Abstract
The effect of dosage of continuous venovenous hemodiafiltration (CVVHDF) on survival in patients with acute renal failure (ARF) is unknown. In this study, 200 critically ill patients with ARF were randomly assigned to receive CVVHDF with prefilter replacement fluid at an effluent rate of either 35 ml/kg per h (high dosage) or 20 ml/kg per h (standard dosage). The primary study outcome, survival to the earlier of either intensive care unit discharge or 30 d, was 49% in the high-dosage arm and 56% in the standard-dosage arm (odds ratio 0.75; 95% confidence interval 0.43 to 1.32; P = 0.32). Among hospital survivors, 69% of those in the high-dosage arm recovered renal function compared with 80% of those in the standard-dosage arm (P = 0.29); therefore, a difference in patient survival or renal recovery was not detected between patients receiving high-dosage or standard-dosage CVVHDF.
The mortality associated with acute renal failure (ARF) in the intensive care unit (ICU) has remained greater than 50% during the past three decades, despite improvements in renal replacement technology.1–5 Continuous renal replacement therapy (CRRT) has emerged as the predominant form of renal replacement therapy (RRT) in the ICU as a result of slow continuous fluid removal, steady acid-base and electrolyte correction, and beneficial effects on hemodynamic stability.6–8 There are no standardized protocols for prescribing or quantifying the adequacy of solute removal with CRRT.5
The available CRRT modalities differ according to whether solute clearance is accomplished primarily by diffusion, convection, or a combination of these techniques. Diffusion clears small molecular weight solutes efficiently across a concentration gradient but is relatively ineffective in clearing larger molecular weight solutes (>5000 Da). Convection removes water by mass transport across a pressure gradient, thereby removing both small and larger molecular weight solutes dissolved in the transported water. There is no consensus as to which one of these clearance techniques is best.
The optimal dosage of CRRT in ICU patients with ARF has not been established. Although three randomized trials using differing CRRT modalities have evaluated the impact of dialysis dosage (defined by effluent rate) on patient survival in this population, they all were single-center studies with differing designs and compared different CRRT strategies.9–12 Continuous venovenous hemodiafiltration (CVVHDF), a CRRT technique that uses both convection and diffusion, is the only CRRT technique used at the University of Alabama at Birmingham (UAB). To determine the impact of CRRT dosage on patient outcomes, we conducted a prospective, randomized study comparing CVVHDF with prefilter replacement fluid using an effluent rate of 35 ml/kg per h versus an effluent rate of 20 ml/kg per h on patient survival in ICU patients with ARF.
RESULTS
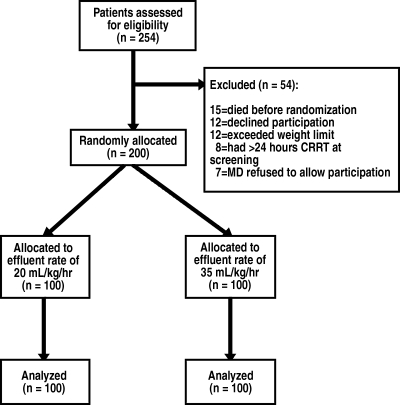
A total of 254 ICU patients with ARF were screened. Two hundred patients were enrolled into the study, with 100 randomly assigned into each treatment arm. Reasons for exclusion are presented in Figure 1. Demographics and clinical characteristics are presented in Table 1. The two arms did not differ in their baseline characteristics, except for the percentage of patients mechanically ventilated, which was significantly higher in the high-dosage arm. Seventy-four patients were in the sepsis + oliguria stratum, 34 in the sepsis + nonoliguria stratum, 53 in the no sepsis + oliguria stratum, and 39 in the no sepsis + nonoliguria stratum. The proportion of patients with oliguria, sepsis, and preexisting chronic kidney disease (CKD; defined as premorbid serum creatinine >1.4 mg/dl) was similar for both treatment arms.
Figure 1.
Trial profile.
Table 1.
Baseline characteristics
| Characteristic | Standard Dosage(20 ml/kg per h) | High Dosage(35 ml/kg per h) | P |
|---|---|---|---|
| Patients (n) | 100 | 100 | |
| Age (yr; mean ± SD) | 62 ± 15 | 58 ± 16 | 0.10 |
| Weight (kg; mean ± SD) | 90 ± 19 | 93 ± 18 | 0.26 |
| Male (%) | 57 | 59 | 0.77 |
| Race (%) | 0.22 | ||
| white | 73 | 65 | |
| black | 27 | 33 | |
| other | 0 | 2 | |
| Premorbid serum creatinine (mg/dl; %) | 0.74 | ||
| ≤1.4 | 46 | 47 | |
| >1.4 | 44 | 40 | |
| unknown | 10 | 13 | |
| Cause of acute kidney injury (%) | 0.33 | ||
| septic shock | 50 | 51 | |
| cardiogenic | 13 | 14 | |
| drug induced | 11 | 13 | |
| postsurgical | 11 | 13 | |
| hypovolemia | 9 | 9 | |
| other | 8 | 8 | |
| Mechanically ventilated (%) | 70 | 85 | 0.01 |
| Required vasopressors (%) | 56 | 62 | 0.38 |
| Oliguric (%) | 63 | 64 | 0.88 |
| Septic (%) | 54 | 54 | 1.00 |
| Severity of illness score (APACHE II; mean ± SD) | 26 ± 7 | 26 ± 6 | 0.33 |
| Renal parameters at CRRT initiation (mean ± SD) | |||
| BUN (mg/dl) | 76 ± 38 | 75 ± 37 | 0.80 |
| serum creatinine (mg/dl) | 4.3 ± 2.2 | 4.2 ± 2.0 | 0.83 |
| urine output (ml/24 h) | 614 ± 800 | 540 ± 621 | 0.46 |
| days from ICU admission to CRRT | 8 ± 11 | 8 ± 14 | 0.64 |
CRRT parameters are described in Table 2. Accounting for the effect of predilution replacement fluid on solute clearance, the mean actual delivered dosage was 29 ml/kg per h in the high-dosage arm and 17 ml/kg per h in the standard-dosage arm (P < 0.001). More than 80% of the target effluent dosage was achieved in 74% of patients in the standard-dosage arm and 79% patients in the high-dosage arm (P = 0.45). Failure to achieve the target dosage was related to dialyzer thrombosis, catheter dysfunction, or ICU absence as a result of procedures. This did not differ between arms. Convective clearance composed 44% of the effluent volume in the high-dosage arm and 43% in the standard-dosage arm. Diffusive clearance composed 56 and 57% of the high- and standard-dosage arms, respectively. Total CRRT duration was 10 ± 9.8 d for the high-dosage arm and 9.7 ± 11.3 d for the standard-dosage arm (P = 0.88; Table 3).
Table 2.
CRRT characteristics by treatment group
| Characteristic | Standard Dosage(20 ml/kg per h) | High Dosage(35 ml/kg per h) | P |
|---|---|---|---|
| Patients (n) | 100 | 100 | |
| Citrate anticoagulation (%) | 90 | 92 | 0.620 |
| Total effluent rate (ml/h; mean ± SD) | 1798 ± 371 | 3237 ± 659 | <0.001 |
| dialysate rate (ml/h; mean ± SD) | 1005 ± 173 | 1831 ± 497 | <0.001 |
| convective rate (ml/h; mean ± SD)a | 793 ± 298 | 1406 ± 252 | <0.001 |
| replacement fluid rate (ml/h; mean ± SD) | 669 ± 288 | 1273 ± 232 | <0.001 |
| fluid removal rate (ml/h; mean ± SD) | 124 ± 66 | 132 ± 78 | 0.440 |
| convective component of effluent, % | 43 | 44 | 0.470 |
| Actual delivered dosage (ml/kg per h) | 17 | 29 | <0.001 |
| Filtration fraction (%; mean ± SD) | 15 ± 6 | 26 ± 6 | <0.001 |
| % patients achieving ≥80% prescribed dosage | 74 | 79 | 0.450 |
Expressed as a combination of replacement fluid rate and fluid removal rate.
Table 3.
Outcomes by treatment group
| Characteristic | Standard Dosage(20 ml/kg per h) | High Dosage(35 ml/kg per h) | P |
|---|---|---|---|
| Total CRRT days | 9.7 ± 11.3 | 10.0 ± 9.8 | 0.68 |
| Total ICU days | 31 ± 36 | 26 ± 26 | 0.34 |
| Total hospital days | 40 ± 39 | 35 ± 30 | 0.54 |
| Survival to ICU discharge or 30 d (%) | 56 | 49 | 0.32 |
| ICU survival (%) | 45 | 40 | 0.47 |
| Hospital survival (%) | 40 | 36 | 0.56 |
| ICU renal recovery (%) | |||
| all patients | 37 | 28 | 0.17 |
| survivors | 69 | 63 | 0.65 |
| Hospital renal recovery (%) | |||
| all patients | 41 | 29 | 0.75 |
| survivors | 80 | 69 | 0.29 |
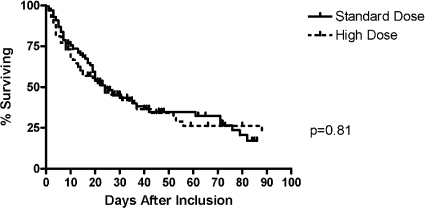
The primary study outcome, survival to the earlier of either ICU discharge or 30 d, was 49% in the high-dosage arm and 56% in the standard-dosage arm (odds ratio 0.75; 95% confidence interval 0.43 to 1.32; P = 0.32). Twenty-one percent of patients in the low-dosage arm and 22% in the high-dosage arm met the end point of survival to 30 d (P = 1.0). Thirty-five percent in the low-dosage arm and 27% in the high-dosage arm met the end point of survival to ICU discharge (P = 0.29). There were no differences in ICU survival, hospital survival, or renal recovery at either ICU discharge or hospital discharge between treatment arms (Table 3, Figure 2). Within each of the four strata of sepsis and oliguria, dosage did not significantly alter survival or renal recovery. There was a trend toward improved overall survival in patients with nonoliguria + nonsepsis compared with patients with sepsis + oliguria, but this was not statistically significant. Eleven percent of patients in the standard-dosage arm and 5% in the high-dosage arm were transitioned to intermittent hemodialysis (IHD) while in the ICU (P = 0.19). At the primary outcome, Acute Physiology and Chronic Health Evaluation (APACHE II) score (P = 0.003), pressor requirement (P = 0.02), and anticoagulation (P = 0.03) remained independent predictors of survival in a multivariate model. At hospital discharge, age (P = 0.05), APACHE II (P = 0.003), serum creatinine at CRRT initiation (P = 0.005), and mechanical ventilation (P = 0.04) were independently associated with survival, whereas pressor use demonstrated a nonstatistically significant trend (P = 0.08).
Figure 2.
Kaplan-Meier analysis of hospital survival rates by prescribed CVVHDF dosage.
DISCUSSION
In this study, treatment with high-dosage CVVHDF (35 ml/kg per h) did not result in a survival advantage over standard dose (20 ml/kg per h) in ICU patients with ARF. There were no differences in ICU survival, hospital survival, CRRT days, and rate of renal recovery between dosage arms.
Systematic efforts to quantify CRRT dosage in patients with ARF have only recently occurred. Three randomized studies evaluated the impact of CRRT dosage, defined by effluent rate, on patient survival (Table 4). Using an end point of 15-d survival after continuous venovenous hemofiltration (CVVH) discontinuation, a purely convective CRRT modality, Ronco et al.11 found that a dosage of 35 ml/kg per h had a survival benefit compared with 20 ml/kg per h. Postdilution CVVH was initiated relatively early in the course of ARF. The majority of patients had postsurgical ARF, and relatively few had sepsis or preexisting CKD. At least 85% of prescribed dosage was achieved by all patients. For compensation for treatment time interruptions, dosage was increased in subsequent hours to match the targeted dosage.
Table 4.
Comparisons of CRRT dosage studiesa
| Parameter | Ronco et al.11 | Bouman et al.9 | Saudan et al.10 | This Study |
|---|---|---|---|---|
| Patient characteristics | ||||
| n | 425 | 106 | 206 | 200 |
| age (yr; mean) | 61 | 68 | 63 | 60 |
| male (%) | 56 | 59 | 61 | 58 |
| weight (kg; mean) | 68 | – | 73 | 91 |
| presence of CKD (%) | – | Excluded stage 4 CKD | 33 | 42 |
| predominant cause of AKI | Surgical | Cardiosurgical | Sepsis | Sepsis |
| presence of sepsis (%) | 13 | – | 60 | 54 |
| APACHE II score | 23 | 23 | 25 | 26 |
| BUN at initiation (mg/dl; mean) | 53 | Early 46 versus late 105 | 83 | 75 |
| creatinine at initiation (mg/dl; mean) | 3.6 | – | 4.9 | 4.3 |
| CRRT parameters | ||||
| modality | Postdilution CVVH | Postdilution CVVH | Predilution CVVH/CVVHDF | Predilution CVVHDF |
| blood flow (ml/min; mean) | 145 to 207 | 100 to 200 | 100 to 125 | 100 to 150 |
| UF rate (ml/kg per h; mean) | ||||
| group 1 | 20 | 20 (ELV) | 25 (CVVH) | 9 |
| group 2 | 35 | 19 (LLV) | 24 (CVVHDF) | 15 |
| group 3 | 45 | 48 (EHV) | – | – |
| dialysate rate (ml/kg per h; mean) | ||||
| group 1 | – | – | – | 11 |
| group 2 | – | – | 18 (CVVHDF) | 20 |
| group 3 | – | – | – | – |
| effluent rate (ml/kg per h; mean) | ||||
| group 1 | 20 | 20 (ELV) | 25 (CVVH) | 20 |
| group 2 | 35 | 19 (LLV) | 42 (CVVHDF) | 35 |
| group 3 | 45 | 48 (EHV) | – | – |
| Outcomes (%) | ||||
| survival | 15 d after d/c CRRT | 28 d | 28 d | ICU d/c or 30 d |
| group 1 | 41 | 69 | 39 | 56 |
| group 2 | 57 | 75 | 59 | 49 |
| group 3 | 58 | 74 | – | – |
| renal recovery of survivors | 15 d after d/c CRRT | Hospital discharge | 90 d | Hospital discharge |
| group 1 | 95 | 100 | 71 | 80 |
| group 2 | 92 | 100 | 78 | 69 |
| group 3 | 90 | 100 | – | – |
–, not available or not applicable; AKI, acute kidney injury; d/c, discharge; EHV, early high-volume hemofiltration; ELV, early low-volume hemofiltration; LLV, late low-volume hemofiltration; UF, ultrafiltration.
Bouman et al.9 investigated the effect of both dosage and timing of treatment on survival with postdilution CVVH in patients with ARF. Patients (n = 106) were randomly assigned to three arms: Early start (blood urea nitrogen [BUN] <46 mg/dl)/high volume, early start/low volume, and late start (BUN >105 mg/dl)/low volume. Low volume corresponded to a dosage of 25 ml/kg per h; high volume corresponded to a dosage of 48 ml/kg per h. There was no survival difference for either dosage or initiation time; however, because 24-h filter life in the early start/high volume group was a median of 13.6 h versus 16.1 and 24.3 h in the other two groups (P < 0.001), actual delivered therapy in the high-dosage arm was much less than the prescribed dosage. Furthermore, survival was greater than expected (survival at 28 d was 69 to 75% in all groups), raising concerns about the study's being underpowered. Saudan et al.10 randomly assigned 206 patients to CVVH or predilution CVVHDF. The CVVH arm had a prescribed dosage of 25 ml/kg per h, whereas a diffusive component was incorporated in the CVVHDF arm to result in an overall dosage of 42 ml/kg per h. More than 50% of patients had sepsis, and 33% had CKD. Mean CRRT initiation BUN and creatinine were 81 and 4.5 mg/dl, respectively. Survival at 28 d was 39 and 59% in the CVVH and CVVHDF arms, respectively (P = 0.03). Within the first 24 h, more than 80% of the treatment prescribed was delivered in both arms.
We did not detect a significant difference in survival between the high-dosage and standard-dosage arms in this study. This study was designed to detect an absolute 20% difference in survival rates with 80% power. It is possible either that a true difference exists but was not detected because of sample size or that the true survival difference was less than 20%. Second, our patient population also had higher rates of CKD and sepsis, which may have had a negative impact on outcomes. Third, although the targeted high dosage in this study was 35 ml/kg per h, the actual delivered dosage was 29 ml/kg per h. The high-dosage arms in the previous studies prescribed higher dosages, suggesting that a critical dosage threshold may not have been reached in our study. Furthermore, only 77% of the 200 patients achieved greater than 80% of the prescribed dosage. Last, convective clearance is more effective for removal of middle molecules, although the clinical importance of this remains unclear.13 Compared with previous studies, lower convective rates in this study might have resulted in higher mortality in patients with sepsis, although this is speculative.
Renal recovery is an important secondary end point in CRRT outcomes. There were no differences in rates of renal recovery at ICU or hospital discharge for survivors (Table 3), and there was a NS trend toward worsening renal recovery in the high-dosage group. None of the previous three studies had greater rates of renal recovery in the high-dosage arms. Renal recovery is influenced by multiple factors, including preexisting CKD and nephrotoxins.
Taken together, these studies (including this one) underscore the difficulty of CRRT research in the ICU. Factors such as sepsis, preexisting CKD, delivered dosage, solute clearance techniques (diffusion versus convection), and time of initiation all are important characteristics that may influence outcome and limit generalizing conclusions. All four CRRT studies discussed thus far are also single-center studies. There are two large, multicenter, randomized trials in progress that should help to resolve some of these issues.
In conclusion, we did not observe a significant difference in either survival to ICU discharge or 30 d between a CVVHDF dosage of 35 versus 20 ml/kg per h. Large, multicenter trials will help to address more definitively the impact of CRRT dosage on the survival of ICU patients with ARF.
CONCISE METHODS
Patients
Patients were recruited from the medical and surgical ICU at UAB from August 1, 2003, through March 20, 2006. The main criterion for study inclusion was a clinical diagnosis of ARF in the ICU, defined by at least one of the following: (1) Volume overload despite diuretics, (2) oliguria (urine output <200 ml/12 h) despite fluid resuscitation and diuretics, (3) anuria (urine output <50 ml/12 h), (4) azotemia (BUN ≥80 mg/dl), or (5) hyperkalemia (K+ ≥6.5 mmol/L) and/or an increase in serum creatinine >2.5 mg/dl from normal values or a sustained rise in serum creatinine of ≥1 mg/dl over baseline. Patients were excluded when they had ESRD, when they had had previous IHD, or when they had >24 h of CRRT at time of enrollment. Patients were also excluded when they weighed >125 or <50 kg because of limitations of the Prisma machine to deliver study doses for those weights. The study protocol was approved by the local institutional review board. Written informed consent was obtained by B.S.S. from all study participants or from their next of kin or legal guardian. Patients were followed prospectively from time of enrollment through hospital discharge.
Treatment Assignments
CVVHDF was initiated at the discretion of the consulting nephrologists, without consideration of the patient's eligibility for this study. CVVHDF was performed with the COBE Prisma (Lakewood, CO) M100 set and AN69 dialyzer (effective surface area 0.9 m2) through a double-lumen 12F catheter inserted into the internal jugular, subclavian, or femoral vein. Hemodiafiltration was accomplished using blood flow rates of 100 to 150 ml/min and predilution replacement fluid. Regional citrate or no anticoagulation was used at the consulting nephrologists' judgment.
Patients were randomly assigned to the treatment dosage by a computer-generated block randomization scheme, using a 1:1 ratio between treatment dosages. Treatment assignments were kept in numbered, sealed envelopes that were opened at the time of enrollment. The treatment assignments were stratified by sepsis and oliguria to ensure balanced randomization, because both parameters are independent predictors of patient survival.5,14 The four stratification categories were (1) sepsis + oliguria, (2) sepsis + nonoliguria, (3) nonsepsis + oliguria, and (4) nonsepsis + nonoliguria. Each time a patient was enrolled, the next available envelope was opened by the study coordinator and the allocated treatment communicated to the consulting nephrologists. Blinding was impossible for logistic reasons.
In all CRRT modalities, the “effluent” represents the end product of filtration and comprises the ultrafiltrate in convective therapies, the spent dialysate in diffusive therapies, and the sum of both in combined therapies. CRRT solute clearance is determined by the ratio between the concentration of the solute in the effluent and in the plasma multiplied by the effluent rate. Because urea is a small molecular weight solute, it reaches complete equilibrium in the effluent; thus, the ratio of the concentration of urea in the effluent to plasma is 1. Urea clearance becomes equal to the effluent rate, provided that the replacement fluid is given after dilution. For this study, the prescribed amount of effluent was used as proxy for treatment dosage. The two treatment dosages were an effluent rate of 20 ml/kg per h (standard) or 35 ml/kg per h (high).
On the Prisma, effluent rate (ml/h) is the sum of the replacement fluid rate, dialysate rate, and fluid removal rate. For example, a 70-kg patient assigned to the high dosage would require an effluent rate of 2450 ml/h (70 kg × 35 ml/kg per h). The replacement fluid rate, dialysate rate, and fluid removal rate for that patient would be adjusted to achieve an effluent rate of 2450 ml/h per d for the study duration. Dosage was calculated only once per patient and based on the patient's actual body weight on the day of CVVHDF initiation. This dosage remained constant throughout the treatment period and was not adjusted for body weight changes. Convective clearance is the sum of the replacement fluid and fluid removal rates. Diffusive clearance equals the dialysate rate. Every attempt was made to divide the effluent rate equally between convective and diffusive clearances. The actual delivered dosage of CVVHDF was measured directly by obtaining effluent BUN and creatinine levels daily. Total time of actual CRRT treatment (minutes/24 h period) was recorded daily, along with time off CRRT secondary to clotting, procedures, or other events. No compensation was made for therapy interruptions; however, interruptions were factored into the average percentage of prescribed therapy achieved.
Patients were transitioned to IHD at the judgment of the treating nephrologists. This usually occurred when the patient was dialysis dependent and transferred from the ICU to the ward or when the patient was being mobilized in the ICU. Dosage and timing of IHD were decided by the treating nephrologists.
Outcome Measurements
The primary outcome measure was survival to the earlier of either ICU discharge or 30 d. Secondary end points included renal recovery at ICU discharge, renal recovery at hospital discharge, ICU survival, hospital survival, ICU length of stay, and hospital length of stay. Renal recovery was defined as freedom from any RRT after CRRT discontinuation. Subanalyses were performed for each of the strata.
Statistical Analyses
Sample size calculation was based on a power analysis that assumed an expected improvement in patient survival of 20% in the high-dosage arm, compared with the standard-dosage arm. On the basis of preliminary data demonstrating 65% mortality in this patient population at UAB, we calculated that 200 patients would be needed to detect an absolute survival difference of 20%, assuming a power of 80%, a significance level of 5%, and a two-sided χ2 test.
Analysis was done on an intention-to-treat basis. The primary analysis of the study compared the proportion of patients who survived to the earlier of either ICU discharge or 30 d in each study arm. The two proportions were compared using the Pearson χ2 test or Fisher exact test when the χ2 test was not valid. The secondary analyses compared the proportion of patients who recovered renal function at ICU discharge and at hospital discharge, ICU survival, hospital survival, and hospital length of stay, using similar methods as the primary analysis. Baseline characteristics and outcome measures were compared using the two-group t test or the Wilcoxon rank-sum test for continuous variables and the Pearson χ2 test or Fisher exact test for categorical variables.
Logistic regression analysis was used to determine which baseline factors were associated with (significant predictors of) survival to ICU discharge or 30 d, hospital survival, and ICU survival. The Kaplan-Meier method was used to obtain hospital survival estimates by prescribed CVVHDF dosage, and the log-rank test was used to compare survival curves of the two dosage arms. All statistical tests were two-sided and performed using a significance level of 5%. Statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
DISCLOSURES
None.
Acknowledgments
This study was supported by a 2002 Pfizer Scholars in Clinical Epidemiology Grant and by General Clinical Research Center grant M01 RR-00032 from the National Center for Research Resources.
This work was presented in abstract form at the annual CRRT meeting; March 7 to 10, 2007; San Diego, CA.
We thank Drs. Michael Allon and Anupam Agarwal for their critical appraisal and thoughtful suggestions and Dr. Earl Francis Cook, Jr., for his mentorship.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Dialysis Dosage in Acute Kidney Injury: Still a Conundrum?” on pages 1046–1048.
REFERENCES
- 1.Bellomo R, Ronco C: Continuous renal replacement therapy in the intensive care unit. Intensive Care Med 25: 781–789, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Turney JH: Acute renal failure: A dangerous condition. JAMA 275: 1516–1517, 1996 [PubMed] [Google Scholar]
- 3.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ: Acute renal failure in intensive care units: Causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med 24: 192–198, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Liano F, Junco E, Pascual J, Madero R, Verde E: The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int Suppl 66: S16–S24, 1998 [PubMed] [Google Scholar]
- 5.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Jones CH, Richardson D, Goutcher E, Newstead CG, Will EJ, Cohen AT, Davison AM: Continuous venovenous high-flux dialysis in multiorgan failure: A 5-year single-center experience. Am J Kidney Dis 31: 227–233, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Ronco C, Bellomo R: Critical Care Nephrology, Dordrecht, Kluwer Academic Publishers, 1998
- 8.Bellomo R, Mansfield D, Rumble S, Shapiro J, Parkin G, Boyce N: A comparison of conventional dialytic therapy and acute continuous hemodiafiltration in the management of acute renal failure in the critically ill. Ren Fail 15: 595–602, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J: Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: A prospective, randomized trial. Crit Care Med 30: 2205–2211, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, Martin PY: Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int 70: 1312–1317, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G: Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: A prospective randomised trial. Lancet 356: 26–30, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Schiffl H, Lang SM, Fischer R: Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 346: 305–310, 2002 [DOI] [PubMed] [Google Scholar]
- 13.John S, Eckardt KU: Renal replacement therapy in the treatment of acute renal failure-intermittent and continuous. Semin Dial 19: 455–464, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]