Abstract
Epithelial differentiation proceeds in at least two steps: Conversion of a nonepithelial cell into an epithelial sheet followed by terminal differentiation into the mature epithelial phenotype. It was recently discovered that the extracellular matrix (ECM) protein hensin is able to convert a renal intercalated cell line from a flat, squamous shape into a cuboidal or columnar epithelium. Global knockout of hensin in mice results in embryonic lethality at the time that the first columnar cells appear. Here, antibodies that either activate or block integrin β1 were used to demonstrate that activation of integrin αvβ1 causes deposition of hensin in the ECM. Once hensin polymerizes and deposits into the ECM, it binds to integrin α6 and mediates the conversion of epithelial cells to a cuboidal phenotype capable of apical endocytosis; therefore, multiple integrins play a role in the terminal differentiation of the intercalated cell: αvβ1 generates polymerized hensin, and another set of integrins (containing α6) mediates signals between hensin and the interior of the cells.
Epithelia, the earliest differentiated cell type to appear during development, constitute an astonishing variety of phenotypes in all metazoans. The abundant variety of epithelial types suggests that epithelial differentiation must proceed in at least two steps; first, a nonepithelial cell differentiates into a “generic” epithelia, characterized by tight and adherens junctions, transepithelial transport, and polarized distribution of membrane proteins and lipids. During development, these “generic” epithelia terminally differentiate into a mature phenotype characterized by distinct cell shapes, specialized apical structures (e.g., microvilli, microplicae), and specialized functions (apical endocytosis, regulated exocytosis), thus producing a myriad of epithelial phenotypes.1 Whereas proper terminal differentiation of various epithelial phenotypes is critical for tissue-specific functions, a blockade in epithelial differentiation is thought to cause carcinomas.
We had previously found that when rabbits were fed an acid diet, the number of HCO3-secreting β intercalated cells decreased while the number of acid-secreting α intercalated cells increased and proposed that the β cell converted to the α form.2 To study the molecular basis of this, we established a β intercalated conditionally immortalized cell line.3 This clonal cell line reproduced all of the functions of the β intercalated cell including transepithelial HCO3 secretion, apical Cl:HCO3 exchange, and the absence of apical endocytosis; however, despite much effort, the cells did not convert to acid-secreting cells after lowering of the pH of the basolateral medium. We then discovered that the phenotype of these cells is controlled by seeding density.4,5 When these cells are seeded at subconfluent density on filters and allowed to form a tight monolayer, they develop into flat epithelia that have tight and adherens junctions, polarized domains, and transepithelial HCO3 secretion transport but none of the characteristics of terminally differentiated epithelia. When the same cells are seeded at superconfluent density, they develop into tall cuboidal epithelia with abundant microvilli and vigorous apical endocytosis. These cells resembled the α intercalated cells and secreted H+ into the apical medium. We point out that seeding density here acts as a developmental “switch,” in that after both low-density and high-density cells are allowed to reach confluence, the phenotype of the high-density cells resembles α intercalated cells and that of the low-density cells resembles β intercalated cells. It is the density at the time of seeding that seemed to be the critical event.
We discovered that high-density cells deposited a novel protein into the extracellular matrix (ECM) that when purified was able to “instruct” low-density cells into assuming the high-density phenotype. Other ECM proteins such as fibronectin, collagen, laminin, or matrigel were ineffective in converting the low-density cells to cuboidal epithelial phenotype, demonstrating the specific role of hensin in epithelial differentiation.5 When we sequenced its cDNA, we found that it was composed of several domains, including SRCR, CUB, and ZP domains,6 and was expressed in most epithelia but in various alternately spliced forms. We termed this protein hensin, but many other names exist for its alternately spliced isoforms, including DMBT1,7 gp-340,8 CRP-ductin,9 vomeroglandin,10 and muclin.11 DMBT1 is the human orthologue of hensin and was found to be deleted in a large fraction of malignant brain tumors7 and later in most epithelial tumors and thus considered to be a tumor suppressor gene.12–14
Global deletion of hensin was embryonic lethal at the early stage of embryonic day 4.5.15 Hensin is expressed in the early embryo in the embryonic stem cells and then becomes concentrated in one of the earliest epithelia, the primitive endoderm, and later the visceral endoderm. In particular, it becomes concentrated in the distal visceral endoderm, a few cells located at the tip of the egg cylinder, which, unlike the rest of the embryonic visceral endoderm, is cuboidal in shape. These cells then migrate proximally to establish the anterior visceral endoderm, thereby establishing the anterior posterior axis.16 In addition, hensin is expressed in the extra-embryonic visceral endoderm, which is entirely composed of cuboidal epithelia. We found that ES cells seeded on hensin (but not on other ECM proteins) became cuboidal epithelia with all of the characteristics of differentiated visceral endoderm.15 These results in combination with our extensive findings in the cell culture system demonstrate that hensin is critical for the differentiation of cuboidal epithelia in vitro and in vivo.
Previous studies have shown that hensin is synthesized and secreted as a monomer by low-density cells, but in high-density cells it is polymerized in the ECM.17 In addition, hensin is present in a pattern compatible with ECM deposition in the mature columnar epithelia of the intestinal villus and prostate luminal cells but not in the less differentiated epithelia of the crypt of the small intestine and basal cells of the prostate.5 Preliminary studies with negative staining electron microscopy show that ECM hensin forms 50- to 100-nm-long fibers composed of several fibrils (S.V. and Q.A.-A., unpublished observations). Hensin seems to undergo a complex polymerization path that requires the presence of at least two other proteins, galectin3 and a cis-trans peptidyl prolyl isomerase17–19; however, when 35S-labeled monomeric hensin was added to high-density cells, it became polymerized, suggesting that a cell-surface event (similar to what occurs during fibronectin fibril formation20 and not a biosynthetic intracellular event (e.g., that seen in collagen assembly21) causes the polymerization of hensin. Here we purified receptor proteins that bind hensin and found that they were integrins. We found that activation of integrin αvβ1 by the high-density state is a critical step in the polymerization of hensin. Binding of hensin to integrin α6 caused the conversion of the cell into a cuboidal epithelium.
RESULTS
Identification of Basolateral Membrane Proteins that Bind to Hensin
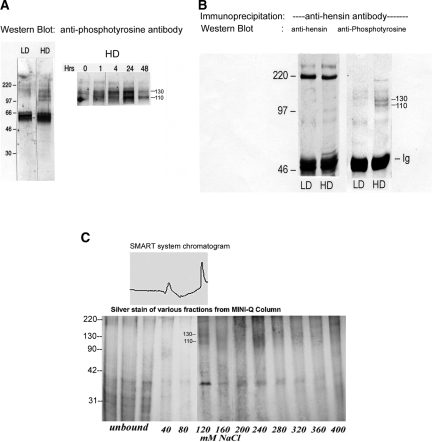
Our previous studies showed that both β and α intercalated cells expressed and secreted hensin, as did the intercalated cell line seeded at high or low density; however, the only form of hensin that causes the conversion is the ECM form of hensin. We measured its apparent molecular weight using sucrose density gradients and found it to be several million Daltons. Hence, we conclude that one mechanism of deposition of hensin in the ECM is polymerization, a “standard” theme in ECM biology. To investigate the mechanism of polymerization, we reasoned that as in the case of fibronectin, hensin might bind to a cell surface protein that then aids in the formation of multimers. Because hensin is deposited in the basement membrane, it would be expected to have basolateral receptors; therefore, we isolated basolateral proteins from cell cultures using biotinylation followed by avidin affinity chromatography. When these proteins were probed with antiphosphotyrosine antibodies, two basolateral proteins (Mr 110 and 130 kD) were tyrosine-phosphorylated in high-density cells but not in low-density cells (Figure 1A). Indeed, we found that tyrosine phosphorylation is likely to be involved in the signal transduction mechanisms mediating hensin action, because genistein, a nonspecific tyrosine kinase inhibitor, blocked the columnarization induced by hensin, whereas several broad-spectrum serine-threonine kinase inhibitors, namely staurosporin, H7, H8, and H89, had no effect (data not shown).
Figure 1.
Identification of basolateral membrane proteins that interact with hensin. (A) Equal amounts of basolateral membrane proteins from low- (LD) and high-density (HD) cells were immunoblotted with anti-phosphotyrosine antibody (left). Two prominent protein bands at 110 and 130 kD were visible only in the HD phenotypes but not in the LD phenotypes (left). These two bands were also seen in HD cell lysates purified by wheat germ agglutinin and probed with anti-phosphotyrosine antibody, indicating these are glycosylated proteins (right). Maximal phosphorylation of these two bands was observed after 24 h of seeding (right). (B) Chemical cross-linking of the basolateral surface followed by immunoprecipitation with hensin antibodies. Only samples from HD cells showed 110- and 130-kD bands when probed with anti-phosphotyrosine antibody. The blots were stripped and reprobed with anti-hensin antibody showing equal amounts of hensin in both LD and HD samples (220-kD band). (C) Glycosylated membrane proteins from HD cells, purified using a Wheat Germ Lectin column, were adsorbed onto Mini-Q resin ion-exchange column and eluted with salt. The 110-/130-kD tyrosine-phosphorylated protein bands eluted at approximately 120 mM NaCl (corresponding to the second peak in the top panel). These bands were excised and analyzed using MALDI-TOF.
In other studies, we exposed cell lysates to wheat germ agglutinin beads to isolate the glycosylated proteins and found that phosphorylated proteins of similar size were present in high-density cell lysates (Figure 1A, right). Furthermore, the phosphorylation of these two proteins was maximal at approximately 24 h after seeding and declined after 48 h of seeding (Figure 1A, right) which coincided with the time course of hensin deposition in the ECM.17 Similarly, membrane fraction of the basolateral proteins obtained from ultracentrifugation methods also provided similar results (data not shown). These results suggested that the 110- and 130-kD proteins identified by these diverse approaches are candidates for hensin-interacting proteins.
To verify that these protein bands were indeed receptors for hensin, we cross-linked hensin to basolateral membrane proteins using cleavable, membrane-impermeable chemical cross-linker DTSSP (Pierce, Rockford, IL) and subjected equal amounts of cell lysate proteins (200 μg) to immunoprecipitation with anti-hensin antibodies followed by immunoblotting with anti-phosphotyrosine antibodies. Although both low-density and high-density cell lysates immunoprecipitated equal amounts of hensin (Figure 1B, left), two tyrosine-phosphorylated protein bands of molecular masses 110 and 130 kD appeared only in the high-density lane and not in the low-density lane (Figure 1B, right). This result demonstrated that the two basolateral membrane proteins that were phosphorylated only in the high-density phenotype also directly interacted with hensin in the high-density phenotypes.
To purify these putative hensin receptors, we solubilized membrane fractions of the high-density cells with 3% NP-40 and subjected them to wheat germ agglutinin chromatography followed by Mini-Q resin ion exchange chromatography. Elution with a buffer containing increasing NaCl concentration showed that p110/p130 eluted from the Mini-Q column at a concentration of 120 mM NaCl (Figure 1C). Purification was monitored in parallel using silver staining and anti-phosphotyrosine Western blotting (data not shown). The purified fractions were run on an SDS-PAGE gel, and the p130 bands were excised. After tryptic digestion and micro-clean-up procedures, matrix assisted laser desorption ionization-reflectron time of flight mass spectrometry (MALDI-ReTOF) analysis of the peptide pools was carried out. Subsequently, mass fingerprinting was carried out on the top experimental masses (m/z) from the MALDI-ReTOF, and peptide sequences of selected peptides were obtained by nano-electronspray tandem mass spectrometry. These procedures resulted in the identification of peptides “LGFGSFIDK” and “SLGTDDLMNEMR,” which matched with mouse integrin β1 (SwissProt P90055), and another peptide, “LNGFEIFAR,” which matched with the chicken integrin αv precursor (SwissProt P26008). The p110 band was very faint and was not suitable for MALDI-TOF analysis; however, our studies with integrin β1 antibodies, discussed next, strongly suggest that p110 band is a glycosylation variant of p130.
Integrin Expression in Clone C Cells
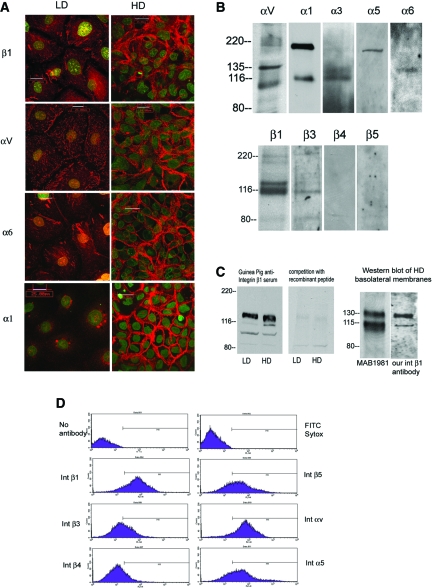
We investigated the expression of α and β subunits of integrins using various approaches. We faced some difficulty because our cell line is derived from rabbit kidneys and most integrin reagents were generated against mouse and human proteins. Only three integrins, αv, β1, and α6, were identified by all methods used: Immunocytochemistry, immunoblot, immunoprecipitation, and flow cytometry. For the others, one or another method gave positive results. Expression of some integrins such as integrin β4 was not identified by any of these methods. We observed positive immunostaining only for integrins α1, α3, αv, α6, and β1 (Figure 2A). To test for the expression of other integrins, we used antibodies to the cytoplasmic domains, which are more conserved across species in Western blotting and immunoprecipitation experiments. We were able to identify the expression of integrins α3, αv, β1, and β3 (Figure 2Β) by immunoprecipitating the biotinylated cell lysates of high-density cells with these cytoplasmic domain antibodies. In addition, Western blots of biotin-streptavidin purified basolateral membranes using these antibodies identified the expression of α1, α5, and β3 integrins by clone C cells (Figure 2B). Integrin β4 and β5 antibodies did not give positive results with these approaches (Figure 2B). Furthermore, we used flow cytometry with integrin antibodies that were known to identify positively various integrin subunits. These results (Figure 2D) identified the expression of integrin β5 by clone C cells as well as confirming the expression of integrins β1, β3, α5, and αv identified by other methods described previously. Integrin β4 expression was equivocal by this method.
Figure 2.
Integrin expression in clone C cells. (A) Expression patterns of integrin β1, αv, α6, and α1 in confluent monolayers of clone C cells seeded at LD and HD. Integrin staining is depicted in red, and nuclear staining with Sytox is shown in green. Integrin α3 staining was also observed by immunostaining but not depicted here. (B) Integrin expression in clone C cells was investigated by basolateral biotinylation followed by immunoprecipitation with integrin antibodies as indicated and Western blotted with anti-streptavidin antibodies (for αv and α3 in the top panel and all lanes in the bottom panel). In addition, Western blots were performed on biotin-streptavidin–purified basolateral membranes from HD cells with integrin α1, α5, and α6. (C) Western blots of cell lysates from LD and HD cells using the polyclonal antibody directed against extracellular domain of rabbit integrin β1 (left). (Right) Western blot using the same antibody in the presence of 600 ng/ml recombinant peptide immunogen. (D) Flow cytometry analysis of clone C cells with various integrin antibodies as indicated in the labels.
To produce a specific antibody for rabbit integrin β1, we cloned and expressed the extracellular domain of integrin β1 from the rabbit cell line (GenBank accession no. gi: 28565527) and generated antibodies against this extracellular fragment of rabbit integrin. Our polyclonal antibodies recognized 110- and 130-kD bands in cell lysates of both high- and low-density cells that were competitively inhibited by recombinant peptides (Figure 2C, left). When basolateral membranes from high-density cells were probed with these polyclonal antibodies, they recognized two bands of molecular weights 110 and 130 kD (Figure 2C, right), similar to the bands recognized by MAB1981, a commercial anti–integrin β1 antibody that has been characterized previously to recognize rabbit integrin β1 (product detail from Chemicon, Temecula, CA).
Integrin β1 Interacts with Hensin
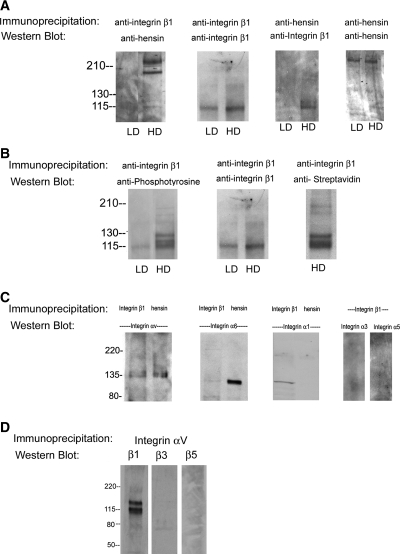
We cross-linked the basolateral surface and immunoprecipitated equal amounts of solubilized membrane proteins (140 μg) from low- and high-density cells with anti–integrin β1 antibodies and blotted the immunoprecipitate with anti-hensin antibodies. We found that hensin was present only in the integrin β1 immunoprecipitates from high-density cells but not low-density cells (Figure 3A, first panel). In control studies, when the nitrocellulose membranes from this study were stripped of primary anti-hensin antibodies and reprobed with a different integrin β1 antibody, we observed the presence of integrin β1 in both low- and high-density cells, although more abundant in high-density cells (Figure 3A, second panel). Identical results were obtained with converse experiments in which integrin β1 immunoprecipitates were first probed with anti–integrin β1 antibody and the membrane was stripped and reprobed with anti-hensin antibody.
Figure 3.
Integrin β1 binds to hensin and integrin αV and gets tyrosine phosphorylated in high density cells. (A) In the first panel, 140 μg of DTSSP–cross-linked cell protein from LD and HD phenotypes were immunoprecipitated with anti–integrin β1 CSAT antibodies, and the blots were probed with guinea pig anti-hensin antibody. In control experiment, membrane containing integrin β1 immunoprecipitates described in the first panel was stripped and reprobed with a different anti–integrin β1 antibody MAB1965 (second panel). Chemically cross-linked cell lysates from LD and HD cells were immunoprecipitated with goat anti-hensin/DMBT1 antibody and blotted with anti–integrin β1 MAB1965 antibody (third panel). Control experiment in which the same samples were probed with guinea pig anti-hensin antibody (fourth panel). (B) Tyrosine phosphorylation of integrin β1. (Left) Anti-phosphotyrosine Western blot of integrin β1 immunoprecipitates (described in A) from LD and HD. (Middle) Control Western blot with integrin β1 antibody. (Right) A streptavidin Western blot of biotinylated basolateral membrane proteins (from HD) that were immunoprecipitated with anti–integrin β1 antibody. (C) Interaction of integrin β1 with integrin α subunits was examined by Western blotting of integrin β1 immunoprecipitates with antibodies to integrin αv, α6, and α1 (left lane in first three panels) and α3 and α5 (last two lanes). Interaction of hensin with integrin α subunits was examined by Western blotting of hensin immunoprecipitates with antibodies to integrin αv, α6, and α1 (right lane in first three panels) from HD cells. (D) Interaction of integrin αv with integrin β subunits was examined by Western blotting of integrin αv immunoprecipitates with integrin β1 (first lane), integrin β3 (second lane), and integrin β5 (third lane) antibodies.
Further confirmation of the interaction of integrin β1 with hensin was obtained when immunoprecipitation of the DTSSP–cross-linked basolateral membrane proteins from low- and high-density cells with anti-hensin antibodies followed by Western blot with anti–integrin β1 antibody revealed the presence of integrin β1 (Figure 3A, third panel). We used a commercially available anti-hensin antibody (made against the human orthologue of hensin DMBT1) for immunoprecipitation in this study. We found that stripping and reprobing this membrane using an antibody generated against rabbit hensin SRCR domains 6 and 7 recognized a protein of the appropriate molecular weight (230 kD) on Western blot (Figure 3A, fourth panel). These studies demonstrate that hensin and β1 integrin interact in high-density cells but not low-density cells.
Integrin β1 Is Tyrosine-Phosphorylated in High-Density Cells
When equal amounts (140 μg) of the DTSSP–cross-linked basolateral membrane proteins from low-density and high-density cells described in the studies of Figure 3A were immunoprecipitated with anti–integrin β1 antibodies and probed with anti-phosphotyrosine antibodies, we observed a doublet band near 110 kD and another band around 130 kD; no such bands were seen in samples from low-density cells (Figure 3B, left). This is an extension of the studies mentioned in Figure 3A and as described previously; when these samples were probed with integrin β1 antibody, we observed the presence of integrin β1 in both low- and high-density cells. We point out that although the anti–integrin β1 antibody MAB1965 used in these studies recognizes only a single band at approximately 110 kD in Western blot, we confirmed that all three bands recognized by anti-phosphotyrosine antibody in this experiment are authentic integrin bands using several methods. When biotinylated basolateral membrane proteins were immunoprecipitated with the same anti–integrin β1 and the membranes were probed with streptavidin, we observed three bands identical to the bands observed with anti-phosphotyrosine Western blot (Figure 2B, first lane, bottom). In addition, although MAB1965 recognizes only a single band at approximately 110 kD in immunoblotting, we observed that our polyclonal antibody against rabbit integrin β1 and another anti–integrin β1 monoclonal antibody MAB1981 both recognize bands of molecular masses identical to the ones recognized by the anti-phosphotyrosine antibodies (Figure 2C, right). Others have also found that antibodies to integrin β1 recognize several species of bands that might be due to different posttranslational modification22,23; however, as to the role of tyrosine phosphorylation of integrin β1, we have no evidence it is either necessary or sufficient for the conversion of low-density to high-density phenotype.
Hensin and Integrin β1 Interact with Integrin αv in High-Density Cells
To examine which α integrin binds to β1, we immunoprecipitated cell lysates from monolayers of high-density cells (chemically cross-linked on the basolateral surface), with anti–integrin β1 antibodies and blotted the material with various anti–α integrin antibodies. We found that αv integrin (135-kD bands) but not α1, α6, α3, and α5 integrins were present in the immunoprecipitate (Figure 3C, left lanes of first three and last two panels). Note that the α1 antibodies recognize a 115-kD unknown β chain in addition to the authentic 180-kD α1 integrin in high-density cell lysates (Figure 2B) as mentioned by the manufacturer (Chemicon). Similarly, immunoprecipitation of the same material with anti-hensin antibodies showed that only αv integrins were present in the immunoprecipitate but not α1 integrin (Figure 3B, second lanes of the first three panels). In summary, these results demonstrate that αv integrins interact with integrin β1 and hensin in the basolateral membranes of high-density cells.
It is known that integrin αv binds to other β integrins, and we tested for this interaction by cross-linking the basolateral surface and immunoprecipitating with integrin αv followed by blotting with antibodies to the cytoplasmic domains of integrin β1, β3, and β5 (Figure 3D). Only β1 was pulled down (lane 1), once again demonstrating its interaction with αv. The absence of integrin β3 band (lane 2) demonstrates that integrin β3 does not interact with αv in clone C cells, because we have been able to identify integrin β3 in basolateral membranes of high-density cells (Figure 2B); however, a similar conclusion could not be made for integrin β5 (lane 3), because we were unable to find antibodies that could detect this integrin in our cell lines using immunoprecipitation or Western blot.
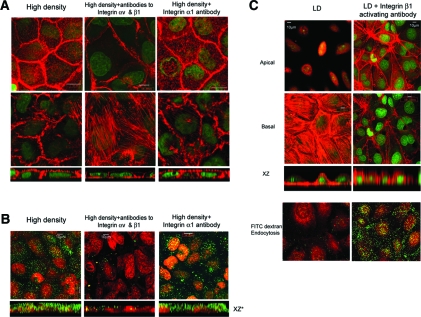
Integrins αv and β1 Play a Critical Role in Terminal Differentiation
To test the effect of integrins αv and β1 signaling in epithelial differentiation directly, we cultured clone C cells at high density (superconfluent) in the presence of blocking antibodies to integrin β124 and integrin αv chosen for their known ability to inhibit integrin functions in diverse assays.25 Cells were counted and an identical number of cells were seeded in each filter. The use of one of these antibodies (either αv or β1) alone produced a partial although demonstrable effect, and we show here the effect of addition of both antibodies together. High-density cells cultured for 3 to 5 d with function-blocking antibodies to integrins αv and β1 lost all of the characteristics of terminally differentiated epithelia; they were no longer cuboidal, becoming short and flat (Table 1, Figure 4A). They also lost most of their subapical actin (Figure 4A, bottom, XZ optical sections). They also developed stress fibers on the basal surface (Figure 4A, middle). To test for specificity, we cultured the cells with integrin α1 (an integrin expressed by these cells) function-blocking antibodies26 and found that these antibodies had no effect on the cell shape or subapical actin staining (Table 1, Figure 4A); however, ECM hensin not only produces a change in cell shape but also causes the development of microvilli and induces new genes such as cytokeratin 19 and villin.4,5 Furthermore, high-density cells have vigorous apical endocytosis, which was completely absent in low-density cells.4 When cells were seeded at high density in the presence of blocking antibodies to αv and to β1 integrins, there was also an inhibition of apical endocytosis (Figure 4B), whereas treatment with antibodies to α1 integrin failed to affect apical endocytosis.
Table 1.
Effect of anti-integrin antibodies on cell height and cross-sectional areaa
| Parameter | n | Height (μ ± SEM) | P | Area (μ ± SEM) | P |
|---|---|---|---|---|---|
| Integrin blockade of HD cells (ANOVA) | |||||
| HD | 6 | 9.190 ± 0.070 | 206.17 ± 5.38 | ||
| + anti-αvβ1 | 6 | 5.248 ± 0.070 | <0.001 | 479.50 ± 6.23 | <0.001 |
| + anti-α1 | 6 | 9.140 ± 0.120 | >0.05 (NS) | 211.83 ± 8.48 | >0.05 (NS) |
| + anti-α6 | 6 | 7.795 ± 0.090 | <0.001 | 313.95 ± 5.57 | <0.001 |
| LD with activating anti–integrin β1 antibodies (unpaired t test) | |||||
| LD | 6 | 5.230 ± 0.050 | 989.08 ± 6.30 | ||
| + anti-β1 | 6 | 8.850 ± 0.040 | <0.0001b | 407.15 ± 13.00 | <0.0001b |
| Integrin blockade of LD cells seeded on polymerized hensin (ANOVA) | |||||
| LD | 5 | 5.110 ± 0.040 | 1011.00 ± 7.70 | ||
| LD on hensin | 5 | 9.320 ± 0.080 | 470.23 ± 7.80 | ||
| + anti-αvβ1 | 5 | 9.210 ± 0.020 | >0.05 (NS)c | 472.65 ± 5.40 | >0.05 (NS)c |
| + anti-α1 | 5 | 9.270 ± 0.020 | >0.05 (NS)c | 458.10 ± 5.00 | >0.05 (NS)c |
| + anti-α6 | 5 | 5.490 ± 0.050 | <0.001c | 2139.30 ± 14.00 | <0.001c |
Results obtained from the measurements of cell height and surface area from confocal images of phalloidin/Sytox green–stained cells as described in the Concise Methods section. HD, high-density; LD, low-density; n, number of independent experiments.
Two-tailed.
Versus LD on hensin.
Figure 4.
Role of integrin αv and β1 in terminal differentiation of clone C epithelia. (A) Clone C cells cultured at high density in media containing integrin αv and β1 function-blocking antibodies or integrin α1 function-blocking antibody. Apical and basal actin stained with phalloidin (red) and imaged by confocal microscopy showing en face and XZ optical sections. (B) HD cells cultured with or without integrin function-blocking antibodies as indicated in the labels were examined for their ability to endocytose fluorescein-dextran. Fluorescein-dextran in green and nuclear stain (Sytox red) in red. (C, top) Clone C cells seeded at low density and allowed to form confluent monolayers, when examined by F-Actin staining, have stress fibers on the basal surface and very little apical actin staining (left); however, when the same experiment is carried out in the presence of integrin β1–activating antibody P4G11, apical actin staining was observed with a marked difference in stress fiber staining on the basal surface. (Bottom) LD cells cultured in the absence or presence of integrin β1–activating antibody P4G11 were examined for their ability to endocytose green fluorescence (FITC) dextran. Nucleus was visualized with Sytox Red dye.
One possible effect of these blocking antibodies is to prevent a large number of cells from binding to the filters, thereby generating a spurious “low density” condition. To examine this, we estimated the number of cells that attached to the filter in high-density cells cultured in the absence and presence of integrin function-blocking antibodies by quantifying the total genomic DNA after 3 and 6 h of seeding. After 3 h of seeding, the amount of DNA in high-density cells was 1.51 μg/filter, whereas in high-density cells cultured in the presence of integrin β1 antibodies, it was 1.54 μg/filter; in high-density cells cultured with both αv and β1 antibodies, it was 1.48 μg/filter, and in high-density cells cultured with integrin α6 antibodies, it was 1.54 μg/filter. These results showed that the blocking antibodies did not significantly alter the spreading or attachment on the filter. After culturing the cells for 6 h, the amount of DNA increased (1.84 μg/filter in high-density cells; 1.99 μg/per filter in high-density cells + integrin αvβ1; i.e., the cells proliferated, but there was still no effect of addition of the antibodies on the cell density). Clearly, the antibodies did not prevent the cells from binding to the filter, and hence the observed phenotype change is in response to the antibody itself rather than to antibody-induced reduction in cell attachment.
To confirm further the role of integrin β1 in columnarization, we used a commercially available, well-documented integrin β1 activating antibody, clone P4G11 (MAB1951; Chemicon). It has been demonstrated that P4G11 reacts with calcium-dependent epitopes on the integrin β1 subunit and that when bound to integrin β1, P4G11 activates β1-dependent binding to ECM substrates.27 Culturing low-density cells with this activating antibody for 3 to 5 d reproduced all of the characteristics of the high-density phenotype, including development of columnarization, subapical actin, and apical endocytosis (Table 1, Figure 4C).
Given that the cross-sectional area of the cells after treatment with the activating antibodies was smaller (Table 1), this raises an interesting question. Did the activating antibody induce proliferation at such a high rate as to convert the low-density cells to a high-density phenotype? To test this, we seeded an identical number of cells (at low density), resuspended them in culture medium with or without integrin β1–activating antibody on two identical transwell filters, and extracted their DNA after 18 h (after the cells had adhered) and found that cells seeded without the activating β1 antibody had 78 ng of DNA per filter, whereas those with the antibody had 79 ng per filter. This study confirmed that the number of cells seeded was indeed identical and showed that the antibody had no effect on the adhesion of cells to the filter. In other studies, we cultured these cells for 5 d while replacing the antibody-containing medium every day and analyzed the DNA content after 5 d. We found that the total DNA content was 360 ng per filter for the control cells and 369 ng per filter for the cells cultured with integrin β1–activating antibodies, demonstrating that the cells cultured in the presence and absence of integrin β1–activating antibodies proliferated at the same rate.
αv and β1 Integrins Mediate Deposition of Hensin in the ECM
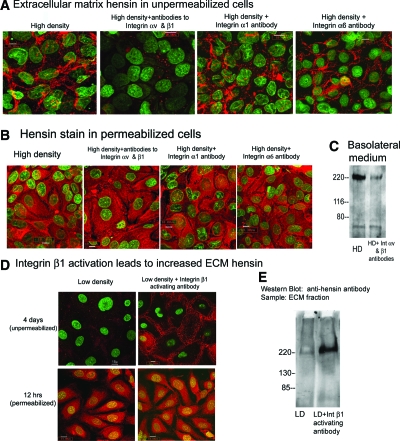
Given that only the ECM form of hensin could cause terminal differentiation, one could postulate that the mechanism of action of αvβ1 integrins was either to cause deposition of hensin into the ECM, where it could signal through binding to its receptor, or else the integrins might be the signaling receptors themselves. To distinguish between these possibilities, we stained unfixed and unpermeabilized clone C cells seeded at high density and cultured for 3 d with anti-hensin antibodies and found that these control cells showed deposition of hensin in the ECM (Figure 5A). In these studies, anti-hensin antibodies were added before the cells were permeabilized; hence, the staining pattern represents hensin localized in the ECM. When these cells were cultured with function-blocking antibodies to integrins αv and β1, very little, if any, ECM hensin was deposited (Figure 5A). Function-blocking antibodies to integrin α628 and integrin α1 had no effect on the deposition of extracellular hensin (Figure 5A). In addition, we found that when the cells were permeabilized and then stained with hensin, the amount of intracellular hensin was as abundant as that in the control high-density cells (Figure 5B), demonstrating that the hensin synthesis is not likely to be affected by integrin function-blocking antibodies. To test whether the blocking antibodies reduced the secretion of hensin into the basolateral media, we immunoprecipitated hensin from the basolateral medium of high-density cells exposed to the blocking antibodies for 3 d and found that indeed the blocking antibodies reduced the amount of hensin in these media (Figure 5C). In summary, these results demonstrate that the function-blocking antibodies to integrins αv and β1 prevented hensin polymerization at least in part by reducing the secretion of hensin into the basolateral medium.
Figure 5.
Integrins αv and β1 mediate ECM deposition of hensin. (A) ECM hensin staining: Clone C cells seeded at high density were cultured with various function-blocking integrin antibodies and exposed to anti-hensin antibodies (followed by rhodamine secondary antibodies) before fixation or permeabilization. Nuclear staining is shown in green. (B) Control experiment to examine intracellular hensin: Clone C cells seeded at high density were cultured with function-blocking integrin antibodies and exposed to anti-hensin antibodies after fixation and permeabilization. (C) Hensin secretion in basolateral medium: Equal amounts of combined basolateral medium from HD cells cultured in the presence and absence of integrin αv–and β1–blocking antibodies collected for 3 d was precleared with agarose beads, immunoprecipitated with anti-hensin (DMBT1) antibodies, and probed with anti-hensin (SRCR6/7) antibodies. (D) Clone C cells seeded at low density and cultured in the absence or presence of activating antibodies to integrin β1 and stained with hensin antibodies before (top) and after (bottom) permeabilization. (E) ECM was extracted from clone C cells seeded at identical low densities but cultured in the absence or presence of integrin β1–activating antibodies for 5 d. The ECM samples were then probed with Western blotting with guinea pig anti-hensin antibody.
To confirm the role of integrin β1 in hensin polymerization, we cultured clone C cells seeded at low density in the presence or absence of the integrin β1–activating antibody for 4 d and examined the deposition of extracellular hensin as described previously. We found that hensin was deposited in the ECM in these cells compared with the control low-density cells (Figure 5D, top); however, hensin synthesis (assayed by the amount of intracellular hensin present after 12 h of seeding) was not affected by the addition of integrin β1–activating antibodies (Figure 5D, bottom). We also extracted the ECM from these cells and probed them by Western blots and found that the integrin β1–activating antibody caused deposition of hensin, whereas control cells had little or no ECM hensin (Figure 5E). These results in the aggregate demonstrate that the high-density state activates αvβ1 integrins, which then causes deposition of extracellular hensin.
Integrin α6 Is a Candidate Receptor that Mediates Hensin Signaling
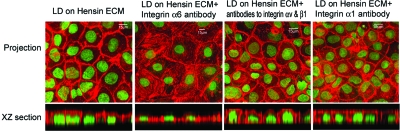
To study the signaling events produced by ECM hensin, we cultured cells at low density on previously polymerized hensin (obtained from the ECM of high-density cells)4,5 and compared the effect of function-blocking antibodies with integrins αv, β1, α1, and α6 in altering the phenotype. Antibodies to integrins αv and β1 or integrin α1 did not inhibit the development of any of the terminal differentiation features caused by polymerized hensin; neither the increase in cell height (Table 1) nor the development of subapical actin cytoskeleton stimulated by polymerized hensin (Figure 6). These results extend the results demonstrating that αvβ1 integrins mediate deposition of hensin in the ECM but play no role in hensin signaling. Remarkably, the function-blocking antibody to integrin α6 completely prevented the development of subapical cytoskeleton and the disappearance of basal stress fibers (Figure 6). These results identified integrin α6 as a potential receptor for polymerized hensin that could mediate hensin signaling to cause phenotypic changes leading to terminal differentiation. In addition, we observed that the clustering of integrin α6 is altered when cultured on hensin matrix compared with culturing on matrigel (data not shown). This result, in addition to the interaction of integrin α6 with hensin (Figure 3B) and the specific blockade of ECM hensin induced columnarization by function-blocking antibodies to integrin α6 (Figure 6, Table 1), demonstrates that hensin binds to α6 and signals to the cell interior. Furthermore, this result confirms our conclusion that integrin αvβ1 is involved in the deposition of hensin in the ECM rather than inducing direct signaling to the cell interior.
Figure 6.
Integrin α6 is a candidate for transmitting signals from ECM hensin to the cell interior. Effect of function-blocking α6 integrin antibodies on clone c cells seeded at low density on preformed hensin matrix and cultured for 5 d. Monolayers were then stained with Phalloidin (red) and Sytox (green), and the combined projection of all confocal sections is presented in these panels. XZ section represents a typical cross-section through the monolayer.
To study further the role of α6, we immunoprecipitated basolateral membrane proteins with anti-hensin antibodies and blotted with integrin α6 antibodies. We found that hensin and α6 formed a complex (Figure 3B, second lane in second panel); however, integrin α6, unlike αv, did not form a complex with β1 integrin. Although it is possible that integrin β4 might interact with α6 in our cell line, we were unable to test this possibility because we could not detect integrin β4 using antibodies directed against cytoplasmic or extracellular domains of β4 with immunoprecipitation or Western blot approaches. Hence, at present, we do not know which β integrin binds to α6.
DISCUSSION
Integrins have been implicated in a variety of cellular functions, including cell motility, proliferation, and survival, in addition to their initial discovery as adhesion receptors for various components of the ECM.29 Their role in the control of differentiation is mentioned,30 but we believe that the results of this article represent a most compelling case for the involvement of integrins in the determination of the cuboidal epithelial phenotype. Differentiation of epithelia is associated with the development of a number of characteristics that include a distinct cell shape, the appearance of apical membrane specializations such as microvilli, and the development of apical endocytosis or exocytosis of specific secretory granules. Whether a unique signaling mechanism is involved in the development of all of these characteristics is unclear, but we show here that integrin signaling results in the development of all of these characteristics in the particular case of the differentiation of the intercalated cell in vitro; however, our results suggest the involvement of integrins in at least two pathways: One involves signaling by αvβ1 integrin resulting in the polymerization of hensin, and the second transduces the signal by which polymeric hensin fibers bind to integrin α6 and signal to produce these changes.
Integrins are well-known receptors for a number of ECM proteins. An important finding of this work has been the identification of integrin α6 as the receptor for hensin's role in columnarization. This integrin, often present in desmosomes, usually associates with β1 or β4 integrins and is expressed widely and early during development.
Although the polymerization of hensin was discovered in an in vitro system, it also occurs in vivo. We had shown that in the cortical collecting tubule of the kidney (the original source of the cell line used here), acid treatment leads to polymerization and deposition of hensin underneath the β intercalated cells in situ, similar to what we found in the cell culture model.31 Furthermore, hensin-blocking antibodies prevented the conversion of β to α intercalated cells in response to acidosis. In addition, in the intestine and prostate, hensin is present in a position compatible with deposition in the ECM in the mature columnar epithelia of the intestinal villus but not in the primitive epithelia of the crypt of the small intestine and colon and similarly in the luminal cells of the prostate but not in the basal primitive cells.5 These studies as well as those of the global deletion of hensin mentioned at the beginning of this article demonstrate that polymerization of hensin is a necessary event in columnar/cuboidal epithelial development.
The role of integrins in differentiation of epithelia has recently been studied in a number of systems since the introduction of genetic deletion studies. The results have been complex, reflecting the multiplicity of ligands that can bind to integrins and the multiplicity of integrins that can substitute for each other in their various functions; however, global deletion of integrin β1 leads to embryonic lethality β1,32 remarkably at the same stage as that induced by global deletion of hensin. Recent studies unequivocally demonstrate that conditional deletion of integrin β1 in the intestinal epithelium leads to inhibition of epithelial differentiation and increased proliferation leading to postnatal lethality.33 Our results provide a possible mechanism by which integrin β1 deletion leads to inhibition of epithelial differentiation. The role integrin β1 in differentiation has also been observed in other systems. For example, deletion of β1 integrin from the breast epithelium results in aberrant differentiation.34 There were anomalies in acinar formation, and although the integrin β1 null cells in vitro remained associated with each other, they did not attach to the ECM and failed to synthesize milk proteins adequately in response to prolactin.
Does tyrosine phosphorylation play any role in integrin β1 activation? Our findings simply show that there was a simultaneous tyrosine phosphorylation and activation of integrin β1 in high-density cells. We do not have any specific results that implicate tyrosine phosphorylation itself in the activation of integrin β1. Previous studies have suggested that the cytoplasmic tail of integrins becomes tyrosine-phosphorylated and that this was necessary for its role in cell migration35; however, recent studies in which these tyrosines were mutated to phenylalanines in transgenic mice showed that this did not result in any change in the viability of these mice, but replacement of the tyrosine with alanines caused the expected lethality.36 These unexpected complexities clearly reveal that more work needs to be done before one can conclude anything about the role of tyrosine phosphorylation in β1 integrin function.
CONCISE METHODS
Cell Culture
Stock cultures of clone C of β intercalated cells established from rabbit kidney cortex were maintained as described previously.4 Passages 7 through 12 were routinely used. The cells were trypsinized and seeded on polycarbonate filters (pore size 0.4 μm; Corning Incorporated, Corning, NY) at a density of 2 × 104 cells/cm2 (low density) or 106 cells/cm2 (high density) and transferred to 40°C to inactivate the T antigen.
Integrin Activation and Function-Blocking Studies
In the studies aimed at integrin function blocking and activation, culture medium with antibodies (described previously) was replaced daily for 3 to 5 d. For function-blocking studies with integrin αvβ1, 20 μg/ml anti-integrin β1 MAB1965 and 10 μg/ml anti-integrin αV (MAB1980) were diluted in the same culture medium. For integrin β1 activation studies, low-density cells were cultured for 5 d with MAB1951 at a concentration of 10 μg/ml (medium replaced daily). In studies in which low-density cells were cultured on hensin matrix, hensin-coated transwell filters were prepared as reported previously.5 For function-blocking studies in superconfluent (high-density) cells and activation studies in low-density cells, identical number of cells were seeded.
DNA Quantification
DNA was isolated from cells cultured on transwell filters using Classica Genomic DNA isolation kit by Lambda Biotech (St. Louis, MO) according to the manufacturer's protocol. DNA concentration was determined by reading the absorption at 260 and 280 nm in a Lightwave S2000 UV/Vis spectrophotometer (WPA, Cambridge, UK) and using the standard formula to obtain DNA concentration from OD at 260 nm.
Hensin Antibodies
Guinea pig anti-hensin antibodies against SRCR6–7 domains were obtained as described previously.37 This antibody recognized hensin by immunoblot, immunoprecipitation, and immunofluorescence, as described previously.5,18 In immunoprecipitation experiments, goat polyclonal anti-DMBT1/hensin antibody from Hypromatrix (Worcester, MA) was used. This antibody has been used in several studies by other authors and has been characterized to recognize rabbit hensin in our laboratory. It is important to note that whereas our anti-hensin antibodies recognize the partially glycosylated form of hensin at approximately 200 kD in addition to the glycosylated 230-kD protein,17 the commercial antibody recognizes only a single band at 230 kD.
Integrin β1 Antibodies
Many of the antibodies used in our studies were obtained from Upstate/Chemicon. We primarily used three anti–integrin β1 antibodies: MAB1981, MAB1965, and MAB1951. MAB1981 was used for immunocytochemistry (1:100), and MAB1965 was used in Western blotting (1:500) and function blocking (20 μg/ml). MAB1951 was used in integrin β1 activation studies at a concentration of 1:100. Both MAB1981 and MAB1951 have been preciously demonstrated to recognize rabbit integrin β1, according to the product description of Chemicon. Immunoprecipitation of integrin β1 was achieved using CSAT mAb38 raised against chicken integrin β1 (provided by Dr. Eugene Marcantonio, Columbia University, New York, NY).
Cloning of Rabbit Integrin β1 Extracellular Domain and Antibody Generation against Rabbit Integrin β1
Since most of the commercial antibodies were either rabbit polyclonal or mAb generated against human integrin β1, we cloned an extracellular fragment of integrin β1 from rabbit and generated antibodies against that fragment. Because there was no previously reported sequence for rabbit integrin β1, we carefully designed the primers for cloning the extracellular domain of integrin β1. We carried out multiple sequence alignments of integrin β1 nucleotide sequences from cow (AF468058), pig (AF192528), human (HSFNRB), cat (FCU27351), and chicken (CHKINT) and, similarly, multiple alignments of integrin β1 protein sequences from mouse (ITB1_MOUSE), rat (ITB1_RAT), cow (ITB1_BOVIN), cat (ITB1_FELCA), human (ITB1_HUMAN), and Xenopus (ITB1_XENLA). From these multiple alignments, several stretches of highly homologous sequences were identified and searched for their similarity to other known proteins in the database using Blast. Sequences that were unique to integrin β1 were chosen, and the primers were designed from these sequences. We successfully generated a 158-residue N-terminal peptide beginning from residue 33 of the mouse sequence using reverse transcriptase–PCR. The cloned fragment was inserted into pTrc-His2 TOPO vector and expressed in TOP10 bacteria. The cloned sequence was verified using sequence analysis and was deposited in the NCBI GenBank database (GenBank accession no. gi: 28565527 or AY195896). Peptides purified using the ProBond purification kit were examined with gel electrophoresis, and pure peptides were used to generate polyclonal serum in guinea pigs. These antibodies were used in some immunocytochemistry experiments, and they gave identical results to the commercial MAB1981 antibodies obtained from Chemicon.
Other Antibodies
Other antibodies that were used are against integrin αV (MAB1980; function-blocking 10 μg/ml; immunocytochemistry 1:50, Western blot 1:250), integrin α6 (MAB1378; clone GoH3; function blocking (20 μg/ml); immunocytochemistry 1:100), and integrin α1 (MAB1973; function blocking 5 μg/ml; immunocytochemistry 1:100). In addition, antibodies against integrin β4 (MAB2058, MAB2060; SC-6628-Santa Cruz Biotechnology, Santa Cruz, CA), integrin β3 (MAB1957; cat. no. 553343-BD Pharmingen, San Jose, CA), integrin α2 (MAB1950), integrin α3 (MAB 1952), and integrin α4 (MAB16983) were also used in immunostaining experiments. Anti-phosphotyrosine 4G10 antibodies were purchased from Upstate and were used at a concentration of 1:1000 in immunoblotting experiments. In addition, AB1920 (flow cytometry and Western blot of integrin α5), MAB2290 (immunostaining, Western blot, and immunoprecipitation of integrin α3), AB1926 (flow cytometry of integrin β3), cytoplasmic domain antibody sc-6626 (Santa Cruz Biotechnology; immunoprecipitation and Western blot of integrin β3), MAB1964 (flow cytometry of integrin β4), sc-6628 (immunoprecipitation and Western blot of integrin β4), AB1926 (flow cytometry of integrin b5), and sc-5402 (immunoprecipitation and Western blot of integrin β5) were used. All antibodies with prefix AB and MAB are from Millipore (Billerica, MA).
Basolateral Biotinylation Experiments
Monolayer cultures were washed, and cell-impermeable EZ-Link Sulfo-NHS-SS-Biotin (Pierce) at a concentration of 0.5 to 1.0 mg/ml in PBS (pH 8.0) was added to the basolateral surface. After 30 min of incubation at room temperature, cell lysates were extracted from the filters using a lysis buffer3,15 containing 5 mM sodium orthovanadate, 5 mM sodium fluoride, “Complete” protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), and 1% glycerol. Cell lysates were centrifuged, and the protein concentration of the supernatant was determined using Pierce BCA protein assay system. Equal quantities of protein (typically 200 μg) were incubated with streptavidin-agarose beads (1 h), and the beads are washed extensively with the lysis buffer, high-salt buffer containing 500 mM NaCl, 50 mM Tris (pH 7.8), 0.1% NP40, and 5 mM EDTA. The biotinylated cell surface proteins bound to the streptavidin beads were extracted from the beads by boiling them briefly in SDS-PAGE sample buffer and run on a 7.5% Tris-HCl precast gel and then immunoblotted with anti-phosphotyrosine antibody (1:1000).
Cross-Linking and Immunoprecipitation Experiments
Cross-linking was performed similar to basolateral biotinylation, except with the addition of 0.5 mg/ml membrane-impermeable, cleavable cross-linker DTSSP (Pierce) instead of biotin. Equal quantities of cell lysates, typically 140 μg from low- and high-density cells, were immunoprecipitated with anti-hensin antibodies (1:500) or anti-integrin β1 CSAT antibodies (1:20). Immunoprecipitation was performed using standard protocol described previously.15 In some immunoprecipitation experiments, ProFound Mammalian Co-Immunoprecipitation Kit (Pierce) was used as per the supplier's protocol. SDS-PAGE and Western blotting were carried out using standard protocols.5,17 Protein bands were visualized using ECL Western blotting system (Amersham Biosciences, Piscataway, NJ). Restore Western Blot stripping buffer (Pierce) was used for stripping and reprobing membranes.
Purification of Cell Surface Proteins that Interact with Hensin
Cell lysates from 40 T75 flasks of high-density cells were subjected to ultracentrifugation (100,000 × g, 2 h), and the membrane pellets were solubilized with a buffer containing 3% NP40 and 0.5% SDS. Solubilized membrane fractions were then diluted to a final concentration of 0.3% NP40 and bound to Wheat Germ Lectin Sepharose 6MB beads (Sigma) for 2 h (4°C). After washing the beads with Buffer A with 0.3% NP40, glycosylated membrane proteins were eluted off the beads using 20 mM HEPES (pH 7.4) containing 300 mM N-acetyl-d-glucosamine, 2 mM EDTA, 1 mM EGTA, 5 mM sodium orthovanadate, and 5 mM sodium fluoride (all from Sigma). Wheat Germ Lectin–purified protein samples were transferred to a buffer containing 20 mM HEPES, 1 mM EDTA, 0.5 mM EGTA, and 0.1% NP40 (Buffer C) using dialysis and were applied onto pre-equilibrated Mini-Q mounted in a SMART microseparation System (Pharmacia, Piscataway, NJ). Buffer C and Buffer C + 0.8 M NaCl were used to establish a step gradient, and samples were eluted in 0.5-ml samples and monitored by detector. Fractions corresponding to the highest peak (approximately 120 mM NaCl) corresponded to the appearance of 110- and 130-kD proteins on the silver stain (and demonstrated to retain tyrosine phosphorylation in test experiments).
Coomassie stain of the combined peak fractions run on a 7.5% Tris-HCl minigels revealed the presence of clean 110-kD (faint) and 130-kD (strong) bands. These bands were excised, and tryptic digests were carried out. For mass fingerprinting of proteins in each preparation, 100% of the generated peptides were subjected to a micro-clean-up procedure using 2 μl of bed volume of Poros 50 R2 reversed-phase beads. Mass spectrometry (MALDI-ReTOF) on two peptide pools (16 and 30% MeCN) recovered from the RP-microtip column using a Bruker REFLEX III instrument with delayed extraction. For mass fingerprinting, top “major” experimental masses (m/z) combined from MALDI-ReTOF experiments were used to search nonredundant database (693,878 entries; NCBI; Bethesda, MD), using the PeptideSearch (M. Mann, University of Southern Denmark, Odense, Denmark) algorithm. Mass spectrometry–based sequencing (ESI-MS/MS) of selected peptides from partially fractionated pools was done using a PE-SCIEX API300 triple quadrupole instrument, fitted with a continuous flow nano-electrospray source (“JaFIS”). The p110 band was very faint and was not included in the analysis; however, we subsequently verified that integrin β1 identified from these results exists in a110-kD form in addition to the 130-kD form (see the Results section).
Immunocytochemistry
Immunocytochemistry was performed as described previously.5 For F-actin staining, the filters were incubated with rhodamine-phalloidin (Molecular Probes, Eugene, OR). Stained monolayers were viewed using an LSM 510 Meta laser-scanning microscope equipped with Axiovert 200M (Carl Zeiss, Thornwood, NY). Images were collected with 0.6- to 1-μm thickness optical sections and analyzed by the Zeiss LSM5 Image Browser and LSM-PC(410) software. The final images were processed with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA). In the figures that show apical versus basal actin staining, the top two to three apical sections and the bottom two to three basal sections (0.6 μ each) were projected together. For most other pictures, images from all confocal sections were projected together.
For visualization of ECM hensin staining, cells were incubated with anti-hensin antibody followed by rhodamine-conjugated anti-guinea pig antibody, washed, and then fixed with cold methanol. Nuclear staining with Sytox Dye (Molecular Probes) was performed after fixation. Function blocking and activation experiments were carried out at least two to three times, and changes observed represent more than 60% of the monolayer.
Analysis of the Shape and Height of Clone C Cells
All analyses of cell size and height were performed with Zeiss 410 laser scanning microscope software (Carl Zeiss) as described previously.5 Ten to 15 individual measurements were recorded for each sample. The experiments were repeated six to seven times. ANOVA and unpaired t test statistics were performed on the mean values of heights and area from each individual experiment, and results are tabulated in Table 1.
Flow Cytometry
Flow cytometry was performed using standard procedures. High-density cells were removed from the cell culture plates by mild treatment with trypsin-EDTA, washed, and exposed to primary antibodies followed by secondary antibodies. Controls without the addition of any antibody or only secondary antibody were also used. Finally, the cells were incubated with propidium iodide for 5 min, washed and filtered through tubes with cell strainer caps and analyzed using BD FACSCalibur system. Dead cells with propidium iodide staining were excluded from analysis.
Apical Endocytosis
Monolayer cell cultures were exposed to 10 mg/ml FITC-dextran FD-40 (Sigma) dissolved in bicarbonate-free culture medium on the apical side only. The cells were incubated for 10 min at 40 or 4°C and then washed with PBS containing 1 mM CaCl2 and 1 mM MgCl2 five times at 4°C. The filters were then fixed with periodate/lysine paraformaldehyde fixative for 15 min at 4°C and exposed to 1:10,000 Sytox Red for 5 min. There was no uptake of FITC dextran at 4°C (data not shown). Images were acquired with laser scanning microscope LSM 510 Meta as described previously. For providing a quantitative overview of apical endocytosis, the three-dimensional projection utility with Make Sequence command was used to make a three-dimensional sequence of all of the sections collected for each field, and the compressed three-dimensional projection that represents the entire apical surface of the field is presented as XZ* section in the figures.
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institutes of Health grant DK-20999 to Q.A. The Columbia Confocal Facility is supported by National Institutes of Health grant RR-10506.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Integrins, Extracellular Matrix, and Terminal Differentiation of Renal Epithelial Cells,” on pages 1043–1044.
References
- 1.Vijayakumar S, Takito J, Gao X, Schwartz GJ, Al-Awqati Q: Differentiation of columnar epithelia: The hensin pathway. J Cell Science 119: 4797–4801, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz GJ, Barasch J, Al-Awqati Q: Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Edwards JC, van Adelsberg J, Rater M, Herzlinger D, Lebowitz J, Al-Awqati Q: Conditional immortalization of bicarbonate-secreting intercalated cells from rabbit. Am J Physiol 263: C521–C529, 1992 [DOI] [PubMed] [Google Scholar]
- 4.van Adelsberg J, Edwards JC, Takito J, Kiss B, Al-Awqati Q: An induced extracellular matrix protein reverses the polarity of band 3 in intercalated epithelial cells. Cell 76: 1053–1061, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Vijayakumar S, Takito J, Hikita C, Al-Awqati Q: Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cells: Processes that resemble terminal differentiation. J Cell Biol 144: 1057–1067, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takito J, Yan L, Ma J, Hikita C, Vijayakumar S, Warburton D, Al-Awqati Q: Hensin, the polarity reversal protein, is encoded by DMBT1, a gene frequently deleted in malignant gliomas. Am J Physiol 277: F277–F289, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Mollenhauer J, Wiemann S, Scheurlen W, Korn B, Hayashi Y, Wilgenbus KK, von Deimling A, Poustka A: DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3–26.1 is deleted in malignant brain tumours. Nat Genet 17: 32–39, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Holmskov U, Mollenhauer J, Madsen J, Vitved L, Gronlund J, Tornoe I, Kliem A, Reid KB, Poustka A, Skjodt K: Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D. Proc Natl Acad Sci U S A 96: 10794–10799, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng H, Bjerknes M, Chen H: CRP-ductin: A gene expressed in intestinal crypts and in pancreatic and hepatic ducts. Anat Rec 244: 327–343, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Li XJ, Snyder SH: Molecular cloning of Ebnerin, a von Ebner'squosquo; apos; ys gland protein associated with taste buds. J Biol Chem 270: 17674–17679, 1995 [DOI] [PubMed] [Google Scholar]
- 11.De Lisle RC, Ziemer D: Processing of pro-Muclin and divergent trafficking of its products to zymogen granules and the apical plasma membrane of pancreatic acinar cells. Eur J Cell Biol 79: 892–904, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Somerville RP, Shoshan Y, Eng C, Barnett G, Miller D, Cowell JK: Molecular analysis of two putative tumour suppressor genes, PTEN and DMBT1, which have been implicated in glioblastoma multiforme disease progression. Oncogene 17: 1755–1757, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Mori M, Shiraishi T, Tanaka S, Yamagata M, Mafune K, Tanaka Y, Ueo H, Barnard GF, Sugimachi K: Lack of DMBT1 expression in esophageal, gastric and colon cancers. Br J Cancer 79: 211–213, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai MA, Moriya T, Imai FL, Shiiba M, Bukawa H, Yokoe H, Uzawa K, Tanzawa H: Down-regulation of DMBT1 gene expression in human oral squamous cell carcinoma. Int J Mol Med 15: 585–589, 2005 [PubMed] [Google Scholar]
- 15.Takito J, Al-Awqati Q: Conversion of ES cells to columnar epithelia by hensin and to squamous epithelia by laminin. J Cell Biol 166: 1093–1102, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivas S, Rodriguez T, Clements M, Smith JC, Beddington RS: Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development 131: 1157–1164, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hikita C, Takito J, Vijayakumar S, Al-Awqati Q: Only multimeric hensin located in the extracellular matrix can induce apical endocytosis and reverse the polarity of intercalated cells. J Biol Chem 274: 17671–17676, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Hikita C, Vijayakumar S, Takito J, Erdjument-Bromage H, Tempst P, Al-Awqati Q: Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J Cell Biol 151: 1235–1246, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe S, Tsuruoka S, Vijayakumar S, Fischer G, Zhang Y, Fujimura A, Al-Awqati Q, Schwartz GJ: Cyclosporin A produces distal renal tubular acidosis by blocking peptidyl prolyl cis-trans isomerase activity of cyclophilin. Am J Physiol Renal Physiol 288: F40–F47, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Mao Y, Schwarzbauer JE: Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 24: 389–399, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hulmes DJ: Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol 137: 2–10, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Yan Z, Chen M, Perucho M, Friedman E: Oncogenic Ki-ras but not oncogenic Ha-ras blocks integrin beta1-chain maturation in colon epithelial cells. J Biol Chem 272: 30928–30936, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Clement M, Rocher J, Loirand G, Le Pendu J: Expression of sialyl-Tn epitopes on beta1 integrin alters epithelial cell phenotype, proliferation and haptotaxis. J Cell Sci 117: 5059–5069, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ni H, Wilkins J: Localisation of a novel adhesion blocking epitope on the human integrin b1 chain. Cell Adhes Commun 5: 257–271, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Friedlander DR, Zagzag D, Shiff B, Cohen H, Allen JC, Kelly PJ, Grumet M: Migration of brain tumor cells on extracellular matrix proteins in vitro correlates with tumor type and grade and involves alphaV and beta1 integrins. Cancer Res 56: 1939–1947, 1996 [PubMed] [Google Scholar]
- 26.Fabbri M, Castellani P, Gotwals PJ, Kotelianski V, Zardi L, Zocchi MR: Regulation of laminin 1-induced pancreatic beta-cell differentiation by alpha6 integrin and alpha-dystroglycan. Tissue Antigens 48: 47–51, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Wayner EA, Gil SG, Murphy GF, Wilke MS, Carter WG: Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for alpha 3 beta 1 positive T lymphocytes. J Cell Biol 121: 1141–1152, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida EA, Huovila AP, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG, White JM: Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell 81: 1095–1104, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Giancotti FG, Tarone G: Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 19: 173–206, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Bokel C, Brown NH: Integrins in development: Moving on, responding to, and sticking to the extracellular matrix. Dev Cell 3: 311–321, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Tsuruoka S, Vijayakumar S, Petrovic S, Mian A, Al-Awqati Q: Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J Clin Invest 109: 89–99, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fassler R, Meyer M: Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev 9: 1896–1908, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK: Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol 175: 505–514, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH: Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 171: 717–728, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai T, Zhang Q, Fassler R, Mosher DF: Modulation of beta1A integrin functions by tyrosine residues in the beta1 cytoplasmic domain. J Cell Biol 141: 527–538, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czuchra A, Meyer H, Legate KR, Brakebusch C, Fassler R: Genetic analysis of beta1 integrin “activation motifs” in mice. J Cell Biol 174: 889–899, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takito J, Hikita C, Al-Awqati Q: Hensin, a new collecting duct protein involved in the in vitro plasticity of intercalated cell polarity. J Clin Invest 98: 2325–2331, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briesewitz R, Epstein MR, Marcantonio EE: The expression of native and truncated forms of the human integrin a1 subunit. J Biol Chem 268: 2989–2996, 1993 [PubMed] [Google Scholar]