Abstract
Chemokines are instrumental in macrophage- and T cell–dependent diseases. The chemokine CCL2 promotes kidney disease in two models of immune-mediated nephritis (MRL-Faslpr mice and the nephrotoxic serum nephritis model), but evidence suggests that multiple chemokines are involved. For identification of additional therapeutic targets for immune-mediated nephritis, chemokine ligands and receptors in CCL2−/− and wild-type (WT) MRL-Faslpr kidneys were profiled. The focus was on intrarenal chemokine ligand/receptor pairs that were highly upregulated downstream of CCL2; the chemokine CXCL10 and its cognate receptor, CXCR3, stood out as potential therapeutic targets. However, renal disease was not suppressed in CXCL10−/− MRL-Faslpr mice, and CXCL10−/− C57BL/6 mice were not protected from nephrotoxic serum nephritis compared with WT mice. Because CXCR3 engages with the ligand CXCL9, CXCR3−/−, CXCL9−/−, and CXCL10−/− B6 mice were compared with WT mice with nephrotoxic serum nephritis. Kidney disease, measured by loss of renal function and histopathology, was suppressed in both CXCR3−/− and CXCL9−/− mice but not in CXCL10−/− mice. With nephrotoxic serum nephritis, CXCR3−/− and CXCL9−/− mice had fewer intrarenal activated T cells and activated macrophages. Both IgG glomerular deposits and antigen-specific IgG in serum were reduced in these mice, suggesting that although CXCR3 and CXCL9 initiate nephritis through cell-mediated events, renal inflammation may be sustained by their regulation of IgG. It is concluded that specific blockade of CXCL9 or CXCR3 may be a potential therapeutic target for human immune-mediated kidney diseases.
Chemokines are instrumental in the recruitment, migration, and effector functions of immune cells during inflammation.1–4 Chemokine ligands engaging with their cognate receptors promote the influx of leukocytes into the kidney, a hallmark of nephritis.5,6 Although multiple chemokines share the same receptor, they are not necessarily redundant.7 Because chemokine ligand/receptors are induced during inflammation, these molecules are appealing therapeutic targets for nephritis.
Macrophages (Mø) and T cells in the kidney mediate inflammation.8 MRL-Faslpr mice and nephrotoxic serum nephritis (NSN) are two distinct T cell–and Mø-dependent mouse models of immune-mediated nephritis.9 We previously determined that CCL2 (monocyte chemoattractant protein-1) is pivotal in promoting renal disease in these models. Using CCL2−/− MRL-Faslpr mice, we established that tubular/interstitial and glomerular disease is suppressed.10 By comparison, tubular/interstitial but not glomerular disease is suppressed in CCL2−/− mice during NSN.10,11 In each model, Mø and T cells are no longer recruited to sites in the interstitium adjacent to tubular epithelial cells (TEC), the major source of CCL2 in WT mice with nephritis10,11; however, Mø and T cells remain in perivascular areas lacking CCL2 but rich in CCL5 (RANTES), a chemokine capable of inciting local renal inflammation in MRL-Faslpr mice.12 This suggests that multiple chemokines dictate the tempo and locale of kidney disease.
To identify the chemokines along with CCL2 that are instrumental in T cell–and Mø-mediated nephritis, we extensively profiled kidneys of CCL2−/− and wild-type (WT) MRL-Faslpr mice during the development of lupus nephritis. We identified a highly expressed chemokine ligand/receptor pair instrumental in attracting T cells during inflammation, CXCL10 (IP-10)/CXCR3.13–16 We report that CXCR3 is expressed on intrarenal activated Mø in addition to T cells during experimental immune-mediated kidney disease. Furthermore, the expression of CXCR3 on T cells and Mø seems to mediate their recruitment into the kidneys expressing CXCL9 during NSN. Finally, we determined that CXCR3 and one, CXCL9, but not another, CXCL10, of its ligands promote T cell–and Mø-dependent nephritis. Thus, CXCL9 and CXCR3 are potential therapeutic targets for immune-mediated kidney illnesses.
RESULTS
Multiple Chemokine Ligand/Receptors Are Upregulated in MRL-Faslpr Nephritic Kidneys
To identify potential therapeutic chemokine ligand/receptor targets in MRL-Faslpr mice, we compared intrarenal chemokine ligand/receptor transcripts in mice before (2 mo of age) and after (5 mo of age) onset of nephritis. The majority of intrarenal chemokine ligands (18 of 23) and chemokine receptors (10 of 16) we evaluated increased with advancing nephritis (Supplemental Figure 1). We focused on the groups with the highest increase in intrarenal transcript expression. Within this group of chemokine ligands, we detected an increase in CXCL10 (nine-fold), CXCL9 (45-fold), CXCL11 (10-fold), CXCL13 (133-fold), CCL5 (16-fold), CCL20 (seven-fold), and CX3CL1 (two-fold), and within the group of chemokine receptors, we detected an increase in CXCR3 (nine-fold), CXCR4 (six-fold), CXCR5 (42-fold), CCR2 (10-fold), and CX3CR1 (six-fold; Supplemental Figure 1). Of note, the chemokine ligand/receptor transcript levels in the MRL-Faslpr and B6 kidneys at 2 mo of age were similar.
Identifying Chemokine Ligand/Receptors Other than CCL2 that Are Expressed in Nephritic MRL-Faslpr Kidneys
CCL2 promotes lupus nephritis in MRL-Faslpr mice.10 To identify chemokines other than CLL2 that are central to MRL-Faslpr nephritis, we compared chemokine ligand/receptor transcripts in CCL2−/− and WT MRL-Faslpr kidneys. Most (>80%) chemokine ligand/receptor transcripts that were upregulated in WT MRL-Faslpr nephritic kidneys were suppressed in CCL2−/− MRL-Faslpr kidneys (Supplemental Figure 2). One possible interpretation is that within the hierarchical pattern of chemokine ligand/receptor expression regulating immune responses, CCL2 is proximal in the chemokine cascade leading to nephritis in MRL-Faslpr mice; therefore, our goal was to focus on the chemokine ligand/receptors that maybe expressed “downstream” of CCL2 in MRL-Faslpr kidneys during nephritis.
Mø and TEC Are Sources of Intrarenal CXCL10 Expression during Lupus Nephritis in MRL-Faslpr Mice
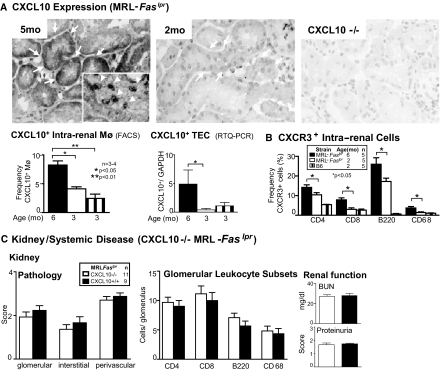
CXCL10 engaging with its receptor CXCR3 is a potent T cell chemoattractant.15 Because CXCL10 is among the most highly upregulated chemokines and may be downstream of CCL2, we explored the role of CXCL10 in MRL-Faslpr mice. Alveolar Mø express CXCL10,17 and TEC are a rich source of multiple chemokines in MRL-Faslpr mice10; therefore, Mø and TEC were prime candidates in our attempt to identify sources of CXCL10. We detected CXCL10 in TEC (Figure 1A), leukocytes (Figure 1A, inset), and endothelial cells (data not shown) in MRL-Faslpr mice with nephritis. CXCL10−/− MRL-Faslpr kidneys served as negative controls. The frequency of CXCL10+ Mø increased (two-fold) in MRL-Faslpr kidneys from 3 to 6 mo of age with advancing nephritis as determined by FACS analysis (Figure 1A). By comparison, the frequency of CXCL10+ T cells (CD4+, CD8+) in MRL-Faslpr kidneys, before and during nephritis, was minimal (approximately 2%; data not shown). Similarly, the expression of CXCL10 in primary TEC derived from MRL-Faslpr mice increased (more than nine-fold) as determined by real-time PCR (Figure 1A). Of note, CXCL10+ Mø and TEC in MRL-Faslpr kidneys were similar to B6 kidneys (3 mo of age). Thus, Mø and TEC are intrarenal sources of CXCL10 during nephritis in MRL-Faslpr mice.
Figure 1.
Despite an increase in intrarenal Mø and TEC expressing CXCL10, and CXCR3 expressing T cells and Mø in MRL-Faslpr nephritic kidneys, CXCL10−/− MRL-Faslpr mice are not protected from renal disease. (A) CXCL10 expression in the kidney was examined by immunostaining, flow cytometry (Mø), and real-time PCR (TEC). CXCL10−/− mice served as negative control. (B) CXCR3 expression was assessed on CD4+, CD8+, B220+ T cells and Mø (CD68+) in kidneys of MRL-Faslpr mice by flow cytometry. B6 mice at 2 mo of age served as normal controls. (C) Renal function, pathology, and leukocytic infiltrates are similar in CXCL10−/− MRL-Faslpr mice as compared with MRL-Faslpr mice. Data are means ± SEM.
Frequency of CXCR3+ T Cells and Mø Is Increased in MRL-Faslpr Nephritic Kidneys
Because there is an increase in the frequency of intrarenal CXCL10+ Mø and TEC, we probed for the expression of CXCR3. The frequency of CD4+, CD8+, and B220+ (unique double-negative) T cells and Mø (CD68+) expressing CXCR3 in the kidneys of MRL-Faslpr mice increased with nephritis (6 mo of age) as compared with non-nephritic MRL-Faslpr and B6 mice (2 mo of age; Figure 1B). The increased frequency of CXCR3+ T cells and Mø was not limited to the kidney; these leukocytes increased in their spleens (p < 0.05; data not shown). Taken together, an increase in intrarenal and extrarenal CXCR3+ T cell subsets and Mø is associated with a rise in intrarenal CXCL10 in nephritic MRL-Faslpr mice.
Nephritis, Systemic Disease, and Survival Are not Altered in CXCL10−/− MRL-Faslpr Mice
To determine whether CXCL10 is central to lupus nephritis and the systemic illness, we compared CXCL10−/− and WT MRL-Faslpr strains (6 mo of age). The severity of glomerular, interstitial, and perivascular pathology was similar in CXCL10−/− and WT MRL-Faslpr mice (Figure 1C). Similarly, we did not detect a difference in the number (CD68+, CD4+, CD8+, B220+ cells) and activation stages (CD69+, IFN-γ+; data not shown) of infiltrating leukocytes and renal function (Figure 1C). Furthermore, we did not detect a difference in survival in CXCL10−/− MRL-Faslpr mice (data not shown). Thus, despite enhanced intrarenal expression of CXCL10 and CXCR3 during nephritis, CXCL10 is not central to disease in MRL-Faslpr mice.
CXCR3 and Its Ligands, CXCL9, CXCL10, and CXCL11, Are Upregulated during NSN
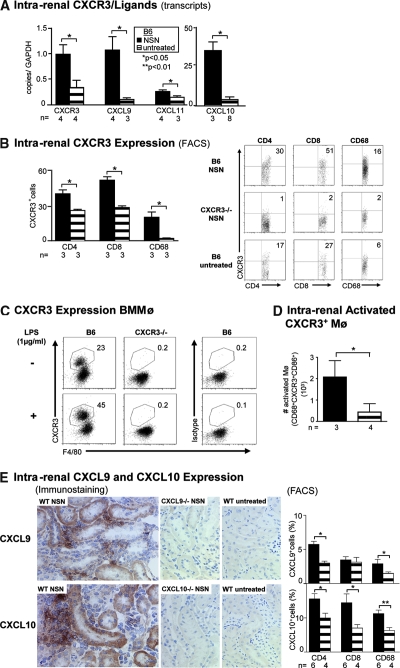
Our results suggest that CXCL10 is not central to lupus nephritis; however, it is possible that CXCR3 engaging with other ligands (CXCL9, CXCL11) promotes T cell–and Mø-mediated renal diseases. To explore this possibility, we selected a rapidly induced T cell–and Mø-dependent nephritis, NSN. Using this model, we evaluated the expression of CXCR3 and its ligands. Intrarenal CXCR3, CXCL10, CXCL9, and CXCL11 transcripts increased during NSN (Figure 2A). Thus, as in the MRL-Faslpr nephritic kidneys, intrarenal CXCR3 and its ligands are upregulated during NSN.
Figure 2.
T cells and activated Mø increase during NSN, whereas TEC are a primary source of CXCL9 and CXCL10 during NSN (day 14). (A) Expression of CXCR3 and its ligands CXCL10, CXCL9, and CXCL11 during NSN were evaluated by real-time PCR. (B) The frequency of intrarenal T cells (CD4+ and CD8+) and Mø expressing CXCR3. Note representative FACS plots of CXCR3 expression on T cells and Mø in WT and control CXCR3−/− mice during NSN. (C) The frequency of cultured BMMø expressing CXCR3 rises in response to stimulation with LPS. To verify staining specificity, we analyzed CXCR3−/− BMMø stimulated with LPS and B6 BMMø stimulated with LPS and stained with an isotype control. These data are representative of three samples and two additional experiments that used isotype controls alone. (D) Intrarenal activated Mø increased during NSN. We determined the total number of intrarenal activated (CD86+) CXCR3+ Mø by multiplying the frequency by the total number of Mø. (E) CXCL9 and CXCL10 are mainly expressed by TEC during NSN (n = 4/group). Intrarenal CXCL9+ CD4+ and CD68+ cells and CXCL10+ CD4+, CD8+, and CD68+ cells increase during NSN. We analyzed 0.5 to 1 × 106 cells (B, D, and E) and 2.5 × 105 cells (C) by FACS analysis. Data are means ± SEM.
To identify the leukocyte populations expressing CXCR3 and its ligands, we probed CD68+ and T cells (CD4+, CD8+) for CXCR3 expression. Of note, a similar percentage of intrarenal CD68+ and CD11b+ cells expressed CXCR3; therefore, we refer to these CD68+ cells as Mø (data not shown). The frequency of intrarenal CD4+, CD8+ T cells, and Mø expressing CXCR3 increased during NSN compared with untreated mice (Figure 2B). We verified CXCR3 antibody (Ab) specificity using CXCR3−/− mice with NSN. Although it is widely appreciated that CXCR3 is expressed on activated T cells, reports of CXCR3+ Mø are limited.15,18 To evaluate further CXCR3+ Mø, we determined that there is an increased frequency of CXCR3+ bone marrow macrophages (BMMø), (F4/80+; Figure 2C) and CD11b+ (data not shown) after stimulation with LPS (Figure 2C) and IFN-γ (data not shown) in WT mice. The specificity of CXCR3 Ab was verified by using CXCR3−/− BMMø and an isotype control. Because activated Mø induce apoptosis of renal parenchymal cells,11 we evaluated the frequency of activated Mø during NSN. Intrarenal CXCR3+-activated Mø increased during NSN as compared with untreated mice (Figure 2D). Thus, intrarenal CXCR3+-activated Mø and T cells are upregulated during NSN.
We next determined the intrarenal locale of the ligands (CXCL9, CXCL10) binding to CXCR3 during NSN. Because there is a functional loss of CXCL11 protein as a result of a missense mutation at 36 bp (G/A) and a single-nucleotide deletion mutation at 39 bp (C/−) in the gene for CXCL11 in B6 mice (Genbank sequence accession no. NT_109320; A.D.L. et al., unpublished observations), we evaluated CXCL9 and CXCL10. CXCL9 is expressed on the majority of TEC and absent on CXCL9−/− kidneys during NSN and in untreated mice (Figure 2E). In addition, a small population of leukocytes express CXCL9 (Figure 2E). These intrarenal CXCL9+ leukocytes that increased during NSN are CD4+ and CD68+ cells (Figure 2E). Similarly, CXCL10 is expressed primarily on TEC and to a lesser extent on leukocytes and is absent in CXCL10−/− control kidneys during NSN and in untreated mice (Figure 2E). The increase in intrarenal CXCL10+ leukocytes during NSN is in CD4+, CD8+, and CD68+ cells (Figure 2E). Although the frequency of these intrarenal CXCL10+ leukocytes is somewhat higher than in CXCL9+ cells, it was substantially (>2×) lower than in CXCR3+ cells (Figure 2, B and E). Thus, intrarenal CXCR3 is primarily expressed on activated T cells and Mø, whereas the ligands CXCL9 and CXCL10 are largely generated by TEC during NSN. Of note, CXCL9 and CXCL10 expression and CXCR3+ leukocytes are rarely detected in glomeruli during NSN (data not shown). This pattern of enhanced CXCR3 and its cognate ligand expression during NSN is consistent with our findings in MRL-Faslpr kidneys.
CXCR3 and CXCL9 but not CXCL10 Promote NSN
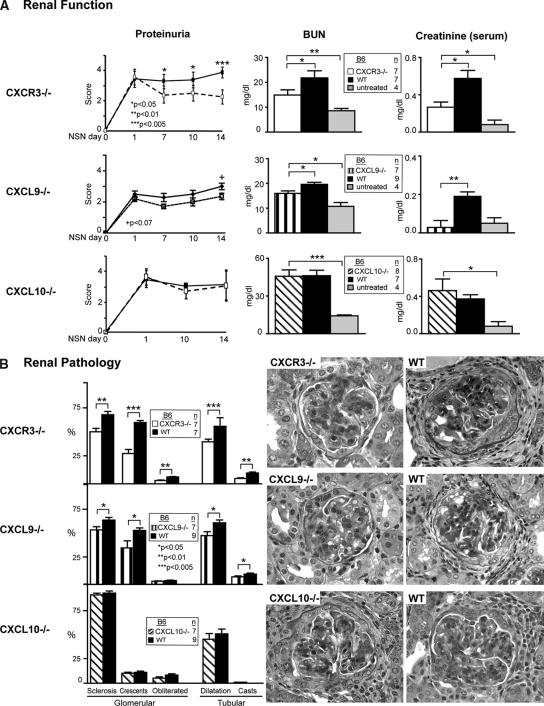
To determine whether CXCR3 regulates intrarenal T cell–and Mø-mediated renal disease via ligands other than CXCL10, we compared CXCR3−/−, CXCL9−/−, and CXCL10−/− mice with WT mice during NSN. Because CXCR3−/−, CXCL9−/−, and CXCL10−/− mouse strains do not express a functional CXCL11, for simplicity, we refer to each strain as the single unique “knockout.” CXCR3−/− mice were protected from the loss of renal function (Figure 3A). By comparison, renal function in CXCL10−/− mice was similar to WT mice during NSN (Figure 3A). Consistent with preserving renal function, renal pathology in CXCR3−/− but not in CXCL10−/− mice was reduced (Figure 3B). Tubular and glomerular pathology were blunted in CXCR3−/− but not in CXCL10−/− mice. Thus, CXCR3 but not CXCL10 promotes NSN, suggesting that another CXCR3 ligand is instrumental in immune-mediated nephritis.
Figure 3.
CXCR3−/− and CXCL9−/− but not CXCL10−/− mice are protected from loss of renal function and renal pathology during NSN (day 14). (A) The rise in proteinuria, blood urea nitrogen (BUN; day 14), and serum creatinine (day 14) was suppressed in CXCR3−/− and CXCL9−/− but not CXCL10−/− mice as compared with WT mice during NSN. Data are means ± SEM; n = 4 to 9/group. (B) Tubular and glomerular pathology is reduced in CXCR3−/− and CXCL9−/− mice as compared with WT mice. In contrast, tubular and glomerular pathology is similar in CXCL10−/− mice and WT mice during NSN. Representative photomicrographs are shown. Data are means ± SEM; n = 7 to 9/group. Magnification, ×40.
To determine whether CXCL9, another ligand for CXCR3, promotes immune-mediated nephritis, we compared CXCL9−/− and WT mice during NSN. Renal function (proteinuria, blood urea nitrogen, and serum creatinine) was improved in CXCL9−/− mice (Figure 3A). Similarly, renal pathology (glomerular and tubular) was suppressed in CXCL9−/− mice (Figure 3B, Supplemental Figure 3). Notably, renal function (Figure 3A) and pathology (data not shown) in CXCR3−/− and CXCL9−/− mice did not return to baseline (untreated mice). This suggests that CXCR3 and one, CXCL9, but not another, CXCL10, of its ligands promote immune-mediated nephritis.
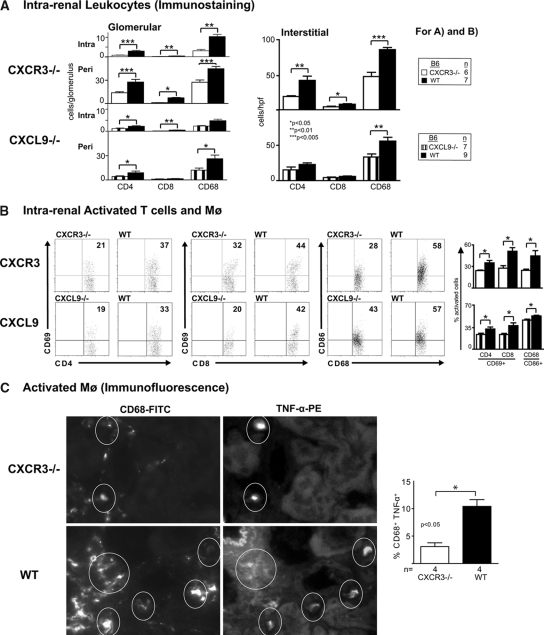
Intrarenal Activated T Cells and Mø Are Decreased in CXCR3−/− and CXCL9−/− Mice during NSN
Because CXCR3 is expressed on intrarenal T cells (CD4+, CD8+) and Mø during NSN, we hypothesized these intrarenal leukocytes decrease in CXCR3−/− as compared with WT mice. Fewer CD4+, CD8+, and Mø were detected in the glomeruli (intra-, periglomerular) and interstitium in CXCR3−/− mice (Figure 4A). A similar decrease in CD4+ T cells and Mø occurred in the CXCL9−/− kidneys, whereas we did not detect a reduction in these leukocytic populations in CXCL10−/− mice (data not shown).
Figure 4.
Intrarenal activated T cells and activated Mø are suppressed in CXCR3−/− and CXCL9−/− mice as compared with WT mice during NSN (day 14). (A) The number of CD4+ and CD8+ T cells and Mø detected by immunostaining is diminished within glomeruli (intra-, periglomerular) and in the interstitium in CXCR3−/− and CXCL9−/− mice as compared with WT mice during NSN. (B) We detected a suppression of activated (CD69+) CD4+ and CD8+ T cells and activated (CD86+) Mø in CXCR3−/− (top) and CXCL9−/− (bottom) mice as compared with WT mice during NSN. We analyzed 0.5 to 1.0 × 106 cells by FACS (n = 6 to 9/group). (C) We evaluated kidneys from CXCR3−/− and WT mice for the presence of activated Mø (CD68+, TNF-α+) during NSN using immunofluorescence. Representative photomicrographs illustrate CD68+ cells expressing TNF-α+ (circled) in the CXCR3−/− compared with WT kidneys. Data are means ± SEM; n = 4/group.
CXCR3 is expressed on activated T cells19 and Mø; therefore, we compared these intrarenal leukocytes in CXCR3−/− mice with WT mice during NSN. We detected a decreased frequency of activated (CD69+) CD4+ and CD8+ T cells and activated (CD86+) Mø in CXCR3−/− mice during NSN (Figure 4B). In support of this decrease in activated Mø, the frequency of TNF-α+ Mø was diminished (Figure 4C, circled, immunofluorescence). Similarly, CXCL9−/− mice had a reduced frequency of activated T cells and Mø (Figure 4B). Thus, CXCR3 engaging with CXCL9 fosters an intrarenal accumulation of activated CD4+, CD8+ T cells and Mø during NSN.
Serum Antigen-Specific IgG Isotypes and Glomerular IgG Deposits Are Suppressed in CXCR3−/− and CXCL9−/− Mice during NSN
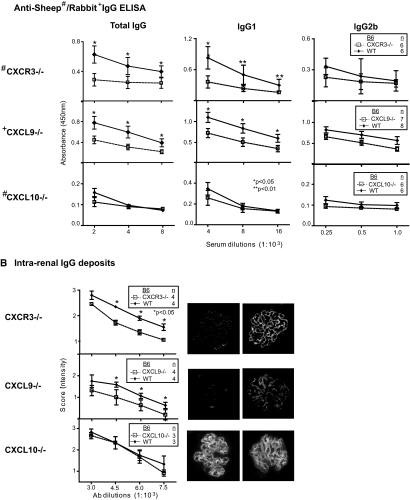
Elevated Ig and Ig class switching are hallmarks of autoimmune disease20,21; therefore, we determined whether the attenuated severity of kidney disease in CXCR3−/− and CXCL9−/− mice was related to a reduction in circulating levels of antigen-specific IgG isotypes during NSN. For this purpose, we measured the anti-sheep and -rabbit IgG isotypes in the sera of CXCR3−/− and CXCL9−/− mice, respectively, in comparison with WT mice during NSN. We detected a suppression of IgG and IgG1 and not IgG2b at multiple titers (2.0 to 8.0 × 103) in CXCR3−/− and CXCL9−/− mice (Figure 5A). Because serum isotypes were attenuated in CXCR3−/− and CXL9−/− mice, we hypothesized that the reduced renal pathology resulted from decreased deposition of IgG within glomeruli of CXCR3−/− and CXCL9−/− mice. We detected a reduction in IgG deposits at multiple dilutions (3.0 to 7.5 × 103) in CXCR3−/− and CXCL9−/− glomeruli (Figure 5B). Contrastingly, circulating anti-sheep IgG isotypes and glomerular IgG deposits in CXCL10−/− did not differ from WT mice. Taken together, this suggests CXCR3 engaging with CXCL9 may promote renal disease by increasing systemic IgG and IgG deposits within glomeruli.
Figure 5.
Decreased serum anti-sheep/rabbit IgG isotype Ab and IgG deposits in CXCR3−/− and CXCL9−/− mice. We detected a decrease in serum total anti-sheep IgG and IgG1 (n = 6/group) and glomerular IgG deposits (n = 4/group) in CXCR3−/− compared with WT mice. Similarly, we detected a reduction in serum total anti-rabbit IgG and IgG1 (n = 7 to 8/group) and in glomerular IgG deposits (n = 4/group) in CXCL9−/− compared with WT mice. In contrast, serum anti-sheep IgG isotypes (n = 6/group) and IgG glomerular deposits (n = 4/group) in CXCL10−/− mice did not differ from WT mice. Data are means ± SEM.
DISCUSSION
Chemokines are tempting therapeutic targets for a wide range of diseases, because they are specifically upregulated during inflammation. Mø and T cells are instrumental in mediating renal inflammation; thus, chemokines that regulate Mø and T cells within the kidney are potential therapeutic targets for nephritis. Therefore, we extensively profiled MRL-Faslpr kidneys before and during Mø- and T cell–dependent lupus nephritis. Although previous reports claimed that a few chemokine ligands (four of nine) and chemokine receptors (three of six) are upregulated in MRL-Faslpr kidneys, we now report that the majority of chemokine ligands (18 of 23) and chemokine receptors (10 of 16) that we profiled were upregulated in MRL-Faslpr mice with lupus nephritis.6,22 This difference in our findings may be related to the larger panel of chemokine ligand/receptors in our experiments and the detection system (ribonuclease protection assay22 versus real-time PCR, our study). We previously established that CCL2 is required to promote Mø- and T cell–dependent lupus nephritis in MRL-Faslpr mice.10 We now report that the rise in the panel of chemokine ligand/receptors that increase in MRL-Faslpr mice is suppressed in CCL2−/− MRL-Faslpr mice. This may suggest that CCL2 is a “proximal master switch” in the chemokine cascade; however, increased expression of a specific chemokine during disease does not necessarily indicate that it promotes inflammation; it may not have an impact or even thwart inflammation. Therefore, we investigated the impact of CXCR3 and its ligands, CXCL9 and CXCL10, during Mø and T cell immune–mediated nephritis (NSN). Of note, we could not analyze the impact of CXCL11 during NSN because the protein for this remaining CXCR3 ligand is not expressed in B6 mice. We now report that CXCR3 and CXCL9 but not CXCL10 are central to Mø- and T cell–dependent kidney disease.
The impact of CXCL10 in autoimmune and kidney disease is controversial. Whereas Ab blockade of CXCL10 in experimental insulin-dependent diabetes23 and adjuvant arthritis24 abrogates disease, deleting CXCL10 does not diminish experimental autoimmune encephalomyelitis, although it lowers disease threshold.25 Thus, the consequences of CXCL10 engaging with CXCR3 in autoimmune diseases are complex and yet not fully understood. Furthermore, the impact of CXCL10 on glomerular and tubulointerstitial renal disease is unclear. In induced forms of renal injury, puromycin aminonucleoside–and anti-nephrin Ab–induced nephropathy,26 Ab blockade of CXCL10 exacerbated proteinuria and podocyte injury. Conversely, Ab blockade of CXCL10 in a model of renal endothelial injury reduced interstitial T cell infiltration and improved renal function; however, pathology was not evaluated.27 We now report that despite the abundant protein expression of intrarenal CXCL10 on TEC and leukocyte infiltrates, CXCL10 is not instrumental in two distinct models of immune-mediated renal disease, MRL-Faslpr mice and NSN. This indicates that CXCL10 alone is not central to immune-mediated kidney disease.
Because it is possible that other ligands alone or together with CXCL10 promote immune-mediated kidney disease, we compared CXCR3−/− and CXCL9−/− mice during NSN with WT mice. CXCR3 and CXCL9 but not CXCL10 are central to immune-mediated kidney disease. This is the first report indicating that CXCL9−/− mice are protected from nephritis. Our findings are in agreement with recent studies reporting that CXCR3−/− mice develop less severe nephritis than WT mice during NSN.28 In fact, CXCR3 has been shown to incite nonimmune-induced renal inflammation because CXCR3 orchestrates the recruitment of T cells instrumental in mediating renal ischemic injury.16 Thus, CXCR3 may be a potential therapeutic target for a broad array of kidney diseases. Our findings are intriguing; although CXCL9 and CXCL10 protein expression is similar in locale and intensity during NSN, the functional impact of these two CXCR3 ligands differ. This differential impact on nephritis may be related to distinct bioavailability of CXCL9 and CXCL10. The preferential contribution of one CXCR3 ligand to disease pathology has been seen in other inflammatory models of disease, such as CXCL10 in mouse hepatitis virus and Dengue virus encephalitis29,30 and CXCL9 in herpes simplex virus-1 ocular infection and cytomegalovirus infection.31,32 Regardless of the exact mechanisms, our findings indicate that selective blockade of CXCL9 and CXCR3 is a potential therapeutic strategy to combat immune-mediated renal diseases.
Although it is well appreciated that activated T cells express CXCR3, we now report that activated and resting Mø express CXCR3 during kidney disease. The frequency of intrarenal CD68+ cells along with CD4+ and CD8+ T cells expressing CXCR3 increased during NSN. This rise in CXCR3+ Mø in the kidney is consistent with studies reporting that a low percentage of CXCR3+ monocytes in the normal circulation increases in patients with rheumatoid arthritis.33 Furthermore, we determined that whereas some primary cultured BMMø express CXCR3, the frequency increases after IFN-γ stimulation and is even more robust after LPS stimulation. This suggests that whereas low numbers of resting Mø express CXCR3, the increase in CXCR3+ Mø is a reflection of a rise in activated Mø during renal inflammation. Consistent with this concept, the total number of intrarenal activated CXCR3+ Mø increases during NSN as compared with untreated mice. Furthermore, CXCR3 is responsible for recruiting these activated Mø, because there is a decrease in intrarenal activated Mø, along with activated CD4+ and CD8+ T cells, in CXCR3−/− during NSN. This suggests that activated Mø bearing CXCR3 are central to inducing renal injury, because activated Mø release mediators that initiate TEC apoptosis.11 Thus, we provide the first evidence that CXCR3 is instrumental in recruiting activated Mø, as well as T cells, into the kidney, that are responsible for inciting renal injury.
Humoral and cell-driven immune mechanisms mediate kidney disease.20 We investigated whether the suppression in renal disease in the CXCR3−/− and CXCL9−/− mice during NSN was related to a decrease in pathogenic Ab within the kidney. We detected a reduction in total serum antigen-specific IgG and IgG1 Ab in CXCR3−/− and CXCL9−/− mice during NSN. Our finding is reminiscent of reduced Ab against a bacterial pathogen (Francisella tularensis) in CXCL9−/− mice34; however, our data are not in agreement with the recent study of CXCR3−/− mice, indicating that there is no difference in total mouse anti-sheep IgG or anti-sheep IgG1 during NSN (day 8).28 This difference may be related to the assay methods, the day of analysis, or other variables related to inducing NSN. Nevertheless, our data support the concept that CXCR3 and CXCL9 enhance Ab production during immune responses. The regulation of serum IgG and glomerular deposits by CXCR3 and CXCL9 remains unclear. We speculate that distinct patterns of CXCR3+CD4+ T cells and CXCL9 expression in germinal centers during inflammation and/or the expression of CXCR3 on a subset of memory B cells and plasma cells may regulate serum IgG and, in turn, glomerular deposits.35,36 Notably, the decline in antigen-specific IgG in CXCR3−/− and CXCL9−/− serum correlates with diminished IgG deposits within glomeruli; therefore, suppressed renal pathology and improved renal function may, in part, result from a reduced intrarenal deposition of pathogenic Ab in CXCR3−/− and CXCL9−/− mice during NSN. In addition, we did not detect CXCL9 and CXCL10 expression and CXCR3+ leukocytes in glomeruli of WT mice during NSN. Thus, the reduction in proteinuria in CXCR3−/− and CXCL9−/− mice during the later stages of NSN may be related to the suppression of humoral immune responses. This is consistent with findings indicating that although the generation of autologous murine IgG against the nephrotoxic Ab is not essential for disease initiation, it may contribute to sustaining renal dysfunction.37 This may indicate that although CXCR3 and CXCL9 promote the initiation of NSN via cell-mediated events, their regulation of the production and deposition of pathogenic Ab may be responsible for sustaining immune-mediated renal disease.
In conclusion, CXCR3 and one, CXCL9, but not another, CXCL10, of its ligands mediate activated T cells and Mø recruitment into the kidney and enhance Ab deposition in glomeruli that may, in turn, promote immune-mediated kidney disease. This suggests that blocking CXCR3 and CXCL9 is a potential therapeutic target for human immune-mediated kidney diseases.
CONCISE METHODS
Mice
We purchased MRL/MpJ-Faslpr/Faslpr (MRL-Faslpr) and C57BL/6 (B6) from the Jackson Laboratory (Bar Harbor, ME). Mice were housed and bred in our pathogen-free animal facility. CXCL9 null (−/−), CXCL10−/−, CXCR3−/− (B6 background), and CCL2−/− (MRL-Faslpr background) mice were generated as described previously.4,10,34,38 We generated CXCL10−/− MRL-Faslpr mice using a backcross-intercross scheme. MRL-Faslpr mice were mated with CXCL10−/− (B6 background) mice to yield heterozygous F1 offspring. We intercrossed F1 mice and screened the progeny for disrupted and intact CXCL10 and the Faslpr mutation in tail genomic DNA. The DNA was assessed by PCR using oligonucleotide primers that recognized the normal CXCL10 gene: Sense (5′-TCC CTC CCG TAA CCA CAC AGT AAA T-3′) and antisense (5′-GCG GAT AGA CTC TGC TTT CAC TTT GG-3′) and Neo gene sense (5′-TGG ATG TGG AAT GTG TGC GAG-3′) and antisense (5-TTT CAC TTT GG-3′). Gel analysis of the PCR products identified the CXCL10 and Neo gene fragments at 342 and 640 bp, respectively. The Faslpr mutation was identified as previously reported.39 After five generations of backcross matings (N5), we analyzed and compared CXCL10−/− MRL-Faslpr mice with age- and gender-matched WT MRL-Faslpr littermates. The number of mice analyzed is specified in each figure. We compared these strains at the N5 generation, because we previously established that there are sufficient MRL-Faslpr background genes to result in consistent phenotypic changes characteristic of MRL-Faslpr mice.40 Use of mice in our study was reviewed and approved by the Standing Committee on Animals in the Harvard Medical School in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Inducing NSN
We prepared nephrotoxic serum by immunizing sheep with a particulate fraction of mouse glomerular basement membrane from B6 mice kidneys, as described previously.41 To induce NSN, we primed CXCL10−/−, CXCR3−/−, and WT male mice (6 wk of age) by injecting 0.5 mg of sheep IgG in Freund's complete adjuvant (Sigma Chemical Co., St. Louis, MO) subcutaneously in each flank. We challenged these mice 7 d later with an intravenous injection of nephrotoxic serum (15 μl/g body wt). We used nephrotoxic serum from rabbits to immunize CXCL9−/− and WT mice as described previously.42 Mice were killed 14 d after challenge and analyzed. The number of mice in each group is specified within each figure.
Intrarenal Chemokine and Chemokine Receptor Transcripts
We analyzed the expression of chemokines (CXCL10, CXCL9, CXCL11, CXCL12, CCL13, CCL5, CCL28, CCL22, CCL24, CCL1, CCL17, CX3CL1, CCL25, CCL2, CCL8, CCL7, CCL3, CXCL2, CCL28α, CCL4, CCL28β, CCL27, CCL21, CXCL16, and CXCL1) and chemokine receptors (CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8, CCR9, CCR10, and CX3CR1) in MRL-Faslpr mice by using real-time, two-step, quantitative PCR, as described previously.43 Primers were designed, as previously reported.44 To detect CXCL10 expression during NSN, we used primers designed by Applied Biosystems (Foster City, CA). To detect CXCL9, CXCL11, and CXCR3 expression during NSN, we used the following primers: glyceraldehyde-3-phosphate dehydrogenase sense 5′-CAT GGC CTC CAA GGA GTA AG-3′ and antisense 5′-CCT AGG CCC CTC CTG TTA TT-3′; CXCL9 sense 5′-TCC TTT TGG GCA TCA TCT TC-3′ and antisense 5′-TTC CCC CTC TTT TGC TTT TT-3′; CXCL11 sense 5′-AGT AAC GGC TGC GAC AAA GT-3′ and antisense 5′-GCA TGT TCC AAG ACA GCA GA-3′; and CXCR3 sense 5′-TGA GAC AAC TGA GGC CTC CTA-3′ and antisense 5′-TCT TGC TCC CCA GTT GAT G-3′ (Invitrogen, Carlsbad CA). We evaluated expression as previously reported.45
Isolation of TEC and BMMø
We isolated and cultured TEC from B6 and MRL-Faslpr mice as previously reported,46 and we isolated and cultured BMMø from B6 mice as described previously.47
Flow Cytometry
We prepared and stained single-cell suspensions from kidneys, spleens, or primary cultured TEC/BMMø as described previously.48 We collected 0.5 to 1.0 × 106 total kidney or spleen cells and 0.5 to 1.0 × 105 of cultured cells using a FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed data using Flowjo software (Tree Star, Palo Alto, CA).
Antibodies
We used the following Ab from eBioscience (San Diego, CA) for FACS analysis: FITC-conjugated anti-CD4 (L3T4), anti-CD8 (53-6.7), anti-B220 (RA3-6B2), and anti-CD45.2 (104); PE-conjugated anti-CD4, anti-CD45.2, anti-CD69 (H1.2F3), and anti-CD86 (GL1); PE-Cy5–conjugated anti-CD8 and anti–TCR-β chain (H57-597); and allophycocyanin-conjugated anti-CD4, anti-CD45.2, and anti-F4/80 (BM8). We used FITC- and allophycocyanin-conjugated anti-CD68 Ab (FA11; Serotec, Oxford, UK). We used purified rabbit anti-mouse CXCR3 Ab (Zymed, San Francisco, CA), purified goat anti-mouse CXCL9 Ab (R&D Systems, Minneapolis, MN), and rabbit anti-mouse CXCL10 Ab (PeproTech, Rocky Hill, NJ). The following isotype-specific Ab were used for controls: Rat-IgG2a (BR2a), rat-IgG2b (KLH/G2b-1-2), and rabbit-IgG (eBioscience). As secondary PE- or allophycocyanin-conjugated Ab, we used goat anti-rabbit (Jackson ImmunoResearch Laboratories; West Grove, PA) and biotin-conjugated rabbit anti-goat Ab (Vector Laboratories, Burlingame, CA). To detect biotin-conjugated secondary Ab, we used streptavidin PE or allophycocyanin (Jackson ImmunoResearch Laboratories). We identified renal proximal tubules by binding of fluorescein-conjugated lotus lectin (Vector Laboratories).49,50
Histopathology
We fixed kidneys in 10% formalin and prepared and stained paraffin sections with the periodic acid Schiff reagent. Slides were coded before grading the renal pathology.
MRL-Faslpr Mice.
We evaluated renal (glomerular, tubular, interstitial, and perivascular) pathology on a scale of 0 (normal) to 3 (severe) as described previously.45
NSN.
We evaluated CXCL9−/−, CXCL10−/−, CXCR3−/−, and B6 kidneys for glomerular and tubular damage as described previously.11
Gross Pathology
We evaluated skin lesions monthly from 2 to 6 mo of age using a scoring system previously described.10
Immunohistochemistry
We stained cryostat-cut kidney sections for the presence of Mø with anti-CD68 Ab (FA-11; Serotec) and for T cells with anti-CD4 Ab (RM4-5), anti-CD8 Ab (53-6.7), and anti-B220 (RA3–6B2) rat anti-mouse Ab (Pharmingen, San Diego, CA) according to a previously described immunoperoxidase method.51 To evaluate CXCL9 and CXCL10 expression in the kidney, we stained frozen sections with goat anti-mouse CXCL9 and rabbit anti-mouse CXCL10. The following isotype-specific Ab were used for controls: Rat-IgG2a (R35–95), rat IgG2b (R35-38), rabbit IgG (eBioscience), and goat IgG (Southern Biotechnology, Birmingham, AL). The secondary Ab for immunostaining was biotin-conjugated rabbit anti-rat IgG (Vector Laboratories). The immunostaining was analyzed by counting for the presence of CD68+, CD4+, CD8+, and B220+ cells within and surrounding glomeruli and in the interstitium in 10 randomly selected high-power fields. Of note, we have determined that the B220+ cells in the kidney of MRL-Faslpr mice are the unique double-negative (CD4−CD8− double-negative) T cells and are not B cells.52
To determine the number of activated Mø in the kidney during NSN, we fixed frozen kidney sections in paraformaldehyde, stained them with rat anti-mouse TNF-α–PE (MP6-XT22; eBioscience) and anti–CD68-FITC (FA-11; Serotec), and analyzed these sections using a fluorescence microscope. The frequency (%) of activated Mø was assessed by enumerating the number of CD68+TNF-α+ cells within the total number of Mø (CD68+) in five high-power fields.
Renal Function
We assessed urine proteins semiquantitatively by dipstick analysis as described previously.45 We measured blood urea nitrogen levels using a colorimetric analysis kit (Infinity; Thermo Electron, Melbourne, Australia) and serum creatinine using the creatinine reagent kit and Creatinine Analyzer 2 (Beckman Coulter, Galway, Ireland) according to the manufacturer's instructions.
Survival
We compared survival of the CXCL10−/− MRL-Faslpr and WT MRL-Faslpr mice using similar numbers of males and females from birth to 10 mo of age.
Serum Anti-Sheep and Anti-Rabbit IgG Isotypes
We measured the levels of mouse anti-sheep IgG Ab (Sigma Chemical Co.) and anti-rabbit IgG (Jackson ImmunoResearch Laboratories) by ELISA using sera collected at day 14 during NSN as described previously.53 We detected bound mouse anti-sheep/anti-rabbit IgG, IgG1, and IgG2b using peroxidase-conjugated rabbit anti-mouse IgG Ab (1:5000; Southern Biotechnology).
IgG Deposits within the Kidney
We evaluated IgG deposits within renal glomeruli as described previously.54 We evaluated stained sections by scoring 20 glomeruli as either positive or negative and graded the amount (severity) of deposits in 20 positive glomeruli per specimen on a scale of 0 to 3 using multiple dilutions (1:3000, 1:4500, 1:6000, and 1:7500) of the detection Ab (fluorescein-conjugated goat anti-mouse IgG; MP Biomedicals, Aurora, OH).40
Statistical Analyses
The data are means ± SEM and were analyzed by GraphPad Prism 4.0 (GraphPad, San Diego, CA). We used the nonparametric Mann-Whitney U test to determine differences among groups. Survival curves were compared and analyzed using the log-rank test.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants DK 52369 (V.R.K.), DK 56848 (V.R.K.), DK 36149 (V.R.K.), KO1 AR 051367 (T.K.M.), and RO1 CA 069212 (A.D.L.); by Deutsche Forschungsgemeinschaft grants ME-3194/1-1 (J.M.) and ZE-711/1 (G.Z.); and the Research Grants Council of Hong Kong HKU 7592/06 (H.Y.L.).
We thank Dr. Craig Gerard for providing CXCR3−/− mice, Dr. Tanya Mayadas for the rabbit anti–glomerular basement membrane anti-serum, and Dr. Julie Lucas for editorial assistance.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Sallusto F, Mackay CR, Lanzavecchia A: The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol 18: 593–620, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Rossi D, Zlotnik A: The biology of chemokines and their receptors. Annu Rev Immunol 18: 217–242, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Segerer S, Cui Y, Hudkins KL, Goodpaster T, Eitner F, Mack M, Schlondorff D, Alpers CE: Expression of the chemokine monocyte chemoattractant protein-1 and its receptor chemokine receptor 2 in human crescentic glomerulonephritis. J Am Soc Nephrol 11: 2231–2242, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD: IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 168: 3195–3204, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Anders HJ, Vielhauer V, Schlondorff D: Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int 63: 401–415, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Vielhauer V, Anders HJ, Schlondorff D: Chemokines and chemokine receptors as therapeutic targets in lupus nephritis. Semin Nephrol 27: 81–97, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Premack BA, Schall TJ: Chemokine receptors: Gateways to inflammation and infection. Nat Med 2: 1174–1178, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Hooke DH, Gee DC, Atkins RC: Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int 31: 964–972, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Kelley VE, Roths JB: Interaction of mutant lpr gene with background strain influences renal disease. Clin Immunol Immunopathol 37: 220–229, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR: Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med 190: 1813–1824, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR: Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest 103: 73–80, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore KJ, Wada T, Barbee SD, Kelley VR: Gene transfer of RANTES elicits autoimmune renal injury in MRL-Fas(lpr) mice. Kidney Int 53: 1631–1641, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Luster AD, Ravetch JV: Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J Exp Med 166: 1084–1097, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, Rosa R, Di Salvo J, Mudgett J, Peterson LB, Wicker LS, DeMartino JA: Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol 73: 771–780, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Callahan MK, Huang D, Ransohoff RM: Chemokine receptor CXCR3: An unexpected enigma. Curr Top Dev Biol 68: 149–181, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Fiorina P, Ansari MJ, Jurewicz M, Barry M, Ricchiuti V, Smith RN, Shea S, Means TK, Auchincloss H Jr, Luster AD, Sayegh MH, Abdi R: Role of CXC chemokine receptor 3 pathway in renal ischemic injury. J Am Soc Nephrol 17: 716–723, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Shiozawa F, Kasama T, Yajima N, Odai T, Isozaki T, Matsunawa M, Yoda Y, Negishi M, Ide H, Adachi M: Enhanced expression of interferon-inducible protein 10 associated with Th1 profiles of chemokine receptor in autoimmune pulmonary inflammation of MRL/lpr mice. Arthritis Res Ther 6: R78–R86, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, Kovac AD, Gerard C, Boddeke HW, Nitsch R, Kettenmann H: CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci 24: 8500–8509, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR: The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 101: 746–754, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean EG, Wilson GR, Li M, Edgtton KL, O'Sullivan KM, Hudson BG, Holdsworth SR, Kitching AR: Experimental autoimmune Goodpasture'squosquo; apos; ys disease: A pathogenetic role for both effector cells and antibody in injury. Kidney Int 67: 566–575, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Izui S, Fossati-Jimack L, da Silveira SA, Moll T: Isotype-dependent pathogenicity of autoantibodies: Analysis in experimental autoimmune hemolytic anemia. Springer Semin Immunopathol 23: 433–445, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Perez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlondorff D: Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol 12: 1369–1382, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB: Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol 171: 6838–6845, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Salomon I, Netzer N, Wildbaum G, Schif-Zuck S, Maor G, Karin N: Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J Immunol 169: 2685–2693, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Klein RS, Izikson L, Means T, Gibson HD, Lin E, Sobel RA, Weiner HL, Luster AD: IFN-inducible protein 10/CXC chemokine ligand 10-independent induction of experimental autoimmune encephalomyelitis. J Immunol 172: 550–559, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Han GD, Suzuki K, Koike H, Yoneyama H, Narumi S, Shimizu F, Kawachi H: IFN-inducible protein-10 plays a pivotal role in maintaining slit-diaphragm function by regulating podocyte cell-cycle balance. J Am Soc Nephrol 17: 442–453, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Panzer U, Steinmetz OM, Reinking RR, Meyer TN, Fehr S, Schneider A, Zahner G, Wolf G, Helmchen U, Schaerli P, Stahl RA, Thaiss F: Compartment-specific expression and function of the chemokine IP-10/CXCL10 in a model of renal endothelial microvascular injury. J Am Soc Nephrol 17: 454–464, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Panzer U, Steinmetz OM, Paust HJ, Meyer-Schwesinger C, Peters A, Turner JE, Zahner G, Heymann F, Kurts C, Hopfer H, Helmchen U, Haag F, Schneider A, Stahl RA: Chemokine receptor CXCR3 mediates T cell recruitment and tissue injury in nephrotoxic nephritis in mice. J Am Soc Nephrol 18: 2071–2084, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Liu MT, Keirstead HS, Lane TE: Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol 167: 4091–4097, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Hsieh MF, Lai SL, Chen JP, Sung JM, Lin YL, Wu-Hsieh BA, Gerard C, Luster A, Liao F: Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol 177: 1855–1863, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Wuest T, Farber J, Luster A, Carr DJ: CD4+ T cell migration into the cornea is reduced in CXCL9 deficient but not CXCL10 deficient mice following herpes simplex virus type 1 infection. Cell Immunol 243: 83–89, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salazar-Mather TP, Hamilton TA, Biron CA: A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Invest 105: 985–993, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katschke KJ Jr, Rottman JB, Ruth JH, Qin S, Wu L, LaRosa G, Ponath P, Park CC, Pope RM, Koch AE: Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum 44: 1022–1032, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Park MK, Amichay D, Love P, Wick E, Liao F, Grinberg A, Rabin RL, Zhang HH, Gebeyehu S, Wright TM, Iwasaki A, Weng Y, DeMartino JA, Elkins KL, Farber JM: The CXC chemokine murine monokine induced by IFN-gamma (CXC chemokine ligand 9) is made by APCs, targets lymphocytes including activated B cells, and supports antibody responses to a bacterial pathogen in vivo. J Immunol 169: 1433–1443, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Rabin RL, Alston MA, Sircus JC, Knollmann-Ritschel B, Moratz C, Ngo D, Farber JM: CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J Immunol 171: 2812–2824, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA: Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood 105: 3965–3971, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Rosenkranz AR, Knight S, Sethi S, Alexander SI, Cotran RS, Mayadas TN: Regulatory interactions of alphabeta and gammadelta T cells in glomerulonephritis. Kidney Int 58: 1055–1066, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD: Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med 193: 975–980, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Zhou T, He J, Mountz JD: Autoimmune disease in mice due to integration of an endogenous retrovirus in an apoptosis gene. J Exp Med 178: 461–468, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kikawada E, Lenda DM, Kelley VR: IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol 170: 3915–3925, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Tipping PG, Cornthwaite LJ, Holdsworth SR: Beta 2 integrin independent neutrophil recruitment and injury in anti-GBM glomerulonephritis in rabbits. Immunol Cell Biol 72: 471–479, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Vielhauer V, Stavrakis G, Mayadas TN: Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest 115: 1199–1209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdi R, Means TK, Ito T, Smith RN, Najafian N, Jurewicz M, Tchipachvili V, Charo I, Auchincloss H Jr, Sayegh MH, Luster AD: Differential role of CCR2 in islet and heart allograft rejection: Tissue specificity of chemokine/chemokine receptor function in vivo. J Immunol 172: 767–775, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD: The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol 170: 5165–5175, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Lenda DM, Stanley ER, Kelley VR: Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J Immunol 173: 4744–4754, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Wuthrich RP, Glimcher LH, Yui MA, Jevnikar AM, Dumas SE, Kelley VE: MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int 37: 783–792, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH: Macrophages in streptozotocin-induced diabetic nephropathy: Potential role in renal fibrosis. Nephrol Dial Transplant 19: 2987–2996, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Lenda D, Kikawada E, Stanley ES, Kelley VR: Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1-deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol 170: 3254–3262, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Faraggiana T, Malchiodi F, Prado A, Churg J: Lectin-peroxidase conjugate reactivity in normal human kidney. J Histochem Cytochem 30: 451–458, 1982 [DOI] [PubMed] [Google Scholar]
- 50.Swinford AE, Bernstein J, Toriello HV, Higgins JV: Renal tubular dysgenesis: Delayed onset of oligohydramnios. Am J Med Genet 32: 127–132, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Moore KJ, Naito T, Martin C, Kelley VR: Enhanced response of macrophages to CSF-1 in autoimmune mice: A gene transfer strategy. J Immunol 157: 433–440, 1996 [PubMed] [Google Scholar]
- 52.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR: IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL- Fas(lpr) mice. J Immunol 161: 494–503, 1998 [PubMed] [Google Scholar]
- 53.Topham PS, Csizmadia V, Soler D, Hines D, Gerard CJ, Salant DJ, Hancock WW: Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis. J Clin Invest 104: 1549–1557, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinoshita K, Tesch G, Schwarting A, Maron R, Sharpe AH, Kelley VR: Costimulation by B7–1 and B7–2 is required for autoimmune disease in MRL-Faslpr mice. J Immunol 164: 6046–6056, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.