polymorphisms and atherogenesis. N. Engl. J. Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 73.Kleinrath T., Gassner C., Lackner P., Thurnher M., Ramoner R. Interleukin-4 promoter polymorphisms: a genetic prognostic factor for survival in metastatic renal cell carcinoma. J. Clin. Oncol. 2007;25:845–851. doi: 10.1200/JCO.2006.07.8154. [DOI] [PubMed] [Google Scholar]
- 74.Cartron G., et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 75.Weng W.K., Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 76.Taskinen M., Karjalainen-Lindsberg M.L., Nyman H., Eerola L.M., Leppa S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin. Cancer Res. 2007;13:5784–5789. doi: 10.1158/1078-0432.CCR-07-0778. [DOI] [PubMed] [Google Scholar]
- 77.Montagna D., et al. Emergence of antitumor cytolytic T cells is associated with maintenance of hematologic remission in children with acute myeloid leukemia. Blood. 2006;108:3843–3850. doi: 10.1182/blood-2006-05-021535. [DOI] [PubMed] [Google Scholar]
- 78.Chen C.I.-U., Maecker H.T., Lee P.P. Development and dynamics of robust T cell responses to CML under imatinib treatment. Blood. 2008 doi: 10.1182/blood-2007-12-128397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Padua R.A., et al. PML-RARA-targeted DNA vaccine induces protective immunity in a mouse model of leukemia. Nat. Med. 2003;9:1413–1417. doi: 10.1038/nm949. [DOI] [PubMed] [Google Scholar]
- 80.Robin M., et al. Frequent antibody production against RARalpha in both APL mice and patients. Blood. 2006;108:1972–1974. doi: 10.1182/blood-2006-03-013177. [DOI] [PubMed] [Google Scholar]
- 81.Galanis E., Frytak S., Rowland K.M., Jr., Sloan J.A., Lennon V.A. Neuronal autoantibody titers in the course of small-cell lung carcinoma and platinum-associated neuropathy. Cancer Immunol. Immunother. 1999;48:85–90. doi: 10.1007/s002620050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antonia S.J., et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin. Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 83.Gribben J.G., et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin. Cancer Res. 2005;11:4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 84.Yu J.S., et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 85.Wheeler C.J., Das A., Liu G., Yu J.S., Black K.L. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin. Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 86.Liu G., et al. Cytotoxic T cell targeting of TRP-2 sensitizes human malignant glioma to chemotherapy. Oncogene. 2005;24:5226–5234. doi: 10.1038/sj.onc.1208519. [DOI] [PubMed] [Google Scholar]
- 87.Hsueh E.C., et al. Androgen blockade enhances response to melanoma vaccine. J. Surg. Res. 2003;110:393–398. doi: 10.1016/S0022-4804(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 88.Sanda M.G., et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/S0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 89.Arlen P.M., et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin. Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noguchi M., et al. Immunological evaluation of individualized peptide vaccination with a low dose of estramustine for HLA-A24+ HRPC patients. Prostate. 2005;63:1–12. doi: 10.1002/pros.20157. [DOI] [PubMed] [Google Scholar]
- 91.Weihrauch M.R., et al. Phase I/II combined chemoimmunotherapy with carcinoembryonic antigen-derived HLA-A2-restricted CAP-1 peptide and irinotecan, 5-fluorouracil, and leucovorin in patients with primary metastatic colorectal cancer. Clin. Cancer Res. 2005;11:5993–6001. doi: 10.1158/1078-0432.CCR-05-0018. [DOI] [PubMed] [Google Scholar]
- 92.Foon K.A., et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J. Clin. Oncol. 1999;17:2889–2895. doi: 10.1200/JCO.1999.17.9.2889. [DOI] [PubMed] [Google Scholar]
- 93.Dave S.S., et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N. Engl. J. Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 94.Ladoire S., et al. Pathological complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating Foxp3+ regulatory T cells. . Clin. Cancer Res. 2008;14:2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 95.Elliott B., et al. Long-term protective effect of mature DC-LAMP+ dendritic cell accumulation in sentinel lymph nodes containing micrometastatic melanoma. Clin. Cancer Res. 2007;13:3825–3830. doi: 10.1158/1078-0432.CCR-07-0358. [DOI] [PubMed] [Google Scholar]
- 96.De Santo C., et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Serafini P., et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melani C., Sangaletti S., Barazzetta F.M., Werb Z., Colombo M.P. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bulanova E., et al. Soluble interleukin IL-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J. Biol. Chem. 2007;282:13167–13179. doi: 10.1074/jbc.M610036200. [DOI] [PubMed] [Google Scholar]
- 100.Zagozdzon R., et al. Effective chemo-immunotherapy of L1210 leukemia in vivo using interleukin-12 combined with doxorubicin but not with cyclophosphamide, paclitaxel or cisplatin. Int. J. Cancer. 1998;77:720–727. doi: 10.1002/(SICI)1097-0215(19980831)77:5<720::AID-IJC10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 101.Nowak A.K., Robinson B.W., Lake R.A. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 102.North R.J. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ehrke M.J., et al. Doxorubicin plus tumor necrosis factor alpha combination treatments in EL4-lymphoma-bearing C57BL/6 mice. Cancer Immunol. Immunother. 1998;45:287–298. doi: 10.1007/s002620050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.D’Agostini C., et al. Antitumour effect of OM-174 and cyclophosphamide on murine B16 melanoma in different experimental conditions. Int. Immunopharmacol. 2005;5:1205–1212. doi: 10.1016/j.intimp.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 105.Gold J.E., Zachary D.T., Osband M.E. Adoptive transfer of ex vivo-activated memory T-cell subsets with cyclophosphamide provides effective tumor-specific chemoimmunotherapy of advanced metastatic murine melanoma and carcinoma. Int. J. Cancer. 1995;61:580–586. doi: 10.1002/ijc.2910610424. [DOI] [PubMed] [Google Scholar]
- 106.Bourquin C., Schreiber S., Beck S., Hartmann G., Endres S. Immunotherapy with dendritic cells and CpG oligonucleotides can be combined with chemotherapy without loss of efficacy in a mouse model of colon cancer. Int. J. Cancer. 2006;118:2790–2795. doi: 10.1002/ijc.21681. [DOI] [PubMed] [Google Scholar]
- 107.Iigo M., Tsuda H., Moriyama M. Enhanced therapeutic effects of anti-tumour agents against growth and metastasis of colon carcinoma 26 when given in combination with interferon and interleukin-2. Clin. Exp. Metastasis. 1994;12:368–374. doi: 10.1007/BF01755880. [DOI] [PubMed] [Google Scholar]
- 108.Schwartz H.S., Grindey G.B. Adriamycin and daunorubicin: a comparison of antitumor activities and tissue uptake in mice following immunosuppression. Cancer Res. 1973;33:1837–1844. [PubMed] [Google Scholar]
- 109.Plotnikov A., Niego B., Ophir R., Korenstein R., Keisari Y. Effective treatment of mouse metastatic prostate cancer by low electric field enhanced chemotherapy. Prostate. 2006;66:1620–1630. doi: 10.1002/pros.20435. [DOI] [PubMed] [Google Scholar]
- 110.Slater L.M., et al. Etoposide induction of tumor immunity in Lewis lung cancer. Cancer Chemother. Pharmacol. 2001;48:327–332. doi: 10.1007/s002800100357. [DOI] [PubMed] [Google Scholar]
- 111.Saxton M.L., et al. Adoptive transfer of anti-CD3-activated CD4+ T cells plus cyclophosphamide and liposome-encapsulated interleukin-2 cure murine MC-38 and 3LL tumors and establish tumor-specific immunity. Blood. 1997;89:2529–2536. [PubMed] [Google Scholar]
- 112.Garaci E., Mastino A., Pica F., Favalli C. Combination treatment using thymosin alpha 1 and interferon after cyclophosphamide is able to cure Lewis lung carcinoma in mice. Cancer Immunol. Immunother. 1990;32:154–160. doi: 10.1007/BF01771450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ophir R., et al. Thymic humoral factor-gamma 2 (THF-gamma 2) immunotherapy reduces the metastatic load and restores immunocompetence in 3LL tumor-bearing mice receiving anticancer chemotherapy. Immunopharmacol. Immunotoxicol. 1996;18:209–236. doi: 10.3109/08923979609052733. [DOI] [PubMed] [Google Scholar]
- 114.Mokyr M.B., Barker E., Weiskirch L.M., Takesue B.Y., Pyle J.M. Importance of Lyt 2+ T-cells in the curative effectiveness of a low dose of melphalan for mice bearing a large MOPC-315 tumor. Cancer Res. 1989;49:4597–4606. [PubMed] [Google Scholar]
- 115.Evans R., Fuller J.A., Christianson G., Krupke D.M., Troutt A.B. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: the potential role of NK cell subpopulations. Cell. Immunol. 1997;179:66–73. doi: 10.1006/cimm.1997.1132. [DOI] [PubMed] [Google Scholar]
- 116.Asao T., Shibata H.R., Batist G., Brodt P. Eradication of hepatic metastases of carcinoma H-59 by combination chemoimmunotherapy with liposomal muramyl tripeptide, 5-fluorouracil, and leucovorin. Cancer Res. 1992;52:6254–6257. [PubMed] [Google Scholar]
- 117.Iida S., et al. Combination chemo-immunotherapy of murine solid tumor with OK-432, G-CSF, IL-2, and chemotherapeutics. Int. J. Immunopharmacol. 1995;17:973–980. doi: 10.1016/0192-0561(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 118.Garaci E., et al. Antitumor effect of thymosin alpha 1/interleukin-2 or thymosin alpha 1/interferon alpha,beta following cyclophosphamide in mice injected with highly metastatic Friend erythroleukemia cells. J. Immunother. Emphasis Tumor Immunol. 1993;13:7–17. doi: 10.1097/00002371-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 119.Golab J., et al. Potentiatied anti-tumor effectiveness of combined therapy with interleukin-12 and mitoxantrone of L1210 leukemia in vivo. Oncol. Rep. 2000;7:177–181. doi: 10.3892/or.7.1.177. [DOI] [PubMed] [Google Scholar]
- 120.Marini G., et al. The effect of adjuvant prednisone combined with CMF on patterns of relapse and occurrence of second malignancies in patients with breast cancer. International (Ludwig) Breast Cancer Study Group. Ann. Oncol. 1996;7:245–250. doi: 10.1093/oxfordjournals.annonc.a010567. [DOI] [PubMed] [Google Scholar]
- 121.Bang S., et al. Differences in immune cells engaged in cell-mediated immunity after chemotherapy for far advanced pancreatic cancer. Pancreas. 2006;32:29–36. doi: 10.1097/01.mpa.0000191651.32420.41. [DOI] [PubMed] [Google Scholar]
- 122.Orlando L., et al. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer. 2006;6:225. doi: 10.1186/1471-2407-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morgan R.A., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Atzpodien J., et al. Interleukin-2/interferon-alpha2a/13-retinoic acid-based chemoimmunotherapy in advanced renal cell carcinoma: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Br. J. Cancer. 2006;95:463–469. doi: 10.1038/sj.bjc.6603271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kasamon Y.L., et al. Phase I study of low-dose interleukin-2, fludarabine, and cyclophosphamide for previously untreated indolent lymphoma and chronic lymphocytic leukemia. Clin. Cancer Res. 2005;11:8413–8417. doi: 10.1158/1078-0432.CCR-05-1612. [DOI] [PubMed] [Google Scholar]
- 126.Fricke I., et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 127.Miralles P., et al. 2007Prognosis of AIDS-related systemic non-Hodgkin lymphoma treated with chemotherapy and highly active antiretroviral therapy depends exclusively on tumor-related factors. J. Acquir. Immune Defic. Syndr. 44167–173. . 10.1097/QAI.0b013e31802bb5d0 [DOI] [PubMed] [Google Scholar]