Abstract
Uric acid may mediate aspects of the relationship between hypertension and kidney disease via renal vasoconstriction and systemic hypertension. To investigate the relationship between uric acid and subsequent reduced kidney function, limited-access data of 13,338 participants with intact kidney function in two community-based cohorts, the Atherosclerosis Risks in Communities and the Cardiovascular Health Study, were pooled. Mean baseline serum uric acid was 5.9 ± 1.5 mg/dl, mean baseline serum creatinine was 0.9 ± 0.2 mg/dl, and mean baseline estimated GFR was 90.4 ± 19.4 ml/min/1.73 m2. During 8.5 ± 0.9 yr of follow-up, 712 (5.6%) had incident kidney disease defined by GFR decrease (≥15 ml/min/1.73 m2 with final GFR <60 ml/min/1.73 m2), while 302 (2.3%) individuals had incident kidney disease defined by creatinine increase (≥0.4 mg/dl with final serum creatinine >1.4 mg/dl in men and 1.2 mg/dl in women). In GFR- and creatinine-based logistic regression models, baseline uric acid level was associated with increased risk for incident kidney disease (odds ratio 1.07 [95% confidence interval 1.01 to 1.14] and 1.11 [95% confidence interval 1.02 to 1.21] per 1-mg/dl increase in uric acid, respectively), after adjustment for age, gender, race, diabetes, systolic BP, hypertension, cardiovascular disease, left ventricular hypertrophy, smoking, alcohol use, education, lipids, albumin, hematocrit, baseline kidney function and cohort; therefore, elevated serum uric acid level is a modest, independent risk factor for incident kidney disease in the general population.
Hypertension and diabetes are the most common causes of chronic kidney disease (CKD) in the United States.1 Elevated levels of serum uric acid are associated with the development of hypertension and are common in individuals with diabetes and diabetes risk factors.2,3 Accordingly, uric acid, via effects on renal vasoconstriction and systemic hypertension, may mediate aspects of the relationship between hypertension and kidney disease.
Cohort studies have shown mixed results on whether uric acid is an independent risk factor for the development of kidney disease.4,5 Ishani et al.,4 evaluating long-term follow-up of the Multiple Risk Factor Intervention Trial (MRFIT), found that higher uric acid levels were associated with the development of ESRD in men but that this result was attenuated when individuals with baseline CKD (estimated GFR [eGFR] <60 ml/min per 1.73 m2 or proteinuria) were excluded. This suggests that uric acid may be a marker of diminished kidney function rather than a cause of incipient kidney disease.4 Chonchol et al.5 evaluated the Cardiovascular Health Study (CHS) population of individuals who were ≥65 yr of age and found that there was a strong cross-sectional association between baseline uric acid levels and baseline kidney function. In longitudinal analyses, individuals with uric acid levels >5.9 mg/dl had increased risk for eGFR decline ≥3 ml/min per 1.73 m2 annually; however, there was no significant association between uric acid level and incident CKD defined by eGFR <60 ml/min per 1.73 m2 at the final study examination.
In this study, we pooled individual participant data from two community-based cohorts, the Atherosclerosis Risk in Communities (ARIC) Study and CHS, to obtain a generalizable population to investigate the relationship between uric acid and incident kidney disease. By focusing on a diverse population spanning a wider age range, concentrating on individuals who have final eGFR ≤60 ml/min per 1.73 m2 and accounting for mortality, we will be able to expand on previous findings and evaluate whether serum uric acid levels are associated with incident kidney disease.
RESULTS
Among 13,338 individuals, mean age was 57.4 ± 9.0 yr and 7549 (56.6%) were female. The mean serum uric acid level was 5.9 ± 1.5 mg/dl (5.3 ± 1.4 mg/dl in women and 6.5 ± 1.3 mg/dl in men). Mean baseline serum creatinine was 0.9 ± 0.2 mg/dl, and mean baseline eGFR was 90.4 ± 19.4 ml/min per 1.73 m2 (Table 1).
Table 1.
Baseline characteristics and final kidney function by gender-specific quartile of uric acid for the creatinine-based cohorta
| Characteristic | Uric Acid Quartile
|
Totals (n = 13,338) | |||
|---|---|---|---|---|---|
| 1 (≤5.6 mg/dl in Men, ≤4.4 mg/dl in Women) (n = 3460; 25.9%) | 2 (5.6 to ≤6.4 mg/dl in Men, 4.4 to ≤5.2 mg/dl in Women) (n = 3394; 25.4%) | 3 (6.4 to ≤7.4 mg/dl in Men, 5.2 to ≤6.1 mg/dl in Women) (n = 3317; 24.9%) | 4 (>7.4 mg/dl in Men, >6.1 mg/dl in Women) (n = 3167; 23.7%) | ||
| Demographics | |||||
| age (yr; mean ± SD) | 58.1 ± 9.9 | 57.4 ± 9.1 | 57.1 ± 8.5 | 57.0 ± 8.3 | 57.4 ± 9.0 |
| female (%) | 57.4 | 58.3 | 53.3 | 57.4 | 56.6b |
| black (%) | 13.8 | 15.8 | 18.1 | 27.4 | 18.0 |
| high school graduate (%) | 82.2 | 82.3 | 81.0 | 76.2 | 80.5 |
| ARIC (%) | 73.6 | 79.9 | 83.0 | 85.3 | 80.3 |
| Medical history (%) | |||||
| diabetes | 7.0 | 5.7 | 5.7 | 7.7 | 6.5b |
| hypertension | 28.9 | 33.0 | 38.1 | 59.2 | 39.4 |
| CVD | 11.4 | 12.1 | 12.4 | 15.1 | 12.7 |
| current smoker | 22.5 | 19.8 | 18.1 | 17.0 | 19.4 |
| current alcohol use | 57.8 | 59.6 | 59.5 | 55.8 | 58.2b |
| diuretic use | 7.2 | 10.7 | 16.2 | 34.5 | 16.8 |
| ACEI use | 3.4 | 5.1 | 4.6 | 8.1 | 5.0b |
| Physical findings | |||||
| SBP (mmHg; mean ± SD) | 120.1 ± 19.2 | 121.2 ± 19.0 | 121.9 ± 18.3 | 125.7 ± 17.7 | 122.2 ± 18.7 |
| DBP (mmHg; mean ± SD) | 70.7 ± 10.6 | 71.8 ± 10.4 | 72.9 ± 10.6 | 74.9 ± 10.9 | 72.5 ± 10.7 |
| LVH (%) | 1.1 | 1.2 | 1.3 | 2.7 | 1.5 |
| BMI (kg/m2; mean ± SD) | 25.1 ± 3.8 | 26.4 ± 4.4 | 27.8 ± 4.6 | 29.9 ± 5.4 | 27.2 ± 4.9 |
| Laboratory results | |||||
| serum creatinine (mg/dl; mean ± SD) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 |
| eGFR (ml/min per 1.73 m2; mean ± SD) | 94.8 ± 19.6 | 90.8 ± 19.0 | 89.2 ± 18.7 | 86.5 ± 19.3 | 90.4 ± 19.4 |
| hematocrit (%; mean ± SD) | 41.3 ± 3.8 | 41.5 ± 3.8 | 42.0 ± 3.7 | 42.1 ± 3.9 | 41.7 ± 3.8 |
| total cholesterol (mg/dl; mean ± SD) | 208.3 ± 37.7 | 212.0 ± 39.1 | 216.1 ± 41.0 | 219.9 ± 43.0 | 213.9 ± 40.4 |
| HDL cholesterol (mg/dl; mean ± SD) | 57.0 ± 17.2 | 54.1 ± 16.7 | 50.9 ± 16.3 | 48.2 ± 15.3 | 52.7 ± 16.8 |
| albumin (g/dl; mean ± SD) | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.3 |
| Final laboratory results (mean ± SD) | |||||
| serum creatinine (mg/dl) | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.4 | 0.9 ± 0.3 |
| eGFR (ml/min per 1.73 m2) | 84.6 ± 18.3 | 82.0 ± 17.6 | 81.4 ± 17.7 | 79.3 ± 20.2 | 81.9 ± 18.6 |
| Incident kidney disease (%) | |||||
| creatinine based | 1.9 | 1.7 | 1.7 | 3.9 | 2.3 |
| eGFR based | 4.6 | 4.7 | 4.9 | 8.2 | 5.5 |
Final eGFR is based on the smaller cohort of 12,819 individuals, because more individuals were excluded for reduced baseline eGFR. Angiotensin-converting enzyme inhibitor (ACEI) use is for the CHS cohort only. Incident kidney disease defined by creatinine incorporates 13,338 individuals; incident kidney disease defined by eGFR incorporates 12,819 individuals. ARIC, Atherosclerosis Risk in Communities; CVD, cardiovascular disease; DBP, diastolic BP; LVH, left ventricular hypertrophy.
P < 0.0001 for trend for all comparisons across groups except
P < 0.01.
eGFR-Based Analyses
During mean follow-up of 8.5 ± 0.9 yr, mean baseline uric acid was 6.2 ± 1.6 mg/dl in the 712 (5.6%) individuals who developed kidney disease versus 5.8 ± 1.5 mg/dl in the 12,107 individuals who did not develop kidney disease (P < 0.0001). Baseline serum uric acid level was associated with a significantly increased risk for developing kidney disease in univariate and multivariable analyses (odds ratio [OR] 1.16 [95% confidence interval (CI) 1.11 to 1.22] and OR 1.07 [95% CI 1.01 to 1.14] per 1-mg/dl increase, respectively; Tables 2 and 3). Gender-specific models revealed an OR of 1.10 (95% CI 1.01 to 1.18) in women and 1.05 (95% CI 0.96 to 1.16) in men; however, the interaction term with gender was not statistically significant (P = 0.8).
Table 2.
Sequentially built logistic regression models for the primary study outcome of incident kidney diseasea
| Parameter | Model 1 (Uric Acid Only) | Model 2 (Model 1 + Kidney Function) | Model 3 (Model 2 + Demographic Data) | Model 4 (Model 3 + History and Labs) | Model 5 (Model 4 + SBP and Hypertension) | Model 6 (Model 5 + BMI and Diuretic Use) |
|---|---|---|---|---|---|---|
| eGFR | 1.16 (1.11 to 1.22) | 1.09 (1.04 to 1.15) | 1.12 (1.06 to 1.19) | 1.12 (1.05 to 1.18) | 1.07 (1.01 to 1.14) | 1.07 (1.01 to 1.15) |
| Creatinine | 1.29 (1.20 to 1.39) | 1.13 (1.04 to 1.22) | 1.15 (1.06 to 1.25) | 1.15 (1.06 to 1.25) | 1.11 (1.02 to 1.21) | 1.11 (1.01 to 1.22) |
OR and 95% CI are those associated with each 1-mg/dl rise in baseline serum uric acid in each model.
Table 3.
Multivariable model for incident kidney diseasea
| Parameter | Creatinine Based (OR [95% CI]) | eGFR Based (OR [95% CI]) |
|---|---|---|
| Serum uric acid (per 1-mg/dl increase) | 1.11 (1.02 to 1.21) | 1.07 (1.01 to 1.14) |
| Age (per 10-yr increase) | 1.76 (1.40 to 2.20) | 1.97 (1.69 to 2.30) |
| Female gender | 1.24 (0.87 to 1.76) | 1.17 (0.94 to 1.45) |
| Black race | 1.22 (0.89 to 1.68) | 0.85 (0.67 to 1.09) |
| History of CVD | 1.78 (1.34 to 2.36) | 1.54 (1.26 to 1.89) |
| History of diabetes | 3.17 (2.34 to 4.29) | 2.22 (1.74 to 2.84) |
| History of hypertension | 1.36 (1.01 to 1.85) | 1.42 (1.17 to 1.73) |
| Current smoking | 1.60 (1.18 to 2.18) | 1.45 (1.17 to 1.79) |
| Current alcohol | 0.80 (0.61 to 1.03) | 0.79 (0.67 to 0.94) |
| High school graduate | 0.82 (0.63 to 1.07) | 0.95 (0.78 to 1.15) |
| SBP (per 10-mmHg increase) | 1.22 (1.15 to 1.30) | 1.18 (1.13 to 1.24) |
| Total cholesterol (per 10-mg/dl increase) | 1.01 (0.98 to 1.04) | 1.01 (0.99 to 1.03) |
| HDL cholesterol (per 5-mg/dl increase) | 0.96 (0.92 to 1.01) | 1.00 (0.97 to 1.03) |
| Baseline kidney function | 1.36 (1.26 to 1.47) | 0.96 (0.96 to 0.97) |
| Serum albumin (per 1-g/dl increase) | 0.48 (0.30 to 0.76) | 0.83 (0.60 to 1.13) |
| Hematocrit (per 2% increase) | 0.93 (0.86 to 1.00) | 0.93 (0.88 to 0.98) |
| ARIC (versus CHS) | 2.56 (1.60 to 4.09) | 4.43 (3.22 to 6.07) |
Creatinine-based outcomes are defined by an increase in serum creatinine of ≥0.4 mg/dl where baseline serum creatinine was ≤1.4 mg/dl in men and ≤1.2 mg/dl in women and final serum creatinine was above these levels. eGFR-based outcomes are defined by a decrease in eGFR of ≥15 ml/min per 1.73 m2 where the initial eGFR was ≥60 ml/min per 1.73 m2 and the final eGFR was <60 ml/min per 1.73 m2. Baseline kidney function is assessed by serum creatinine in creatinine-based models with the OR reflecting each 0.1-mg/dl rise and by eGFR in eGFR-based models with OR reflecting each 10-ml/min per 1.73 m2 rise.
Creatinine-Based Analyses
There were 302 (2.3%) individuals who had a rise in serum creatinine ≥0.4 mg/dl with final serum creatinine levels >1.4 mg/dl in men and >1.2 mg/dl in women. In those who developed kidney disease, mean baseline uric acid was 6.5 ± 1.7 versus 5.9 ± 1.5 mg/dl in those who did not develop kidney disease (P < 0.0001). In univariate and multivariable analyses, baseline serum uric acid level was associated with a significantly increased risk for developing kidney disease (OR 1.29 [95% CI 1.20 to 1.39] and OR 1.11 [95% CI 1.02 to 1.21] per 1-mg/dl increase, respectively; Tables 2 and 3). Gender-specific models revealed an OR of 1.19 (95% CI 1.05 to 1.36) in women and 1.05 (95% CI 0.92 to 1.19) in men; however, the interaction term with gender was not statistically significant (P = 0.2).
Sensitivity Analyses
Among 18,966 individuals with complete baseline data and without baseline CKD, 5628 were missing serum creatinine at their final study examination. Among these individuals, 831 (14.8%) had a final examination but no laboratory results. These individuals were predominantly from CHS (n = 757, 91.1%) and had a higher prevalence of baseline cardiovascular disease (CVD; 22% versus 18%; P = 0.001) and diabetes (13% versus 9%; P < 0.01) than individuals from CHS who completed the study. There were 2915 (15.4%) individuals alive at the final study visit without data; these individuals were predominantly from ARIC (92%), were disproportionately black (35%), and had a higher prevalence of both baseline CVD and CVD risk factors than individuals who completed the study.
There were 1882 (9.9%) deaths before the final examination. Elevated uric acid was a significant risk factor for the composite outcome of death or incident kidney disease (hazard ratio [HR] 1.09 [95% CI 1.06 to 1.13] per 1-mg/dl increase in uric acid). When the eGFR-based outcome was used, there were 1668 (11.5%) deaths before the final examination. Elevated serum uric acid was a significant risk factor for the composite outcome of death or incident kidney disease (HR 1.08; 95% CI 1.05 to 1.11 per 1-mg/dl increase; Table 4).
Table 4.
Multivariable model for the composite outcome of incident kidney disease and deatha
| Parameter | Creatinine Based (HR [95% CI]) | eGFR Based (HR [95% CI]) |
|---|---|---|
| Serum uric acid (per 1-mg/dl increase) | 1.09 (1.06 to 1.13) | 1.08 (1.05 to 1.12) |
| Age (per 10-yr increase) | 2.33 (2.16 to 2.52) | 2.34 (2.17 to 2.53) |
| Female gender | 0.71 (0.63 to 0.80) | 0.77 (0.69 to 0.85) |
| Black race | 1.43 (1.27 to 1.62) | 1.32 (1.17 to 1.48) |
| History of CVD | 2.01 (1.83 to 2.21) | 1.88 (1.72 to 2.06) |
| History of diabetes | 2.16 (1.94 to 2.41) | 2.03 (1.82 to 2.62) |
| History of hypertension | 1.15 (1.03 to 1.28) | 1.16 (1.05 to 1.29) |
| Current smoking | 2.28 (2.06 to 2.52) | 2.06 (1.87 to 2.27) |
| Current alcohol | 0.88 (0.80 to 0.96) | 0.89 (0.81 to 0.97) |
| High school graduate | 0.71 (0.65 to 0.78) | 0.76 (0.69 to 0.83) |
| SBP (per 10-mmHg increase) | 1.11 (1.08 to 1.13) | 1.11 (1.09 to 1.13) |
| Total cholesterol (per 10-mg/dl increase) | 1.01 (1.00 to 1.02) | 1.01 (1.00 to 1.02) |
| HDL cholesterol (per 5-mg/dl increase) | 1.00 (0.98 to 1.01) | 1.00 (0.99 to 1.02) |
| Baseline kidney function | 1.04 (1.01 to 1.06) | 0.95 (0.93 to 0.98) |
| Serum albumin (per 1-g/dl increase) | 0.52 (0.44 to 0.60) | 0.57 (0.49 to 0.67) |
| Hematocrit (per 2% increase) | 0.99 (0.97 to 1.02) | 0.98 (0.96 to 1.01) |
| ARIC (versus CHS) | 0.97 (0.82 to 1.15) | 0.95 (0.80 to 1.12) |
Creatinine-based outcomes are defined by an increase in serum creatinine of ≥0.4 mg/dl where baseline serum creatinine was ≤1.4 mg/dl in men and ≤1.2 mg/dl in women and final serum creatinine was above these levels. eGFR-based outcomes are defined by a decrease in eGFR of ≥15 ml/min per 1.73 m2 where the initial eGFR was ≥60 ml/min per 1.73 m2 and the final eGFR was <60 ml/min per 1.73 m2. Baseline kidney function is assessed by serum creatinine in creatinine-based models with the OR reflecting each 0.1-mg/dl rise and by eGFR in eGFR-based models with OR reflecting each 10-ml/min per 1.73 m2 rise.
In multivariable analyses not adjusted for systolic BP (SBP) and history of hypertension, uric acid was a significant risk factor for development of kidney disease in both eGFR- and creatinine-based models (OR 1.15 [95% CI 1.08 to 1.22] and OR 1.17 [95% CI 1.08 to 1.27] per 1-mg/dl rise, respectively). In multivariable analyses including body mass index (BMI), uric acid remained significant with the magnitude of effect unchanged (Table 2). In study-specific analyses, uric acid was associated with similar risk in each study (eGFR-based outcomes OR 1.06 [95% CI 0.99 to 1.13] and 1.09 [95% CI 0.95 to 1.26], and for creatinine-based outcomes OR 1.12 [95% CI 1.01 to 1.24] and OR 1.09 [95% CI 0.91 to 1.30], for ARIC and CHS, respectively). Notably, when the composite outcome of death and kidney disease was examined, the term for study (comparing ARIC with CHS) was no longer significant (HR 0.97; 95% CI 0.82 to 1.15), suggesting that study difference is a function of increased mortality in CHS. Finally, in multivariable analyses that included diuretic use, uric acid remained significant, whereas diuretic use was NS (Table 2).
DISCUSSION
In this study, we demonstrated that elevated serum uric acid level is an independent, albeit modest, risk factor for incident kidney disease in a generalizable US population over a lengthy follow-up period. Several other studies reported an association between baseline uric acid and kidney function decline. A 2-yr study of Japanese residents of Okinawa demonstrated that higher baseline uric acid level was associated with an increase in the number of individuals with elevated serum creatinine over 2 yr but not with a change in creatinine clearance as estimated by the Cockroft-Gault equation.6 The authors did not explain this discrepant finding. These same investigators also identified uric acid in women in Okinawa as an independent predictor of kidney failure requiring kidney replacement therapy during a 7-yr period; results in men did not achieve statistical significance.7 In our study, we noted a significant risk for incident kidney disease associated with higher uric acid levels in women and a trend in men; however, the interaction between gender and uric acid was NS, indicating that this result was statistically comparable in both men and women.
Chonchol et al.5 evaluated the association between hyperuricemia and progression of kidney disease in the CHS, demonstrating a 14% (OR 1.14; 95% CI 1.04 to 1.24 per 1-mg/dl rise in uric acid) increase in kidney disease progression, defined by eGFR decline ≥3 ml/min per 1.73 m2/yr, but no relationship between baseline serum uric acid levels and incident CKD (OR 1.00; 95% CI 0.89 to 1.14). The results in our study differ, perhaps reflecting the limitations of serum creatinine–based GFR estimates in the elderly, particularly at high eGFR levels. In the elderly, muscle mass is less predictable at any given level of serum creatinine, making interpretation of serum creatinine levels more challenging.8,9 Uric acid, reflecting its handling by the kidney, may improve the predictive ability of creatinine-based GFR estimation in the elderly, particularly at lower serum creatinine levels (and therefore higher eGFR levels). For these reasons, given the heterogeneity of kidney function at relatively normal-appearing serum creatinine and eGFR levels in the elderly, the use of a definition for incident kidney disease that relied only on a final eGFR level below the threshold of 60 ml/min per 1.73 m2 without requiring sufficient eGFR decline may introduce enough imprecision to bias results in the Chonchol study to the null. Our approach, requiring both a change in kidney function and crossing a threshold associated with CKD to define incident kidney disease, may address this imprecision.
Uric acid may be associated with CKD through several mechanisms: (1) Uric acid may be directly toxic to the kidney; (2) elevated uric acid may exacerbate other risk factors for kidney disease, specifically hypertension; or (3) uric acid may be a marker of the severity of other risk factors, including those attributable to or associated with diabetes and the metabolic syndrome. In animal studies, mild hyperuricemia caused direct kidney toxicity, manifest by renal vasoconstriction and systemic hypertension as well as tubulointerstitial injury not accounted for by uric acid crystal deposition within the kidney.10–12 Uric acid may cause these conditions through inhibition of endothelial nitric oxide bioavailability, activation of the renin-angiotensin system, and/or direct effects on endothelial cells and vascular smooth muscle cells.3 Clinical support for this hypothesis is provided by data from the Health Professionals' Follow-up Study, which suggested that elevated serum uric acid is a risk factor for development of hypertension in younger men.13
Both direct kidney injury and preexisting systemic hypertension may lead to incident kidney disease associated with uric acid. Given that systemic hypertension is an exceedingly common condition with multifactorial etiology, it is not possible to determine whether uric acid–induced hypertension is causing kidney disease or whether kidney disease is causing both worse hypertension and retention of uric acid. Accordingly, the results of our sensitivity analyses are informative; we demonstrated that OR associated with the term for uric acid were higher in models that did not include hypertension or SBP, suggesting that some but not all of the effect of uric acid on development of kidney disease may be mediated by systemic hypertension.
Finally, uric acid may be a marker of kidney risk rather than a direct contributor to kidney injury. Elevated serum uric acid is strongly associated with both prevalent kidney disease and CKD risk factors, including the metabolic syndrome, likely reflecting both renal handling of uric acid and collinearity among uric acid, obesity, hypertension, diabetes, and other lifestyle characteristics.14 In cross-sectional studies examining US adults as well as US children and adolescents enrolled in the Third National Health and Nutrition Examination Survey (NHANES III), the prevalence of the metabolic syndrome increased substantially with increasing levels of serum uric acid14,15; however, the association between uric acid and CKD risk factors does not require components of the metabolic syndrome to be present; a recent evaluation of MRFIT data examined the association between incident hypertension and baseline serum uric acid levels >7 mg/dl in men without diabetes or metabolic syndrome and found an 81% increased risk for incident hypertension after adjustment for comorbid conditions, baseline BP, and kidney function.16 We attempted to account for these associations in sensitivity analyses in which we included BMI. Notably, this did not affect the modest but statistically significant independent association between baseline serum uric acid level and incident kidney disease; therefore, although it is still possible that elevated uric acid may solely be a marker of risk for development of kidney disease, elevated serum uric acid itself likely does contribute to development of kidney disease.
This study does have several weaknesses. We lack data on baseline proteinuria and allopurinol use. Although proteinuria is a powerful predictor of development of kidney disease, hyperuricemia in theory would result in a nonproteinuric or minimally proteinuric kidney disease because uric acid is hypothesized to cause kidney insult primarily via preglomerular vasoconstriction.17 In the case of allopurinol, it is likely that if uric acid is directly toxic to the kidney, then use of allopurinol would not significantly affect the results, whereas, if uric acid is a marker of kidney risk, then lack of allopurinol data would likely bias the results to the null. Notably, Chonchol et al.5 used medication data not available in the limited access CHS data set and found no significant difference in baseline allopurinol use across uric acid quintiles.
Strengths of this study include use of a racially diverse, community-based population with age at enrollment ranging from 45 to >90 yr. This permits generalizability to the US population. In addition, both CHS and ARIC have detailed ascertainment of baseline risk factors and outcomes.
In conclusion, we have demonstrated that elevated serum uric acid level is an independent risk factor for development of kidney disease and the composite of incident kidney disease and mortality in a generalizable population. Future studies should examine the effects of modifying serum uric acid levels on these outcomes.
CONCISE METHODS
Study Population
Individual participant data were pooled from two community-based, longitudinal, limited-access data sets: ARIC and CHS.18,19 From 1987 to 1989, ARIC recruited 15,792 participants between 45 and 64 yr of age from four communities. From 1989 to 1990, CHS recruited 5201 participants who were age ≥65 yr and randomly selected from Medicare eligibility files in four communities. In both studies, follow-up occurred at 3- to 4-yr intervals. An additional 687 black participants were recruited in CHS from 1992 to 1993 (year 5); they were not included here because of limited follow-up. Final serum creatinine measurement occurred at visit 4 in ARIC (1996 to 1998) and year 9 in CHS (1996 to 1997). Details of recruitment and follow-up for the studies are described elsewhere.18,19
Kidney Function
Baseline serum creatinine was assessed for 15,582 (99%) participants in ARIC and in 5716 (97%) participants in CHS. We indirectly calibrated creatinine values from ARIC and CHS to mean NHANES III values for a given age, race, and gender, resulting in adjustments of −0.24 mg/dl in ARIC and −0.11 mg/dl in CHS.20 Baseline creatinine values were determined by subtracting these adjustments from measured values. We adjusted for changes in laboratory measurements over time using published calibration factors: In ARIC, 0.18 mg/dl was added to visit 4 measurements; in CHS, 0.11 mg/dl was subtracted from year 9 measurements.21,22 eGFR was calculated with the four-variable Modification of Diet in Renal Disease (MDRD) Study equation.23
Other Variables
Baseline serum uric acid was measured on the Kodak Ektachem 700 Analyzer in CHS and by the Uricase method in ARIC, both at central laboratories. Other baseline characteristics included demographics (age, gender, race, and education status), lifestyle characteristics (smoking and alcohol intake), medication use, medical history (diabetes, hypertension, and CVD), physical examination findings (height, weight, SBP and diastolic BP, and electrocardiogram results), and laboratory variables (total cholesterol, HDL cholesterol, albumin, glucose, and hematocrit). Race was white or black. Education level was dichotomized by high school graduation status. Smoking and alcohol were dichotomized by current use. Diabetes was defined by medication use or fasting glucose level ≥126 mg/dl. Baseline CVD was defined by previous myocardial infarction, previous stroke or transient ischemic attack, coronary revascularization, or angina and intermittent claudication on the basis of Rose questionnaires. Hypertension was defined by SBP ≥140 mmHg, diastolic ≥90 mmHg, or antihypertensive medication use. Left ventricular hypertrophy (LVH) was defined on the basis of electrocardiogram criteria.24 BMI was calculated as (weight in kg/height in m2). Diuretic use included thiazide, loop, and potassium-sparing diuretics, because the limited access data did not universally differentiate diuretic class.
Study Outcomes
Incident kidney disease was defined using both a serum creatinine–based definition and an eGFR-based definition: (1) eGFR decrease of ≥15 ml/min per 1.73 m2 in participants with baseline eGFR ≥60 ml/min per 1.73 m2 and final eGFR below this threshold and (2) serum creatinine increase ≥0.4 mg/dl, where baseline serum creatinine was <1.4 mg/dl in men and <1.2 mg/dl in women and final serum creatinine exceeded these levels.21,25,26
Study Sample
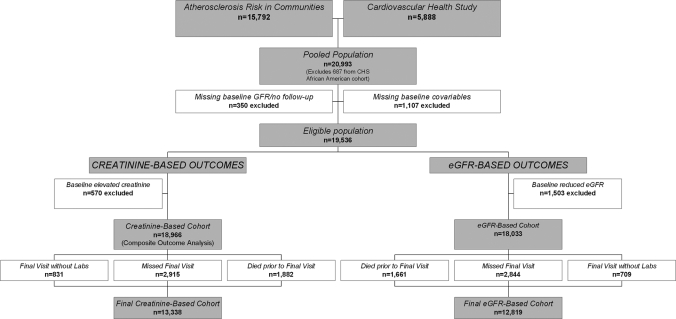
From a pooled sample of 21,680 individuals, we excluded the 687 newly recruited participants from year 5 of CHS, 340 with missing baseline creatinine, age, gender, or race data and 10 missing all follow-up data. Another 1107 individuals were excluded for other missing baseline data, the majority of which was baseline CVD (556 individuals, all from ARIC) and LVH (209 individuals). A total of 570 individuals were excluded for reduced baseline kidney function in creatinine-based analyses (serum creatinine <1.4 mg/dl in men and <1.2 mg/dl in women) and 1503 in eGFR-based analyses (eGFR <60 ml/min per 1.73 m2). A total of 5628 and 5214 individuals had missing creatinine levels at the final examination in the creatinine- and eGFR-based cohorts, respectively (Figure 1). Baseline characteristics are presented for the creatinine-based cohort.
Figure 1.
Development of the study cohort for the eGFR- and serum creatinine–based analyses.
Statistical Analyses
Baseline characteristics were compared using ANOVA or χ2 tests. Logistic regression was used to assess the relationship between baseline uric acid and study outcomes after adjustment for a priori identified covariates (age, gender, race, diabetes, history of hypertension, CVD, smoking, alcohol use, level of education, SBP, LVH, baseline kidney function [creatinine or eGFR], total and HDL cholesterol, albumin, hematocrit, and study). We did not use proportional hazards models for primary analyses, because we cannot determine specific time at which an individual's kidney function reached a level consistent with CKD. Interactions between uric acid and covariates were tested.
Sensitivity Analyses
To test validity, we compared individuals who had only baseline serum creatinine levels with individuals who had baseline and final levels. Because many individuals missing final creatinine levels died prior to the final study examination, we used Cox proportional hazards models to assess whether baseline uric acid influenced the composite outcome of incident kidney disease defined by creatinine or death. We estimated time to kidney disease as the duration between the initial and final creatinine measurements and censored those alive but missing their final evaluation at the expected time of this evaluation.
Because uric acid's effects on developing kidney disease may be mediated by hypertension, we performed sensitivity analyses excluding baseline history of hypertension and SBP from analyses.12 Because BMI may influence the development of kidney disease through its association with elevated uric acid levels, hypertension and diabetes, the effects of additional adjustment for BMI were evaluated in sensitivity analyses that previously adjusted for lipids and diabetes.27 Because the study term was significant, we performed subgroup analysis by study of origin. We also performed analyses adjusting for diuretic use. We did not have data on allopurinol use and were unable to adjust for this. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
DISCLOSURES
None.
Acknowledgments
This study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R21 DK068310, K23 DK71636, and T32 DK007777) and Amgen Inc. (Thousand Oaks, CA). Study sponsors were not involved in data analysis or interpretation of findings.
A poster based on this article was presented at the annual meeting of the American Society of Nephrology; October 31–November 5, 2007; San Francisco, CA.
Published online ahead of print. Publication date available at www.jasn.org.
The ARIC Study and CHS are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the individual study investigators. This manuscript was not prepared in collaboration with the study investigators and does not necessarily reflect the opinions or views of the study investigators or the NHLBI.
REFERENCES
- 1.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 2.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML: Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 42: 474–480, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Cirillo P, Sato W, Reungjui S, Heinig M, Gersch M, Sautin Y, Nakagawa T, Johnson RJ: Uric Acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol 17[Suppl 3]: S165–S168, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD: Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 17: 1444–1452, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Conchol MB, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, Kestenbaum B, Carney JK, Fried LF: Relationship of uric acid with progression of kidney disease. Am J Kidney Dis 50: 239–247, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S: Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res 24: 691–697, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S: Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 44: 642–650, 2004 [PubMed] [Google Scholar]
- 8.Odden MC, Chertow GM, Fried LF, Newman AB, Connelly S, Angleman S, Harris TB, Simonsick EM, Shlipak MG: Cystatin C and measures of physical function in elderly adults: The Health, Aging, and Body Composition Study. Am J Epidemiol 164: 1180–1189, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ: Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Lozada LG, Tapia E, Avila-Casado C, Soto V, Franco M, Santamaria J, Nakagawa T, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J: Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol 283: F1105–F1110, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ: A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Forman JP, Choi H, Curhan GC: Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol 18: 287–292, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Choi HK, Ford ES: Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 120: 442–447, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Li C, Cook S, Choi HK: Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 115: 2526–2532, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Krishnan E, Kwoh CK, Schumacher HR, Kuller L: Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension 49: 298–303, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Feig DI, Rodriguez-Iturbe B, Nakagawa T, Johnson RJ: Nephron number, uric acid, and renal microvascular disease in the pathogenesis of essential hypertension. Hypertension 48: 25–26, 2006 [DOI] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 19.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A: The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1: 263–276, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Cardiovascular outcomes and all-cause mortality: Exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis 48: 392–401, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Merkin SS, Coresh J, Roux AV, Taylor HA, Powe NR: Area socioeconomic status and progressive CKD: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 46: 203–213, 2005 [DOI] [PubMed] [Google Scholar]
- 22.The Cardiovascular Health Study. Available at: http://www.chs-nhlbi.org. Accessed March 13, 2007
- 23.Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 24.Weiner DE, Tighiouart H, Vlagopoulos PT, Griffith J, Salem DN, Levey AS, Sarnak MJ: Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol 16: 1803–1810, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Edwards MS, Wilson DB, Craven TE, Stafford J, Fried LF, Wong TY, Klein R, Burke GL, Hansen KJ: Associations between retinal microvascular abnormalities and declining renal function in the elderly population: The Cardiovascular Health Study. Am J Kidney Dis 46: 214–224, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem DN, Sarnak MJ, Weiner DE: Cardiovascular disease and subsequent kidney disease. Arch Intern Med 167: 1130–1136, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kurella M, Lo JC, Chertow GM: Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 16: 2134–2140, 2005 [DOI] [PubMed] [Google Scholar]