Abstract
Calcineurin inhibitor (CNI) nephrotoxicity is a major concern after renal transplantation. To investigate the safety and efficacy of a CNI-free immunosuppressive regimen, 132 live-donor renal transplant recipients were included in a prospective, randomized controlled trial. All patients received induction therapy with basiliximab and steroids. The patients were randomized to a maintenance immunosuppression regimen that included steroids, sirolimus, and either low-dose tacrolimus or mycophenolate mofetil (MMF). Over a mean follow-up period of approximately 5 yr, patient and graft survival did not significantly differ between the two maintenance regimens. Patient survival was 93.8% and 98.5% in the tacrolimus/sirolimus and MMF/sirolimus groups, respectively, and graft survival was 83% and 88%, respectively. However, the MMF/sirolimus group had significantly better renal function, calculated by Cockcroft-Gault, from the second year post-transplant until the last follow-up. In addition, this group was less likely to require a change in their primary immunosuppression regimen than the tacrolimus/sirolimus group (20.8% versus 53.8%, P = 0.001). The safety profile was similar between groups. In summary, after long-term follow-up, a CNI-free maintenance regimen consisting of sirolimus, MMF, and steroids was both safe and efficacious among low to moderate immunologic risk renal transplant recipients.
Avoidance or at least minimization of the nephrotoxic effect of the calcineurin inhibitors (CNIs) is indeed a worthy goal; however, it must be approached with great care.
The current CNIs in use, including cyclosporine (CsA) and tacrolimus (Tac), are, in addition to being nephrotoxic, associated with detrimental effects on cardiovascular risk factors, such as hypertension, dyslipidemia, and glucose intolerance. Besides, development of chronic allograft nephropathy (CAN) is common in recipients treated with CNI.1–3 Recently, it was found that especially long-term treatment with CNI drugs results in fibrotic scarring of transplanted kidneys, an effect present in about 25% of the patients already during the first 6 mo post-transplant.4
The introduction of potent immunosuppressive alternatives to CNI drugs, such as mycophenolate mofetil (MMF), mTOR inhibitors (mammalian target of rapamycin, sirolimus, everolimus), and the anti-CD25 antibodies (anti-IL-2 receptor antibodies daclizumab and basilixiamb) challenge investigations of less toxic immunosuppressive protocols. MMF has been shown to be a well-tolerated and effective alternative in patients with CsA-induced nephrotoxicity, not only allowing CsA dose reduction but also full CsA withdrawal in selected patients resulting in significantly improved renal function.5–8 MMF also has a preferred profile on blood pressure, lipids, and glucose metabolism.
CNI avoidance might translate into improved graft and patient survival as long as the overall immunosuppression is adequate. CNI-free regimens based on daclizumab induction followed by MMF and steroid maintenance have shown 1-yr graft survival of more than 95%; however, acute rejection rate was unacceptably high in these patients with average immunogenic risk.9,10
The complementary properties of sirolimus (SRL) and MMF may provide the rationale for their combination in induction and maintenance regimens. SRL, an mTOR inhibitor, inhibits cell proliferation driven by growth factors; and MMF, a reversible inhibitor of inosine monophosphate dehydrogenase, acts as an antiproliferative drug. Early experiences with the use of the SRL, MMF, and steroid combination yielded insufficient prophylaxis of acute rejection.11,12 However, the introduction of induction therapy with monoclonal or polyclonal antilymphocyte antibodies to the SRL/MMF and steroid combination brings an efficient acute rejection prophylaxis while improving renal function and/or reducing chronic allograft nephropathy.13,14
However, a recent review article of an organ-by-organ review of Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients data showed a decline in the number of renal transplant patients who were discharged on regimens containing SRL down to 16% in 2004. Moreover, the use of the SRL/MMF combination has remained less than 1%.15
We have previously reported on the safe and efficient use of SRL/MMF combination regimen together with steroids and basiliximab induction therapy in a short-term prospective randomized trial.14 We hereby report on the safety and efficacy of the longer-term use of such a regimen in living donor renal transplant recipients.
RESULTS
Demography
Demographic and baseline characteristics were previously outlined.14 Both groups were homogeneous regarding recipients' age, sex, body weight, original kidney disease, pretransplant hypertension, urinary bilharziasis, hepatitis C virus antibody test, donors' age, and human leukocyte antigen matching.
Patient and Graft Outcome
Mean follow-up period was 62.6 ± 13 and 63.5 ± 9.9 mo for group A and B, respectively (range, 55 to 75 mo). Higher patient and graft survivals were noted among group B patients, although it does not rank to statistical significance (Figures 1and 2; Table 1).
Figure 1.
Patient survival relative to the primary immunosuppression in groups A (Tac) and B (MMF).
Figure 2.
Graft survival relative to the primary immunosuppression in groups A (Tac) and B (MMF).
Table 1.
Patient and study outcome
| Group A (Tac) (N = 65) | Group B (MMF) (N = 67) | P | |
|---|---|---|---|
| Mean follow-up, mo (mean ± SD) | 62.6 ± 13 | 63.5 ± 9.9 | 0.337 |
| Condition at last follow-up, no. (%) | |||
| living with functioning graft | 54 (83) | 59 (88) | 0.415 |
| living on dialysis | 7 (10.8) | 7 (10.5) | 0.952 |
| died with functioning graft | 4 (6.2) | 1 (1.5) | 0.161 |
| Secondary immunosuppression, no. (%) | 35 (53.8) | 14 (20.8) | 0.001 |
Group A.
Four patients died. The cause of death in 2 of them was miliary tuberculosis (TB), 3 and 6 mo post-transplantation. The cause of death in the others was most probably attributed to a cardiovascular cause being reported at home, 31 and 32 mo post-transplantation. Seven patients returned back to dialysis throughout the whole follow-up period. Recurrence of original kidney disease (anti-glomerular basement membrane nephritis and focal segmental glomerulosclerosis [FSGS]) was responsible for two graft failures, 4 and 7 mo post-transplantation. One patient had thrombotic microangiopathy complicated by transplant glomerulopathy and FSGS and put on dialysis 5 mo post-transplantation. Another patient had recurrent acute rejections (3 episodes) and complicated by transplant glomerulopathy resulting from minimal change disease and returned to dialysis 24 mo post-transplantation. Chronic allograft nephropathy was responsible for graft failure in the last 3 cases (28, 42, and 60 mo post-transplantation).
Group B.
One patient died of septicemia caused by recurrent bacterial infections secondary to multiple anal abscesses and fistulae 7 mo post-transplantation. Similar to group A, 7 patients return back to dialysis during the whole follow-up period. Recurrence of original kidney disease (FSGS and membranoproliferative glomerulonephritis) was responsible for two graft failures, 8 and 43 mo post-transplantation, respectively. De novo glomerulopathy (FSGS) was responsible for one graft failure 21 mo post-transplantation. Chronic allograft nephropathy was responsible for graft failure in the last 4 cases (19, 44, 57, and 60 mo post-transplantation).
Immunosuppressive Drugs
After the first 3 post-transplant months, mean SRL levels were kept within the targeted therapeutic windows throughout the whole follow-up period. After the first 2 yr, there was no statistically significant difference of SRL levels between either group as the targeted window became the same (Figure 3). Subsequently, mean SRL doses were close to each other in both groups. During the fifth year, mean SRL dose was 2.27 ± 1.14 mg/d and 2.28 ± 1.11 mg/d in group A and B, respectively.
Figure 3.
Sirolimus trough levels (ng/ml) in both groups.
Mean Tac doses and levels were kept within the targeted windows among group A patients. Fifth year mean Tac dose value was 2.37 ± 2.62 mg/d and mean level value was 3.82 ± 1.11 ng/ml. Mean MMF doses were progressively reduced over time down to 1.5 ± 0.36 g/d during last follow-up year among group B patients.
Secondary Immunosuppression
Statistically significant higher change rate of the primary immunosuppressive regimen was encountered among group A patients as shown in Table 2. In 21 patients of group A, Tac was replaced by MMF, in essence cross over to group B, because of biopsy-proven acute Tac toxicity despite optimum level (2 cases), chronic tubulointerstitial fibrosis of moderate degree (8 cases), recurrent severe diarrhea complicated by marked body weight reduction and frequent hospital admissions (6 cases), and uncontrolled newly discovered diabetic state (5 cases).
Table 2.
Secondary immunosuppression regimens in both groups
| Indications | Group A (Tac) (N = 65) | Group B (MMF) (N = 67) | P |
|---|---|---|---|
| Conversion to sirolimus-based regimens (crossover) | |||
| acute Tac toxicity | 2 | 0 | 0.148 |
| tubule-interstitial fibrosis (moderate degree) | 8 | 0 | 0.003 |
| chronic diarrhea | 6 | 0 | 0.013 |
| uncontrolled new DM | 5 | 0 | 0.021 |
| leucopenia | 0 | 3 | 0.244 |
| Conversion to non–sirolimus-based regimens | |||
| proteinuria | 5 | 3 | 0.439 |
| surgical cause | 3 | 0 | 0.075 |
| elevated liver enzymes | 1 | 0 | 0.308 |
| seek pregnancy | 1 | 4 | 0.182 |
| leucopenia | 2 | 0 | 0.148 |
| pulmonary TB | 2 | 2 | 0.975 |
| interstitial pneumonitis | 0 | 1 | 0.323 |
| intolerance | 0 | 1 | 0.323 |
DM, diabetes mellitus; TB, tuberculitis.
In another 14 patients of the same group, SRL was completely withdrawn and replaced by either MMF, due to proteinuria (5 cases), surgical complications (3 cases), high liver enzymes (1 case), or replaced by azathioprine in one female who was seeking pregnancy. Two patients were maintained on steroids and Tac only because of persistent leucopenia despite therapeutic SRL blood level; and finally, 2 patients were maintained on steroids and MMF because of pulmonary TB.
Among group B patients, MMF was replaced by Tac in 3 patients, in essence cross over to group A, because of persistent leucopenia with optimum SRL level.
In another 11 patients, SRL was withdrawn and replaced by Tac because of proteinuria (3 patients), interstitial pneumonitis (1 case), and intolerance (1 case). In 4 females, SRL and MMF were replaced by Tac and azathioprine for the sake of getting pregnant; and finally, 2 patients with pulmonary TB were maintained on steroids and MMF only.
Graft Function
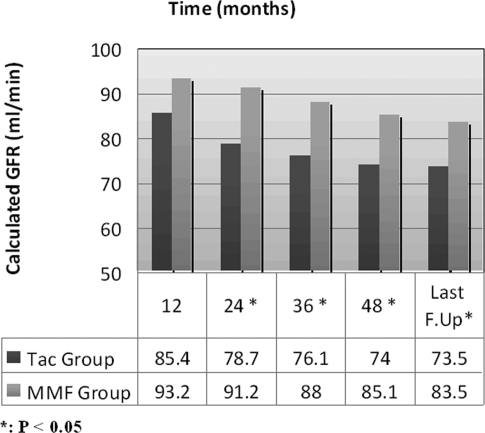
Based on intention-to-treat analysis, statistically significant better renal allograft function was encountered among group B patients from the second post-transplant year until the last follow-up, as measured by calculated glomerular filtration rate (GFR), in comparison with group A (Figure 4).
Figure 4.
Graft function based on intention-to-treat analysis (all cases are included) as estimated by calculated GFR (ml/min).
By exclusion of those with secondary immunosuppression (35 in group A and 14 in group B), no significant difference of renal function was found, although group B patients still have higher calculated GFRs at all time periods (Figure 5).
Figure 5.
Graft function based on maintenance of primary immunosuppressive regimen (all cases with secondary immunosuppression are excluded) as estimated by calculated GFR (ml/min).
Histopathologic Findings
No acute rejection episodes were encountered beyond the second year of follow-up. Meanwhile, CAN of various degrees was diagnosed among 11 and 9 patients of group A and B, respectively (Table 3). Two of those with moderate CAN and all of those with marked CAN returned back to dialysis.
Table 3.
Pathologic diagnosis of event graft biopsies (last 3 yr)
| Value | Group A | Group B | P |
|---|---|---|---|
| Chronic allograft nephropathy | |||
| mild degree | 4 | 5 | |
| moderate degree | 6 | 3 | 0.576 |
| marked degree | 1 | 1 | |
| Focal segmental glomerulosclerosis | 1 | 0 |
On studying the impact of chronic allograft damage index (CADI) obtained from protocol graft biopsies (originally 86 cases) at the end of the first post-transplant year,14 we opted to classify whole patients of the two groups together into nearly equal two divisions: those with CADI score equal 0, 1, or 2 and those whose the score equals 4, 5, 6, or 7. Table 4 shows higher calculated GFR values among the first group, which approaches significance at the last follow-up.
Table 4.
Impact of CADI score on calculated GFR (ml/min)
| CADI Score = 0 to 2
|
CADI Score = 3 to 7
|
P | |||
|---|---|---|---|---|---|
| No. | GFR | No. | GFR | ||
| 1 yr | 44 | 93.74 ± 19.91 | 38 | 88.45 ± 26.40 | 0.305 |
| 2 yr | 44 | 89.32 ± 23.85 | 37 | 82.48 ± 26.37 | 0.225 |
| 3 yr | 43 | 89.28 ± 27.54 | 37 | 79.94 ± 26.04 | 0.125 |
| 4 yr | 42 | 83.82 ± 24.27 | 34 | 74.32 ± 26.33 | 0.107 |
| 5 yr | 41 | 85.25 ± 24.83 | 32 | 74.25 ± 26.73 | 0.074 |
Among group B patients, studying the impact of each of the six elements of the CADI score (mesangial matrix increase, glomerular sclerosis, tubular atrophy, interstitial inflammation, interstitial fibrosis, and vascular intimal proliferation) on subsequent renal allograft function revealed that interstitial inflammation (which affected 30.2% of group B patients at 1 yr) was the only element found to have significant impact on GFR throughout all time points after the first year (Table 5).
Table 5.
Impact of presence or absence of interstitial inflammation of 1-yr protocol biopsies on subsequent GFR (ml/min) among group B patients
| Absent
|
Present
|
P | |||
|---|---|---|---|---|---|
| No. | GFR | No. | GFR | ||
| 1 yr | 30 | 97.7 ± 22.2 | 12 | 86.5 ± 22.2 | 0.146 |
| 2 yr | 29 | 96.5 ± 21.1 | 12 | 77.0 ± 21.9 | 0.011a |
| 3 yr | 29 | 95.4 ± 22.3 | 12 | 78.0 ± 21.4 | 0.027a |
| 4 yr | 29 | 88.0 ± 23.0 | 11 | 67.5 ± 21.5 | 0.014a |
| 5 yr | 29 | 86.8 ± 23.9 | 9 | 68.8 ± 21.1 | 0.048a |
P < 0.05.
Adverse Events
No significant difference was found regarding the incidence or the number of antihypertensive medications used in each group, nor in the hematologic complications throughout the follow-up period (Table 6). The most significant infectious complication was the development of tuberculosis among 6 and 4 patients in group A and B, respectively. No significant difference was encountered regarding the incidence of diabetes mellitus, elevated liver enzymes, osteonecrosis, proteinuria, or surgical complications. Group A shows significant higher incidence of chronic diarrhea, whereas group B shows significant higher incidence of hyperlipidemia and herpes zoster infection. No single case of malignancy was diagnosed in either group.
Table 6.
Adverse events
| Group A (Tac) | Group B (MMF) | P | |
|---|---|---|---|
| Hypertension, no. (%) of patients | 33 (61.1) | 45 (76.3) | 0.055 |
| No. of drugs | |||
| 1 drug | 18 | 29 | |
| 2 drugs | 12 | 13 | |
| 3 drugs | 3 | 3 | 0.284 |
| Diabetes mellitus | 19 | 15 | 0.369 |
| Anemia (Hb ≤ 10 g/dl) | 8 | 11 | 0.501 |
| Thrombocytopenia | 5 | 2 | 0.228 |
| Infections | |||
| herpes zoster | 1 | 7 | 0.032a |
| tuberculosis | 6 | 4 | 0.479 |
| Elevated liver enzymes | |||
| 1st and 2nd yr | 28 | 34 | 0.377 |
| 3rd, 4th, and 5th yr | 10 | 13 | 0.543 |
| Osteonecrosis | 4 | 7 | 0.372 |
| Chronic diarrhea | 15 | 4 | 0.005a |
| Hyperlipidemia | |||
| 1st and 2nd yr | 38 | 53 | 0.010a |
| 3rd, 4th, and 5th yr | 19 | 35 | 0.007a |
| Proteinuria | |||
| 1 to 3 g/day | 7 | 13 | |
| ≥3 g/day | 13 | 12 | 0.351 |
| Surgical | |||
| 1st and 2nd yr | |||
| lymphocele | 4 | 7 | |
| urinary fistula | 1 | 2 | 0.127 |
| wound complication | 7 | 11 | |
| 3rd, 4th, and 5th yr | |||
| renal stone | 0 | 1 | |
| ovarian cyst | 1 | 1 | |
| herniorrhaphy | 2 | 3 | |
| skin ulcer | 1 | 1 | 0.543 |
P < 0.05.
DISCUSSION
Based on data of the Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients, which identified trends that have evolved over the past decade in the use of immunosuppression for recipients of solid organ transplants, Tac/MMF combination was found to be the most frequently used maintenance immunosuppression combination at 1 and 2 yr after kidney transplantation. At 1 yr after transplantation in 2003, 51% of patients were receiving Tac/MMF combination, 17% were receiving CsA/MMF, 8% Tac/SRL, and only 1% on SRL/MMF combination regimen. Moreover, a surprisingly low percentage of patients continued their original immunosuppressive discharge regimen through the first 3 yr following transplantation. Among patients transplanted in 2001, the change rate for Tac/MMF combination at 3 yr was 43% and that for SRL/MMF combination was up to 65%.15
We have previously reported on the 2-yr safety and efficacy profile of SRL/steroids based regimens in combination with either low-dose Tac (group A) or MMF (group B) together with basiliximab induction therapy among live-donor renal allotransplant recipients in a prospective randomized study, and it was shown that the SRL/MMF regimen was safe regarding patient and graft survival and was associated with significant better renal function at 2 yr than the Tac/SRL regimen.14 We hereby report on the long-term safety and efficacy profile of the same randomized patients.
Throughout the follow-up period from the third to fifth year, 2 group A patients had sudden death most probably secondary to cardiovascular cause. During the same period, 3 and 4 patients in group A and B, respectively, returned back to dialysis mostly secondary to the development of CAN. Therefore, it was noted that early causes of death among our patients were infectious and late causes were cardiovascular, whereas early causes of graft failure were mostly the result of recurrent original kidney disease and transplant glomerulopathy and late causes were the result of CAN. Slightly lower rates of patient and graft survival for the SRL/MMF arm (87.1% and 83.9%, respectively) were obtained by Flechner et al.16 in a 5-yr prospective randomized study of either SRL/MMF or CsA/MMF with steroids and basiliximab induction in adult renal allotransplant recipients.
Our group B patients show significant lower change rate of the primary immunosuppression compared with group A through the whole follow-up period, being observed in 14 patients (20.8%), 4 of which were seeking pregnancy. This comes in accordance with Flechner et al.16 about low change rates of primary SRL/MMF regimens. Our main indications for SRL withdrawal, in addition to pregnancy, were development of proteinuria in view of several recent reports linking SRL to proteinuria17,18 and tuberculous infection, leukopenia, and surgical causes. Group A unique indications for change of the primary regimen were mainly related to combined SRL/Tac nephrotoxicity (e.g., pathologic diagnosis of acute Tac toxicity despite optimum blood level and/or moderate tubulointerstitial fibrosis), GIT toxicity (e.g., chronic diarrhea with marked weight reduction), and presumed islet cell toxicity19 (e.g., newly discovered difficult controlled diabetes mellitus [DM]).
Based on intention-to-treat analysis, statistically significant higher renal function obtained at 2 yr among SRL/MMF patients,14 was maintained until the last follow-up period, a finding that comes in accordance with all short-term12,13,20 and long-term studies16; however, more recently, Larson et al.,21 in a comparison of TAC/MMF versus SRL/MMF, showed that the iothalamate clearance at 1 mo was higher in the SRL/MMF than in the TAC/MMF group (67 ± 18 ml/min versus 58 ± 17 ml/min); however, this difference was lost at 1 yr because of the unexpected loss of GFR in the SRL/MMF group. Differences in renal function between Larson's and other studies may be explained by the different anti-calcineurinic drugs used in the control group because it seems that CsA induces more profound renal hemodynamic changes than TAC, at least at the current target levels for clinical immunosuppression.22
By exclusion of those with secondary immunosuppression, group A patients could obtain comparable renal function with group B patients after 5 yr, however, with high exclusion rate that exceeds 50%.
By classification of patients according to stages of chronic kidney diseases,23 it was found that about one half and one third of SRL/MMF patients enjoyed quite normal renal function by 3 and 5 yr, respectively.
Among our patients, who are of low to moderate immunologic risk, we reported reduced incidence of acute rejection among group B patients, in the first post-transplant year down to13.5%,14 a finding that comes in accordance with all trials using SRL/MMF with steroids and induction therapy in which acute rejection incidence varies from 6.5%,13 to 13%.21 Moreover, even in patients with high immunologic risk, Lo et al.20 reported 12-mo incidence of as low as 7%.
At the end of the first post-transplant year, CADI score was not significantly different between either group.14 On studying the impact of CADI score on the subsequent renal function among both groups to define a cut off value, it was found that CADI scores of 0,1or 2 were associated with nearly significant better renal function at 5 yr. Moreover, on studying the impact of each of the 6 elements of the score24 on eventual renal function in both groups, interstitial inflammation among group B patients (which affected 30.2% of patients at 1 yr) was the only element found to have significant impact on GFR throughout all time points after the first year.
The safety profile of both groups has markedly improved after the first 2 post-transplant years, as the immunosuppressive doses were reduced as well as the targeted therapeutic window of SRL. Therefore, low incidence of hematologic complications, apart from renal impairment induced anemia, and low surgical complications were encountered. Similarly, the incidence of infectious complications was low apart from TB, which was encountered among 10 cases (7.6%) of our patients. In a previous report on 1200 renal transplant population from the same locality, the incidence of post-transplant TB infection was 3.8%.25 A higher incidence among our series may be explained by the use of more potent immunosuppressive medications, improper pretransplant screening, new epidemic, or combination of these factors. Early (before 1 yr) and aggressive presentation of TB (miliary form) was associated with higher mortality rates. In view of such a life-threatening disease, a policy of handling immunosuppressive drugs among recent cases could show some success, by which all group A or group B affected patients were converted to a dual regimen consisting of steroids and MMF so as to reduce net immunosuppression and avoid drug interactions of SRL and Tac with anti-TB drugs.
No case of solid organ or skin cancer was diagnosed in either group, a finding that supports the hypothesis of Luan et al.,26 that SRL might prevent rather than promote tumor progression.
In summary, a calcineurin-inhibitor free immunosuppressive regimen, consisting of steroids, SRL, and MMF together with monoclonal antibody induction therapy, has proven to be both safe and effective among low to moderate immunologic risk renal allotransplant patients after long-term follow-up. Avoidance of early high change rates of the regimen could be achieved by proper SRL dosages through strict follow-up of SRL blood levels. SRL administration should be delayed in certain patients at increased risk of side effects (e.g., high body mass index, delayed graft function). Administration of this regimen among high immunologic risk patients has proved success on short term, awaiting longer-term follow-up.
CONCISE METHODS
Patients
Between May 2001 and January 2003, a total of 132 patients with end-stage renal disease, who received a live donor renal allo-transplant at the Urology and Nephrology Center, Mansoura University, Egypt, were the subjects of this study. Exclusion criteria included patients requiring second renal transplantation, patients younger than 18 yr, cases with pretransplant chemistries demonstrating a total serum cholesterol greater than 300 mg/dl, triglycerides greater than 400 mg/dl, white blood cell count less than 4000/mm3 or platelets less than 150 000/mm3, patients with pretransplant positive lymphocytotoxic cross-match test, and those with more than 50% DR mismatch.
Immunosuppression Protocol
All patients in both groups received basiliximab (Simulect, Novartis, Basel, Switzerland) 20 mg intravenously at surgery and on day 4 postoperative. Patients in both groups received intravenous methyl prednisolone 500 mg one day before and on day of surgery. Oral prednisolone was then given at a dose of 1 mg/kg per day, which is then gradually tapered down to 0.1 mg/kg by the 10th mo post-transplantation.
Group A patients received SRL solution (Rapamune, WyethAyerst, Princeton, NJ) within 24 h after completion of surgery in a dose of 10 mg/d orally (single morning dose) for 3 d and then maintained on 5 mg/d. Further doses were concentration controlled to keep 24 h whole blood trough level between 6 and 12 ng/ml. Tacrolimus (Prograf, Fujisawa Pharmaceutical, Osaka, Japan) was also administered to this group of patients on the third day postoperative, provided that creatinine clearance is more than 50 ml/min. Tacrolimus was started at a dose of 0.03 mg/kg per day in two equally divided doses targeting 12 h whole blood trough level of 3 to 7 ng/ml.
Group B patients received SRL and maintained on single oral morning dose of 10 mg/d targeting 24 h whole blood trough level between 10 and 15 ng/ml. Mycophenolate Mofetil (Cellcept, Hoffman-La Roche, Nutley, NJ) 1 g twice daily was begun the morning after surgery. Patients remained on this dose unless side effects, such as gastrointestinal toxicity or leucopenia, necessitated dose reduction.
No standard antimicrobial prophylaxis therapy was adopted in this study.
Therapeutic Drug Monitoring
Serum trough levels of SRL were evaluated by high performance liquid chromatography with mass spectroscopy detection through the first 2-yr transplant period, after which the microparticle enzyme immunoassay method was used.27 Two years post-transplantation, SRL trough level was maintained between 5 and 10 ng/ml in both groups. Tac trough levels were evaluated using a microparticulate enzyme immunoassay method and maintained between 3 and 7 ng/ml.
Clinical Assessment
The patients were assessed clinically with particular emphasis on blood pressure measurement. Hypertension was defined according to the JNC vΙΙ report.28 The number of antihypertensive drugs was reported for every patient to express severity of hypertension. New-onset DM was defined as post-transplant hyperglycemia necessitating treatment with oral hypoglycemic agents or insulin injections. Clinical tolerance to the given medications was assessed, which included the safety profile and the occurrence of any adverse events.
Laboratory Investigations
These included complete urine analysis, serum electrolytes, liver function tests, fasting blood sugar, uric acid, calcium, phosphorus, complete lipid profile, and hematologic profile. Renal allograft function was evaluated by the estimation of serum creatinine and calculated GFR using the Cockroft-Gault formula.29
Evaluation of Graft Biopsies
Event (for cause) biopsy was carried out in case of nephrotic range proteinuria or episodes of renal dysfunction (25% increase in creatinine from baseline) for which histopathologic examination was performed according to the Banff Schema (1997).30
Study Outcomes
The study outcomes consisted of efficacy profiles, toxicity profiles, and renal allograft pathology profiles. The efficacy profiles were patient and graft survival, biopsy-confirmed acute rejection episodes, and graft function. The toxicity profiles included serious adverse events, malignancies, infections, wound complications, and metabolic complications. Renal allograft pathology was evaluated using the Banff schema (1997) for event biopsies.
Statistical Analysis
t test was used to compare between the two groups in continuous data. χ2 was used to compare categorical variables. The survival of the patients and the grafts was computed using the Kaplan-Meier method31 on an intention-to-treat basis. Differences in survival were calculated by the log-rank test.32 For all of the above tests, a P value ≤0.05 was considered as significant.
DISCLOSURES
None.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Myers BD: Cyclosporine nephrotoxicity. Kidney Int 30: 964–974, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Bertani T: Renal vascular and thrombotic effects of cyclosporine. Am J Kidney Dis 13: 261–272, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Bennett WM, Houghton DC, Buss WC: Cyclosporine-induced renal dysfunction: Correlations between cellular events and whole kidney function. J Am Soc Nephrol 1: 1212–1219, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Borrows RJ, Fung CL, O'squosquo; apos; yConnell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ducloux D, Fournier V, Bresson-Vautrin C, Rebibou JM, Billerey C, Saint-Hiller Y, Chalpoin JM: Mycophenolate mofetil in renal transplant recipients with cyclosporine-associated nephrotoxicity: A preliminary report. Transplantation 65: 1504–1506, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Hueso M, Bover J, Seron D, Gil-Vernet S, Sabate I, Fulladosa X, Ramos R, Coll O, Alsina J, Grynio JM: Low-dose cyclosporine and mycophenolate mofetil in renal allograft recipients with suboptimal renal function. Transplantation 66: 1727–1731, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Keunecke C, Rothenpieler U, Zanker B, Schneeberger H, Illner WD, Theodorakis J, Stangl M, Land W: Mycophenolate mofetil monotherapy: an example of a safe nephrotoxicity/atherogenicity-free immunosuppressive maintenance regimen in a selected group of kidney-transplanted patients. Transplant Proc 32[Suppl]: S6–S8, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Schrama YC, Joles JA, van Tol A, Boer P, Koomans HA, Hene RJ: Conversion to mycophenolate mofetil in conjunction with stepwise withdrawal of cyclosporine in stable renal transplant recipients. Transplantation 69: 376–383, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Vincenti F, Ramos E, Brattstrom C, Cho S, Ekberg H, Grinyo J, Johnson R, Kuypers D, Stuart F, Khanna A, Navarro M, Nashan B: Multicenter trial exploring calcineurin inhibitors avoidance in renal transplantation. Transplantation 71: 1282–1287, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Tran HT, Acharya MK, McKay DB, Sayegh MH, Carpenter CB, Auchincloss HJR, Kirkman RL, Milford EL: Avoidance of cyclosporine in renal transplantation: Effects of daclizumab, mycophenolate mofetil, and steroids. J Am Soc Nephrol 11: 1903–1909, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Groth CG, Backman L, Morales JM: Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine: Sirolimus European Renal Transplant Study Group. Transplantation 67: 1036–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Kreis H, Cisterne JM, Land W: Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 69: 1252–1260, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastorianni B, Savas K, Cook DJ, Novick AC: Kidney transplantation without calcineurin inhibitor drugs: A prospective randomized trial of sirolimus versus cyclosporine. Transplantation 74: 1070–1076, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Hamdy AF, El-Agroudy A, Bakr MA, Mostafa A, El-baz M, El-Shahawy M, Ghoneim MA: Comparison of sirolimus with low-dose tacrolimus versus sirolimus-based calcineurin inhibitor-free regimen in live donor renal transplantation. Am J Transplant 5: 2531–2538, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, Leichtman AB: Immunosuppression: Evolution in practice and trends, 1994–2004. Am J Transplant 6: 1111–1131, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Flechner S, Goldfarb D, Solez K, Modlin C, Mastroianni B, Savas K, Babineau D, Kurian S, Salomon D, Novick A, Cook DJ: Kidney transplantation with sirolimus and mycophenolate mofetil-based immunosuppression: 5-year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 83: 883–892, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Diekmann F, Gutierrez-Dalmau A, Lopez S, Cofan F, Esforzado N, Ricart MJ, Rossich E, Saval N, Torregrosa J, Oppenheimer F, Campistol JM: Influence of sirolimus on proteinuria in de novo kidney transplantation with expanded criteria donors: Comparison of two CNI-free protocols. Nephrol Dial Transplant 22: 2316–2321, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Stephany B, Augustine J, Krishnamurthi V, Goldfarb D, Flechner S, Braun W, Hricik D, Dennis V, Poggio E: Differences in proteinuria and graft function in de novo sirolimus-based vs. calcineurin inhibitor-based immunosuppression in live donor kidney transplantation. Transplantation 82: 368–374, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hricik DE, Anton HA, Knauss TC, Rodriguez V, Seaman D, Siegel C, Valente J, Schulak JA: Outcomes of African American kidney transplant recipients treated with sirolimus, tacrolimus and corticosteroids. Transplantation 74: 189–193, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lo A, Egidi MF, Gaber L, Amiri H, Vera S, Nezakatgoo N, Gaber O: Comparison of sirolimus-based calcineurin inhibitor-sparing and calcineurin inhibitor-free regimens in cadaveric renal transplantation. Transplantation 77: 1228–1235, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, Gloor JM, Cosio FG, Lund WJ, Kremers WK, Nyberg SL, Ishitani MB, Prieto M, Velosa JA: Complete avoidance of calcineurin inhibitors in renal transplantation: A randomized trial comparing sirolimus and tacrolimus. Am J Transplant 6: 514–522, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Klein IH, Abrahams A, van Ede T, Hene RJ, Koomans HA, Ligtenberg G: Different effects of tacrolimus and cyclosporine on renal hemodynamics and blood pressure in healthy subjects. Transplantation 73: 732–736, 2002 [DOI] [PubMed] [Google Scholar]
- 23.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 24.Isoniemi HM, Krogerus I, Von Willebrand E, Taskinen E, Ahonen J, Hayry P: Histopathological findings in well-functioning, long term renal allografts. Kidney Int 41: 155–160, 1992 [DOI] [PubMed] [Google Scholar]
- 25.El-Agroudy A, Refaie A, Moussa O, Ghoneim M: Tuberculosis in Egyptian kidney transplant recipients: Study of clinical course and outcome. J Nephrol 16: 404–411, 2003 [PubMed] [Google Scholar]
- 26.Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M: Rapamycin blocks tumour progression: Unlinking immunosuppression from antitumour efficacy. Transplantation 73: 1565–1572, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Wilson DH, Book B, Brunet M, Engelmayer J, Gaulier JM, Luthe H, Marquet P, Moscato D, Mosso R, Oellerich M, Pescovitz M, Ramanathan L, Schmid R, Stickle D, Sarkozi L, Wallemacq P: Multicenter evaluation of an immunoassay for the measurement of sirolimus on the Abbott IMx analyzer. Transplantation 78[Suppl 1]: 477–478, 2004 [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ: The Seventh Report Joint National Committee on Prevention, Detection Evaluation and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Cockroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 1227–1236, 1976 [DOI] [PubMed] [Google Scholar]
- 30.Racusen L, Solez K, Colvin R, Bonsib SM, Castro MC, Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P: Non parametric estimation from incomplete observation. J Am Stat Assoc 53: 457–481, 1958 [Google Scholar]
- 32.Peto R, Peto J: Asymptotically efficient rank invariant test procedures. J R Stat Soc 135: 185–207, 1972 [Google Scholar]