Abstract
The response to tissue injury involves the coordination of inflammatory and repair processes. IL-6 expression correlates with the onset and severity of acute kidney injury (AKI), but its contribution to pathogenesis remains unclear. This study established a critical role for IL-6 in both the inflammatory response and the resolution of AKI. IL-6–deficient mice were resistant to HgCl2-induced AKI compared with wild-type mice. The accumulation of peritubular neutrophils was lower in IL-6–deficient mice than in wild-type mice, and neutrophil depletion before HgCl2 administration in wild-type mice significantly reduced AKI; these results demonstrate the critical role of IL-6 signaling in the injurious inflammatory process in AKI. Renal IL-6 expression and STAT3 activation in renal tubular epithelial cells significantly increased during the development of injury, suggesting active IL-6 signaling. Although a lack of renal IL-6 receptors (IL-6R) precludes the activation of classical signaling pathways, IL-6 can stimulate target cells together with a soluble form of the IL-6R (sIL-6R) in a process termed trans-signaling. During injury, serum sIL-6R levels increased three-fold, suggesting a possible role for IL-6 trans-signaling in AKI. Stimulation of IL-6 trans-signaling with an IL-6/sIL-6R fusion protein activated STAT3 in renal tubular epithelium and prevented AKI. IL-6/sIL-6R reduced lipid peroxidation after injury, suggesting that its protective effect may be largely mediated through amelioration of oxidative stress. In summary, IL-6 simultaneously promotes an injurious inflammatory response and, through a mechanism of trans-signaling, protects the kidney from further injury.
Acute kidney injury (AKI) is a major source of morbidity and mortality in hospitalized patients, complicating the course of 5% of hospital admissions and 30% of intensive care unit admissions.1 Approximately 40% of patients with renal disease present with AKI mostly as a result of development of acute tubular necrosis (ATN).1 ATN is also the final common pathway of severe renal dysfunction in patients who have diseases of nonrenal origin; therefore, an understanding of the pathophysiologic principles and pathways of ATN is central to the development of potential means of prevention or treatment. The induction of IL-6 expression has been observed during the development of AKI both in humans2 and in experimental animal models3,4; however, the role of IL-6 in AKI has not been clearly established.
IL-6 is a pleiotropic cytokine that has long been described as having both pro- and anti-inflammatory properties.5,6 IL-6 is produced in copious amounts by endothelial cells in response to proinflammatory signals including TNF-α7 and hypoxia,8 and is also a common response to tissue injury and organ failure.9,10 On target cells, IL-6 acts by binding to a specific cognate receptor (IL-6R), which triggers gp130 and leads to the activation of the Jak/STAT signaling pathway and in particular the activation of STAT3.11 Unlike the ubiquitously expressed gp130, the cellular distribution of IL-6R is limited to a few cell types, including hepatocytes, and some leukocyte subpopulations, including monocytes, neutrophils, and some T cells and B cells6; however, IL-6R also exists in a soluble form (sIL-6R) that upon binding to IL-6 stimulates cells via direct interaction with gp130.6,12 Of importance, then, in a process called IL-6 trans-signaling, the IL-6/sIL-6R complex acts as an agonist on cell types that, although expressing gp130, would not inherently respond to IL-6 alone.6,12
This study was designed to elucidate the role of IL-6 in AKI. The results of this study reveal that an IL-6–mediated inflammatory response contributes in part toward the generation of renal injury; however, the results also demonstrate that IL-6 trans-signaling mediates the induction of a protective response to renal injury. Moreover, we demonstrate that activation of gp130 by the administration of an IL-6/sIL-6R fusion protein, called Hyper-IL-6, prevents the onset of AKI and significantly enhances survival.
RESULTS
IL-6 and STAT3 Signaling Increase after HgCl2-Induced Renal Injury
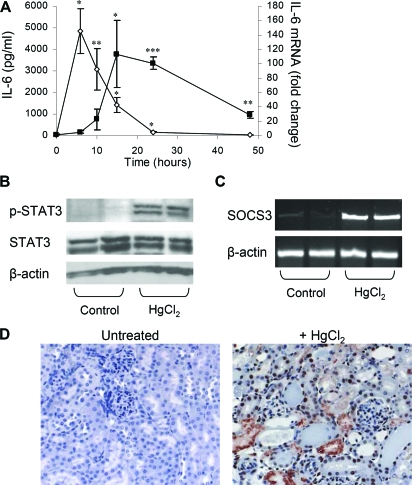
Administration of mercury-containing compounds such as mercuric chloride (HgCl2) is a well-established model for the study of nephrotoxin-induced AKI.13 Administration of HgCl2 to mice resulted in a rapid and substantial elevation of serum IL-6 protein levels that rose to peak levels by 6 h (Figure 1A), concurrent with the development of AKI. Quantitative analysis of IL-6 mRNA in the kidney showed that IL-6 expression was strongly induced in the kidney (113-fold) and correlated with the manifestation of renal injury, reaching peak levels at 15 h and persisting more than 24 h, when serum IL-6 levels had largely diminished. Increased levels of IL-6 mRNA were also observed in the liver and moderately so in the spleen (Supplemental Figure 1). Together with the rise in IL-6 levels, we also observed a five-fold increase in pSTAT3 (Tyr705) levels (0.23 ± 0.07 versus 0.04 ± 0.06 OD units for HgCl2 versus control mice; P = 0.05) and upregulation of SOCS3 mRNA in the kidney, consistent with an induction of IL-6 signaling after HgCl2-induced injury (Figure 1, B and C). Immunohistochemical staining showed that activated STAT3 was present in a variety of cell types and predominantly so in renal tubular epithelial cells (pSTAT3 positive tubular epithelial cells = 144.3 ± 21 versus 1.2 ± 0.9 per high-power field for HgCl2 and untreated mice, respectively; P < 0.001; Figure 1D).
Figure 1.
HgCl2 induces IL-6 expression and STAT3 activation. (A) IL-6 ELISA analysis of serum samples from mice treated with HgCl2 (6 mg/kg). Data are means ± SEM (n = 3 to 6 mice per time point). *P ≅ 0.004, **P ≅ 0.01 versus 0 h. Real-time PCR analysis of IL-6 mRNA in kidney taken from mice after treatment with HgCl2 (6 mg/kg; n = 3 to 6 mice per time point). *P < 0.05, **P ≅ 0.003, ***P ≅ 0.0001 versus 0 h. (B) Western blot analysis of p-STAT3 and STAT3 in the kidney 6 h after HgCl2-induced injury. (C) RT-PCR analysis of SOCS3 mRNA induction 6 h after HgCl2-induced injury. (D) p-STAT3 immunostaining in the kidney 24 h after HgCl2-induced injury reveals extensive STAT3 activation (red nuclear staining) in renal tubular epithelial cells. Magnification, ×200.
IL-6 Mediates a Proinflammatory Response that Exacerbates Renal Injury
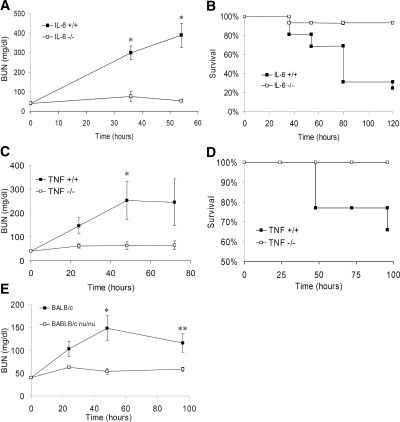
To determine the physiologic role of IL-6 in the development of renal injury, we compared the sensitivity of IL-6–deficient (IL-6−/−) and wild-type (IL-6+/+) mice with HgCl2-induced AKI. IL-6+/+ mice treated with HgCl2 developed AKI manifested by an elevation of blood urea levels culminating in mortality of up to 70% of treated animals. In contrast, IL-6−/− mice showed remarkable resistance to HgCl2 toxicity (Figure 2A) and better survival (Figure 2B). A dose-response analysis of HgCl2 toxicity showed that whereas IL-6−/− mice were significantly more resistant to HgCl2 toxicity than IL-6+/+ mice at dosages ranging from 5 to 7 mg/kg, higher dosages of HgCl2 (≥8 mg/kg) led to levels of renal failure that were indistinguishable between the two strains (blood urea nitrogen [BUN] at 24 h = 210 ± 160 (n = 5) versus 281 ± 188 mg/dl (n = 6) in IL-6−/− and IL-6+/+ mice, respectively; NS). These results indicated that IL-6 participates as a proinflammatory agent contributing toward the development of AKI.
Figure 2.
IL-6−/−, TNF-α−/−, and immune-deficient mice are partially resistant to HgCl2-induced AKI. (A) Renal function as indicated by BUN levels in IL-6+/+ and IL-6−/− mice administered HgCl2 (6 mg/kg). Data are means ± SEM of surviving mice. *P ≅ 0.001 versus IL-6+/+ mice (n = 14 to 15). (B) Kaplan-Meier survival plot of mice treated in A; log rank test, P ≅ 0.001 IL-6−/− versus IL-6+/+ mice. (C) Renal function in TNF-α+/+ (TNF+/+) and TNF-α−/− (TNF−/−) mice after HgCl2 administration. Data are means ± SEM of surviving mice. *P ≅ 0.04 (n = 5 to 9). (D) Kaplan-Meier survival plot of mice treated in C; log rank test, P ≅ 0.04 TNF+/+ versus TNF−/− mice. (E) Renal function as indicated by BUN levels in BALB/c and BALB/c nu/nu mice. Data are means ± SEM. *P ≅ 0.008, **P ≅ 0.03 (n = 8). No mortality was observed.
To determine whether other inflammatory pathways mediated HgCl2-induced AKI, we examined the renal toxicity of HgCl2 in TNF-α–deficient (TNFα−/−) mice. Similar to the IL-6−/− mice, TNF-α−/− mice displayed relative resistance to HgCl2-induced AKI and a corresponding reduction in mortality (Figure 2, C and D). Interestingly, serum IL-6 levels resulting from HgCl2 administration in TNF-α−/− mice were not diminished in comparison with those of the wild-type mice (serum IL-6 = 2575 ± 830 pg/ml versus 2735 ± 3980 pg/ml in TNF-α+/+ [n = 4] and TNF-α−/− mice [n = 5], respectively; NS), indicating that HgCl2-induced IL-6 expression is not TNF-α dependent.
Various leukocyte subsets, including lymphocytes and neutrophils, have been implicated as key cellular mediators in the development of renal ischemic/reperfusion injury.14,15 To examine further the role of the inflammatory response, in particular that of T lymphocytes in HgCl2-induced AKI, we compared the sensitivity of athymic BALB/c nu/nu mice versus wild-type BALB/c mice to HgCl2 toxicity. The results of this analysis (Figure 2E) showed that BALB/c nu/nu mice, like IL-6−/− mice and TNF-α−/− mice, were significantly more resistant to moderate dosages of HgCl2 than wild-type BALB/c mice. Similar results were also obtained in a single experiment comparing HgCl2-induced AKI in SCID/bg versus wild-type BALB/c mice (BUN at 48 h = 41.8 ± 3.5 versus 437.5 ± 216.2 mg/dl, in SCID/bg [n = 6] versus wild-type [n = 8] mice, respectively; P < 0.003; mortality at 120 h 25 versus 66%, respectively).
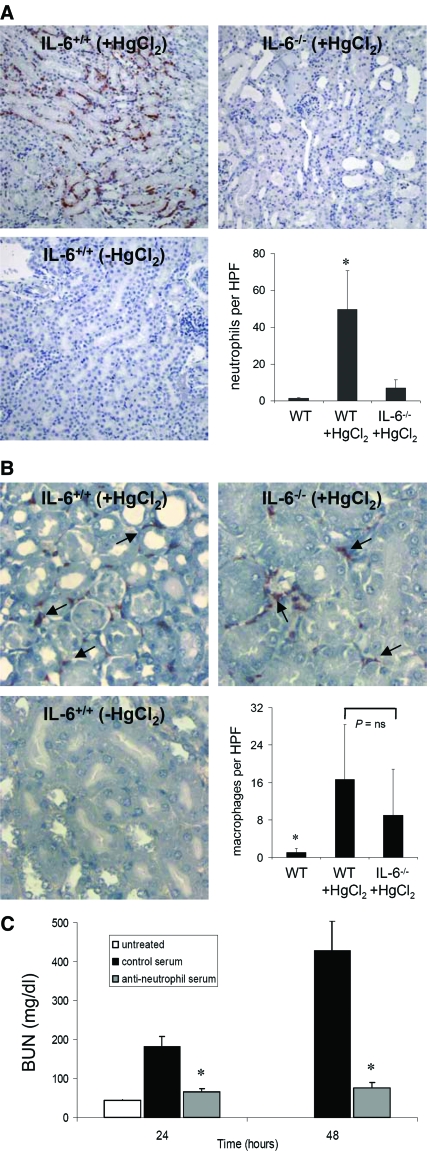
Neutrophils participate in the development of AKI and are considered to be a source of exacerbation of injury in ischemic- and sepsis-induced AKI.16–18 To determine whether neutrophil accumulation was related to IL-6 expression in HgCl2-induced AKI, we compared neutrophil infiltration to kidneys in wild-type and IL-6−/− mice after HgCl2-induced injury. Twenty-four hours after HgCl2 administration, robust accumulation of neutrophils within the peritubular capillaries and interstitium was observed in wild-type mice but was significantly less in IL-6−/− mice (Figure 3A). This difference was also apparent in the few IL-6−/− mice displaying more extensive morphologic injury. Substantial macrophage infiltration to the inner cortex was also observed after HgCl2-induced injury but did not seem to be IL-6 dependent (Figure 3B). A corresponding infiltration of CD3+ T cells after HgCl2-induced injury was not observed in either wild-type or IL-6−/− mice (data not shown). Depletion of neutrophils before HgCl2 administration dramatically prevented the development of AKI (creatinine at 24 h 1.10 ± 0.21 versus <0.5 mg/dl in control [n = 7] and antineutrophil serum–treated [n = 5] mice, respectively; P = 0.01; Figure 3C). Thus, IL-6 intrinsically links the cellular inflammatory response to the development of AKI.
Figure 3.
Neutrophilic infiltration to the renal parenchyma after injury is IL-6 dependent and promotes renal injury. (A) Few peritubular neutrophils are present at baseline in IL-6+/+ mice. Accumulation of neutrophils in peritubular capillaries at the inner cortex and outer medulla accompanied by neutrophilic extravasation into the renal interstitium is evident 24 h after HgCl2 administration. Peritubular accumulation of neutrophils in IL-6−/− mice after HgCl2 administration was significantly diminished compared with IL-6+/+ mice but not significantly different than in naïve IL-6+/+ mice. Quantification of renal neutrophilic infiltration in IL-6+/+ and IL-6−/− mice 24 h after HgCl2 administration is shown. Data are means ± SEM. *P ≅ 0.04 (n = 7) versus other groups. (B) Macrophage infiltration after AKI is not IL-6 dependent. Staining and quantification of renal macrophages in the inner cortex in IL-6+/+ and IL-6−/− mice 24 h after HgCl2 administration. Data are mean ± SEM. *P < 0.05 (n = 7) versus other groups. (C) Effect of neutrophil depletion on HgCl2-induced AKI. BUN levels in antineutrophil serum (□), control serum (▪), and untreated (baseline; □) are shown. Data are means ± SEM. *P ≅ 0.003 and ≅ 0.01 versus control serum + HgCl2–treated and untreated mice, respectively. HPF, high-power field. Magnification, ×200.
In the Absence of IL-6R, IL-6 Trans-Signaling but not Classical Signaling Mediates a Protective Response to Kidney Injury
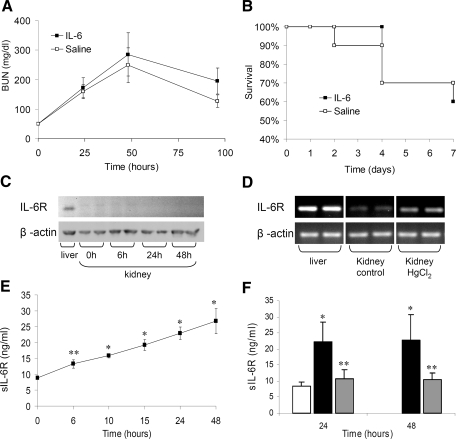
IL-6 is known to have pleiotropic effects, participating in both inflammatory processes and tissue protection.19–23 We therefore questioned whether pretreatment of mice with IL-6 would affect HgCl2-induced AKI. Mice treated with IL-6 before HgCl2 administration displayed BUN levels and a mortality rate similar to those of saline-treated control mice (Figure 4, A and B). Prolonged exposure to IL-6 by hydrodynamics-based in vivo transfection of the IL-6 expression plasmid phAAT-IL-6 did not affect HgCl2-induced AKI (data not shown).
Figure 4.
The absence of IL-6R expression in murine kidney precludes protection by IL-6 treatment, but sIL-6R is elevated after AKI. (A) Pretreatment with exogenous human IL-6 fails to protect mice from HgCl2-induced AKI. Renal function as indicated by BUN levels measured in mice treated with human IL-6 protein (20 μg, intravenously) or normal saline 4 h before administration of HgCl2 (6 mg/kg). Data are means ± SEM of surviving mice. P = NS, IL-6 treated versus control (n = 10). (B) Kaplan-Meier plot of survival in mice treated in A. Log rank test, P = NS. (C) Western blot analysis of IL-6R from normal murine liver and kidney and from kidney after HgCl2. The blots were stripped and reprobed for β-actin protein as a loading control. (D) IL-6R mRNA analysis by RT-PCR of RNA extracts from normal liver, normal kidney, and kidney tissue 6 h after HgCl2 administration. RT-PCR analysis of β-actin mRNA is shown for comparison. OD analysis: 141.7 ± 2.9 (liver), 28.8 ± 3.4 (normal kidney), and 58.7 ± 2.8 (kidney after HgCl2); P ≅ 0.008 for normal kidney versus kidney after HgCl2. (E) Serum sIL-6R ELISA analysis from HgCl2-treated (6 mg/kg) mice. Data are means ± SEM (n = 6 [48 h] to 12 mice [24 h]).*P < 0.00001, **P ≅ 0.002 versus 0 h. (F) Effect of neutrophil depletion on sIL-6R production after HgCl2-induced AKI. sIL-6R ELISA analysis on serum samples from antineutrophil serum (□) and control serum–treated mice (▪) after HgCl2 administration and from untreated mice (□) is shown. Data are means ± SD. *P ≅ 0.01 versus other groups; **P ≅ 0.01 versus control serum and P = NS versus untreated mice.
Because responsiveness to IL-6 via the classical IL-6 signaling pathway depends on the expression of IL-6R on the target cell, we examined IL-6R expression in the renal parenchyma. Western blot and reverse transcriptase–PCR (RT-PCR) analyses of tissue extracts showed that the IL-6R protein and mRNA are expressed at very low levels in the normal mouse kidney in comparison with the liver, which was used as a positive control (Figure 4C). Six hours after treatment with HgCl2, a slight but clear increase in IL-6R mRNA was evident; however, no change in the level of IL-6R protein was apparent at this or later times (Figure 4, C and D). Thus, the absence of IL-6R in the kidney precludes the activation of gp130 in the renal parenchyma via the classical IL-6 signaling pathway.
To account for the remarkable increase in STAT3 activation after renal injury (Figure 1, B and D), we postulated that gp130 signaling through a mechanism of sIL-6R–mediated trans-signaling may occur. Analysis of serum sIL-6R levels revealed a three-fold increase in sIL-6R during the 48 h after HgCl2 administration (Figure 4E), which, together with the concurrent rise in IL-6 expression (Figure 1A), strongly points to the presence of IL-6 trans-signaling after kidney injury. Receptor shedding by activated neutrophils has recently been attributed as being a significant source of sIL-6R production.24 Neutrophil depletion before HgCl2 administration significantly diminished the sIL-6R levels (Figure 4F), suggesting that the rise in sIL-6R levels during HgCl2-induced injury is substantially due to IL-6R shedding by neutrophils.
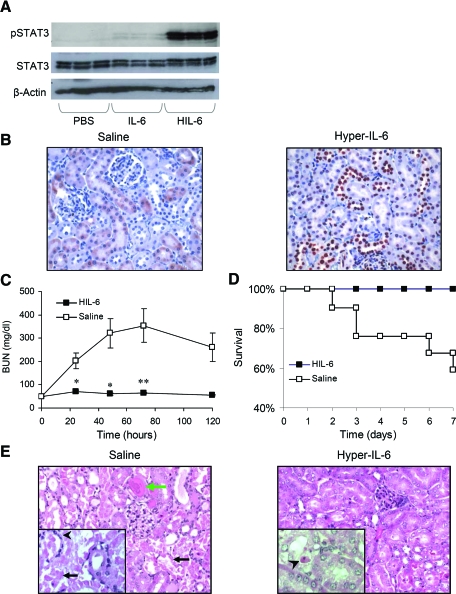
To determine the effect of IL-6 trans-signaling on the kidney in the absence of injury, we administered to mice an injection of the IL-6/sIL-6R fusion protein Hyper-IL-6 (HIL-6).25 Analysis of renal STAT3 activation as a marker of IL-6 signaling clearly demonstrated that treatment with HIL-6, as opposed to IL-6, strongly stimulates gp130 signaling in the kidney (Figure 5A). pSTAT3 immunostaining after HIL-6 treatment revealed substantial nuclear staining in epithelial cells of the distal and proximal renal tubules (Figure 5B).
Figure 5.
HIL-6 treatment protects mice from HgCl2-induced AKI. (A) Western blot analysis of p-STAT3 (Tyr 705) and STAT3 in the kidney after treatment with PBS, IL-6 (20 μg), or HIL-6 (4 μg) demonstrates the effect of HIL-6 on gp130 stimulation in the kidney. (B) pSTAT3 (Tyr705) immunostaining of kidney sections 1 h after HIL-6 treatment reveals strong STAT3 activation (expressed as nuclear staining), particularly in the distal tubules, medulla and glomeruli (strong staining), and proximal tubules (mild staining) (pSTAT3-positive tubular epithelial cells 307.7 ± 36.3 versus 0.0 ± 0.0 per HPF for HIL-6–and saline-treated mice, respectively; P ≅ 0.007). (C) Renal function in mice treated with HIL-6 or normal saline 4 h before administration of HgCl2. Data are means ± SEM. *P ≅ 0.002; **P ≅ 0.005 (n = 10). (D) Kaplan-Meier survival plot of mice treated as in B; log rank test, P ≅ 0.007, HIL-6 (n = 19) versus saline (n = 21). (E) Hematoxylin and eosin staining of paraffin-embedded renal tissue shows histologic changes 24 h after HgCl2 administration in mice pretreated with normal saline or HIL-6 (8 μg, intravenously) 4 h before HgCl2 administration. Saline-pretreated mice show extensive necrosis of tubular epithelial cells (black arrows), dilation of the tubular lumina filled with proteinaceous material (green arrow), and accumulation of neutrophils in the peritubular capillaries (arrowhead). HIL-6–pretreated mice display subtle tubular injury with occasional apoptotic tubular epithelial cells (arrowhead). Magnifications: ×400 in B and ×200 in E, inset; ×400 in E.
We next assessed the effect of HIL-6 on the development of AKI. Mice treated with HIL-6 protein before HgCl2 administration were dramatically resistant to AKI (serum creatinine at 48 h 1.55 ± 0.684 mg/dl [n = 13] versus <0.5 mg/dl in saline- and HIL-6–treated mice, respectively; P = 0.001; Figure 5C). Moreover, whereas HgCl2-treated mice displayed significant mortality, all mice treated with HIL-6 survived (Figure 5D). Morphologic analysis of renal tissue 24 h after HgCl2-induced AKI (Figure 5E) revealed extensive necrosis of proximal tubules and widespread areas with signs of tubular injury (Table 1). In contrast, the HIL-6–treated mice exhibited significantly less evidence of tubular injury, presenting with mild focal tubular dilation and occasional apoptotic epithelial cells.
Table 1.
Histological analysis of control and HIL-6–treated micea
| Parameter | HgCl2(n = 5) | HgCl2 + HIL-6(n = 5) | P |
|---|---|---|---|
| No injury (%) | 23.6 ± 6.4 | 55.3 ± 20.7 | <0.010 |
| Reversible injury (%) | 42.5 ± 7.0 | 33.8 ± 19.1 | NS |
| Necrosis (%) | 34.0 ± 10.3 | 11.3 ± 2.4 | <0.001 |
Renal histology is expressed as percentage area examined presenting the corresponding finding. Data are means ± SD.
HIL-6 Induces Antioxidant Factors in the Kidney
The mechanism of HgCl2-induced ATN involves oxidative stress, leading to apoptosis and necrosis of the proximal tubular epithelial cells.26 To elucidate the mechanism of HIL-6–induced protection, we analyzed the effect of HIL-6 on the expression of antiapoptotic and oxidative stress–related factors. RT-PCR analysis of kidney RNA revealed that whereas gp130 mRNA was constitutively expressed in the kidney, SOCS3 mRNA was strongly induced by HIL-6 but not by IL-6 (Supplemental Figure 2). In contrast, no substantial changes were observed in the mRNA or protein levels of antiapoptotic factors Bcl-2, Bcl-xL, or FLIP after any of the treatments tested.
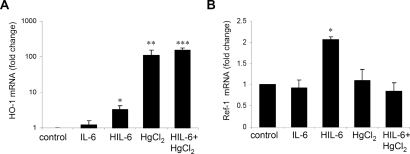
The nephrotoxicity of HgCl2 is largely attributed to the generation of reactive oxygen species.13 In response to oxidant injury, mammalian cells are known to upregulate a number of redox-responsive genes, including heme oxygenase (HO-1) and apurinic endonuclease/redox effector factor (Ref-1).27–29 HIL-6 treatment strongly upregulated HO-1 mRNA (approximately 3.5-fold; Figure 6A). HO-1 was also strongly upregulated by HgCl2 (approximately 112-fold) and substantially more in mice administered HIL-6 and HgCl2 treatments (approximately 155-fold) despite the dramatic reduction in renal injury. Ref-1 mRNA expression increased two-fold after treatment with HIL-6 (Figure 6B); however, contrary to our expectations, it was unaffected after HgCl2 administration, with or without HIL-6 treatment.
Figure 6.
Effect of HIL-6 and IL-6 on the expression of oxidative stress response genes in normal and HgCl2-treated mice. (A) Real-time PCR analysis of HO-1 mRNA. *P ≅ 0.0006 versus control, **P ≅ 0.002; versus control; ***P ≅ 0.0001 versus control and P ≅ 0.03 versus HgCl2, (n = 4 to 7). (B) Real-time PCR analysis of Ref-1 mRNA. *P = 0.0002 versus other groups (n = 4 to 7).
To determine the importance of HO-1 to HIL-6–induced protection, we analyzed the effect of HIL-6 in the presence of the specific competitive inhibitor of HO-1 activity, tin mesoprotoporphyrin (SnMP). Administration of SnMP, however, did not prevent the HIL-6–generated resistance to HgCl2-induced injury (BUN at 24 h 103 ± 43, 47 ± 10, and 44 ± 10 mg/dl [n = 8] in saline-treated, HIL-6–treated, and HIL-6+SnMP-treated mice, respectively; P < 0.003 for HIL-6 versus saline P = 0.54 for HIL-6 versus HIL-6+SnMP). As a positive control for the effect of SnMP, we tested whether SnMP would block HIL-6 protection of glycerol-induced abrupt rhabdomyolysis, in which HO-1 is essential to renal protection.29 Mice transfected with the HIL-6 expression plasmid phAAT-HIL-6 were dramatically resistant to glycerol-induced AKI, and SnMP significantly blocked the protective effect of HIL-6 (Supplemental Figure 3). SnMP also strongly exacerbated renal injury in control transfected mice, although SnMP per se was not nephrotoxic (data not shown). Thus, HIL-6–induced resistance to glycerol- but not HgCl2-induced AKI is HO-1 dependent.
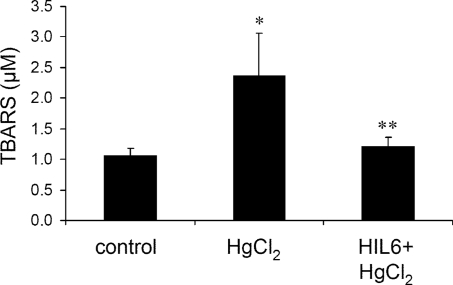
These results suggested that HIL-6–induced protection against AKI is largely mediated by mechanisms that reduce oxidative stress. Measurement of the lipid peroxidation end product malondialdehyde (MDA) by thiobarbituric acid reactive substances analysis did not show a significant increase in MDA levels in kidney extracts after HgCl2-induced injury (data not shown), in agreement with previous studies performed on mice.30,31 In contrast, HgCl2 significantly elevated serum MDA levels (Figure 7), which strongly correlated with levels of AKI (correlation coefficient r = 0.9521, P < 0.0001). HIL-6 treatment maintained serum MDA at nearly baseline levels after HgCl2-induced injury, indicating that prevention of oxidative stress may be an important mechanism through which HIL-6 ameliorates renal injury.
Figure 7.
HIL-6 prevents HgCl2-induced oxidative stress. Thiobarbituric acid reactive substances analysis of serum samples from mice 48 h after HgCl2-induced injury and mice treated with HIL-6 4 h before HgCl2 administration. Data are as means ± SD. *P ≅ 0.0002, HgCl2 (n = 13) versus control (n = 6); **P ≅ 0.0002, HIL-6+HgCl2 (n = 8) versus HgCl2.
DISCUSSION
Recent studies have shown a strong correlation between IL-6 expression and AKI. The novel findings of this study indicate that IL-6 mediates two functions during the induction of AKI: A cytokine-dependent cell-mediated immune response that exacerbates renal injury and a protective response in tubular epithelial cells that ameliorates injury and maintains renal function.
The results of this study demonstrate that IL-6 is critical to the inflammatory response to renal injury. The salient observations supporting this conclusion are, first, that IL-6 deficiency diminishes neutrophil accumulation after injury and renders mice relatively resistant to injury, and, second, that neutrophil depletion in wild-type mice significantly reduced HgCl2-induced injury. In this respect, ischemia-induced AKI is similar to both HgCl2- and endotoxemia-induced AKI regarding neutrophil infiltration but dissimilar regarding the contribution of neutrophils to injury.16,17,32,33 Together, these observations support the notion that local IL-6 expression is an intrinsic element in the cell-mediated inflammatory response that contributes significantly to HgCl2-induced AKI (Figure 8A).
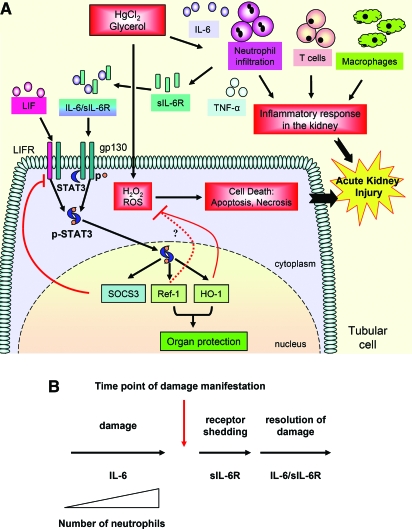
Figure 8.
Schematic view of the role of IL-6 and gp130 signaling in AKI. (A) IL-6 stimulates an immune-mediated inflammatory response involving neutrophil infiltration to the renal parenchyma that ultimately exacerbates renal injury. Stimulation of gp130 by IL-6 trans-signaling and leukemia inhibitory factor (LIF) or by therapeutic intervention with HIL-6 induces resistance to injury in tubular epithelial cells by activation of STAT3 and upregulation of redox-related genes including HO-1 and Ref-1. HO-1 is essential for protection against glycerol-induced but not HgCl2-induced AKI. These proteins may collaborate to prevent oxidative stress and reduce renal injury. ROS, reactive oxygen species. (B) A model for the dual role of gp-130 signaling in AKI. IL-6 promotes neutrophil infiltration via membrane-bound IL-6R and exacerbates renal injury. Neutrophils can release their membrane-bound IL-6R by shedding and promote protection by gp130 trans-signaling. This provides the basis of the dual role of IL-6 and IL-6/sIL-6R in tissue damage and protection.
Whereas in other examples of organ failure IL-6 induces resistance to injury,20,34–36 this study and previous reports37 showed that IL-6 treatment does not prevent AKI. As shown here, the lack of IL-6R expression in the kidney precludes an IL-6–mediated protective response through the classical signaling pathway. We postulated that STAT3 activation observed after injury in the absence of IL-6R expression occurs through a mechanism of IL-6 trans-signaling. Support for this notion is found in the three-fold increase in serum sIL-6R levels after renal injury, which may result through receptor shedding by infiltrating neutrophils, as shown previously6,24 and as indicated by neutrophil depletion studies performed here. Stimulation of trans-signaling using HIL-6 dramatically reduced renal injury and maintained renal function, demonstrating that IL-6 trans-signaling functions to induce protection in response to renal injury. Multiple factors may participate simultaneously to induce gp130 signaling and activate STAT3 after renal injury, including IL-11 and leukemia inhibitory factor, which are also induced after renal injury.4,38 This study provides the first demonstration that gp130 signaling and STAT3 activation in the kidney function to ameliorate AKI. That similar findings were observed in two independent and dissimilar models of AKI suggest that IL-6 trans-signaling produces a broad protective response to various types of injury.
To understand the mechanism(s) by which gp130 activation prevents renal injury, we examined two molecular pathways associated with HgCl2-induced renal injury: Apoptosis and oxidative stress. The notion that amelioration of renal injury may be controlled through antiapoptotic factors is appealing because it is known that IL-6 and STAT3 upregulate antiapoptotic factors, including Bcl-2 and Bcl-xL,39,40 which are reportedly upregulated after exposure to HgCl2.13 In this study, a clear effect on these antiapoptotic factors was not observed at early times after either HIL-6 or HgCl2 administration, although such effects cannot be ruled out at later times.
Oxidative stress is a cardinal element of HgCl2-induced renal injury, leading to lipid peroxidation41 and upregulation of redox-sensitive genes.13,42–44 HIL-6 significantly upregulated both redox-sensitive genes analyzed here, HO-1 and Ref-1; however, their importance in the protective effect has only been partially elucidated. Our results show that HIL-6–mediated protection to glycerol-induced AKI but not HgCl2-induced AKI largely depends on HO-1 activity. This is consistent with the importance of HO-1 in AKI, as previously reported,13,29 and indicates that alternative factors must be responsible for the HIL-6–mediated protection against HgCl2-induced injury. Whether Ref-1 is essential for the HIL-6–induced protective response has yet to be determined. HIL-6 treatment also dramatically reduced the levels of lipid peroxidation, indicating that induction of antioxidative stress–related factors may be of particular importance in the protective mechanism. Taken together, these results suggest that gp130-mediated protection against AKI is largely mediated by mechanisms that ameliorate oxidative stress.
This study shows that IL-6 and gp130 signaling is an important physiologic response to renal injury. We propose a general model (Figure 8B) in which renal injury induces local and systemic elevation of IL-6 that promotes neutrophilic infiltration and exacerbates renal injury. The neutrophils can release their membrane-bound IL-6R,24 which, via IL-6 trans-signaling, activates STAT3 in the renal epithelial cells. Trans-signaling functions to render protection against oxidative stress and further injury in the surrounding tissue and promotes resolution of injury. This may represent a universal dual role of IL-6 classical and trans-signaling in other types of tissue injury as well. Because in many cases AKI is a consequence of an unrelated therapeutic intervention or the result of a clinically related episode, it can, in practice, be anticipated and is therefore potentially preventable. This study directly supports the conclusion that an HIL-6–based therapeutic strategy protects the kidney from injury and can facilitate maintenance of renal function during stress.
CONCISE METHODS
Reagents
All chemicals were from Sigma-Aldrich Chemicals (St. Louis, MO), unless otherwise stated. Recombinant human IL-6 was from PeproTech (Rocky Hill, NJ). HIL-6 produced by a stably transfected CHO cell line was purified as described previously.25 A fresh aliquot was used for each experiment. SnMP was purchased from Frontier Scientific (Logan, UT).
Animals
Male BALB/c and C57BL/6 mice (19 to 21 g) were purchased from Harlan Laboratories (Jerusalem, Israel). IL-6−/− mice and TNF−/− mice, both on a C57BL/6 background, were bred in-house from breeding pairs originally purchased from the Jackson Laboratory (Bar Harbor, ME).45,46 Weight-matched C57BL/6 control mice were used in all experiments involving IL-6−/− or TNF-α−/− mice. BALB/c nu/nu mice and SCID/bg were purchased from Harlan UK (Blackthorn, Bicester, Oxon, England). Procedures and maintenance (see Supplemental Concise Methods) were performed in accordance with the Institutional Animal Care and Use Committee–approved animal treatment protocol (license no. OPRR-A01-5011).
Mercury-induced AKI was induced by intraperitoneal injection of a freshly prepared solution of HgCl2 (6 mg/kg) dissolved in PBS.41 Glycerol-induced AKI was induced in anesthetized (Isoflurane) BALB/c mice by injection of 50% glycerol, at a total dosage of 8 ml/kg body wt, one-half dose injected into the anterior thigh muscle of each hind leg. HO-1 was inhibited by administration of SnMP,13 as described in the Supplemental Concise Methods.
Neutrophil Depletion
Circulating neutrophils were depleted by intraperitoneal injection of 0.2 ml of rabbit antineutrophil serum (Accurate Chemical and Scientific Corp, Westbury, NY.) 24 h before HgCl2 administration.47 This procedure removed 83 ± 17% of polymorphonuclear cells from the peripheral circulation. Previous studies reported that treatment with this antibody does not have a significant effect on other leukocyte subpopulations in the peripheral circulation.47,48
In Vivo DNA Transfection
Animals were treated by hydrodynamics-based in vivo plasmid DNA transfection49,50 with phAAT-IL-6 (10 μg), phAAT-HIL-6 (2.5 μg), or a control plasmid, pGEM-7 (20 μg). Details of plasmid DNA construction and preparation are in the Supplemental Concise Methods.
Determination of BUN and Creatinine
Blood samples were obtained by tail-vein bleeding. BUN levels and creatinine were determined in heparinized serum using the Reflotron system and Urea or Creatinine test strips (Roche Diagnostics, Basel, Switzerland).
Histologic and Immunohistochemical Analysis
Kidneys were removed 24 to 48 h after HgCl2 administration and fixed in 4% buffered formaldehyde, followed by 80% ethanol, and embedded in paraffin blocks. Tissue sections were stained with hematoxylin and eosin. Neutrophils were stained with rat anti-mouse neutrophil antibody (Serotec, Oxford, England) diluted 1:3000 followed by biotinylated rabbit anti-rat (Dako, Glostrup, Denmark), and developed with horseradish peroxidase (HRP)-streptavidin (Invitrogen, Carlsbad, CA) using 3-amino-9-ethyl-carbazol (AEC) (Dako). Phospho-STAT3 was stained using monoclonal rabbit anti-mouse pSTAT3 (Tyr 705; Cell Signaling, Danvers, MA) diluted 1:500, followed by biotinylated goat anti-rabbit (Jackson Laboratory) diluted 1:5000, amplified using Tyramide Signal Amplification kit (PerkinElmer, Boston, MA) and developed with AEC. Macrophages were stained using rat anti-mouse F4/80 antigen (Serotec) diluted 1:200, followed by anti-rat HRP Histofine (Nichirei, Tokyo, Japan) and developed with AEC. CD3+ cells were stained using rat anti-human CD3 antigen (Serotec) diluted 1:200, followed by biotinylated rabbit anti-rat diluted 1:50 and developed with HRP-streptavidin using AEC. The number of positively stained cells per high-power field (Magnification, ×400) by immunohistochemistry was counted in 20 fields for each sample from coded specimens. Morphologic analysis of tubular injury was performed as described in the Supplemental Concise Methods.
Western Blot Analysis
Protein extracts were prepared from tissue samples (approximately 100 mg) by homogenization in 1 ml of whole-cell lysis buffer (1% NP-40, 10 mM Tris [pH 7.8], 150 mM NaCl, 40 mM EDTA, 10 mM Na-pyrophosphate, 10 mM NaF, 1 mM PMSF, 4 mM orthovanadate, 1 μg/ml pepstatin A, and 2 μg/ml leupeptin). Protein extracts for analysis of IL-6R were prepared by homogenization in m-PER buffer (Pierce, Rockford, IL). Protein extracts (50 μg) were separated by PAGE and subjected to Western blot analysis. For analysis of murine IL-6R, Western blots were probed with goat anti-mouse IL-6R antibody (R&D Systems, Minneapolis, MN) followed by rabbit anti-goat antibody (Zymed) and anti-rabbit HRP polymer (Dako) and developed using the ECL-Plus Western blotting Detection System (GE Healthcare, Upsala, Sweden). For analysis of pSTAT3 and STAT3, Western blots were probed with mouse monoclonal anti-phosphorylated STAT3 (sc-8059) and mouse monoclonal anti-STAT3 (sc-8019; Santa Cruz Biotechnology, Santa Cruz, CA), respectively. As a loading control, the blots were stripped with 0.1 M glycine (pH 2.8) and reprobed with a monoclonal anti–β-actin antibody, clone AC-74 (Sigma), and developed with HRP Envision (Dako).
IL-6 and sIL-6R ELISA
IL-6 and sIL-6R levels were determined using a mouse IL-6 and a mouse sIL-6R DuoSet ELISA kit (R&D Systems) according to the manufacturer's instructions on serum samples that were collected and frozen at −20°C until analysis.
RNA Extraction, RT-PCR, and Real-Time PCR Analysis
RNA samples were prepared from approximately 100 mg of snap-frozen tissue by homogenization in Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions using a Polytron high-speed homogenizer, followed by treatment with DNA-free DNase (Ambion, Austin, TX). The primer sequences and details for RT-PCR and real-time PCR analysis are provided in the Supplemental Concise Methods.
Lipid Peroxidation Analysis
MDA, an end product of lipid peroxidation, was analyzed in serum and tissue samples by measurement of thiobarbituric acid reactive substances as described previously.51,52 A detailed description is provided in the Supplemental Concise Methods.
Statistical Analyses
Mortality data were compared using the Kaplan-Meier survival procedure and the log rank (Mantel-Cox) test to compare equality of survival distributions, with P ≤ 0.05 considered statistically significant. Comparison of mean serum creatinine levels was performed using a nonparametric Mann-Whitney test, in which all values less than the level of detection (<0.5 mg/dl) were arbitrarily set at 0.5 mg/dl. All other comparisons were subjected to the t test, with P ≤ 0.05 considered statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
These studies were supported by a grant from the Israel Science Foundation (Jerusalem, Israel; ISF 853/04) to J.H.A. and E.G. and by a grant from the Deutsche Forschungsgemeinschaft (Bonn, Germany; SFB415) to S.R.J. E.G. is supported by the Israeli Ministry of Science through a grant to the National Gene Therapy Knowledge Center and through EC grants LSHB-CT-2004-512034 and LSHB-CT-2005-018961. E.G. and J.H.A. are also supported by grants from the Blum, the Harold Grinspoon, the Horowitz, and the Wolfson Foundations.
Parts of this study were presented at the American Society of Nephrology Renal Week Conference; November 14 through 19, 2006; San Diego, CA.
We thank Amnon Peled for invaluable advice, Deborah Olam and Carol Levy for expert assistance in the care and breeding of IL-6–deficient mice, Ido Weiss and Hadas Shoham for TNF-α–deficient mice, Ariel Orfaig for assistance with computer graphics, and Tally Bdolah-Abraham for assistance with statistical analyses.
Published online ahead of print. Publication date available at www.jasn.org.
Y.N.-A. and D.B. contributed equally to this study.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Brady HR, Brenner BM: Acute renal failure. In: Harrison's Principles of Internal Medicine, 16th Ed., edited by Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, New York, McGraw-Hill, 2005, pp 1644–1653
- 2.Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA: Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 65: 1357–1365, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Vaidya VS, Shankar K, Lock EA, Dixon D, Mehendale HM: Molecular mechanisms of renal tissue repair in survival from acute renal tubule necrosis: Role of ERK1/2 pathway. Toxicol Pathol 31: 604–618, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Lemay S, Rabb H, Postler G, Singh AK: Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation 69: 959–963, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Gadient RA, Patterson PH: Leukemia inhibitory factor, Interleukin 6, and other cytokines using the GP130 transducing receptor: Roles in inflammation and injury. Stem Cells 17: 127–137, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM: The soluble interleukin 6 receptor: Mechanisms of production and implications in disease. FASEB J 15: 43–58, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Shalaby MR, Waage A, Espevik T: Cytokine regulation of interleukin 6 production by human endothelial cells. Cell Immunol 121: 372–382, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L, Stern D: Induction of interleukin 6 (IL-6) by hypoxia in vascular cells: Central role of the binding site for nuclear factor-IL-6. J Biol Chem 270: 11463–11471, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Sekiyama KD, Yoshiba M, Thomson AW: Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol 98: 71–77, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacGowan GA, Mann DL, Kormos RL, Feldman AM, Murali S: Circulating interleukin-6 in severe heart failure. Am J Cardiol 79: 1128–1131, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Peters M, Muller AM, Rose-John S: Interleukin-6 and soluble interleukin-6 receptor: Direct stimulation of gp130 and hematopoiesis. Blood 92: 3495–3504, 1998 [PubMed] [Google Scholar]
- 12.Rose-John S, Heinrich PC: Soluble receptors for cytokines and growth factors: Generation and biological function. Biochem J 300: 281–290, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nath KA, Croatt AJ, Likely S, Behrens TW, Warden D: Renal oxidant injury and oxidant response induced by mercury. Kidney Int 50: 1032–1043, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Friedewald JJ, Rabb H: Inflammatory cells in ischemic acute renal failure. Kidney Int 66: 486–491, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, De Broe ME: T cells as mediators in renal ischemia/reperfusion injury. Kidney Int 66: 491–496, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, Cuzzocrea S, Thiemermann C: Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther 312: 1170–1178, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Singbartl K, Bockhorn SG, Zarbock A, Schmolke M, Van Aken H: T cells modulate neutrophil-dependent acute renal failure during endotoxemia: Critical role for CD28. J Am Soc Nephrol 16: 720–728, 2005 [DOI] [PubMed] [Google Scholar]
- 18.De Greef KE, Ysebaert DK, Persy V, Vercauteren SR, De Broe ME: ICAM-1 expression and leukocyte accumulation in inner stripe of outer medulla in early phase of ischemic compared to HgCl2-induced ARF. Kidney Int 63: 1697–1707, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H: T cell activation-associated hepatic injury: Mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med 179: 1529–1537, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camargo CA Jr, Madden JF, Gao W, Selvan RS, Clavien PA: Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology 26: 1513–1520, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Yoshizawa K, Naruto M, Ida N: Injection time of interleukin-6 determines fatal outcome in experimental endotoxin shock. J Interferon Cytokine Res 16: 995–1000, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Galun E, Zeira E, Pappo O, Peters M, Rose-John S: Liver regeneration induced by a designer human IL-6/sIL-6R fusion protein reverses severe hepatocellular injury. FASEB J 14: 1979–1987, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hecht N, Pappo O, Shouval D, Rose-John S, Galun E, Axelrod JH: Hyper-IL-6 gene therapy reverses fulminant hepatic failure. Mol Ther 3: 683–687, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Chalaris A, Rabe B, Paliga K, Lange H, Laskay T, Fielding CA, Jones SA, Rose-John S, Scheller J: Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood 110: 1748–1755, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Fischer M, Goldschmitt J, Peschel C, Brakenhoff JP, Kallen KJ, Wollmer A, Grotzinger J, Rose-John S: I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol 15: 142–145, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Stacchiotti A, Borsani E, Rodella L, Rezzani R, Bianchi R, Lavazza A: Dose-dependent mercuric chloride tubular injury in rat kidney. Ultrastruct Pathol 27: 253–259, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG: Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev 55: 551–571, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Evans AR, Limp-Foster M, Kelley MR: Going APE over ref-1. Mutat Res 461: 83–108, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Vogt BA, Shanley TP, Croatt A, Alam J, Johnson KJ, Nath KA: Glomerular inflammation induces resistance to tubular injury in the rat: A novel form of acquired, heme oxygenase-dependent resistance to renal injury. J Clin Invest 98: 2139–2145, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka T, Nishiyama Y, Okada K, Hirota K, Matsui M, Yodoi J, Hiai H, Toyokuni S: Induction and nuclear translocation of thioredoxin by oxidative damage in the mouse kidney: Independence of tubular necrosis and sulfhydryl depletion. Lab Invest 77: 145–155, 1997 [PubMed] [Google Scholar]
- 31.Farina M, Brandao R, de Lara FS, Pagliosa LB, Soares FA, Souza DO, Rocha JB: Profile of nonprotein thiols, lipid peroxidation and delta-aminolevulinate dehydratase activity in mouse kidney and liver in response to acute exposure to mercuric chloride and sodium selenite. Toxicology 184: 179–187, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY: Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL: Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110: 1083–1091, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B: Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology 40: 933–941, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA: Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol 22: 535–542, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Dawn B, Xuan YT, Guo Y, Rezazadeh A, Stein AB, Hunt G, Wu WJ, Tan W, Bolli R: IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res 64: 61–71, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Homsi E, Ribeiro-Alves MA, Lopes de Faria JB, Dias EP: Interleukin-6 stimulates tubular regeneration in rats with glycerol-induced acute renal failure. Nephron 92: 192–199, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Yoshino J, Monkawa T, Tsuji M, Hayashi M, Saruta T: Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J Am Soc Nephrol 14: 3090–3101, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, Okuyama T, Takeda K, Akira S, Ogino T, Irani K, Ozaki M: Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest 112: 989–998, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R: Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem 276: 26605–26613, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Nava M, Romero F, Quiroz Y, Parra G, Bonet L, Rodriguez-Iturbe B: Melatonin attenuates acute renal failure and oxidative stress induced by mercuric chloride in rats. Am J Physiol Renal Physiol 279: F910–F918, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Guillermina G, Adriana TM, Monica EM: The implication of renal glutathione levels in mercuric chloride nephrotoxicity. Toxicology 58: 187–195, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Gstraunthaler G, Pfaller W, Kotanko P: Glutathione depletion and in vitro lipid peroxidation in mercury or maleate induced acute renal failure. Biochem Pharmacol 32: 2969–2972, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Bohets HH, Van Thielen MN, Van der Biest I, Van Landeghem GF, D'squosquo; apos; yHaese PC, Nouwen EJ, De Broe ME, Dierickx PJ: Cytotoxicity of mercury compounds in LLC-PK1, MDCK and human proximal tubular cells. Kidney Int 47: 395–403, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G: Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368: 339–342, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G: Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med 184: 1397–1411, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norman KE, Cotter MJ, Stewart JB, Abbitt KB, Ali M, Wagner BE, Wallace WA, Forlow SB, Hellewell PG: Combined anticoagulant and antiselectin treatments prevent lethal intravascular coagulation. Blood 101: 921–928, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Johnston B, Issekutz TB, Kubes P: The alpha 4-integrin supports leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo. J Exp Med 183: 1995–2006, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu F, Song Y, Liu D: Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 6: 1258–1266, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Zhang G, Budker V, Wolff JA: High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther 10: 1735–1737, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Esterbauer H, Cheeseman KH: Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186: 407–421, 1990 [DOI] [PubMed] [Google Scholar]
- 52.Buege JA, Aust SD: Microsomal lipid peroxidation. Methods Enzymol 52: 302–310, 1978 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.