Abstract
Individuals waiting for a renal transplant experience excessive cardiovascular mortality, which is not fully explained by the prevalence of ischemic heart disease in this population. Overt heart failure is known to increase the mortality of patients with ESRD, but the impact of lesser degrees of ventricular systolic dysfunction is unknown. For examination of the association between left ventricular ejection fraction (LVEF) and mortality of renal transplant candidates, the records of 2718 patients evaluated for transplantation at one institution were reviewed. During 6355 patient-years (median 27 mo) of follow-up, 681 deaths occurred. Patients with systolic dysfunction (LVEF ≤0.40) had significantly lower survival than those with higher systolic function (median 49 ± 3.1 versus 72 ± 4.0 mo; P < 0.001) but had similar survival to patients with ischemia (48 ± 2.5 mo). Multivariate modeling showed that those with systolic dysfunction were nearly twice as likely to die as those with normal systolic function, adjusted for risk factors including diabetes, left ventricular hypertrophy, and ischemia (adjusted hazard ratio 1.7; 95% confidence interval 1.43 to 2.07). In addition, a graded, reverse association between LVEF and mortality was identified. In conclusion, systolic dysfunction is strongly associated with mortality, in a graded manner, in renal transplant candidates.
Cardiovascular mortality is increased in chronic kidney disease (CKD).1 CKD has been proposed as an independent risk factor for cardiac mortality,2 a phenomenon particularly evident among dialysis patients.3 Reports on the mechanisms of cardiac death thus far have drawn associations between CKD and outcomes of atherosclerotic heart disease. Notwithstanding the high prevalence of coronary artery disease in the dialysis population,4 ischemic events would not account for most of the cardiac deaths reported.5 An alternative explanation for the high mortality on the renal transplant waiting list is needed. Overt heart failure (HF) is associated with high mortality rates in dialysis6 and posttransplantation populations.7 HF is commonly described as either diastolic or systolic ventricular dysfunction (SD), the latter been gauged by the left ventricular ejection fraction (LVEF). In the general population, all-cause and cardiac mortality risk increases with declining LVEF.8 Whether lesser degrees of SD, which are often asymptomatic, exert similar impact on mortality in patients who have CKD and are awaiting transplantation is unknown. Furthermore, despite the association between ischemia and SD, previous reports have not differentiated their specific roles to the premature cardiac mortality in the CKD population.
Renal transplant candidates constitute an ideal population to study cardiac disease and mortality because (1) they are most often at stage 4 or 5 CKD at the time of evaluation, (2) the prevalence of cardiovascular disease is markedly increased, (3) follow-up is near complete while patients are on the waiting list, and (4) mortality while on the waiting list is increased. Furthermore, the transplant evaluation process is fairly standardized across centers and focused on identifying cardiovascular disease. Most common, noninvasive techniques are used, including cardiac single-photon emission computed tomography (SPECT), which identifies coronary artery disease by describing myocardial perfusion defects,9 a procedure widely used and validated in the CKD population.10 More recently, gated-SPECT imaging has allowed simultaneous measurement of LVEF and perfusion. This study describes the role of LVEF as an independent risk factor for all-cause and cardiac-related mortality in a population awaiting renal transplantation.
RESULTS
Between January 1, 1997, and June 30, 2004, 5305 individuals were evaluated for kidney transplantation at our institution. We excluded 76 recipients of previous nonrenal allografts and 557 individuals with previous diagnosis of HF. In addition, 458 patients were not considered transplant candidates and never listed. Of the remaining 4214 patients, 2718 (64.5%) fulfilled the American Society of Transplantation guidelines criteria for cardiac imaging and had stress-SPECT imaging completed and comprise the final study cohort. Of these, 446 patients had also undergone two-dimensional echocardiogram (2DE). Demographic data are depicted in Table 1. When compared with those with normal LVEF, the SD group included more men, smokers, left ventricular hypertrophy (LVH), perfusion abnormalities, and longer exposure to dialysis. In contrast, patients with normal LVEF were older, had lower serum albumin and creatinine levels, and had higher body mass index (BMI).
Table 1.
Demographic data according to left ventricular systolic function
| Parameter | SDa(n = 442) | Normal ejection fraction(n = 2276) | Overall(n = 2718) |
|---|---|---|---|
| Diabetes (%) | 67 | 65 | 66 |
| Hypertension (%) | 78 | 76 | 76 |
| Black race (%) | 56 | 51 | 52 |
| Male gender (%) | 75b | 57 | 59 |
| Age (yr; mean ± SD) | 50.0 ± 9.8c | 51.0 ± 10.9 | 51.0 ± 10.7 |
| Low socioeconomic status (%) | 24 | 23 | 24 |
| Body mass index (kg/m2; mean ± SD) | 28.0 ± 5.1c | 29.0 ± 5.4 | 28.0 ± 5.3 |
| Tobacco smoking (%) | 49b | 39 | 41 |
| Dialysis exposure (%) | 87b | 79 | 80 |
| Dialysis (mo; mean ± SD) | 20.0 ± 25.7b | 15.0 ± 22.9 | 16.0 ± 23.5 |
| Previous renal transplant (%) | 8 | 7 | 7 |
| Anemia (%) | 35 | 35 | 35 |
| Left ventricular hypertrophy (%) | 54c | 40 | 44 |
| Perfusion abnormalities on SPECT (%) | 39b | 17 | 22 |
| Serum Albumin (mg/dl; mean ± SD) | 3.5 ± 0.4c,d | 3.4 ± 0.5 | 3.4 ± 0.5 |
| Serum Creatinine (mg/dl; mean ± SD) | 9.1 ± 7.3b | 8.1 ± 4.3 | 3.4 ± 0.5 |
| LVEF by SPECT | 32.0 ± 6.4b | 57.0 ± 8.9 | 53.0 ± 12.5 |
LVEF ≤40%.
P < 0.001 between SD and normal EF.
P < 0.05 between SD and normal EF.
Serum creatinine conversion factor (from mg/dl to μ mol/L): 88.4.
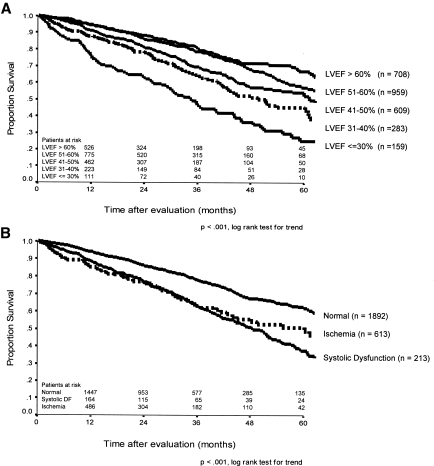
During 6355 patient-years of follow-up, 681 (25%) patients died and 813 (30%) patients received a transplant. The median follow-up for the entire cohort was 27 mo (range 0.3 to 95.8 mo). Death was due to a cardiac event in 43% of the known causes of death. Patients with normal LVEF survived substantially longer on the waiting list than did those in the SD group (73 ± 4.3 versus 44 ± 3.1 mo; P < 0.001). Further categorization of the SD group by LVEF disclosed a stepwise relationship between degrees of SD and mortality (Figure 1A), which was also shown when cardiovascular death was the end point (Figure 2). The proportion of patients removed from the waiting list within the first 2 yr as a result of transplantation was similar between the SD (17%) and control subjects with normal LVEF (23%).
Figure 1.
(A) Overall survival after evaluation according to categories of LVEF. (B) Overall survival according to presence of ischemia or systolic dysfunction (Systolic DF: left ventricular ejection fraction ≤40%) at the time of transplant evaluation.
Figure 2.
Cardiovascular death survival after evaluation, according to categories of LVEF. All other causes of death were considered censoring events.
Univariate analysis of the stratified cohort disclosed a difference in longevity, with median waiting list survival for SD patients of 49 ± 3.1 mo, significantly lower than 72 ± 4.0 mo for control subjects (P < 0.001). Four-year mortality was 54.8% in the SD group. Patients with SD but no evidence of coronary artery disease fared no better than patients in the ischemia group, whose median survival was 48 ± 2.5 mo (P = 0.8; Figure 1B). By excluding the 613 patients with ischemia, a stepwise increase in all-cause mortality was documented with reduced LVEF (reference LVEF 51 to 60%; n = 800): Crude hazard ratio (HR) 2.9 (95% confidence interval [CI] 1.96 to 4.27) for LVEF ≤30% (n = 60), 1.5 (1.09 to 2.09) for LVEF 31 to 40% (n = 153), 1.3 (1.05 to 1.69) for LVEF 41 to 50% (n = 436), and 0.9 (0.68 to 1.12) for LVEF >60% (n = 656). The same pattern was seen with cardiac mortality: Crude HR 6.4 (4.15 to 10.01) for LVEF ≤30%, 3.5 (2.26 to 5.33) for LVEF 31 to 40%, 1.8 (1.16 to 2.67) for LVEF 41 to 50%, and 0.8 (0.51 to 1.37) for LVEF >60%.
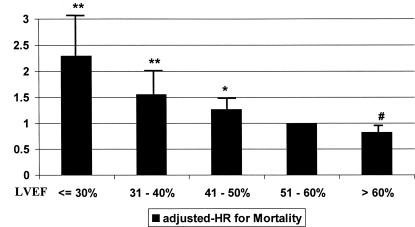
After accounting for the presence of ischemia and all other risk factors, the adjusted HR for SD was 1.7 (95% CI 1.43 to 2.07), an independent association from diabetes, LVH, and abnormal perfusion (Table 2). A stepwise relationship is suggested by the presence of a graded-reverse association between LVEF and mortality (Figure 3). This finding was further confirmed by entering LVEF into the models as a continuous variable (adjusted HR 0.975; 95% CI 0.968 to 0.981; P < 0.001; in other words, for each percent increment in LVEF, the adjusted mortality risk decreased by 2.5%). Further analysis using cardiac death as the outcome of interest showed similar results: adjusted HR for SD 2.9 (95% CI 2.12 to 4.03). The models including the interaction term “ischemia × SD” resulted in adjusted HR not different from the main-effect models. The models built across strata of risk factors showed internal consistency because adjusted HR were similar across strata (dialysis and nondialysis, gender, race, age categories, and ischemia).
Table 2.
Multivariable analysis: Mortality after evaluation
| Parameter | Adjusted HR | 95% CI | P |
|---|---|---|---|
| LVEF ≤30% | 2.3 | 1.79 to 3.06 | <0.001 |
| LVEF 31 to 40% | 1.6 | 1.21 to 1.99 | <0.001 |
| LVEF 41 to 50% | 1.3 | 1.03 to 1.57 | 0.020 |
| LVEF 51 to 60% | 1.0 | Reference | |
| LVEF >60% | 0.8 | 0.65 to 1.05 | 0.100 |
| Diabetes | 1.6 | 1.33 to 1.91 | <0.001 |
| Ischemia | 1.2 | 1.04 to 1.48 | 0.017 |
| Months on dialysis | 1.0 | 1.01 to 1.02 | 0.020 |
| Male gender | 0.8 | 0.70 to 0.98 | 0.025 |
| Hypertension | 0.8 | 0.62 to 0.98 | 0.030 |
| Obesity status | 0.7 | 0.59 to 0.99 | 0.050 |
| Age >50 yr | 1.1 | 0.94 to 1.29 | 0.200 |
| Black race | 0.6 | 0.30 to 1.16 | 0.100 |
| Anemia | 1.1 | 0.94 to 1.29 | 0.200 |
| Low socioeconomic status | 1.1 | 0.93 to 1.31 | 0.300 |
Figure 3.
Adjusted HR for all-cause mortality, according to categories of LVEF. Adjustments were made for age, gender, SES, obesity, race, presence of diabetes, hypertension, LVH, preexisting ischemia, anemia, low albumin level, tobacco smoking, and duration of dialysis. **P < 0.001; *P = 0.02; #P = 0.1.
2DE was performed for 446 (16.4%) of the 2718 patients with SPECT data. There was a significant correlation between the LVEF measurements by SPECT and 2DE (r = 0.74, P < 0.001). The multivariable analysis confirmed the association between SD (LVEF ≤40%; n = 91) by 2DE and mortality (adjusted HR 1.8; 95% CI 1.23 to 2.60) and cardiac mortality (adjusted HR 2.2; 95% CI 1.29 to 3.91).
DISCUSSION
Although the prevalence of cardiovascular disease is markedly increased in the CKD population, a closer analysis of the mortality data suggests that ischemic events are not the predominant cause of death in this population, and other mechanisms may be operative.5 Overt HF in the setting of ESRD portends a dismal outlook with a 3-yr mortality of 50% after diagnosis6 and 80% after hospitalization for HF.11 In a population-based study, the reported all-cause mortality at 4 yr was 9.8% for individuals with LVEF ≤40%.12 Little is known about mortality of patients with advanced-stage CKD and milder degrees of SD or regarding the relative importance of ischemic and nonischemic causes. Unlike previous reports, this analysis focused on asymptomatic individuals without previous diagnoses of HF: The sole reason for testing was to establish transplantation candidacy. This approach allowed us to examine the importance of lesser degrees of SD, adding a “dose-response” dimension that best described the continuum of exposure and avoiding arbitrary and potentially biased cutoff points for LVEF. This is the first work attempting to dissect the role of an ischemic substrate in SD, by using stratified analysis, adjustments via multivariable modeling, and the analysis of two-way interactions. Finally, we used a single standardized technique widely used clinically (SPECT) with validation by another method (2DE), which allows for easy reproducibility of our protocol.
The presence of a relationship between LVEF and mortality in our population was not unexpected; however, the magnitude of such association was not anticipated, because we described an almost six-fold increase in mortality for transplant candidates (54.8% 4-yr all-cause mortality for individuals with LVEF ≤40%) when compared with the general population with similar degree of SD.12 These findings offer clinically important observations for the care of patients with advanced-stages CKD. First, it provides an alternative explanation to the ischemic model as the leading mechanism resulting in the premature death of these individuals. Uremia has been associated with decrease in cardiac contractility function in a rodent model.13 We hope that our report stimulates further research into the mechanisms and therapeutic strategies focusing on myocardial contractility. Second, it identifies a subset of the CKD population (those with SD, with or without ischemia) at significantly higher mortality risk while awaiting transplantation, where the role of medical interventions and devices such as implantable cardiac defibrillators and pacemakers should be studied. Transplantation is considered the therapy of choice for advanced stages of CKD, and improvements or even normalization of LV function within the first year of engraftment have been described14,15; however, because of disparities in the supply of organs and number of listed patients, transplantation is often delayed for months or years, which results in unacceptably high mortality rates in individuals waiting for a kidney. Because the Centers for Medicare and Medicaid Services and the United Network for Organ Sharing are currently evaluating kidney allocation policies in the light of “net benefit,”16 the significance of LV function in influencing outcomes should be studied prospectively.
This observational study has limitations. Whereas ascertainment of outcome was possible, specific causes of death were described as “unknown” in one third of cases, which might have limited some of the analyses. Measurements of LVEF by two modalities were limited to the 446 patients who had undergone 2DE. Nonetheless, it is reassuring to find some correlation between the two techniques. LVEF measurements were not systematically repeated in a large proportion of patients, and no adjustments for interim therapies have been made. For full ascertainment of the presence of coronary artery disease, angiograms should have been performed for all patients with decreased LVEF; however, because of expenses and risks associated with the procedure, this approach seems unfeasible. No assessment of diastolic dysfunction was obtained, thereby preventing the analysis of this contributing factor in mortality. Finally, our data were limited to the period spent on the waiting list; therefore, the impact of transplantation on survival of patients with CKD and SD should be the focus of future research.
In conclusion, we reported a significant association between SD and mortality in patients awaiting renal transplantation. This association was independent of ischemia and other cardiovascular risk factors. The cumulative mortality for patients who had SD and were awaiting transplantation was almost six-fold higher than the reported mortality for similar LVEF in the general population. Widely available and minimally invasive testing could identify this high mortality risk subset of the CKD population.
CONCISE METHODS
This is a single-center observational study using data collected prospectively in a large transplant center. The University of Alabama at Birmingham (UAB) Renal Transplant Database contains detailed information pertaining to all individuals seeking renal transplantation at UAB since 1993. Three research nurses and one supervising data analyst continuously update the patients' files. Several sources of information, including contact with patients and patients' families, referring physicians, UAB and other medical facilities records, death certificates, and the US Social Security Death Master File are used to ascertain mortality correctly.
We excluded all patients with previous heart, lung, or liver transplants and those not considered transplant candidates and thus never placed on the waiting list. Because we focused on patients without clinically manifested HF, we excluded patients with previous diagnosis of congestive HF, cardiomyopathy, or significant cardiac valvulopathy. For the purpose of this study, the evaluation date was the date of measurement of LVEF by SPECT. Survival time was estimated from the date of evaluation to the date of death; date of transplantation; or until December 31, 2005. Mortality as a result of cardiac event was defined by death certificates (primary or contributing cause) or medical records and often confirmed with health care provider or family members.
Pharmacologic stress-SPECT perfusion imaging detects and quantifies ischemic and scar burden. From pooled data of 11 studies, the sensitivity and specificity were 85 and 91%, respectively, in detecting ischemia.9 Furthermore, at UAB since 1997, the SPECT images were acquired in the gated mode, which allows measurement of LVEF. The EF, by gated SPECT, has in multiple studies correlated well with EF measured by other methods such as contrast angiography, 2DE, and magnetic resonance imaging. It has the major advantage of being automated and reproducible.17 Cardiac ischemia and SD in combination portend a worse outcome. Presence of ischemia or scar limits the capacity to recover LV function.18,19 Previous work found that recipients with myocardial perfusion defects had a higher cardiac event rate within 42 mo of transplantation.20 Our institution follows the renal transplant candidates evaluation guidelines put forth by the American Society of Transplantation21; perfusion scans (stress-SPECT) are performed routinely in candidates who are older than 50, in individuals with diabetes for longer than 10 yr, in patients with previous cardiovascular events (CVE), and in patients with at least two of the following risk factors: LVH, family history of ischemic heart disease, dyslipidemia, or significant smoking history. Perfusion scans were performed according to the Guidelines for Nuclear Cardiology Procedures22 and were routinely scheduled at midweek to reduce the influence of volume overload and uremia. All echocardiograms performed during the evaluation period were retrieved.
Following the Guidelines for Nuclear Medicine Procedures,23 LVEF ≤40% defined SD; for the purpose of this study, ischemia was defined by either a stress-SPECT showing a perfusion abnormality (ischemia, scar, or both) or a history of previous CVE including revascularization. Anemia was defined by a hematocrit <33%; socioeconomic status (SES) was categorized by using educational level attained as a surrogate marker (<12 yr of schooling defined low SES); hypertension was defined by a systolic BP >140 mmHg, a diastolic BP >90 mmHg, or the use of antihypertensive drugs. LVH was diagnosed by 12-lead electrocardiogram24 or 2DE.25 BMI was calculated by weight (kg)/square of height (m). Duration of dialysis, age, gender, race, diabetes, serum albumin and creatinine levels, and tobacco smoking history were extracted from the database. The study protocol was reviewed and approved by the institutional review board for human use at UAB.
Statistical Analyses
Distribution of patients' characteristics at evaluation was tested by χ2 analysis or t test as appropriate. Results were described as means ± SD. Event-free survival curves were constructed using the product-limit method (Kaplan-Meier), and differences among survival curves were estimated by the log-rank test. Cox regression modeling was used to estimate crude (univariate) and adjusted (multivariate) risks. Variables entered in the models were the ones described as being associated with cardiovascular mortality: Age, gender, SES, obesity, race, presence of diabetes, hypertension, LVH, preexisting ischemia, anemia, low albumin level, tobacco smoking, and duration of dialysis. Direct visualization of Log [−log (survival time)] versus log (survival time) plots and examination of Martingale residuals26 were used to validate the proportionality of hazard increments assumption. LVEF by SPECT, age, BMI, and duration of dialysis were tested in the models both as a continuous and as categorical variables (LVEF ≤30, 31 to 40, 41 to 50, 51 to 60, or >60%; age <50 or ≥50; BMI <30, 30 to 35, or >35 kg/m2; dialysis >2, 1 to 2, or <1 yr or no dialysis). The decision to describe a variable either as continuous or categorical was based on the best fit of the multivariable models. We attempted to distinguish the impact of ischemia and SD on mortality by performing a stratified analysis in which the study cohort was divided into three groups: (1) SD (LVEF ≤40%) without perfusion abnormality (ischemia or scar) or previous CVE, (2) ischemia (previous CVE or perfusion abnormalities), and (3) normal control subjects (LVEF >40%, no previous CVE, and normal perfusion pattern). We then performed multivariable analysis in which the attributed risk for SD was adjusted by the presence of ischemia and other cardiovascular risk factors. In addition, models including the interaction term “ischemia × LVEF” were built and their HR compared with main-effect models. Subsequent analyses were conducted using cardiac death as the outcome of interest. Internal consistency was assessed by building several models across strata of risk factor categories: Dialysis and nondialysis, male and female, older and younger than 50 yr, black and nonblack, with and without ischemia. Estimated risks were reported as HR with corresponding 95% CI. All P values reported were two-sided. SPSS 11.5 for Windows (SPSS, Chicago, IL) software was used in the analyses.
DISCLOSURES
None.
Acknowledgments
We acknowledge the work of the UAB Renal Transplant Database personnel and transplant coordinators.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Stack AG, Bloemberger WE: Prevalence and clinical correlates of coronary artery disease among new dialysis patients in the United States: A cross-sectional study. J Am Soc Nephrol 12: 1516–1523, 2001 [DOI] [PubMed] [Google Scholar]
- 5.US Renal Data System: Cardiovascular special studies. In: USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2005, pp 174–184
- 6.Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS: Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney Int 47: 884–890, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J: Congestive heart failure in renal transplant recipients: Risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol 13: 1084–1090, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA, for the Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators: Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 112: 3738–3744, 2005 [DOI] [PubMed] [Google Scholar]
- 9.O'Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, Pohost GM, Shaw LJ, Weintraub WS, Winters WL Jr: American College of Cardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J Am Coll Cardiol 36: 326–340, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Dahan M, Viron BM, Faraggi M, Himbert DL, Lagallicier BJ, Kolta AM, Pessione F, Le GD, Gourgon R, Mignon FE: Diagnostic accuracy and prognostic value of combined dipyridamole-exercise thallium imaging in hemodialysis patients. Kidney Int 54: 255–262, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Trespalacios FC, Taylor AJ, Agodoa LY, Bakris GL, Abbott KC: Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis 41: 1267–1277, 2003 [DOI] [PubMed] [Google Scholar]
- 12.McDonagh TA, Cunningham AD, Morrison CE, McMurray JJ, Ford I, Morton JJ, Dargie HJ: Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart 86: 21–26, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy D, Omran E, Periyasamy SM, Nadoor J, Priyadarshi A, Willey JC, Malhotra D, Xie Z, Shapiro JI: Effect of Chronic Renal Failure on Cardiac Contractile Function, Calcium Cycling, and Gene Expression of Proteins Important for Calcium Homeostasis in the Rat. J Am Soc Nephrol 14: 90–97, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Sahagun-Sanchez G, Espinola-Zavaleta N, Lafragua-Contreras M, Chavez PY, Gomez-Nunez N, Keirns C, Romero-Cardenas A, Perez-Grovas H, Acosta JH, Vargas-Barron J: The effect of kidney transplant on cardiac function: An echocardiographic perspective. Echocardiography 18: 457–462, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, Drachenberg C, Papadimitriou J, Brisco MA, Blahut S: Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol 45: 1051–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Stegall MD: The development of kidney allocation policy. Am J Kidney Dis 46: 974–975, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Iskandrian AE, Heo J, Mehta D, Tauxe EL, Yester M, Hall MB, MacGregor JM: Gated SPECT perfusion imaging for the simultaneous assessment of myocardial perfusion and ventricular function in the BARI 2D trial: An initial report from the Nuclear Core Laboratory. J Nucl Cardiol 13: 83–90, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Seghatol FF, Shah DJ, Diluzio S, Bello D, Johnson MR, Cotts WG, O'squosquo; apos; yDonohue JA, Bonow RO, Gheorghiade M, Rigolin VH: Relation between contractile reserve and improvement in left ventricular function with beta-blocker therapy in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 93: 854–859, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Holper EM, Blair J, Selzer F, Detre KM, Jacobs AK, Williams DO, Vlachos H, Wilensky RL, Coady P, Faxon DP: The impact of ejection fraction on outcomes after percutaneous coronary intervention in patients with congestive heart failure: An analysis of the National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry and Dynamic Registry. Am Heart J 151: 69–75, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Patel AD, bo-Auda WS, Davis JM, Zoghbi GJ, Deierhoi MH, Heo J, Iskandrian AE: Prognostic value of myocardial perfusion imaging in predicting outcome after renal transplantation. Am J Cardiol 92: 146–151, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kasiske BL, Cangro CB, Hariharan S, Hricik DE, Kerman RH, Roth D, Rush DN, Vazquez MA, Weir MR: The evaluation of renal transplantation candidates: Clinical practice guidelines. Am J Transplant 1[Suppl 2]: 3–95, 2001 [PubMed] [Google Scholar]
- 22.Travin MI, Heller GV, Johnson LL, Katten D, Ahlberg AW, Isasi CR, Kaplan RC, Taub CC, Demus D: The prognostic value of ECG-gated SPECT imaging in patients undergoing stress Tc-99m sestamibi myocardial perfusion imaging. J Nucl Cardiol 11: 253–262, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Port SC: Imaging guidelines for nuclear cardiology procedures. J Nucl Cardiol 6: G47–G84, 1999 [PubMed] [Google Scholar]
- 24.Goldberg AL: Clinical electrocardiography. In: Clinical Electrocardiography: A Simplified Approach, 7th Ed., edited by Mosby I, St. Louis, 2006, pp 17–28
- 25.Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, Miller DH, Reis G, Alderman MH, Laragh JH: Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol 4: 1222–1230, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Therneau TM, Grambsch PM, Fleming TR: Martingale-based residuals for survival models. Biometrika 77: 147–160, 1990 [Google Scholar]