Abstract
Genetic evidence supports an early role for Notch signaling in the fate of podocytes during glomerular development. Decreased expression of Notch transcriptional targets in developing podocytes after the determination of cell fate suggests that constitutive Notch signaling may oppose podocyte differentiation. This study determined the effects of constitutive Notch signaling on podocyte differentiation by ectopically expressing Notch's intracellular domain (NOTCH-IC), the biologically active, intracellular product of proteolytic cleavage of the Notch receptor, in developing podocytes of transgenic mice. Histologic and molecular analyses revealed normal glomerular morphology and expression of podocyte markers in newborn NOTCH-IC–expressing mice; however, mice developed severe proteinuria and showed evidence of progressive glomerulosclerosis at 2 wk after birth. Features of mature podocytes were lost: Foot processes were effaced; expression of Wt1, Nphs1, and Nphs2 was downregulated; cell-cycle re-entry was induced; and the expression of Pax2 was increased. In contrast, mice with podocyte-specific inactivation of Rbpsuh, which encodes a protein essential for canonical Notch signaling, seemed normal. In addition, the damaging effects of NOTCH-IC expression were prevented in transgenic mice after simultaneous conditional inactivation of Rbpsuh in murine podocytes. These results suggest that Notch signaling is dispensable during terminal differentiation of podocytes but that constitutive (or inappropriate) Notch signaling is deleterious, leading to glomerulosclerosis.
Genetic evidence in mice suggests that podocyte cell fate determination is regulated by Notch signaling during nephrogenesis.1–3 Notch signaling controls cell differentiation in multiple developing organ systems (reviewed by Kadesch4). A key step in Notch receptor activation is proteolytic cleavage of its intracellular domain (NOTCH-IC),5 resulting in NOTCH-IC nuclear translocation and complex formation with the transcriptional repressor, recombination binding protein J-κ (Rbpsuh; Mouse Genome Informatics; also known as RBPJ-κ, CSL, and CBF1).6 NOTCH-IC binding to RBPJ-κ recruits transcriptional co-activators that induce expression of downstream targets, including Hairy/Enhancer of Split (Hes) and Hes-related (Hey) genes7–9 that function as Notch effectors by negatively regulating tissue-specific differentiation.10,11
During proximal nephron specification, it is unclear whether Notch is involved in binary decisions that influence podocyte versus proximal tubular cell fate choices or subsequently functions in podocyte terminal differentiation during glomerular development. The demonstration that the γ-secretase inhibitor N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester (DAPT) does not prevent podocyte Wilms' tumor 1 (WT1) expression in advanced-stage cultured metanephroi argues against a subsequent role for cleavage-dependent Notch activation in podocyte terminal differentiation.2 Consistent with this concept are reports of Notch pathway genes expression patterns in developing podocytes, which reveal a progressive decline in Notch1, Notch2, Hes1, and Hey1 mRNA levels in immature podocytes and absent expression in podocytes at more advanced stages of glomerulogenesis.12–14 Collectively, these data suggest that high levels of Notch signaling in podocyte progenitors may be essential for initiating differentiation, yet lower or absent levels of Notch signaling in developing podocytes may be necessary for terminal differentiation. Conversely, persistent activation of Notch in developing podocytes may oppose terminal differentiation.
To investigate the effect of Notch activation in developing podocytes, we used a tissue-specific, CRE-loxP–mediated approach to express ectopically NOTCH-IC in embryonic podocytes and determined the effect of constitutive Notch signaling on podocyte differentiation and postnatal function. Our results indicate that ectopic NOTCH-IC expression causes loss of glomerular filtration barrier selective permeability, podocyte differentiation defects, and glomerulosclerosis within the first few weeks of life. These effects are completely prevented by blocking canonical Notch signaling through mutational inactivation of Rbpsuh. Our data support the concept that downregulated Notch activity is crucial for podocyte differentiation and suggest a novel pathogenic role for inappropriate podocyte Notch activation in glomerulosclerosis.
RESULTS
Podocyte-Specific Expression of MYCNOTCH-IC in Transgenic Mice
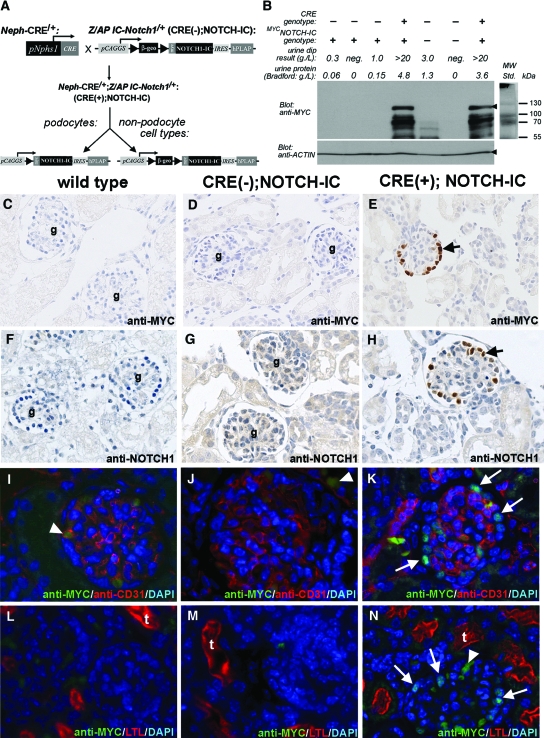
In vivo effects of constitutive Notch signaling in murine podocytes were determined using a CRE-loxP conditional transgenic approach to induce podocyte-specific expression of MYC-tagged Notch intracellular domain (termed MYCNOTCH-IC; Figure 1A). CRE-dependent MYCNOTCH-IC protein expression was demonstrated by Western blot in extracts from Nephrin-CRE/+;NOTCH-IC/+ [CRE(+);NOTCH-IC] isolated mouse glomeruli but not from CRE-negative, NOTCH-IC/+ transgenic [CRE(−);NOTCH-IC] or wild-type mouse littermates (Figure 1B). Immunohistochemistry using anti-MYC or anti-NOTCH1 antibodies to detect MYCNOTCH-IC protein expression in kidney tissue sections showed multiple podocytes staining positively with anti-MYC (Figure 1E, black arrow) or anti-NOTCH1 antibodies (Figure 1H, black arrow). The patterns of anti-MYC or anti-NOTCH1 immunostaining were nuclear, suggesting nuclear localization of MYCNOTCH-IC protein. In contrast, glomeruli of CRE(−);NOTCH-IC and wild-type littermates lacked anti-MYC (Figure 1, C and D, respectively) or anti-NOTCH1 (Figure 1, F and G, respectively) staining in podocyte nuclei. Co-incubation of sections with anti-MYC and anti-WT1 antibodies revealed co-localization of MYCNOTCH-IC and WT1 in many cells, thereby identifying (MYC, WT1)–double-positive cells as MYCNOTCH-IC–expressing podocytes (Figure 5, F and G, and N and O). We quantified MYCNOTCH-IC protein expression in podocytes by counting the number of (MYC, WT1)–double-positive and total WT1-positive cells per glomerulus and observed a progressive increase in the fraction of podocytes expressing MYCNOTCH-IC protein over time. At postnatal day 7 (P7), 21 ± 5% of podocytes were (MYC, WT1)–double-positive (n = 22 glomeruli). By P21, this fraction increased to 35 ± 6% (n = 22 glomeruli) and was 51 ± 6% at P28 (n = 16 glomeruli).
Figure 1.
Podocyte-specific expression of MYCNOTCH-IC in newborn Neph-CRE/+;IC-Notch1/+ transgenic mouse kidneys. (A) Breeding scheme to generate transgenic mice that express MYCNOTCH-IC in podocytes. Neph-CRE/+ mice express CRE in podocytes under control of Nphs1 promoter elements. The modular IC-Notch1 transgene is represented pictorially. pCAGGS, CMV enhancer/chicken β-actin hybrid promoter; ▸, loxP; β-geo, β-galactosidase/neomycin/polyA fusion cassette; MYC, 6X human MYC epitope tag; IRES, internal ribosomal entry site; hPLAP, human placental alkaline phosphatase. (B) Analysis of MYCNOTCH-IC protein expression in lysates of isolated glomeruli as separated by SDS-PAGE. Top and bottom left panels show Western blot results after incubation with anti-MYC and anti–β-actin antibodies, respectively. Black arrowhead in top panel denotes band position corresponding to MYCNOTCH-IC with approximate molecular weight of 111 kD. Black arrowhead in lower panel denotes actin band. Top right panel shows protein ladder (MW Std.) and corresponding protein molecular weights. (C through H) Detection of conditional MYCNOTCH-IC protein in podocytes as revealed by immunohistochemistry using anti-MYC (C through E), and anti-NOTCH1 (F through H) antibodies. Nonserial, representative images are shown of newborn mouse kidney tissue sections from wild-type (C and F), CRE(−);NOTCH-IC(D and G), and CRE(+);NOTCH-IC (E and H) mice. Black arrows denote anti-MYC or anti-NOTCH -stained cells. g, glomeruli. Sections were counterstained with hematoxylin. (I through N) Dual immunofluorescence labeling of mouse kidney tissue sections with either anti-MYC (green) and anti-CD31 (red) antibodies (I through K) or anti-MYC (green) and LTL (red; L through N). Shown are representative images of glomeruli from wild type (I and L), CRE(−);NOTCH-IC (J and M), and CRE(+);NOTCH-IC (K and N) mice. For L through N, anti-MYC staining was originally detected with AlexaFluor 594 secondary antibody, and LTL staining was performed with FITC-LTL. For comparative purposes, corresponding original images (shown in Supplemental Figure 1) were color-adjusted using Photoshop 6.0 to show anti-MYC immunodetection as green and LTL reactivity as red. Sections were counterstained with DAPI. White arrows, anti-MYC–labeled podocytes. White arrowheads, background anti-MYC/anti-mouse IgG immunoreactivity. Magnifications: ×400 in C through H; ×1000 in I through N.
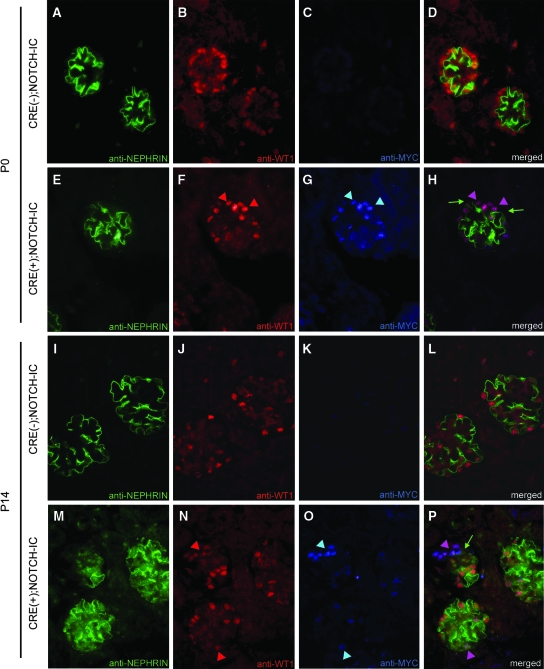
Figure 5.
Decreased NEPHRIN protein expression in MYCNOTCH-IC–expressing podocytes. (A through P) Shown are representative micrographs of glomeruli after triple immunofluorescence labeling of mouse tissue sections with anti-NEPHRIN, anti-WT1, and anti-MYC antibodies. (A through C and I through K) Serial micrographs of P0 (A through C) and P14 (I through K) CRE(−);NOTCH-IC glomeruli after multichannel imaging. (E through G and M through O) Serial micrographs of P0 (E through G) and P14 (M through O) CRE(+);NOTCH-IC obtained by multichannel imaging. (A, E, I, and M) Green channel images showing anti-NEPHRIN immunodetection with Alexa488-conjugated secondary antibody. (B, F, J, and N) Red channel images showing anti-WT1 immunodetection with Alexa594-conjugated secondary antibody. (C, G, K, and O) Blue channel images showing anti-MYC immunodetection with Cy5-conjugated secondary antibody. (D, H, L, and P) Merged images of corresponding micrographs obtained from green, red, and blue channels. (F and N) Red arrowheads, podocytes stained positively with anti-WT1 antibody. (G and O) Blue arrowheads, cells stained positively with anti-MYC antibody. (H and P) Pink arrowheads, cells that show overlapping staining for both anti-WT1 and anti-MYC antibodies [i.e., (anti-WT1, anti-MYC)–double-positive cells] identifying these cells as MYCNOTCH-IC–expressing podocytes. (H, green arrows) Preservation of anti-NEPHRIN staining in vicinity of (anti-WT1, anti-MYC)–double-positive cells at P0. (P, green arrow) Loss of anti-NEPHRIN staining in vicinity of (anti-WT1, anti-MYC)–double-positive cells at P14. Magnification, ×1000.
Occasionally, weak anti-NOTCH1 immunostaining was detected within the glomerular capillary tuft or in the cytoplasm of tubular elements (Figure 1G), raising the possibility that CRE-independent (i.e., leaky) MYCNOTCH-IC expression in nonpodocyte lineages might account for glomerular and tubular anti-NOTCH1 immunostaining in transgenic tissues. To examine this possibility, we performed double-labeling experiments on mouse kidney tissue sections using anti-MYC antibody and either anti-CD31 or Lotus tetragonolobus lectin (LTL) to detect simultaneously MYCNOTCH-IC–expressing cells in glomerular capillary endothelium or proximal tubular epithelium, respectively. MYC-positive cells were detected in glomeruli of CRE(+);NOTCH-IC transgenic mice contiguous but not overlapping with staining for anti-CD31 antibody (Figure 1K). In contrast, MYC-positive cells were not detected in glomeruli of wild-type and CRE(−);NOTCH-IC mice also stained with anti-CD31 antibody (Figure 1, I and J, respectively), suggesting that glomerular capillary endothelium did not exhibit leaky MYCNOTCH-IC expression. Some MYC-positive cells were occasionally detected within capillary lumina outlined by anti-CD31 staining (Figure 1, I and N, white arrowhead); however, because these MYC-positive cells lacked DAPI staining, we suspected that they were anucleate erythrocytes with background staining. We confirmed this by incubating tissues with anti-mouse secondary antibody alone and demonstrated frequent labeling of DAPI-negative cells with anti-mouse secondary within glomerular capillary lumina (Supplementary Figure 1, A through C). Likewise, we did not detect nuclear anti-MYC staining in tubular structures identified by LTL staining in wild-type, CRE(−);NOTCH-IC, and CRE(+);NOTCH-IC mice (Figure 1, L, M, and N, respectively) but observed background staining within tubular cytoplasm (Supplemental Figure 1, D through F). Thus, we concluded that MYCNOTCH-IC expression was restricted to the podocyte lineage in our transgenic system, which afforded the opportunity to determine effects of constitutive NOTCH activation exclusively in podocytes.
Early-Onset Proteinuria in CRE(+);NOTCH-IC Transgenic Mice
Effects of MYCNOTCH-IC expression on postnatal podocyte function as they related to glomerular filtration barrier selective permeability were determined by urine protein analysis. Screening for proteinuria was performed by random urine dipstick testing in newborn (P0), P7, P14, P21, and P42 mice (Table 1). At P0 and P7, the median urine dipstick test results were trace for wild-type, CRE(−);NOTCH-IC, and CRE(+);NOTCH-IC mice. At P14, however, the median test result for CRE(+);NOTCH-IC mice was 3.0 g/L (n = 8 mice) and subsequently was >20 g/L at P21 (n = 8 mice) and P42 (n = 4 mice). In contrast, the median result was consistently trace for CRE(−);NOTCH-IC mice at P14 through P42. For more accurate assessment of protein excretion, random urine protein concentration and corresponding total urine protein-creatinine ratio were determined. Random urine protein concentration measurements correlated with urine dipstick results (Table 1) and were significantly higher in CRE(+);NOTCH-IC mice at P14, P21, and P42 as compared with age-matched CRE(−);NOTCH-IC and wild-type mice [CRE(+);NOTCH-IC urine protein, P versus CRE(−);NOTCH-IC, P versus wild-type: P14: 3535 ± 513 mg/L, P = 0.0001, P < 0.0001; P21: 3273 ± 452 mg/L, P = 0.0005, P = 0.0006; P42: 3179 ± 833 mg/L, P = 0.007, P = 0.01]. Likewise, urine protein:creatinine values for CRE(+);NOTCH-IC mice were higher compared with wild-type or CRE(−);NOTCH-IC mice at P7 and reached statistical significance at P14 [CRE(+);NOTCH-IC urine protein;creatinine mg/mmol, P versus CRE(−);NOTCH-IC, P versus wild-type: P14: 217 ± 51, P = 0.01, P = 0.0005; P21: 2011 ± 464, P = 0.008, P = 0.007; P42: 3359 ± 1567, P = 0.05, P = 0.07]. For CRE(−);NOTCH-IC mice, random urine protein; creatinine ratios were not statistically significantly different from wild-type values, except at P14 (protein:creatinine mg/mmol: wild type: 22 ± 3 versus CRE(−);NOTCH-IC: 67 ± 13, P = 0.002). Because corresponding values at P21 and P42 were not statistically different between these two groups (Table 1), we did not consider our finding of proteinuria in CRE(−);NOTCH-IC mice at P14 to indicate a permanent effect on glomerular permselectivity. We also did not observe proteinuria in CRE-expressing mice that did not inherit the IC-Notch1 transgene (data not shown), suggesting that sustained effects on glomerular permselectivity were dependent on combined inheritance of the IC-Notch1 and Nephrin-CRE alleles. Thus, demonstration of proteinuria in CRE(+);NOTCH-IC mice suggested that constitutive expression of MYCNOTCH-IC in podocytes impaired glomerular filtration barrier selective permeability.
Table 1.
Urine protein quantification by dipstick analysis, protein concentration, and protein-creatinine ratio over time in wild-type, CRE(−);NOTCH-IC, and CRE(+);NOTCH-IC mice
| Parameter | Urine Protein at Postnatal Age
|
||||
|---|---|---|---|---|---|
| P0 | P7 | P14 | P21 | P42 | |
| Wild type | |||||
| median urine dip test (lower quartile, upper quartile) | Trace (tr, tr) | Trace (tr, tr) | Trace (tr, 0.3) | Trace (tr, 0.3) | 0.3 g/L (0.3, 0.3) |
| (n = 3) | (n= 5) | (n = 7) | (n = 4) | (n = 4) | |
| protein (mg/L) | 118 ± 57 | 46 ± 5 | 117 ± 43 | 213 ± 16 | 247 ± 70 |
| protein:creatinine (mg/mmol) | 97 ± 28 | 34 ± 3 | 22 ± 3 | 31 ± 4 | 61 ± 13 |
| (n = 3) | (n = 5) | (n = 7) | (n = 4) | (n = 4) | |
| CRE(−);NOTCH-IC | |||||
| median urine dip test (lower quartile, upper quartile) | Trace (tr, tr) | Trace (tr, 0.3) | Trace (tr, 0.3) | Trace (tr, tr) | Trace (tr, 0.3) |
| (n = 10) | (n = 6) | (n = 13) | (n= 6) | (n = 5) | |
| protein (mg/L) | 24 ± 9 | 52 ± 7 | 177 ± 43 | 123 ± 28 | 367 ± 98 |
| protein:creatinine (mg/mmol) | 54 ± 28 | 18 ± 3a | 67 ± 13a | 64 ± 24 | 108 ± 17 |
| (n = 2) | (n = 3) | (n = 5) | (n = 4) | (n = 5) | |
| CRE(+);NOTCH-IC | |||||
| median urine dip test (lower quartile, upper quartile) | Trace (tr, tr) | Trace (tr, 0.3) | 3.0 (3.0, >20) | >20 g/L (>20, >20) | >20 g/L (>20, >20) |
| (n = 12) | (n = 5) | (n = 8) | (n = 8) | (n = 4) | |
| protein (mg/L) | 17 ± 6 | 281 ± 146 | 3535 ± 513 | 3273 ± 452 | 3179 ± 833 |
| protein:creatinine (mg/mmol) | 31 ± 17 | 138 ± 64 | 217 ± 51a,b | 2011 ± 464a,b | 3359 ± 1567 |
| (n = 4) | (n = 4) | (n = 4) | (n = 5) | (n = 4) | |
P < 0.05 versus wild-type.
P < 0.05 versus CRE(−);NOTCH-IC.
CRE(+);NOTCH-IC Transgenic Mice Develop Progressive Glomerulosclerosis
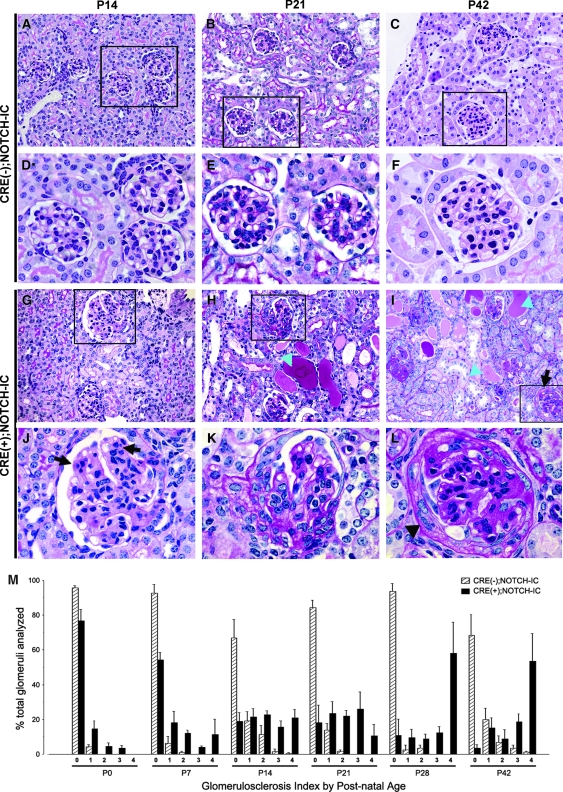
Light microscopy examination of CRE(+);NOTCH-IC newborn kidney tissue sections revealed normal histomorphology of developing and advanced stage glomeruli (data not shown); however, at P14, some CRE(+);NOTCH-IC glomeruli exhibited increased periodic acid-Schiff (PAS) staining and mild mesangial hypercellularity (Figure 2, G and J). In contrast, age-matched CRE(−);NOTCH-IC mice had normal glomerular morphology (Figure 2, A and D). At P21, several CRE(+);NOTCH-IC mouse glomeruli showed intense mesangial PAS staining (Figure 2, B and E, and H and K). Whereas these lesions exhibited reduced caliber of glomerular capillaries, the overall architecture of the glomerular capillary tuft was intact and did not appear collapsed (Figure 2K). Within same sections, the extent of individual glomerular damage was variable; that is, some glomeruli exhibited milder lesions (Figure 2J), whereas others showed more severe lesions (Figure 2K). In addition, sections showed evidence of renal tubular injury (Figure 2H, blue arrowheads). By P42, the majority of CRE(+);NOTCH-IC glomeruli showed marked mesangial matrix expansion, reduced capillary diameter, thickening of Bowman's capsule (Figure 2, C and F, and I and L), and severe tubular damage.
Figure 2.
Histologic progression of glomerular lesions in CRE(+);NOTCH-IC transgenic mice. (A through L) Representative images of PAS-stained tissue sections from P14 (A, D, G, and J), P21 (B, E, H, and K), and P42 (C, F, I, and L) mouse kidneys. (A through F) CRE(−);NOTCH-IC mice; (G through L) CRE(+);NOTCH-IC mice. (D through F and J through L) Corresponding boxed areas. (J) Black arrows show zones of mesangial hypercellularity and matrix increase, with decreased caliber of neighboring capillaries at P14. (K) At P21, glomerulus shows further increase in PAS-stained mesangial matrix and reduced caliber of glomerular capillaries. (L) At P42, glomerulus shows intense PAS-stained mesangial matrix expansion, obliteration of glomerular capillaries, and thickening of parietal epithelium (black arrowhead). (M) Frequency distribution plot showing percentage of total number of glomeruli per tissue section at P0, P7, P14, P21, P28, and P42 (n = 3 mice per age group) with corresponding GS score. ▒, CRE(−);NOTCH-IC mice; ▪, CRE(+);NOTCH-IC mice. SE as shown. Magnifications: ×400 in A through C and G through I; ×800 in D through F and J through L.
To determine the extent of glomerular involvement over time, tissue sections from P0 through P42 mice were semiquantitatively scored for glomerulosclerosis severity (GS score). In CRE(−);NOTCH-IC mice, the median GS score was 0 at all ages (Table 2). GS score ≥1 accounted for less than one third of the total number of glomeruli analyzed at each time point in CRE(−);NOTCH-IC mice (Figure 2M; percentage of glomeruli with GS score ≥1: P0, 4 ± 1%; P7, 7 ± 4%, P14, 33 ± 8%; P21, 16 ± 4%; P28, 6 ± 3%; P42, 32 ± 8%). In contrast, lesions were observed more frequently in CRE(+);NOTCH-IC glomeruli over time (Figure 2M; percentage of CRE(+);NOTCH-IC glomeruli with GS score ≥1: P0, 23 ± 5%; P7, 46 ± 11%, P14, 81 ± 8%; P21, 82 ± 14%; P28, 89 ± 19%; P42, 96 ± 18%) and were more severe (Figure 2M; percentage of CRE(+);NOTCH-IC glomeruli with GS score >2: P0, 8 ± 2%; P7, 28 ± 9%, P14, 60 ± 6%; P21, 59 ± 12%; P28, 80 ± 18%; P42, 81 ± 17%).
Table 2.
Median glomerulosclerosis index score for CRE(+);NOTCH-IC and CRE(−);NOTCH-ICa
| Parameter | Median Glomerulosclerosis Index Score (Lower Quartile, Upper quartile) at Postnatal Age
|
|||||
|---|---|---|---|---|---|---|
| P0 | P7 | P14 | P21 | P28 | P42 | |
| CRE(−);NOTCH-IC | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) |
| (n = no. of glomeruli) | (n = 163) | (n = 361) | (n = 232) | (n = 232) | (n = 108) | (n = 216) |
| CRE(+);NOTCH-IC | 0 (0, 0) | 0 (0, 2) | 2 (1, 3) | 2 (1, 3) | 3 (1, 4) | 3 (2, 4) |
| (n = no. of glomeruli) | (n = 186) | (n = 307) | (n = 183) | (n = 205) | (n = 182) | (n = 209) |
n = 3 mice per age group.
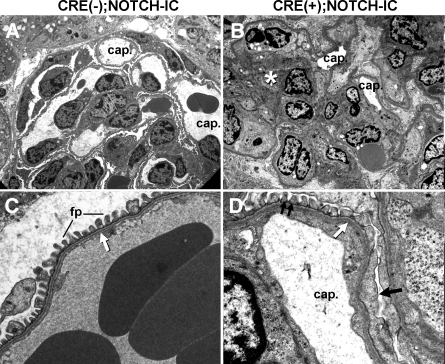
Transmission electron microscopy demonstrated reduced glomerular capillary lumen caliber and increased mesangial matrix in CRE(+);NOTCH-IC glomeruli (Figure 3, A and B). Podocytes showed focal foot process effacement and slit diaphragm loss (Figure 3, C and D, black arrow). The glomerular basement membrane was not thickened or split in regions contiguous with foot process effacement (Figure 3D, white arrow). Immunostaining for type IV collagen α3, α4, and α5 chains was identical between CRE(+) and CRE(−);NOTCH-IC glomeruli (data not shown), suggesting that a primary defect in glomerular basement membrane composition was unlikely to explain glomerular filtration barrier dysfunction in CRE(+);NOTCH-IC mice.
Figure 3.
Transmission electron micrographs (TEM) of CRE(+);NOTCH-IC mouse glomeruli. (A) P42 CRE(−);NOTCH-IC mouse glomerulus. cap., patent capillary lumina. (B) P42 CRE(+);NOTCH-IC mouse glomerulus. cap., decreased capillary lumen diameter. White * denotes mesangial matrix expansion. (C) Podocyte-endothelial interface of a CRE(−);NOTCH-IC glomerular capillary loop. fp, normal foot processes. White arrow, normal glomerular basement membrane. (D) Corresponding interface from a CRE(+);NOTCH-IC glomerulus. Single black arrow, focal foot process effacement; double black arrows, normal-appearing foot processes; white arrow, glomerular basement membrane neither thickened nor split. Magnifications: ×2200 in A and B; ×13,000 in C and D.
Loss of Mature Marker Expression and Cell-Cycle Re-entry in Notch-Activated Podocytes
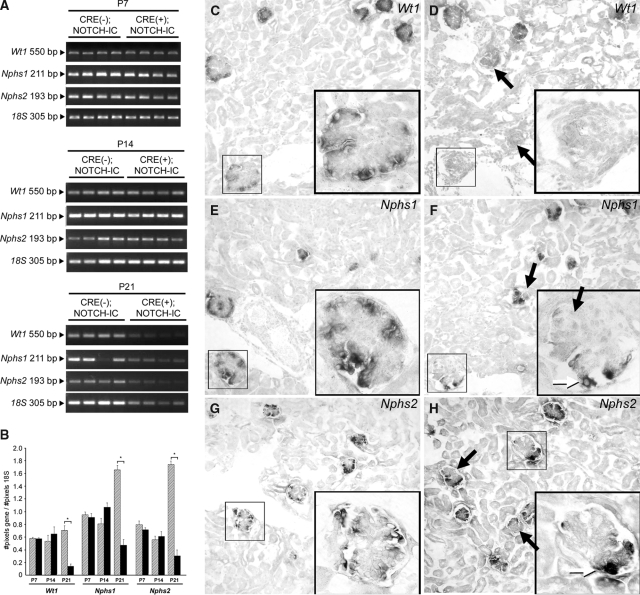
Wt1, Nphs1, and Nphs2 mRNA were initially expressed in glomeruli of newborn CRE(+);NOTCH-IC transgenic mice (data not shown); therefore, we speculated that primary deficiency in these genes was unlikely the cause of glomerulosclerosis in CRE(+);NOTCH-IC mice as suggested for glomerulosclerosis in humans and mice with corresponding loss of function mutations.15–17 However, secondary loss of podocyte-specific gene expression has been shown to accompany glomerulosclerosis in experimental models of nephrosis,18–20 which may be a mechanism of disease progression in these systems. To gain insight into the mechanism of glomerulosclerosis progression in CRE(+);NOTCH-IC mice, we analyzed Wt1, Nphs1, and Nphs2 mRNA expression over time in glomeruli of CRE(+);NOTCH-IC mice by semiquantitative reverse transcriptase–PCR (RT-PCR) on RNA extracted from glomerular isolates. For semiquantification, 18S RNA levels served as an internal reference. At P7, Wt1, Nphs1, and Nphs2 mRNA levels were not significantly different in glomerular extracts from four CRE(+);NOTCH-IC mice compared with four age-matched controls (Figure 4, A, top, and B). Likewise, at P14, corresponding expression levels were comparable in CRE(+) and CRE(−);NOTCH-IC mice (Figure 4, A, middle, and B); however, at P21, Wt1, Nphs1, and Nphs2 mRNA levels were lower in CRE(+);NOTCH-IC compared with CRE(−);NOTCH-IC mice (Figure 4, A, bottom, and B). The fold change in mRNA expression at P21 in CRE(+);NOTCH-IC relative to CRE(−);NOTCH-IC glomeruli for Wt1, Nphs1, and Nphs2 was −4.6 ± 0.3 (P = 0.0005), −3.4 ± 0.2 (P < 0.0001), and −5.5 ± 0.3 (P < 0.0001), respectively. mRNA in situ hybridization revealed decreased Wt1, Nphs1, and Nphs2 expression in some glomeruli (Figure 4, C and D, E and F, and G and H, respectively), reminiscent of the focal pattern of glomerulosclerosis onset in CRE(+);NOTCH-IC kidneys (Figure 2M). Double labeling of renal tissue sections at P7 and P14 with anti-NEPHRIN and anti-MYC antibodies revealed absent NEPHRIN immunostaining in glomerular segments occupied by anti–MYC-positive cells (Figure 5, A through H, and I through P, respectively), suggesting that loss of NEPHRIN represented a change in protein expression within MYCNOTCH-IC-expressing podocytes as opposed to a loss of podocytes per se. Within the same glomerulus, podocytes that did not stain positively with anti-MYC retained NEPHRIN immunoreactivity, suggesting that loss of NEPHRIN depends on MYCNOTCH-IC expression. Taken together with our histologic analysis of CRE(+);NOTCH-IC glomeruli, we concluded that Notch activation induced loss of mature podocyte characteristics, namely morphologic changes in foot process and slit diaphragm architecture and decreased expression of differentiated podocyte markers.
Figure 4.
Glomerular Wt1, Nphs1, and Nphs2 mRNA expression in CRE(+);NOTCH-IC mice. (A and B) Semiquantitative RT-PCR on total RNA extracted from isolated glomeruli of CRE(−);NOTCH-IC and CRE(+);NOTCH-IC mice. (A) Shown are RT-PCR results from P7 (top), P14 (middle), and P21 (bottom) mice after agarose gel electrophoresis. Gene name and corresponding band size are indicated in the left margin. (B) Graphic representation of RT-PCR results by gene name and postnatal age showing mRNA expression as determined by band densitometry. Shown are mean mRNA expression levels calculated as the number of pixels per band for a corresponding gene divided by number of pixels per band for loading control mRNA (18S). ▒, CRE(−);NOTCH-IC; ▪, CRE(+);NOTCH-IC. Error bars denote SE. * P < 0.05. (C through H) Representative, nonserial images of frozen sections from P14 CRE(−);NOTCH-IC (C, E, and G) and CRE(+);NOTCH-IC (D, F, and H) kidneys showing mRNA in situ hybridization using specific nonradioactive riboprobes. (C and D) Wt1. (E and F) Nphs1. (G and H) Nphs2. Corresponding insets are magnified views of regions enclosed by black boxes. Sections are counterstained with Nuclear Fast Red. (D, F, and H) Black arrows, representative glomeruli showing decreased Wt1, Nphs1, and Nphs2 mRNA expression. (D, F, and H insets) Magnified image of representative CRE(+);NOTCH-IC glomeruli showing global decreased Wt1 mRNA expression (D, inset) or segmental decreased Nphs1 mRNA expression (F, inset, black arrow) and Nphs2 mRNA expression (H, inset). (F and H insets) White arrows, some cells within the same glomerulus maintain Nphs1 and Nphs2 expression. Magnification, ×200 in C through H.
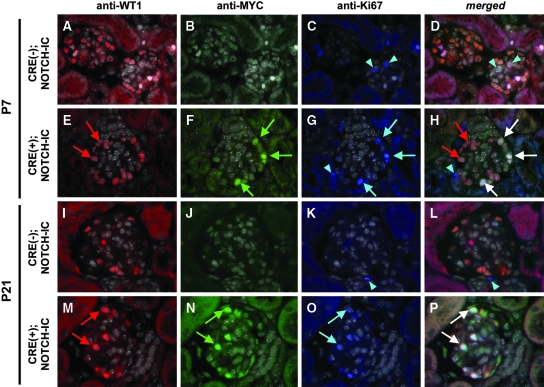
To determine the fate of MYCNOTCH-IC-transformed podocytes, we examined glomeruli for markers of cell-cycle re-entry and podocyte de-differentiation by analyzing expression of Ki67 and Pax2,21,22 respectively. Kidney tissue sections were incubated with anti-WT1, anti-MYC, and anti-Ki67 antibodies to label simultaneously podocytes, MYCNOTCH-IC–expressing cells, and proliferating cells, respectively. Accordingly, we hypothesized that cells staining positively with anti-WT1, anti-MYC, and anti-Ki67 represented proliferating, Notch-IC–transformed podocytes.
Triple staining of sections revealed a subset of podocytes showing simultaneous anti-WT1, anti-MYC, and anti-Ki67 staining as early as P7 (Figure 6, E through H). These data suggested that Notch activation induced cell-cycle re-entry in podocytes at a stage preceding loss of WT1 protein expression. At P21, the majority of WT1-positive cells were co-labeled with anti-MYC and anti-Ki67 antibodies (Figure 6, M through P), suggesting that proliferating podocytes continue to express WT1 protein at P21. Anti-Ki67 staining was not detected in WT1-positive podocytes of P7 or P21 CRE(−);NOTCH-IC glomeruli (Figure 6, A through D, and I through L).
Figure 6.
Analysis of glomerular cell proliferation in CRE;NOTCH-IC transgenic mice. (A through P) Shown are representative micrographs of glomeruli after triple immunofluorescence labeling of mouse tissue sections with anti-WT1, anti-MYC, and anti-Ki67 antibodies. (A through C and I through K) Serial micrographs of P7 (A through C) and P21 (I through K) CRE(−);NOTCH-IC glomeruli after multichannel imaging. (E through G and M through O) Serial micrographs of P7 (E through G) and P21 (M through O) CRE(+);NOTCH-IC obtained by multichannel imaging. (A, E, I, and M) Red channel images showing anti-WT1 immunodetection with Alexa594-conjugated secondary antibody. (B, F, J, and N) Green channel images showing anti-MYC immunodetection with Alexa488-conjugated secondary antibody. (C, G, K, and O) Blue channel images showing anti-Ki67 immunodetection with Cy5-conjugated secondary antibody. (D, H, L, and P) Merged images of corresponding micrographs obtained through green, red, and blue channels. Sections were counterstained with DAPI. DAPI images were converted to grayscale and merged onto corresponding green, red, and blue channel images. (E and M) Red arrows, podocytes stained positively with anti-WT1 antibody. (F and N) Green arrows, cells stained positively with anti-MYC antibody. (C, G, K, and O) Blue arrows and arrowheads, cells stained positively with anti-Ki67 antibody. (D, H, L, and P) White arrows, cells showing overlapping staining with anti-WT1, anti-MYC, and anti-Ki67 antibodies (anti-WT1, anti-MYC, anti-Ki67)–triple-positive cells), identifying these cells as proliferating, MYCNOTCH-IC–transformed podocytes. (H, red arrows) Nonproliferating podocytes as revealed by positive staining with anti-WT1 but absent anti-MYC or anti-Ki67 immunoreactivity. (D, H, and L, blue arrowheads) Ki67-positive cells that lack anti-WT1 or anti-MYC immunoreactivity. Magnification, ×1000.
Effects of MYCNOTCH-IC expression on podocyte cell proliferation over time were determined quantitatively by counting the total number of anti-MYC, anti-Ki67, and anti-WT1–labeled cells per glomerulus at P7, P21, and P28. Results in Table 3 show the percentage of (Ki67, WT1)–double-positive cells, which reveal a quantitative increase in podocyte cell proliferation over time in CRE(+);NOTCH-IC mice. As expected, no proliferating podocytes were observed in CRE(−);NOTCH-IC mice at any age. Within individual glomeruli, the relative proportion of (MYC, WT1)–double-positive cells that were also Ki67-positive [i.e., (MYC+, WT1+, Ki67+)/(total MYC+, WT1+)] was 50 ± 11% (n = 15 glomeruli) at P7, 92 ± 6% (n = 12 glomeruli) at P21, and 69 ± 11% (n = 16 glomeruli) at P28, suggesting that MYCNOTCH-IC–transformed podocytes eventually assume a proliferative fate. Incidentally, anti-Ki67 staining was detected in some cells of CRE(−) and CRE(+);NOTCH-IC glomeruli, which neither stained positively with anti-WT1 or anti-MYC antibodies (Figure 6, C and K, blue arrowheads), signifying these cells of nonpodocyte origin. The mean number of Ki67-positive cells that were (WT1,MYC)–double negative per glomerulus was not significantly different in CRE(+);NOTCH-IC mouse glomeruli compared with age-matched CRE(−);NOTCH-IC glomeruli [mean number (Ki67+ WT1− MYC−) cells per glomerulus, CRE(+);NOTCH-IC versus CRE(−);NOTCH-IC: P7: 0.5 ± 0.2 versus 0.8 ± 0.2, P = 0.34; P21: 0.4 ± 0.2 versus 0.3 ± 0.1, P = 0.75; P28: 0.4 ± 0.3 versus 0.5 ± 0.2, P = 0.80), suggesting that increased cell proliferation in nonpodocyte lineages is not a prominent feature of CRE(+);NOTCH-IC glomerulopathy.
Table 3.
Analysis of podocyte cell proliferation in glomeruli of CRE(+);NOTCH-IC and CRE(−);NOTCH-IC transgenic micea
| Parameter | % Ki67-Positive Podocytes at Postnatal Age
|
||
|---|---|---|---|
| P7 | P21 | P28 | |
| CRE(−);NOTCH-IC | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| (n = no. of glomeruli) | (n = 31) | (n = 27) | (n = 23) |
| CRE(+);NOTCH-IC | 12 ± 4b | 32 ± 6b | 38 ± 8b |
| (n = no. of glomeruli) | (n = 22) | (n = 12) | (n = 16) |
n = 3 mice per age group.
P < 0.001.
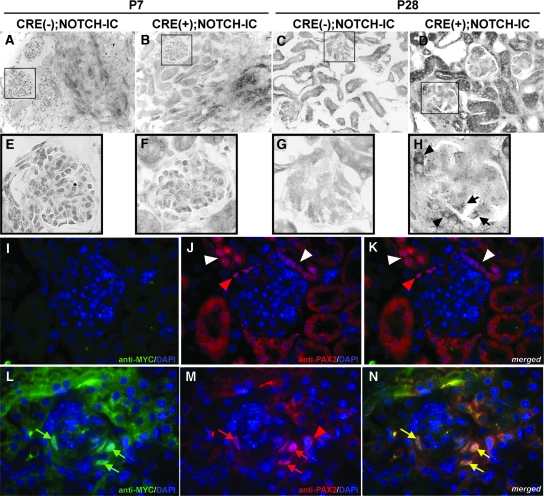
Pax2 expression was evaluated in CRE(+);NOTCH-IC glomeruli, first by mRNA in situ hybridization and second by dual fluorescence immunostaining. Pax2 mRNA transcripts were not detected by in situ hybridization in glomeruli of P7 CRE(−);NOTCH-IC (Figure 7, A and E) and CRE(+);NOTCH-IC mice (Figure 7, B and F); however, at P28, Pax2 mRNA transcripts were detected within the capillary tuft of some CRE(+);NOTCH-IC glomeruli (Figure 7H, black arrows). In contrast, Pax2 mRNA was not detected in glomeruli of P28 CRE(−);NOTCH-IC mice (Figure 7, C and G). To determine whether Pax2 expression was preferentially induced in MYCNOTCH-IC–expressing cells, we performed double-labeling experiments to evaluate simultaneous anti-PAX2 and anti-MYC immunoreactivities in MYCNOTCH-IC–transformed podocytes. CRE(−);NOTCH-IC glomeruli did not demonstrate anti-PAX2 or anti-MYC staining within the glomerular capillary tuft (Figure 7, I through K); however, PAX2-positive cells were detected in Bowman's capsule of CRE(−);NOTCH-IC mice (Figure 7J, red arrowhead) and in adjacent tubular structures (Figure 7J, white arrowhead), suggesting that PAX2 expression was independent of Notch in these lineages. Similarly, PAX2-positive cells in Bowman's capsule of CRE(+);NOTCH-IC glomeruli did not stain positively with anti-MYC (Figure 7M, red arrowhead), further suggesting that PAX2 expression in nonpodocyte cell types was independent of MYCNOTCH-IC expression. In contrast, anti-PAX2 antibody staining was detected in cells residing within the capillary tuft of CRE(+);NOTCH-IC glomeruli (Figure 7M). Simultaneous double-labeling with anti-MYC identified these PAX2-positive cells as MYCNOTCH-IC–expressing podocytes (Figure 7, L and N, green and yellow arrows, respectively), suggesting that induction of PAX2 expression in podocytes depended on presence of Notch. Collectively, these data suggested that constitutive Notch signaling induces podocytes to a proliferative fate with increased expression of PAX2.
Figure 7.
Analysis of Pax2 expression in podocytes of CRE(+);NOTCH-IC transgenic mice. (A through H) Pax2 mRNA in situ hybridization in frozen sections of transgenic mouse kidneys. Shown are representative images from P7 (A, B, E, and F) and P28 (C, D, G, and H) mouse kidneys. (A through D) Low-power images of CRE(−);NOTCH-IC (A and C) and CRE(+);NOTCH-IC (B and D) mouse kidney cryosections. (E through H) Magnified views of CRE(−);NOTCH-IC (E and G) and CRE(+);NOTCH-IC (F and H) glomeruli enclosed by black boxes in corresponding top panels. At P7, Pax2 mRNA transcripts are not detected in glomeruli of CRE(−);NOTCH-IC (A and E) or CRE(+);NOTCH-IC (B and F) mouse kidneys. In contrast, at P28, Pax2 mRNA transcripts are detected in podocytes of CRE(+);NOTCH-IC glomeruli (H, black arrows). Also, Pax2 mRNA is detected in parietal glomerular epithelium (H, black arrowhead). (I through N) Dual immunofluorescence labeling of mouse kidney tissue sections with anti-MYC and anti-PAX2 antibodies. Shown are representative serial micrographs of glomeruli obtained from P28 CRE(−);NOTCH-IC (I through K) and CRE(+);NOTCH-IC (L through N) mouse kidneys imaged by multichannel fluorescence microscopy. (I and L) Green channel images showing anti-MYC immunodetection with Alexa488-conjugated secondary antibody. Green arrows, cells stained positively with anti-MYC antibody. (J and M) Red channel images showing anti-PAX2 immunodetection with Alexa594-conjugated secondary antibody. Red arrows and arrowheads, cells stained positively with anti-PAX2. Sections were counterstained with DAPI, imaged by blue channel, and merged onto corresponding green and red channel images. (K and N) Merged images of corresponding green, red, and blue channel images. (N, yellow arrows) Cells showing overlapping staining with anti-MYC and anti-PAX2 antibodies, identifying these cells as coexpressing MYCNOTCH-IC and PAX2. (K and N, red arrowheads) PAX2-positive cells that do not stain positively with anti-MYC antibody. Magnifications: ×200 in A through D; ×1000 in E through N.
Phenotypic Rescue of CRE(+);NOTCH-IC Mice by Rbpsuh Mutational Inactivation in Podocytes
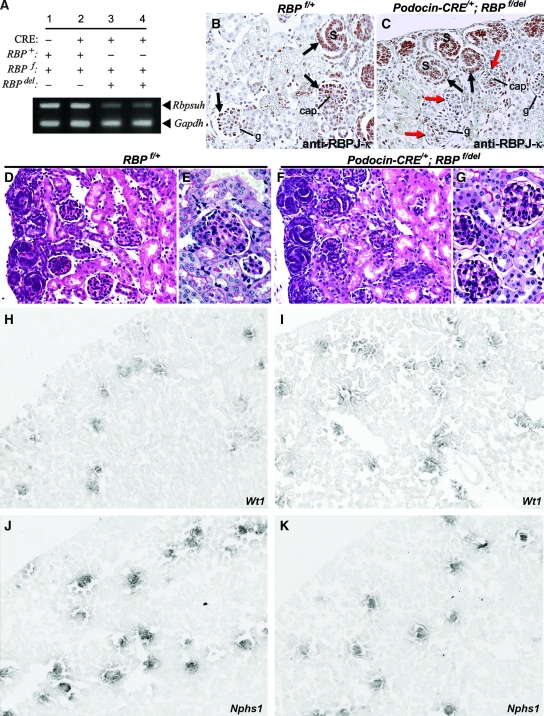
Recognizing that Notch may activate downstream transcriptional events through RBPJ-κ -dependent and -independent mechanisms,23,24 we sought to determine to what extent glomerulopathy could be prevented in CRE(+);NOTCH-IC mice by blocking RBPJ-κ–dependent Notch signaling. Accordingly, we used a genetic approach to induce simultaneously conditional Rbpsuh inactivation and MYCNOTCH-IC expression in podocytes. For purposes of interpreting effects of Rbpsuh loss of function in CRE(+);NOTCH-IC mice, we first determined the individual effect of Rbpsuh inactivation in podocytes. To circumvent embryonic lethality associated with homozygous targeted Rbpsuh deletion in mice,25 we induced podocyte-specific, homozygous mutational inactivation of a conditional null Rbpsuh allele, RBPf.26 Evidence of Rbpsuh mutational inactivation was provided by semiquantitative RT-PCR analysis of newborn kidney cortex total RNA, which showed decreased Rbpsuh mRNA levels in Podocin-CRE/+;RBPf/del newborn mouse kidneys as compared with CRE-negative, RBPf/+ kidneys (Figure 8A). Absent RBPJ-κ protein in podocytes after the capillary loop stage of glomerulogenesis was demonstrated by immunohistochemistry using a monoclonal anti–RBPJ-κ antibody (Figure 8, B and C).
Figure 8.
Phenotypic analysis of podocyte-specific Rbpsuh conditional knockout mice. (A) Semiquantitative, multiplex RT-PCR analysis of Rbpsuh mRNA expression in newborn mouse kidney after podocyte-specific mutational inactivation of Rbpsuh. Total kidney cortex Rbpsuh mRNA was evaluated by comparing the relative intensities of PCR products corresponding to Rbpsuh (top bands) and Gapdh (bottom bands) within each lane. Results shown are representative multiplex RT-PCR for the following genotypes: CRE-negative, RBPf/+ (lane 1), Podocin-CRE/+;RBPf/+ (lane 2), and Podocin-CRE/+;RBPf/del (lanes 3 and 4) mice. (B and C) Representative images of anti–RBPJ-κ antibody staining patterns in tissue sections from CRE-negative RBPf/+ (B) and Podocin-CRE/+;RBPf/del (C) newborn mouse kidneys. Glomerular structures are identified at three representative stages of development: S-shaped bodies (S); capillary loop stage glomeruli (cap.), and more advanced stage (g). Brown-stained nuclei, cells staining positively with anti–RBPJ-κ antibody; black arrows, anti–RBPJ-κ antibody-positive podocytes; red arrows, anti–RBPJ-κ antibody-negative podocytes. (H through M) mRNA in situ hybridization using probes for podocyte-specific markers Wt1 (H and I) and Nphs1 (J and K). Representative images are shown of kidney frozen sections from P21 control (H and J) and CRE-positive;RBPf/del (J and K) mice. Magnification, ×200 in B through G; ×100 in H through K.
On light microscopy, Podocin-CRE/+;RBPf/del newborn and P21 mouse glomeruli appeared morphologically normal (Figure 8, D through G). Molecular analysis of podocyte differentiation as revealed by mRNA in situ hybridization demonstrated normal patterns of Wt1 (Figure 8, H and I) and Nphs1 (Figure 8, J and K) expression in Podocin-CRE/+;RBPf/del mouse glomeruli. Functional assessment of the ability of RBPJ-κ–deficient podocytes to maintain glomerular filtration barrier selective permeability revealed no evidence of urine protein on urinalysis in Podocin-CRE/+;RBPf/del mutant mice. None of six mice screened weekly from birth to P21 exhibited urine protein exceeding 0.3 g/L on dipstick. Collectively, these data suggested that lack of RBPJ-κ–mediated Notch signaling in podocytes from the capillary loop stage does not adversely affect subsequent podocyte differentiation or their functional contribution to glomerular filtration barrier selective permeability. Consequently, this series of experiments afforded us the opportunity to evaluate independently the effects of MYCNOTCH-IC expression when Rbpsuh was simultaneously inactivated.
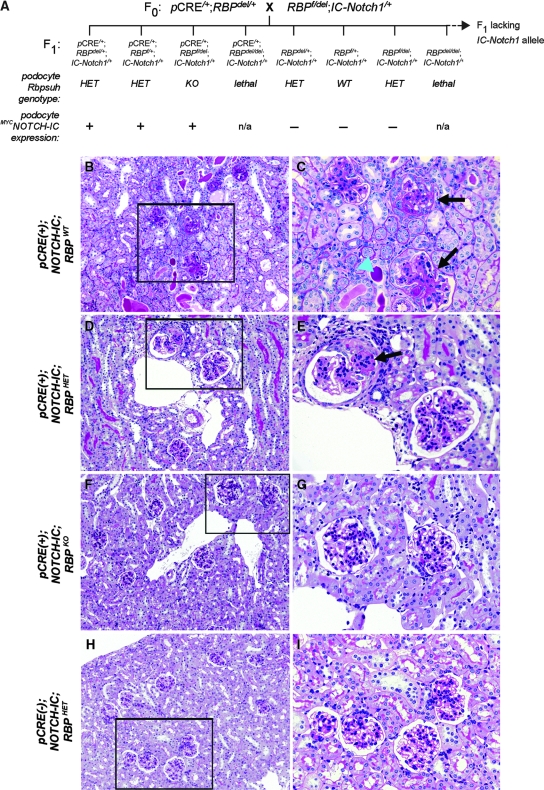
To generate mice in which expression of MYCNOTCH-IC and mutational inactivation of Rbpsuh were simultaneously induced in podocytes, we used a three-stage breeding strategy involving Podocin-CRE/+, RBPf/del, and IC-Notch1/+ mouse lines. This strategy ultimately resulted in the generation of triple-transgenic compound mutant mice with the following genotypes (Figure 9A): (1) Podocin-CRE/+;RBPf/del;IC-Notch1/+ [pCRE(+);NOTCH-IC;RBPKO mice], which renders podocytes deficient for RBPJ-κ; (2) Podocin-CRE/+;(RBPf/+ or RBPdel/+);IC-Notch1/+ [pCRE(+);NOTCH-IC;RBPHET], which express RBPJ-κ in podocytes; and (3) Podocin-CRE/+;IC-Notch1/+ [pCRE(+);NOTCH-IC;RBPWT], which are phenotypically equivalent to Nephrin-CRE/+;IC-Notch1/+ mice. Consequently, we predicted that the full effects of blocking RBPJ-κ–mediated Notch signaling would be evident only in pCRE(+);NOTCH-IC;RBPKO mice.
Figure 9.
Phenotypic rescue of glomerulosclerosis in CRE(+);NOTCH-IC transgenic mice with simultaneous podocyte-specific mutational inactivation of Rbpsuh. (A) Schematic representation of breeding to generate Podocin-CRE/+;RBPf/del;IC-Notch1/+ transgenic mice and associated littermates. Shown are F0 generation and a subset of F1 that harbor the IC-NOTCH1 transgenic allele. Resulting effect of podocyte-specific, CRE-mediated recombination on conditional Rbpsuh allele (RBPf) and MYCNOTCH-IC expression is represented in lanes below corresponding genotypes. pCRE, Podocin-CRE; RBP+, wild type Rbpsuh allele; RBPdel, mutated conditional Rbpsuh allele previously generated by ubiquitous CRE-mediated recombination (see the Concise Methods section). WT, no mutated Rbpsuh alleles; HET, one mutated Rbpsuh allele; KO, two mutated Rbpsuh alleles; +, MYCNOTCH-IC present; −, MYCNOTCH-IC absent. (B, D, F, and H) Representative low-power images of PAS-stained kidney tissue sections from Podocin-CRE/+;IC-Notch1/+;RBP+/+ [pCRE(+);NOTCH-IC;RBPWT; B], Podocin-CRE/+;IC-Notch1/+; RBPf/+ [pCRE(+);NOTCH-IC;RBPHET; D], Podocin-CRE/+;IC-Notch1/+; RBPf/del [pCRE(+);NOTCH-IC;RBPKO; F], and IC-Notch1/+;RBPf/+ (H). (C, E, G, and I) High-power views of marked regions (black box) in corresponding left-hand images. Black arrows, glomeruli with evidence of glomerulosclerosis; blue arrowhead, dilated tubule filled with PAS-positive material. Magnifications: ×400 in B, D, F, and H; ×800 in C, E, G, and I.
pCRE(+);NOTCH-IC;RBPKO, pCRE(+);NOTCH-IC;RBPHET, and pCRE(+);NOTCH-IC;RBPWT mice were tested at P14 for proteinuria by urine dipstick analysis as shown in Table 4. pCRE(−);NOTCH-IC;RBPWT and pCRE(−);NOTCH-IC;RBPHET mice were tested for comparison and showed median test results of 0.3 g/L and trace, respectively. The median test result for eight pCRE(+);NOTCH-IC;RBPWT mice was >20 g/L, reminiscent of the degree of proteinuria exhibited by CRE(+);NOTCH-IC mice. Four of 14 pCRE(+);NOTCH-IC;RBPHET mice showed a dipstick test result of ≥3.0 g/L on urine dipstick at P14, yet the median result was 0.3 g/L. In contrast, none of five pCRE(+);NOTCH-IC;RBPKO mice showed proteinuria >0.3 g/L on dipstick at P14 (median, trace). This analysis suggested that loss of RBPJ-κ prevented the severe selective filtration defect caused by podocyte MYCNOTCH-IC expression.
Table 4.
Urine dipstick results for P14 CRE(+);NOTCH-IC transgenic mice with simultaneous podocyte-specific mutational inactivation of Rbpsuh(RBP).
| Parameter | No. of Mice with Corresponding Urine Dipstick Result
|
|||||
|---|---|---|---|---|---|---|
| Negative | Trace | 0.3 g/L | 1.0 g/L | 3.0 g/L | >20 g/L | |
| CRE(−);NOTCH-IC;RBPWT | 2 | 5 | 6 | 1 | 0 | 0 |
| CRE(−);NOTCH-IC;RBPHET | 1 | 6 | 4 | 0 | 1 | 0 |
| CRE(+);NOTCH-IC;RBPWT | 0 | 0 | 0 | 1 | 3 | 4 |
| CRE(+);NOTCH-IC;RBPHET | 1 | 3 | 6 | 0 | 2 | 2 |
| CRE(+);NOTCH-IC;RBPKO | 0 | 4 | 1 | 0 | 0 | 0 |
Similar to glomerulosclerosis lesions exhibited by Neph-CRE/+;IC-Notch1/+ mice, P42 pCRE(+);NOTCH-IC;RBPWT mouse kidneys showed evidence of severe glomerulosclerosis and tubular damage on light microscopy (Figure 9, B and C). Kidneys of pCRE(+);NOTCH-IC;RBPHET mice, however, were less severely affected, showing less severe glomerular lesions and less extensive tubular damage (Figure 9, D and E). In contrast, kidneys of CRE(+);NOTCH-IC;RBPKO mice showed no evidence of glomerulosclerosis on light microscopy (Figure 9, F and G), and their glomeruli were morphologically indistinguishable from glomeruli of CRE-negative, IC-Notch1/+;RBPHET mice (Figure 9, H and I), which have one mutated RBP allele but do not express MYCNOTCH-IC. On the basis of the normal histologic appearance and lack of proteinuria in pCRE(+);NOTCH-IC;RBPf/del mice, we concluded that glomerulopathy was prevented by blocking RBPJ-κ–dependent Notch signaling in podocytes. These data consequently implicate constitutive RBPJ-κ–dependent Notch signaling as a pathogenic mechanism for podocyte de-differentiation and glomerulosclerosis in mice.
DISCUSSION
Notch signaling is implicated in podocyte cell fate induction.1–3 A subsequent requirement for Notch after induction seems to be less crucial. Our demonstration that podocyte development is normal in mice after conditional mutational inactivation of Rbpsuh in developing podocytes supports this view. Previously, a role for Notch in glomerulogenesis was revealed by phenotypic analyses of mice homozygous for a Notch2 hypomorphic allele (Notch2del1) and in compound heterozygotes involving Notch2del1 and Jag1 (Jag1dDSL), encoding the Notch ligand JAGGED1.27 The glomerular defect in these mutants is characterized by disorganized Wt1-expressing podocytes and either complete absence or dilation of the glomerular capillary tuft. Defective recruitment and maintenance of endothelial and mesangial cells during glomerular capillary tuft formation are postulated as mechanisms for the Notch2del1 phenotype, which may involve altered Vegf expression.27 Our analysis of glomerular development in Rbpsuh conditional knockout mice argues against a role for podocyte Notch signaling in glomerular capillary tuft maintenance because Podocin-CRE/+;RBPf/del mice lack glomerular tuft defects demonstrated by Notch2del1 mutants or by mice deficient for Vegf.28 We cannot rule out the possibility that Notch signaling may be involved in glomerulogenesis at a developmental stage that precedes the onset of CRE expression in our transgenic system (e.g., in podocyte progenitors of S-shaped bodies). Alternatively, it is possible that endothelial cells require the Notch signal cell-autonomously during glomerular capillary tuft formation. This latter possibility may be more precisely ascertained in experiments involving glomerular endothelial-specific inactivation of Rbpsuh to circumvent lethal effects of Rbpsuh deletion in extrarenal endothelium.25
In CRE(+);NOTCH-IC mice, we observed delayed effects on podocyte differentiation that occurred postnatally despite immunohistochemical evidence of nuclear MYCNOTCH-IC protein in developing podocytes. One possible explanation for the apparent delayed response is that levels of nuclear MYCNOTCH-IC in developing podocytes in our transgenic system may be subthreshold for activating Notch-responsive promoters, as previously proposed.29 Alternatively, differentiated podocytes may lack sufficient levels of transcriptional co-activators, which are predicted to decrease the likelihood of NOTCH-IC/RBPJ-κ complexes binding to target promoters.30 It has been shown in developing pancreas that NOTCH activation in endocrine precursor cells prevents their differentiation, whereas activation in mature β cells is without effect.31 Likewise, it would be intriguing to determine whether terminally differentiated podocytes are less sensitive to Notch activation by using inducible genetic systems to delay induction of podocyte MYCNOTCH-IC expression.
Podocytes play a crucial role in generating and maintaining glomerular filtration barrier selective permeability through synthesis of glomerular basement membrane components and formation of slit diaphragms.32 Because we did not observe structural defects in the glomerular basement membrane or altered expression of type IV collagen α1 to α6 subchains (data not shown), it is unlikely that a primary defect in the glomerular basement membrane contributes to loss of filtration barrier selective permeability in CRE(+);NOTCH-IC mice. It is possible that overexpression of MYCNOTCH-IC could exert a dominant negative effect through titration of crucial slit diaphragm components. Because the abnormal phenotype resulting from MYCNOTCH-IC overexpression in murine podocytes is prevented by conditional deletion of Rbpsuh, we favor the likelihood that MYCNOTCH-IC transduces a signal through RBPJ-κ rather than exerting a dominant effect in our studies. Molecular targets of NOTCH-IC/RBPJ-κ–dependent signaling in podocytes are unknown; however, insight into potential downstream candidates may derive from studies in Drosophila myogenesis that show genetic interactions between Notch and the nephrin homolog hibris (hbs) in stabilizing cell–cell adhesion and promoting myoblast fusion.33 Moreover, NOTCH-IC overexpression results in a defective myoblast fusion phenotype,33 which suggests that inappropriate Notch signaling destabilizes cell–cell junctions during Drosophila myogenesis. Further work will be required to determine whether similar interactions between Notch and nephrin are conserved in podocytes and whether inappropriate Notch signaling destabilizes slit diaphragm protein–protein interactions.
Clinical and experimental data support primary podocyte injury as a key step in the development of glomerular disease causing severe proteinuria.34 Histopathologic patterns of glomerulopathy have recently been catalogued according to cause of podocyte injury and quantitative effects on podocyte number.35 Accordingly, podocyte injury resulting in cell depletion through death or detachment is proposed as a mechanism for FSGS, whereas defective podocyte differentiation causes either collapsing glomerulopathy when associated with high rates of cell proliferation rates or mesangial sclerosis when associated with lower rates of cell proliferation.35 Using this taxonomy as a framework offers insight into a potential role for Notch activation in human podocytopathy. For example, CRE(+);NOTCH-IC glomerulopathy shows features of mesangial expansion, moderate podocyte cell proliferation, and altered podocyte differentiation that are most consistent with histopathologic findings in diffuse mesangial sclerosis,21 which is characterized in humans by idiopathic early onset, or in association with WT1 gene mutations affecting DNA binding ability.36 Some glomeruli in CRE(+);NOTCH-IC transgenic mice show focal and segmental sclerotic changes suggestive of FSGS, yet they lack morphologic features of classic FSGS,32 including podocyte hypertrophy, synechiae formation, and apoptosis (data not shown). Likewise, although podocytopathy in CRE(+);NOTCH-IC transgenic mice is associated with loss of mature markers, expression of Ki67, and increased expression of PAX2 as observed in collapsing glomerulopathy and HIV nephropathy,18,22 histologic features of these latter human glomerulopathies, which include glomerular capillary tuft collapse and robust podocyte hyperplasia,18 are lacking in CRE(+);NOTCH-IC glomerulopathy. Alternatively, Notch activation may be a common mechanism in podocytopathies wherein the final histologic outcome depends on the level of Notch signaling in affected podocytes.
A precise role for Notch in human glomerular disease is unknown. Glomerular mesangiolipidosis, the glomerular lesion in Alagille syndrome (OMIM# 118450), a multisystem disorder featuring severe cholestasis caused by JAG1 or NOTCH2 mutations,37 is characterized by lipid deposits within mesangial cells and matrix38 and bears little resemblance to glomerular lesions demonstrated in Notch2del1/+;Jag1+/− mice, which exhibit extrarenal phenotypes observed in humans with Alagille syndrome.39 Because glomerular mesangiolipidosis is reported in conditions of abnormal lipid metabolism,38 glomerulopathy in humans with Alagille syndrome is more likely to be an effect of cholestasis rather than a primary glomerular defect in Notch signaling.
To our knowledge, our study is the first to demonstrate that activated Notch in podocytes causes glomerulosclerosis in transgenic mice. Further studies will be required to examine whether increased expression of Notch ligands, receptors, or downstream transcriptional targets is associated with glomerulosclerosis in human biopsy specimens, which could support a role for Notch activation in human glomerular disease. Molecular and functional studies have suggested synergistic cross-talk between Notch and TGF-β signaling in renal epithelial disease.40,41 Because there is compelling evidence that local production of TGF-β contributes to the pathogenesis of glomerulosclerosis,32 studies examining the extent of interplay between Notch and TGF-β signaling may provide insight into molecular mechanisms involving Notch that contribute to glomerulosclerosis progression. Alternatively, it is tempting to speculate that activation of Notch/RBPJ-κ–dependent signaling may modify transcriptional responses to WT1 as a pathomechanism of podocyte abnormal phenotype.
CONCISE METHODS
Breeding and Genotyping of NOTCH-IC Transgenic and Rbpsuh Mouse Strains
Neph-CRE/+,42 Podocin-CRE/+,43 IC-Notch1/+,44 and RBPf/f26 mice were previously generated. All mouse strains were maintained on mixed backgrounds. CRE and RBPf genotyping was performed by PCR.26,42 IC-Notch1 genotyping was determined by tail-clip lacZ assay.44 RBPdel refers to a ubiquitously deleted Rbpsuh allele obtained by crossing homozygous RBPf/f and heterozygous pCAGGS-CRE/+ mice45 (provided by A. Nagy, Samuel Lunenfeld Research Institute, Toronto, ON, Canada). RBPf/del mice, which are phenotypically normal,26 were generated by breeding RBPf/f and pCAGGS-CRE/+;RBPdel/+ mice. Experiments complied with ethical standards of the Hospital for Sick Children Research Institute Animal Care Committee.
RT-PCR Analysis
Glomerular isolation was performed by magnetic bead microperfusion.46,47 Total RNA was extracted and purified using the RNeasy Micro Kit (Qiagen, Mississauga, ON, Canada). cDNA was generated using SuperScript II Reverse Transcriptase (Invitrogen, Burlington, ON, Canada). PCR was performed as described previously48 using specific primers (Table 5). Semiquantitative analysis was performed by band densitometry using ImageJ.49
Table 5.
Primers used for RT-PCR
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Wt-1 | TGCGGCGTGTATCTGGAG | TTGAAAGGTGAGTGGGAGGAA |
| Nphs1 | CCCAACACTGGAAGAGGTGT | CTGGTCGTAGATTCCCCTTG |
| Nphs2 | TGAGGATGGCGGCTGAGAT | GGTTTGGAGGAACTTGGGT |
| 18S | AAGGGCACCACCAGGAG | GGACATCTAAGGGCATCACAG |
| Rbpsuh | CGGTTTTCCTCAGTCTCCAC | TGCAGTAGTTCTTCCCTTCCA |
| Gapdh | CTCATGACCACAGTCCATGC | CACATTGGGGGTAGGAACAC |
Urine Protein Analysis
Mouse urine was obtained by spontaneous expression. For dipstick analysis, 10 to 20 μl of collected urine was spotted onto Labstix Urinalysis Reagent Strips (Bayer, Toronto, ON, Canada) and evaluated by standard colorimetric assay. Urine protein concentration was measured by Bradford microassay (BioRad, Toronto, ON, Canada). Urine creatinine concentration was determined using the Parameter kit (R&D Systems, Minneapolis, MN).
Renal Histology, Glomerulosclerosis Scoring, and Electron Microscopy
For routine histology, mouse kidneys were fixed in 10% formalin and processed for paraffin sectioning. Representative sections were stained with PAS, imaged by brightfield microscopy, and photographed using an Axioskop microscope (Carl Zeiss, Toronto, ON, Canada).
Glomerulosclerosis scoring was carried out as described previously.50 Sclerosis severity was graded from 0 to 4 as follows: 0, no lesion; 1, 25% of the glomerulus; 2, 25 to 50%; 3, 50 to 75%; and 4, 75% to 100%. Mean GS scores were obtained by averaging scores from all glomeruli on one section.
For electron microscopy, kidney cortex was fixed in 2% glutaraldehyde and processed for epoxy resin embedding and sectioning. Tissue processing and imaging were performed by the Imaging Centre, Toronto Centre for Comparative Models of Human Disease, Mt. Sinai Hospital (Toronto, ON, Canada).
In Situ Hybridization
Nonradioactive mRNA in situ hybridization was performed as described previously.12 Plasmids for RNA probe synthesis were provided by J. Kreidberg (Harvard University, Boston, MA) (mWt1), S. Quaggin (Samuel Lunenfeld Research Institute, Toronto, ON, Canada) (mNphs1), C. Antignac (Hôpital Necker-Enfant Malades, Paris, France) (mNphs2), and G. Dressler (University or Michigan, Ann Arbor, MI) (mPax2).
Immunohistochemistry and Immunofluorescence Multilabeling
Primary antibodies and lectins were mouse monoclonal anti-MYC (1:1000; Invitrogen), goat polyclonal anti-NOTCH1 (C-20; 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-WT1 (1:50; Santa Cruz Biotechnology), rat monoclonal anti-CD31 (1:100; Santa Cruz Biotechnology), guinea pig polyclonal anti-nephrin (1:100; Fitzgerald, Concord, MA), sheep polyclonal anti-Ki67 (1:100; Chemicon, Temecula, CA), rabbit polyclonal anti-PAX2 (1:50; Zymed, San Francisco, CA), rat monoclonal anti–RBPJ-κ (clone T6709; 1:400; Institute of Immunology, Tokyo, Japan), and FITC-conjugated LTL (1:100; Vector Laboratories, Burlington, ON, Canada). Immunoperoxidase staining was performed on formalin-fixed, paraffin-embedded tissue sections. Microwave antigen retrieval was carried out in citrate buffer in four 5-min cycles at medium-high setting (Panasonic NN-S758WC, 950-W maximum output, Panasonic, Mississauga, ON, Canada) followed by 20 min of cooling at room temperature. Unless otherwise specified, blocking was performed in Universal Blocking Reagent (DAKO, Mississauga, ON, Canada). For monoclonal incubations, sections were blocked in 5% rabbit serum for 1 h (rat monoclonals) or with the M.O.M. blocking kit (mouse monoclonals; Vector Laboratories). Primary antibody incubations were carried out at 4°C overnight. Biotin-conjugated secondary antibodies were diluted 1:1000 in blocking reagent and incubated at room temperature. Immunoperoxidase staining was developed using the Vectastain ABC kit (Vector Laboratories). For anti–RBPJ-κ antibody staining, tyramide signal amplification was performed (TSA-Biotin kit; Perkin Elmer, Woodbridge, ON, Canada). Sections were imaged by brightfield microscopy, and digital photographs were obtained as described previously.
Dual and triple immunofluorescence antibody staining was performed on PFA-fixed frozen sections treated with Proteinase K (Roche, Laval, QC, Canada) 20 μg/ml (5 min, 37°C), washed in 0.1% Triton X-100, and blocked with Universal or M.O.M blocking reagent for polyclonal and mouse monoclonal primary antibodies, respectively. Primary antibody incubations were carried out simultaneously. AlexaFluor 488–, AlexaFluor 594–, or Cy5-conjugated secondary antibodies (1:1000; Invitrogen) were used for multi-immunofluorescence labeling. Sections were counterstained with DAPI and imaged by fluorescence microscopy using a Zeiss Axioskop microscope with an EXFO X-Cite120 120-W mercury vapor lamp (Photonics Solutions, Edinburgh, Scotland). Digital photographs were obtained as described previously, and merged images were obtained using Photoshop 6.0 (Adobe Systems, Toronto, ON, Canada).
Statistical Analyses
Statistical analyses were performed by t test using Statview 5.0 (Stat Corp, College Station, TX).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by a Biomedical Research Grant and Biomedical Scholarship from the Kidney Foundation of Canada and Canadian Society of Nephrology and by start-up funds from the Hospital for Sick Children Research Institute.
We thank T. Honjo (Kyoto University, Kyoto, Japan) for providing us with RBPf/f mice. We gratefully acknowledge X. Si, V. Eremina, S.Y. Cui, C.J. Li, P. Jaksa, J. Martin, and D. Segal for analytical and technical assistance in the preparation of this manuscript. Special thanks to D. Holmyard and L. Morikawa, CMHD, Mt. Sinai Hospital, for technical assistance in histopathology. We sincerely thank S. Egan, N. Rosenblum, and L. Robinson (Hospital for Sick Children Research Institute) for critique during manuscript preparation.
Published online ahead of print. Publication date available at www.jasn.org.
A.M.W. and M.Y.J.W. contributed equally to this work.
See related editorial, “Notch Signaling: A Common Pathway of Injury in Podocytopathies?” on pages 1045–1046.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Wang P, Pereira FA, Beasley D, Zheng H: Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development 130: 5019–5029, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R: Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 130: 5031–5042, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R: Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 135: 801–811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadesch T: Notch signaling: The demise of elegant simplicity. Curr Opin Genet Dev 14: 506–512, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Schroeter EH, Kisslinger JA, Kopan R: Notch-1 signaling requires ligand-induced proteolytic release of intracellular domain. Nature 393: 382–386, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A: Signaling downstream of activated mammalian Notch. Nature 377: 355–358, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD: MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet 26: 484–489, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Bailey AM, Posakony JW: Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev 9: 2609–2622, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Jarriault S, Le Bail O, Hirsinger E, Pourquie O, Logeat F, Strong CF, Brou C, Seidah NG, Isra l A: Delta-1 activation of Notch-1 signaling results in HES-1 transactivation. Mol Cell Biol 18: 7423–7431, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R: Hes1 and Hes5 as Notch effectors in mammalian neuronal differentiation. EMBO J 18: 2196–2207, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M: The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18: 901–911, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piscione TD, Wu MY, Quaggin SE: Expression of Hairy/Enhancer of Split genes, Hes1 and Hes5, during murine nephron morphogenesis Gene Expr Patterns 4: 707–711, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Al-Awqati Q: Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol 288: F939–F952, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Leimeister C, Schumacher N, Gessler M: Expression of Notch pathway genes in the embryonic mouse metanephros suggests a role in proximal tubule development. Gene Expr Patterns 3: 595–598, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Barbaux S, Niaudet P, Gubler M-C, Grünfeld J-P, Jaubert F, Kuttenn F, Fékété CN, Souleyreau-Therville N, Thibaud E, Fellous M, McElreavey K: Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 17: 467–470, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Gao F, Maiti S, Sun G, Ordonez NG, Udtha M, Deng JM, Behringer RR, Huff V: The Wt1+/R394W mouse displays glomerulosclerosis and early-onset renal failure characteristic of human Denys-Drash syndrome. Mol Cell Biol 24: 9899–9910, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patek CE, Little MH, Fleming S, Miles C, Charlieu JP, Clarke AR, Miyagawa K, Christie S, Doig J, Harrison DJ, Porteous DJ, Brookes AJ, Hooper ML, Hastie ND: A zinc finger truncation of murine WT1 results in the characteristic urogenital abnormalities of Denys-Drash syndrome. Proc Natl Acad Sci U S A 96: 2931–2936, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barisoni L, Kriz W, Mundel P, D'Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Ohtaka A, Ootaka T, Sato H, Ito S: Phenotypic change of glomerular podocytes in primary focal segmental glomerulosclerosis: Developmental paradigm? Nephrol Dial Transplant 17[Suppl 9]: 11–15, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Guo JK, Menke AL, Gubler MC, Clarke AR, Harrison D, Hammes A, Hastie ND, Schedl A: WT1 is a key regulator of podocyte function: Reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet 11: 651–659, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Jeanpierre C, Dressler GR, Lacoste M, Niaudet P, Gubler MC: WT1 and PAX-2 podocyte expression in Denys-Drash syndrome and isolated diffuse mesangial sclerosis. Am J Pathol 154: 181–192, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Gubler MC, Beaufils H: Dysregulation of podocyte phenotype in idiopathic collapsing glomerulopathy and HIV-associated nephropathy. Nephron 91: 416–423, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Wilson-Rawls J, Molkentin JD, Black BL, Olson EN: Activated notch inhibits myogenic activity of the MADS-Box transcription factor myocyte enhancer factor 2C. Mol Cell Biol 19: 2853–2862, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross DA, Kadesch T: The notch intracellular domain can function as a coactivator for LEF-1. Mol Cell Biol 21: 7537–7544, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T: Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development 121: 3291–3301, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T: Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol 3: 443–450, 2002 [DOI] [PubMed] [Google Scholar]
- 27.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T: Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128: 491–502, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, Quaggin SE: Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol 17: 724–735, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Ong CT, Cheng HT, Chang LW, Ohtsuka T, Kageyama R, Stormo GD, Kopan R: Target selectivity of vertebrate notch proteins: Collaboration between discrete domains and CSL-binding site architecture determines activation probability. J Biol Chem 281: 5106–5119, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Fryer CJ, Lamar E, Turbachova I, Kintner C, Jones KA: Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex. Genes Dev 16: 1397–1411, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA: Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100: 14920–14925, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavenstadt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Artero RD, Castanon I, Baylies MK: The immunoglobulin-like protein hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development 128: 4251–4264, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Klamt B, Koziell A, Poulat F, Wieacker P, Scambler P, Berta P, Gessler M: Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1+/-KTS splice isoforms. Hum Mol Genet 7: 709–714, 1998 [DOI] [PubMed] [Google Scholar]
- 37.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB: NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the Notch signaling pathway. Am J Hum Genet 79: 169–173, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habib R, Dommergues JP, Gubler MC, Hadchouel M, Gautier M, Odievre M, Alagille D: Glomerular mesangiolipidosis in Alagille syndrome (arteriohepatic dysplasia). Pediatr Nephrol 1: 455–464, 1987 [DOI] [PubMed] [Google Scholar]
- 39.McCright B, Lozier J, Gridley T: A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 129: 1075–1082, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP: Integration of TGF-beta/Smad and Jagged1/Notch signaling in epithelial-to-mesenchymal transition. EMBO J 23: 1155–1165, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrissey J, Guo G, Moridaira K, Fitzgerald M, McCracken R, Tolley T, Klahr S: Transforming growth factor-beta induces renal epithelial Jagged-1 expression in fibrotic disease. J Am Soc Nephrol 13: 1499–1508, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE: Glomerular-specific gene excision in vivo. J Am Soc Nephrol 13: 788–793, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Lobe CG: Cre-conditional expression of constitutively active Notch1 in transgenic mice. Genesis 45: 259–265, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Sakai K, Miyazaki J: A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun 237: 318–324, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui S, Li C, Ema M, Weinstein J, Quaggin SE: Rapid isolation of glomeruli coupled with gene expression profiling identifies downstream targets in Pod1 knockout mice. J Am Soc Nephrol 16: 3247–3255, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Zielenski J, Aznarez I, Onay T, Tzounzouris J, Markiewicz D, Tsui L: CFTR mutation detection by multiplex heteroduplex (mHET) analysis on MDE gel. In: Methods in Molecular Medicine: Cystic Fibrosis Methods and Protocols, edited by Skach W, Towata, NJ, Humana Press, 2002, pp 3–19 [DOI] [PubMed]
- 49.Rasband WS: ImageJ. Bethesda, National Institutes of Health, 1997– 2007
- 50.Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB: Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int 59: 1899–1910, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.