Abstract
In addition to its classical role in calcium-phosphate homeostasis, vitamin D has anti-inflammatory effects that may influence vascular disease. This study examined the impact of vitamin D levels on the vascular phenotype in 61 children who had been on dialysis for ≥3 mo and in 40 age-matched control subjects. All dialysis patients were prescribed daily oral 1-α hydroxyvitamin D3. 92% of patients were deficient in 25-hydroxyvitamin D [25(OH)D]. 1,25-dihydroxyvitamin D [1,25(OH)2D] levels were low in 36% and high in 11% of patients. There was a weak correlation between 1α-hydroxyvitamin D3 dosage and 1,25(OH)2D levels. Both carotid intima-media thickness and calcification scores showed a U-shaped distribution across 1,25(OH)2D levels: patients with both low and high 1,25(OH)2D had significantly greater carotid intima-media thickness (P < 0.0001) and calcification (P = 0.0002) than those with normal levels. Low 1,25(OH)2D levels were associated with higher high-sensitivity C-reactive protein (P < 0.0001). Calcification was most frequently observed in patients with the lowest 1,25(OH)2D and the highest high-sensitivity C-reactive protein. In contrast, 25(OH)D levels did not correlate with any vascular measure. In conclusion, both low and high 1,25(OH)2D levels are associated with adverse morphologic changes in large arteries, and this may be mediated through the effects of 1,25(OH)2D on calcium-phosphate homeostasis and inflammation. For optimization of strategies to protect the vasculature of dialysis patients, careful monitoring of 1,25(OH)2D levels may be required.
Cardiovascular disease is the most common cause of death in patients with chronic kidney disease (CKD).1 Structural and functional abnormalities and calcification in the large vessels begin as early as the first decade of life,2,3 contributing to a 30-fold higher mortality1 than in the age-matched population. In patients with CKD, vitamin D deficiency and disorders in mineral metabolism associated with secondary hyperparathyroidism are the most important causes of vascular damage and calcification4–7 and are also associated with abnormal bone turnover states; this is called CKD-mineral and bone disorder.8
Because the failing kidney cannot convert the “nutritional” 25-hydroxyvitamin D3 [25(OH)D] to the biologically active or “hormonal” form 1,25-dihydroxyvitamin D3 [1,25(OH)2D],4 1,25(OH)2D deficiency is widely prevalent in patients with CKD.9,10 Thus, vitamin D analogues are routinely used, but both vitamin D deficiency and supplementation with vitamin D analogues have been implicated as potential risk factors in the development and progression of vascular disease.6,7,11–16
Vitamin D analogues increase calcium (Ca) and also phosphate (PO4) absorption and suppress parathyroid hormone (PTH) secretion via the vitamin D receptor on the parathyroid gland.4,17,18 Inadequate 1,25(OH)2D levels would be expected, therefore, to result in low plasma Ca and hyperparathyroidism, whereas high 1,25(OH)2D levels cause hypercalcemia, hyperphosphatemia, and oversuppression of PTH. In turn, high bone turnover (as a result of hyperparathyroidism) results in an efflux of Ca and PO4 from the bones into the soft tissues,4 whereas low bone turnover (as a result of oversuppression of PTH) results in an inability of the bone to buffer fluxes in serum Ca and PO4.13,17,18 Thus, it is known that optimal 1,25(OH)2D levels are required to manipulate PTH so as to achieve effective parathyroid gland suppression and allow a normal bone turnover, but the effects of 1,25(OH)2D on the vasculature are poorly understood.
In addition to its endocrine effects, vitamin D has important autocrine/paracrine roles: The vitamin D receptor is ubiquitous19 and, importantly, is present in the vascular smooth muscle cells (VSMC),20 cardiomyocytes,9,21 and cells of the monocyte/macrophage lineage.22 1,25(OH)2D has important anti-inflammatory and immunomodulatory effects.15,23,24 Because inflammation plays a key role in vascular damage,25,26 vitamin D may have antiatherogenic effects. Thus, although vitamin D exerts potentially deleterious procalcific effects on the vasculature, its anti-inflammatory properties may confer a significant cardioprotective benefit. In this study, we hypothesize that 1,25(OH)2D levels in the normal range are associated with less vascular damage and calcification in children on dialysis.
RESULTS
The clinical and biochemical characteristics of the patient and control groups are shown in Tables 1 and 2. Of the 61 patients (37 boys), 39 had renal dysplasia, nine had inherited nephropathies, five had cystic kidney disease, four had primary renal tubular disorders, three had renovascular disorders, and one had Wilms' tumor.
Table 1.
Demographic, clinical, anthropometric, and biochemical characteristics of patients and control subjectsa
| Characteristic | Patients (n = 61) | Control Subjects (n = 40) | P |
|---|---|---|---|
| Age (yr; mean ± SD) | 13.4 ± 4.1 | 14.4 ± 3.8 | 0.2900 |
| Gender (male/female) | 37/24 | 22/18 | 0.1900 |
| Race (white/Asian/black/other) | 37/12/9/3 | 27/11/2/ 0 | – |
| eGFR (ml/min per 1.73 m2; mean ± SD) | 8.9 ± 8.0 | 121.0 ± 5.8 | <0.0001 |
| Time in stage 4 CKD (yr; median [range]) | 4.9 (0.2 to 6.8) | – | – |
| Time on dialysis (yr; median [range]) | 1.10 (0.25 to 8.70) | – | – |
| Dialysis modality (PD/HD) | 43/18 | – | – |
| BMI SDS (mean ± SD) | −0.5 ± 1.6 | 0.9 ± 0.9 | <0.0001 |
| SBP index (mean ± SD)b | 1.3 ± 0.3 | 0.9 ± 0.1 | 0.0200 |
| No. on antihypertensive medications | 11 | 0 | – |
| No. on ACEi or ARB | 2 | 0 | – |
| Hemoglobin (g/dl; mean ± SD) | 11.7 ± 1.5 | 13.3 ± 1.1 | 0.0700 |
| Albumin (g/L; mean ± SD) | 38.0 ± 3.0 | 41.0 ± 0.6 | 0.2200 |
| Total cholesterol (mmol/L; mean ± SD) | 4.0 ± 1.1 | 3.3 ± 1.3 | 0.1000 |
| Triglycerides (mmol/L; mean ± SD) | 1.2 ± 1.2 | 0.9 ± 2.2 | 0.6700 |
| No. on statins | 2 | 0 | – |
| Diabetes | 0 | 0 | – |
| Smokers | 0 | 1 | – |
ARB, angiotensin II receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; BMI, body mass index; SBP, systolic BP; SDS, SD score.
BP index = measured BP/95th centile BP for age, gender, and height.
Table 2.
Serum Ca, PO4, and PTH levels and dosages of PO4 binders and alphacalcidol in patients and control subjectsa
| Parameter | Patients (n = 61)
|
Control Subjects (n = 40) | Pb | |
|---|---|---|---|---|
| Exposure from the Onset of Stage 4 CKD | Values at the Time of Study | |||
| Serum PO4 level (mmol/L; mean ± SD) | 1.5 ± 0.7c | 1.4 ± 0.5 | 0.9 ± 0.3 | 0.007 |
| Serum Ca (albumin adjusted; mmol/L; mean ± SD) | 2.4 ± 0.1c | 2.4 ± 0.0 | 2.3 ± 0.2 | 0.190 |
| Serum Ca-PO4 product (mmol2/L2; mean ± SD) | 4.2 ± 0.9c | 4.3 ± 0.4 | 3.3 ± 0.3 | 0.002 |
| Serum iPTH (fold ULN; mean ± SD) | 1.8 ± 1.3c | 1.6 ± 0.9 | ND | – |
| PO4 binders | ||||
| Patients on Ca-based PO4 binders (n [%]) | NA | 52 (88) | – | – |
| Patients on sevelamer ± Ca-based PO4 binders (n [%]) | 9 (12) | – | ||
| Intake of elemental Ca from PO4 binders (g/kg per yr; mean ± SD) | 31.9 ± 11.8d | NA | 0 | – |
| dosage (mg/day; mean ± SD) | NA | 1828 ± 52 | 0 | – |
| Alphacalcidol (μ /kg per yr; mean ± SD) | 19.2 ± 7.3d | NA | 0 | – |
| dosage (μ g/d; mean ± SD) | NA | 0.89 ± 0.3 | 0 | – |
| Parathyroidectomy | 0 | 0 | 0 | – |
ND, not done.
Compares values at the time of the study between patients and control subjects.
All biochemical values in this column are expressed as mean time-averaged values from the onset of stage 4 CKD.
The dosage of elemental calcium intake from PO4 binders and the alphacalcidol dosage are expressed as the cumulative intake from stage 4 CKD and standardized per year of exposure to account for the varied duration since onset of stage 4 CKD.
Vitamin D Levels in Patients and Control Subjects
Levels of 25(OH)D were low in the majority of patients as well as control subjects (Table 3). Despite that all of the patients were prescribed daily oral alphacalcidol, 1,25(OH)2D levels were low (<40 pmol/L) in 22 (36%) and high (>150 pmol/L) in 11 (18%) patients. 1,25(OH)2D levels showed a weak correlation with both the alphacalcidol dosage at the time of the study (P = 0.06, r = 0.25) and the mean time-averaged alphacalcidol dosage (P = 0.06, r = 0.19). No correlation was found between 25(OH)D and 1,25(OH)2D levels (P = 0.4, r = 0.11).
Table 3.
Comparison of vascular measures and vitamin D levels between dialysis and control groups
| Parameter | Dialysis (n = 61) | Control Subjects (n = 40) | P |
|---|---|---|---|
| cIMT (mm; mean ± SD) | 0.48 ± 0.17 | 0.38 ± 0.01 | 0.0010 |
| Aortic PWV (m/s; mean ± SD) | 7.7 ± 1.0 | ND | – |
| No. with cardiac calcification (%) | 13 (21) | ND | |
| Agatston score (median [range]) | 141.2 (0.0 to 2039.0) | – | – |
| coronary arteries | 12 | – | |
| valves | 3 | – | |
| aorta | 2 | – | |
| 25(OH)D (ng/ml; mean ± SD)a | 13.3 ± 10.9 | 28.2 ± 9.9 | <0.0001 |
| <10 (n [%]) | 30 (50) | 0 | |
| 10 to 30 (n [%]) | 26 (42) | 29 (72) | |
| >30 (n [%]) | 5 (8) | 11 (27) | |
| 1,25(OH)2D (pmol/L; median [range]) | 10 (10 to 216) | ND | |
| <40 (n [%]) | 22 (36) | ||
| 40 to 150 (n [%]) | 28 (46) | ||
| >150 (n [%]) | 11 (18) |
To convert 25(OH)D levels to nmol/L, multiply by 2.5.
Vitamin D Levels and Their Clinical and Biochemical Correlations
Patients on peritoneal dialysis (PD; n = 43, 70%) had significantly lower 1,25(OH)2D levels than those on hemodialysis (HD; 46.7 ± 18.8 versus 68.2 ± 35.7 pmol/L; P = 0.02, r = 0.31); 53% of PD and 24% of HD patients had low 1,25(OH)2D levels. There was no correlation with serum albumin levels in the overall cohort, but PD patients with low 1,25(OH)2D had lower serum albumin at the time of the study (35.2 ± 8.0 versus 41.1 ± 3.1; P = 0.04) than those with 1,25(OH)2D in the normal or high range.
1,25(OH)2D showed a linear correlation with both the Ca-PO4 product at the time of the study (P = 0.02, r = 0.28) as well as the mean time-averaged Ca-PO4 product (P = 0.03, r = 0.22; Table 2). Patients with high 1,25(OH)2D had more hypercalcemic episodes (15 versus 6%; P = 0.02). As classically observed, the mean time-averaged intact PTH (iPTH) level inversely correlated with 1,25(OH)2D [P = 0.02, r = 0.36 for mean time-averaged iPTH versus 1,25(OH)2D levels and P = 0.003, r = 0.41 for iPTH levels at the point of study versus 1,25(OH)2D levels]. No correlation was found between 25(OH)D or 1,25(OH)2D levels and the serum Ca, PO4, or alkaline phosphatase measured at any time point. Neither 25(OH)D nor 1,25(OH)2D showed any correlation with the patients' age, gender, race, dialysis vintage, BP, or body mass index. There was no seasonal variation in the levels.
Vitamin D Levels and Correlations with Vascular Measures
Both low and high levels of 1,25(OH)2D were associated with abnormal vascular measures: Patients with 1,25(OH)2D in the normal range had carotid intima-media thickness (cIMT) levels comparable to those in the control subjects (Table 3), but those with 1,25(OH)2D <40 or >150 pmol/L had significantly higher cIMT (P < 0.0001 for quadratic term; Figure 1A). The cardiac calcification score showed a similar relationship to 1,25(OH)2D: Calcification was seen in eight (36%) of 22 patients with 1,25(OH)2D levels <40 pmol/L, five (45%) of 11 patients with levels >150 pmol/L, and only one (3.6%) of 28 patient with levels in the normal range (P = 0.0002 for quadratic term; Figure 1B). No association was found between 1,25(OH)2D and pulse wave velocity (PWV) or between 25(OH)D and any of the vascular measures.
Figure 1.
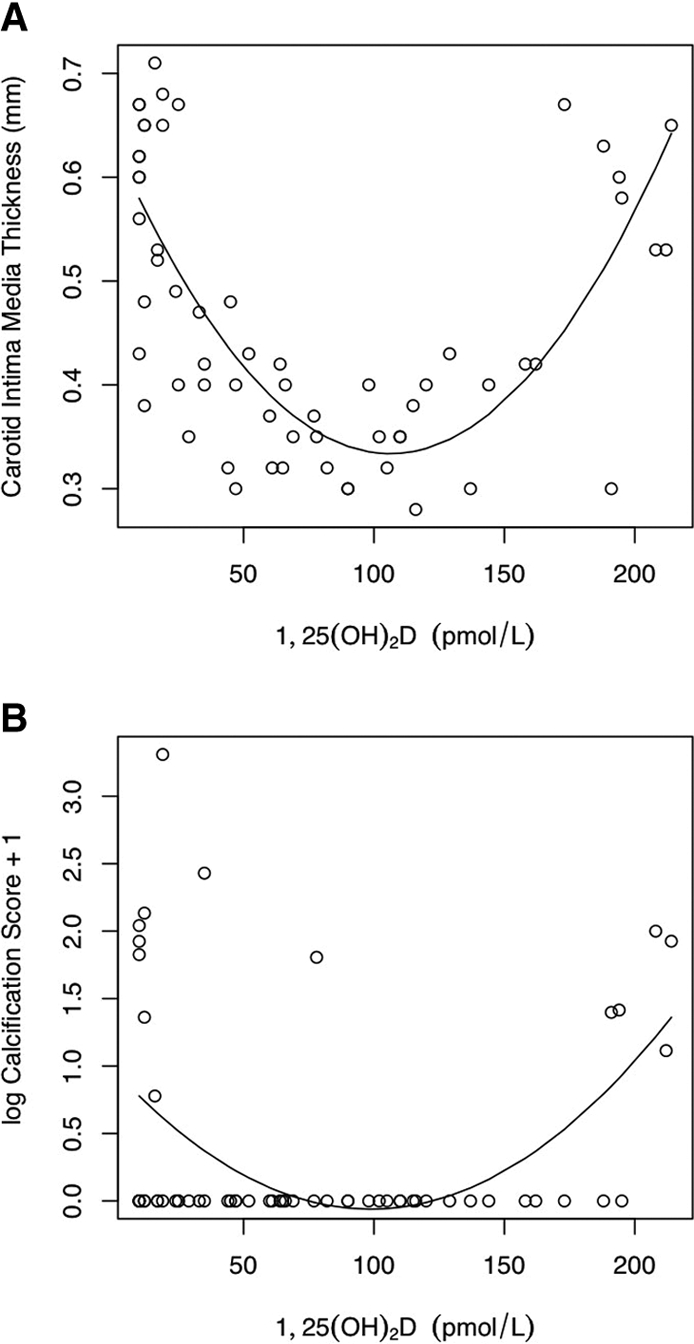
(A) The cIMT showed a significant quadratic relationship with 1,25(OH)2D levels (P < 0.0001). (B) The cardiac calcification score showed a similar significant quadratic relationship (P = 0.0002).
Vitamin D Levels and Correlations with Inflammation
The high-sensitivity C-reactive protein (hs-CRP) level was higher in patients than in control subjects (median 9.3 [range 1.2 to 53] versus 0.43 mg/L [0.1 to 5.02]; P < 0.00001, r = 0.73) and higher in HD than in PD patients (17.9 [1.7 to 54.4] versus 3.2 mg/L [0.3 to 23]; P = 0.01, r = 0.52). None of the patients had active infections at the time of the study. The hs-CRP was significantly higher in patients with calcification (15.3 [0.5 to 54.4] versus 1.35 mg/L [0.3 to 32]; P < 0.0001, r = 0.25; Figure 2) and was independent of age, dialysis vintage, BP, body mass index, lipid profile, cIMT, or PWV. An inverse correlation was seen between 1,25(OH)2D and hs-CRP (P < 0.0001, r = −0.29; Figure 3). Both the prevalence of calcification and the highest calcification scores were found in patients with a combination of low 1,25(OH)2D levels and high hs-CRP, whereas patients with 1,25(OH)2D levels in the normal range had the lowest incidence of calcification (Figure 4). There was no interaction between 1,25(OH)2D and hs-CRP (P = 0.11). There was no association between 25(OH)D and hs-CRP.
Figure 2.

The hs-CRP level was higher in the patients than in the control subjects (P < 0.00001, r = 0.73) and was highest in the patients with calcification than in those without calcification on CT scan (P < 0.0001, r = 0.25). Error bars represent the median and range for each group.
Figure 3.
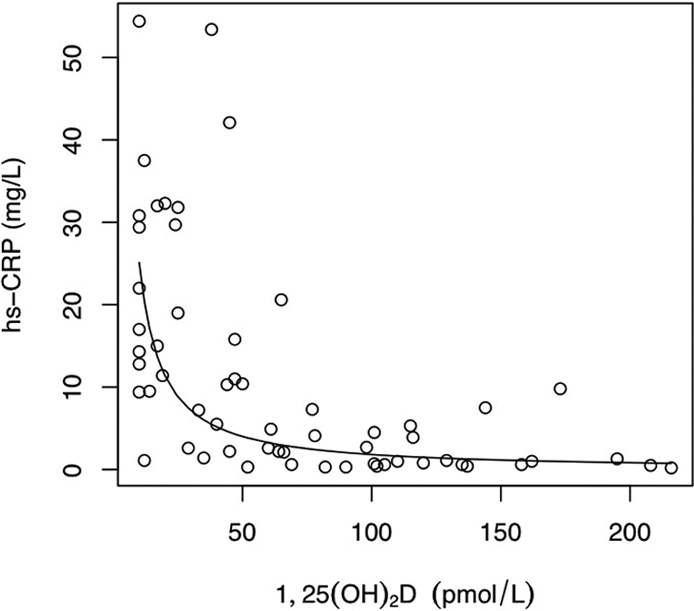
There was an inverse correlation between 1,25(OH)2D and hs-CRP levels (P < 0.0001, r = −0.29). The regression line was fitted on the log-log scale and is shown back-transformed.
Figure 4.
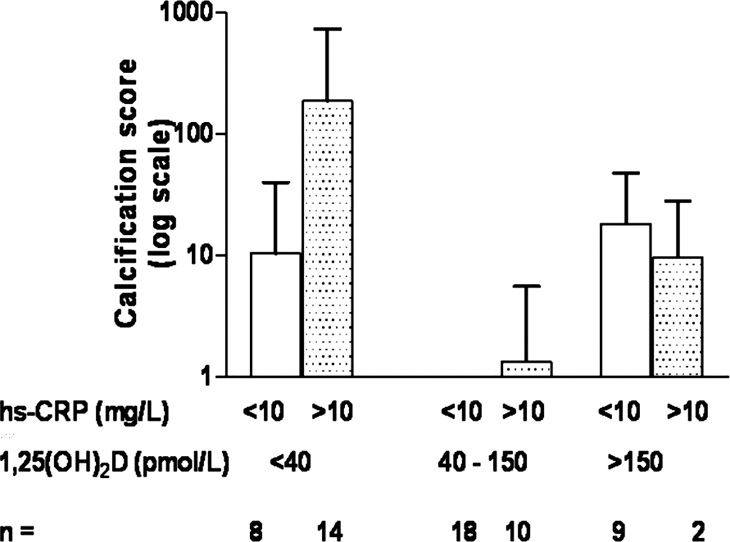
Both the prevalence of calcification and the highest calcification scores were found in patients with a combination of low 1,25(OH)2D levels and high hs-CRP, whereas patients with 1,25(OH)2D levels in the normal range had the lowest incidence of calcification.
Predictors of cIMT and Calcification
In addition to the strong quadratic relationship between cIMT and 1,25(OH)2D and between the calcification score and 1,25(OH)2D, we found univariate associations between cIMT and the mean time-averaged Ca-PO4 product (r = 0.38, P < 0.001), calcification score and the mean time-averaged Ca-PO4 product (r = 0.27, P = 0.02), and calcification score and iPTH levels (r = 0.18, P = 0.04). Significant predictors of the outcome variables, cIMT and calcification score, from univariate analyses were entered into stepwise multiple linear regression analyses (Table 4). 1,25(OH)2D was an independent predictor of cIMT, whereas both 1,25(OH)2D and hs-CRP were significant predictors of the calcification score.
Table 4.
Multivariate analysis for predictors of cIMT and calcification score
| Variables | βa | SE | P | Model R2b |
|---|---|---|---|---|
| cIMT | 57% | |||
| 1,25(OH)2D level | −2.49 | 0.001 | <0.0001 | |
| square of 1,25(OH)2D level (quadratic coefficient) | <0.001 | <0.0001 | ||
| mean time-integrated Ca-PO4 product | 0.32 | 0.090 | 0.0730 | |
| Calcification scorec | 16% | |||
| 1,25(OH)2D level | −9.10 | 4.200 | 0.0400 | |
| square of 1,25(OH)2D level (quadratic coefficient) | 0.04 | 0.020 | 0.0400 | |
| hs-CRP | 11.60 | 4.700 | 0.0200 | |
| mean time-integrated Ca-PO4 product | 10.20 | 1.700 | 0.1600 | |
| iPTH levels | 5.20 | 2.600 | 0.4800 |
Unstandardized regression coefficient; indicates the difference in the outcome variable (cIMT or calcification score) per unit change in the independent variables.
The amount of variance in the dependent variable that can be explained by the model.
Analyzed as loge(calcification score + 1).
DISCUSSION
This is the first study to show that both low and high levels of 1,25(OH)2D are associated with abnormal vascular structure and calcification, possibly through a dual effect on Ca-PO4 homeostasis and inflammation. A significant number of children on dialysis have low levels of 1,25(OH)2D despite daily alphacalcidol supplements, and given the narrow therapeutic window for vitamin D analogues on vascular health, careful monitoring of 1,25(OH)2D levels is recommended in the dialysis population.
A total of 92% of our patients had 25(OH)D deficiency, and studies have shown that the majority of patients with CKD have low 25(OH)D levels9,27; however, unlike other studies in which the patients were naive to vitamin D sterols, all of our patients were prescribed daily alphacalcidol. The “nutritional” form, 25(OH)D, is unaffected by alphacalcidol treatment, so we also measured 1,25(OH)2D, the “hormonal,” or “active,” form of vitamin D produced by 25-hydroxylation of alphacalcidol in the liver,17,18 and found that 36% of the children had low 1,25(OH)2D. Reduced levels of 1,25(OH)2D despite treatment may be due to a loss of albumin-bound vitamin D in the peritoneum, as suspected in our patients, or nonconcordance with treatment. None of our patients had malabsorption syndromes or liver disorders to suggest poor absorption or reduced enzymatic conversion of alphacalcidol. Despite the strong inverse correlation between inflammation and vitamin D and the higher inflammatory status of HD patients, we found lower vitamin D levels in the PD cohort, suggesting that a loss of albumin-bound vitamin D did indeed play a major role. Earlier work from our group showed that in children on dialysis, the dosage of alphacalcidol influenced cIMT and calcification,3 but the lack of a consistent dosage-response relationship as shown in this study suggests that adjustments in the alphacalcidol dosage alone may not be sufficient for optimal management.
The effects of high vitamin D dosages on vascular calcification are widely recognized in clinical studies3,16,28–30 and in vitro work,20,31–33 and this study confirms an increased cIMT and calcification in patients with high 1,25(OH)2D levels. Vitamin D analogues may induce calcification by a number of mechanisms that include enhancing the gastrointestinal absorption of Ca and PO4, oversuppression of PTH leading to adynamic bone disease,13,17,18 and a direct effect on VSMC.20,31–33 An early study by Milliner et al.34 showed that, at autopsy, 60% of children with CKD had soft tissue calcification and 36% had systemic calcinosis, and use of any vitamin D analogue showed the strongest independent association with calcinosis. In addition, both 25(OH)D and 1,25(OH)2D can have a direct effect on the VSMC: 1,25(OH)2D upregulates the vitamin D receptor and induces cellular calcium uptake,31 decreases VSMC proliferation,20 and induces VSMC migration and osteoblastic conversion of the VSMC.32,33 Recent observational studies by Litwin et al.,29 Civilibal et al.,28 and Mitsnefes et al.30 and our own work3 have shown that a high vitamin D dosage adversely affects cIMT3,29,30 and calcification.3,28 Newer vitamin D analogues, such as paricalcitriol and doxercalciferol, are shown to be less calcemic35,36 but have only a marginal survival advantage over calcitriol,11,12 and their effects on the vasculature have not as yet been studied.
Despite the clearly deleterious calciotropic effects of vitamin D, in this study, low levels of 1,25(OH)2D were also associated with increased cIMT and calcification. Low 1,25(OH)2D results in an increase in PTH levels that can promote soft tissue calcification through its effect on calcium absorption and an efflux of Ca and PO4 from a high-turnover bone state.13,17,18 Our findings are supported by two studies of adult HD patients that demonstrated a approximately 20% survival advantage of any vitamin D formulation over no vitamin D treatment.11,12 In a small cohort of Japanese HD patients, Shoji et al.37 showed that the use of 1-α hydroxyvitamin D3 was associated with a 70% lower risk for death from cardiovascular disease as compared with a group not on any vitamin D supplements. London et al.38 recently showed that in a cohort of adult dialysis patients who all were naive to vitamin D analogues, low 25(OH)D and 1,25(OH)2D were associated with greater vessel stiffness and reduced brachial artery distensibility. In an earlier study, we showed that children who were on dialysis and had PTH levels more than two-fold the upper limit of normal (ULN) had higher cIMT, PWV, and calcification than those with PTH levels less than two-fold the ULN3; however, as seen in this study, 1,25(OH)2D levels were associated with cIMT and calcification independent of PTH, suggesting that the biologic consequences of 1,25(OH)2D extend beyond the regulation of Ca-PO4 homeostasis alone.
Anti-inflammatory actions of vitamin D22,26 may also contribute to its effects on the vasculature but have not been explored in clinical studies. Interestingly, we found a strong inverse correlation between hs-CRP and 1,25(OH)2D levels that was associated with vascular calcification but no association between 25(OH)D and hs-CRP. 1-α Hydroxylase is expressed on many cell types such as macrophages, endothelial cells, and dendritic cells, where it acts in an autocrine/paracrine manner independent of the PTH–bone axis and is unaffected by renal failure but may be regulated by immune stimuli. In vitro studies as well as studies in other inflammatory disease states, such as rheumatoid arthritis,39 have shown that vitamin D can influence various aspects of inflammation,40 including inhibition of antigen-presenting cell maturation, downregulation of NF-κB, and modulation of cytokine production to create an anti-inflammatory environment22,41 (increased IL-10 and decreased IL-6, IL-12, and TNF-α), but this is the first clinical study that has found an association between 1,25(OH)2D levels and inflammation; however, it is also possible that inflammation leads to low vitamin D levels. In a cross-sectional study, it would be impossible to discern a cause–effect relationship between inflammation-malnutrition and vitamin D levels, but given the results of in vitro studies that have shown a causal effect of vitamin D on inflammation, it is likely that low vitamin D levels contribute to the proinflammatory milieu in dialysis patients. Studies of patients without renal failure have shown that vitamin D supplementation can suppress serum TNF-α and increase IL-10 levels42: TNF-α promotes atherosclerosis and IL-10 has antiatherogenic properties. Vitamin D also has direct cardioprotective effects such as an antiproliferative effect on cardiomyocytes21 and negative endocrine regulation of the renin-angiotensin system.43
In this cross-sectional study, an association between vitamin D levels and vascular phenotype does not necessarily indicate a cause–effect relationship; however, this study serves as a starting point to stimulate cell biology work and generate hypotheses for randomized, controlled studies of the effects of vitamin D analogues on cardiovascular health. We were unable to find a correlation between hs-CRP levels and cIMT, suggesting that either the study population was too small to demonstrate an effect or inflammation has a greater influence on calcification than on the mechanisms involved in vessel thickening. Unlike the study by London et al.,38 we did not find a correlation between vitamin D levels and vessel stiffness. The greater plasticity of children's vessels and their shorter dialysis vintage may allow for compensatory mechanisms that can maintain normal vessel function in the face of early structural damage to the vessel. Vessels from children provide an ideal model to study uremic influences on the arterial wall, because they do not have the confounding proatherosclerotic risk factors that are prevalent in the adult CKD population.
In conclusion, we have shown, for the first time, that both low and high levels of 1,25(OH)2D are associated with adverse morphologic changes in the large arteries and that the vascular damage may be determined by the effects of vitamin D on Ca-PO4 homeostasis and inflammation. Given the narrow therapeutic window for vitamin D analogues on vascular health, optimal vascular protective strategies in dialysis patients may require careful monitoring of not only the vitamin D dosage but also 1,25(OH)2D levels.
CONCISE METHODS
Study Design
From January 2005 to December 2006, 61 consecutive children (5 to 18 yr) who had been on dialysis for ≥3 mo were recruited. Levels of 25(OH)D, 1,25(OH)2D and hs-CRP were measured and related to cIMT, PWV, and the presence of coronary and valvular calcification on multislice computed tomography (CT) scan. Patients were compared with healthy age- and gender-matched school children who underwent vascular scans in our department as part of a parallel study investigating normal levels of cIMT and PWV in healthy children. From this cohort, we included 40 consecutive children aged 5 to 18 yr with no known medical illnesses or family history of heart disease or diabetes to serve as control subjects in our study, in a 1.5:1 patient:control subject ratio. Of note, the majority of children in the control group were overweight, although no intentional selection bias was involved. Informed written consent was obtained from all parents or caregivers and from children when appropriate. The study was approved by the local research ethics committee.
In our center PD is offered to all children as the initial dialysis modality unless specific contraindications such as recent intra-abdominal surgery, peritoneal infections, or difficult family circumstances preclude it. As a result, our group of patients on HD are often older and have a longer dialysis vintage. All patients in this study were prescribed daily oral 1-α hydroxycholecalciferol (alphacalcidol), titrating the dosage so as to keep the iPTH level less than two-fold the ULN. Hyperphosphatemia was managed by dietary PO4 restriction and calcium-based PO4 binders. Sevelamer, a newer non–calcium-based PO4 binder, was used only for patients with hypercalcemia and persistently high iPTH levels.
Data Collection
Serum Ca, PO4, and iPTH levels and the dosages of elemental calcium intake from phosphate binders and alphacalcidol were recorded at monthly intervals from the start of stage 4 CKD, and mean time-averaged values were calculated (the sum total of the monthly values for each variable was divided by the number of months of exposure to provide a mean time-averaged value). Given the variable amounts of time spent in stage 4 CKD, the biochemical values and medication dosages at the time of the study are expressed separately in Table 2. Because the iPTH assay has changed over time, iPTH is expressed as multiples of the ULN. The number of hypercalcemic episodes (defined as albumin-adjusted serum Ca levels >2.5 mmol/L and expressed as a percentage of the total number of albumin-adjusted serum Ca measurements in each patient) was calculated. In control subjects, a single blood test at the time of the scans was performed.
Vitamin D Levels and hs-CRP
All biochemical data were determined on blood samples taken before the vascular scans and before a midweek session of HD but at varying times throughout the year. There was an interval of 10 to 12 h between alphacalcidol intake and blood sampling. Serum 25(OH)D and 1,25(OH)2D levels were measured by a single technician using the enzyme immunoassay (IDS-OCTEIA 25-HydroxyvitaminD EIA kit; Immunodiagnostics Systems Ltd., Tyne and Wear, UK) and RIA (1,25 dihydroxyvitamin D 125I RIA kit; DiaSorin, Stillwater, MN), respectively. 1,25(OH)2D levels in 120 healthy normal children have been measured and validated in our laboratory (unpublished data), so we measured 25(OH)D levels only in the 40 control subjects. hs-CRP levels were determined by ELISA (EIA test kit; MP Biomedicals, Orangebury, NY) for all patients and control subjects.
Carotid Ultrasonography for cIMT
B-mode ultrasound of both common carotid arteries was performed using a 12-MHz linear array transducer (Vivid 7; GE Medical, Horton, Norway). Longitudinal two-dimensional images of the vessel 1 to 2 cm proximal to the carotid bulb were acquired on the R wave of the electrocardiogram (ECG), frozen in diastole, and analyzed off-line. The cIMT was calculated as the distance between the leading edge of the lumen-intima interface and the media-adventitia interface on the far wall of the artery.
Applanation Tonometry for PWV
Applanation tonometry in the carotid and femoral arteries was performed with a micromanometer (SPC-301; Millar Instruments, Houston, TX) and recorded directly onto a computer running proprietary software (SphygmoCor version 7.0; Scanmed, Gloucestershire, UK). An ECG gated signal and standard distance measurements were used. PWV was computed from contour analysis of 10 consecutive pressure waveforms recorded consecutively in the carotid and femoral arteries to give the aortic PWV. PWV was not measured in the control subjects because permission was not granted for femoral artery measurements.
Multislice-Spiral CT for Cardiac Imaging
Sixteen-slice spiral CT scans were performed (Somatom Sensation 16; Siemens Medical Solutions, Erlangen, Germany) using the standard Ca scoring protocol. Prospective ECG triggering was performed to obtain image acquisition in diastole, using 60% of the RR interval for image reconstruction. The Agatston score44 for each main epicardial coronary artery, cardiac valves, and aorta and an overall score for each patient were determined. In the control group, CT was not undertaken because of radiation concerns.
Statistical Analyses
Results are presented as means ± SD or median and range, depending on the distribution. Comparisons between groups were made using t test or the Mann-Whitney U test as appropriate, and correlations were tested using Pearson or Spearman correlation tests for parametric and nonparametric data, respectively. The two main outcome variables were cIMT and calcification score [transformed to log10(CS + 1) to adjust for skewness]. Factors affecting the two outcome variables were explored using multiple regression analysis. From univariate analyses, variables with P < 0.15 were entered into the stepwise multiple regression analyses. The nonlinear effect of vitamin D levels was fitted by including a quadratic term: The outcome variables were regressed on 1,25(OH)2D and the square of 1,25(OH)2D, and the significance of the squared (quadratic) term was noted. A third outcome measure, hs-CRP, was analyzed after log transformation to account for skewness, and its correlation with 1,25(OH)2D was strengthened when that, too, was log-transformed. The interaction between 1,25(OH)2D and hs-CRP was tested by two way ANOVA. P ≤ 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 12.0.1 (SPSS, Chicago, IL).
DISCLOSURES
None.
Acknowledgments
This study was funded by the Kidney Research Aid Fund, and funding for Court scans was obtained from the Eyck and Strutt scholarship from the British Medical Association. R.S. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
We gratefully acknowledge Dr. Atul Singhal, Childhood Nutrition Research Centre, Institute of Child Health, London, for contributing data on the control subjects.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Kari JA, Donald AE, Vallance DT, Bruckdorfer KR, Leone A, Mullen MJ, Bunce T, Dorado B, Deanfield JE, Rees L: Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int 52: 468–472, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Shroff R, Donald A, Hiorns M, Watson A, Feather S, Milford D, Ellins E, Storry C, Ridout D, Deanfield J, Rees L: Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18: 2996–3003, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Rostand SG, Drueke TB: Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 56: 383–392, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Raggi P, Chasan-Taber S, Bommer J, Holzer H, Burke SK: Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant 19: 1489–1496, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith D, Ritz E, Covic A: Vascular calcification: a stiff challenge for the nephrologist: Does preventing bone disease cause arterial disease? Kidney Int 66: 1315–1333, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Zittermann A: Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol 92: 39–48, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG: Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int 70: 1858–1865, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Brown AJ, Dusso AS, Slatopolsky E: Vitamin D analogues for secondary hyperparathyroidism. Nephrol Dial Transplant 17[Suppl 10]: 10–19, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Querfeld U: Is atherosclerosis accelerated in young patients with end-stage renal disease? The contribution of paediatric nephrology. Nephrol Dial Transplant 17: 719–722, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Towler DA: Calciotropic hormones and arterial physiology: “D”-lightful insights. J Am Soc Nephrol 18: 369–373, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Goldsmith DJ, Covic A, Sambrook PA, Ackrill P: Vascular calcification in long-term haemodialysis patients in a single unit: A retrospective analysis. Nephron 77: 37–43, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Dusso AS, Brown AJ, Slatopolsky E: Vitamin D. Am J Physiol Renal Physiol 289: F8–F28, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Feldman D, Glorieux FH, Pike JW: Vitamin D, 2nd Ed., Elsevier Academic Press, 2005
- 19.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW: The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J Bone Miner Res 13: 325–349, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Carthy EP, Yamashita W, Hsu A, Ooi BS: 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension 13: 954–959, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Xiang G, Seki T, Schuster MD, Witkowski P, Boyle AJ, See F, Martens TP, Kocher A, Sondermeijer H, Krum H, Itescu S: Catalytic degradation of vitamin D up-regulated protein 1 mRNA enhances cardiomyocyte survival and prevents left ventricular remodeling after myocardial ischemia. J Biol Chem 280: 39394–39402, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Mathieu C, Adorini L: The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med 8: 174–179, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Tabata T, Shoji T, Kikunami K, Matsushita Y, Inoue T, Tanaka S, Hino M, Miki T, Nishizawa Y, Morii H: In vivo effect of 1 alpha-hydroxyvitamin D3 on interleukin-2 production in hemodialysis patients. Nephron 50: 295–298, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Tokuda N, Kano M, Meiri H, Nomoto K, Naito S: Calcitriol therapy modulates the cellular immune responses in hemodialysis patients. Am J Nephrol 20: 129–137, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Kopple JD: Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis 38: 1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Ross R: Atherosclerosis: An inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Civilibal M, Caliskan S, Adaletli I, Oflaz H, Sever L, Candan C, Canpolat N, Kasapcopur O, Kuruoglu S, Arisoy N: Coronary artery calcifications in children with end-stage renal disease. Pediatr Nephrol 21: 1426–1433, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Litwin M, Wuhl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Troger J, Mehls O, Schaefer F: Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16: 1494–1500, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: Role of calcium-phosphorus metabolism. J Am Soc Nephrol 16: 2796–2803, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Inoue T, Kawashima H: 1,25-Dihydroxyvitamin D3 stimulates 45Ca2+-uptake by cultured vascular smooth muscle cells derived from rat aorta. Biochem Biophys Res Commun 152: 1388–1394, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Jono S, Nishizawa Y, Shioi A, Morii H: Parathyroid hormone-related peptide as a local regulator of vascular calcification: Its inhibitory action on in vitro calcification by bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 17: 1135–1142, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Jono S, Nishizawa Y, Shioi A, Morii H: 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation 98: 1302–1306, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Milliner DS, Zinsmeister AR, Lieberman E, Landing B: Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int 38: 931–936, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Brown AJ: Vitamin D analogs for secondary hyperparathyroidism: What does the future hold? J Steroid Biochem Mol Biol 103: 578–583, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D: Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 63: 1483–1490, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y: Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant 19: 179–184, 2004 [DOI] [PubMed] [Google Scholar]
- 38.London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F: Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol 18: 613–620, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D: Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis 1. Arthritis Rheum 56: 2143–2149, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Levin A, Li YC: Vitamin D and its analogues: Do they protect against cardiovascular disease in patients with kidney disease? Kidney Int 68: 1973–1981, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R: Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83: 754–759, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Muller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, Bendtzen K: 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine 4: 506–512, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]


