Abstract
Objective
To examine the acute glucose-lowering effects of aerobic exercise in children and adolescents with type 1 diabetes (T1D).
Research Design and Methods
Fifty children and adolescents with T1D (ages 10-<18) were studied during exercise. The 75 minute exercise session consisted of four 15 minute periods of walking on a treadmill to a target heart rate of 140 beats per minute and three 5 minute rest periods. Blood glucose and plasma glucagon, cortisol, growth hormone and norepinephrine concentrations were measured before, during and after exercise.
Results
In most subjects (83%), plasma glucose concentration dropped at least 25% from baseline and 15 (30%) subjects became hypoglycemic (≤60 mg/dL) or were treated for low glucose either during or immediately following the exercise session. The incidence of hypoglycemia and/or treatment for low glucose varied significantly by baseline glucose, occurring in 86% vs. 13% vs. 6% of subjects with baseline values <120, 120-180, and >180 mg/dL, respectively (p<0.001). Exercise-induced increases in growth hormone and norepinephrine concentrations were marginally higher in subjects whose glucose dropped ≤70 mg/dL. Treatment of hypoglycemia with 15 grams of oral glucose resulted in only about a 20 mg/dL rise in glucose concentrations.
Conclusions
In youth with T1D, prolonged moderate aerobic exercise results in a consistent reduction in plasma glucose and the frequent occurrence of hypoglycemia when pre-exercise glucose concentrations are <120 mg/dL. Moreover, treatment with 15 grams of oral glucose is often insufficient to reliably treat hypoglycemia during exercise in these youngsters.
The benefits of exercise in children and adolescents with type 1 diabetes (T1D) have long been recognized. Nevertheless, exercise has potential acute adverse effects that are related to hypo- and hyperglycemic excursions. Hypoglycemia during exercise in a child can be dangerous and decreases a young person’s performance during sports or other activities. Conversely, excessive snacking prior to or during exercise can result in hyperglycemia and negate some metabolic and vascular benefits of exercise. Despite the prominent role that sports and physical activity play in the lives of many youth with T1D, standard recommendations that could guide families and clinicians in the management of glycemia during exercise are lacking.
There are a variety of conditions that need to be considered in understanding hypoglycemia during exercise including the type and duration of exercise, glucose concentrations prior to starting exercise, and the relation of exercise to meals and insulin doses. Guelfi and colleagues (1) compared the effects of exercise on a bicycle ergometer over a 30 minute period with and without brief maximal sprints in 7 children with T1D. Exercise, which was performed in the late morning when the blood glucose was ~200 mg/dL following breakfast and a pre-breakfast insulin dose, resulted in an approximately 25 mg/dL greater fall in blood glucose when exercise did not include the interspersed sprints. There was a larger increase in counter-regulatory hormone concentrations that may have blunted the fall in glucose concentrations when exercise included the sprints. Plasma glucose concentrations <72 mg/dL were observed in 3 of the 14 exercise tests.
We conducted a study to examine the acute glucose-lowering effects of aerobic exercise in 50 youth with T1D. In contrast to the Guelfi study, our subjects exercised in the late afternoon 4 hours after lunch over a 75 minute period at moderate intensity, at the time of day of after school physical activity. In a prior publication, we reported that the mean overnight glucose was lower (131 vs. 154 mg/dL, P=0.003) and the overnight incidence of hypoglycemia greater on the exercise day compared with a control sedentary day (48% versus 28%, P=0.009).(2) Herein, we report the changes in glucose and counter-regulatory hormones during the exercise session compared with those during a control day without exercise.
Research Design and Methods
Details of the protocol are reported elsewhere.(2) Major eligibility criteria included 1) age 10 to <18 years, 2) diagnosis of T1D of at least 18 months duration, 3) stable insulin regimen involving either use of an insulin pump or use of insulin glargine and short-acting insulin for at least one month (NPH or Lente, if part of regimen, used only in the morning), 4) HbA1c ≤10.0%, 5) body mass index (BMI) between the 5th and 95th percentile for age and gender,(3) and 6) no severe hypoglycemia episodes within the past 2 weeks.
Procedures
The study consisted of two, randomized 24-hour clinical research center admissions separated by one to four weeks: one with a 75-minute exercise session in the late afternoon (“exercise day”) and one without exercise (“sedentary day”). Insulin management on both study days were as similar as possible and followed the routine that the subject followed at home on a day without exercise.
On the morning of the exercise day, the subjects walked on a motorized treadmill for 5 to 15 minutes to determine the settings needed to achieve a steady state heart rate of 140 beats per minute (bpm). These treadmill settings were used for the start of the afternoon exercise session at about 4 p.m. A heart rate of 140 bpm is approximately 70% of maximal heart rate, equivalent to 60% maximum aerobic effort. This level of exertion in children and adolescents is considered moderate and aerobic.(4) Subjects were assumed to have a normal cardiovascular response to exercise due to their age and limited duration of diabetes. At 2 and 3 p.m. on both the exercise and sedentary days, the glucose concentrations in blood were measured with a One Touch® Ultra® meter (“Ultra”; LifeScan, Milpitas, CA). These values are expressed as equivalent concentrations in plasma or serum, comparable to central laboratory measurements.(5) At the discretion of the investigator, small correction doses of rapid-acting insulin analog were allowed for high glucose (6 subjects received a correction dose on both days, 3 others on the exercise day, and 5 others on the sedentary day) and 15-30 grams of carbohydrate for low glucose concentrations with the aim of achieving 4 p.m. glucose concentrations between 80-200 mg/dL. On the exercise day at 4 p.m., if the glucose concentration was <80 mg/dL, the subject was given a snack and the start of the exercise was deferred until the glucose was ≥80 mg/dL. No extra insulin was given if the 4 p.m. glucose concentration was >200 mg/dL. In insulin pump patients, the usual basal rate was continued during the exercise session, and was the same rate of infusion as on the sedentary day. Six children were receiving NPH insulin in the morning with the same dose on both days.
The exercise protocol consisted of four 15-minute periods walking on a treadmill at a heart rate of approximately 140 bpm (target range 133-147 bpm) separated by a 5-minute seated rest period. A heart rate monitor (Polar Electro, Kempele, Finland) was worn throughout the exercise session.
Blood samples for central laboratory determination of glucose and counter-regulatory hormone concentrations (with plasma and serum separated promptly) were obtained from an intravenous catheter prior to starting exercise, during each of the 3 rest periods, immediately following exercise completion, and 30 minutes after exercise completion. Glucose concentrations were also checked using the Ultra meter at each time point during and at 15-minute intervals following completion of exercise. If the meter glucose concentration dropped to <60 mg/dL the subject was given 15g of carbohydrate and after 5-15 minutes, the glucose concentration was rechecked. Exercise did not resume until the glucose concentration was >70 mg/dL. On the sedentary day, glucose determinations were made with the Ultra meter at 4 p.m. and approximately 6 p.m.
Serum glucose was measured by the DirecNet Central Laboratory (University of Minnesota) using a hexokinase enzymatic method, which has been proposed as the reference method for measuring glucose. (6,7) Glucagon was measured by a radioimmunoassay (Linco Research, St. Charles, MO) with the primary antibody from guinea pig, and secondary from goat. Lower limit of detection was 20 pg/mL. Coefficients of variation (CVs) were 6.5-8.8% on 3 controls. Cortisol was assayed with a competitive chemiluminescence assay (Bayer Advia Centaur; Bayer HealthCare, Tarrytown, NY), using a polyclonal rabbit antibody and mouse monoclonal antibody coupled with paramagnetic particles. Lower limit of detection was 0.5 μg/dL. CVs were 11-12% on 2 controls. Growth hormone (GH) was measured by a sandwich chemiluminescence assay (DPC Immulite). Monoclonal mouse antibody was coated on the bead with a rabbit polyclonal antibody in the reagent. Lower limit of detection was 0.1 ng/mL. CVs were 5.9-9.1%.
Norepinephrine and epinephrine were measured at the Mayo Clinic Laboratory (Rochester, MN) using a reverse phase (C18) HPLC column to separate norepinephrine and epinephrine, which were detected coulometrically, using an ESA Coulochem II instrument. Lower limit of detection is 10 pg/mL. CVs were 7-11% and 6-7%, respectively, on 3 controls. Unlike norepinephrine, many baseline epinephrine values were below the assay’s detection limit which may have been due in part to the fact that plasma samples were not collected in tubes containing preservatives. Consequently, only norepinephrine results will be reported.
Statistical Analysis
Hypoglycemia was defined as a laboratory glucose concentration ≤60 mg/dL and/or treatment for low glucose based on the Ultra meter concentration available at the time. Laboratory glucose concentrations were used for analysis whenever available. When a laboratory glucose was unavailable, the Ultra meter concentration was used instead. Two subjects were excluded from the analysis of glucose change during exercise because a snack was given prior to the start of exercise (due to an Ultra concentration <80 mg/dL) and a baseline glucose concentration was not recorded after the snack.
Exercise vs. sedentary day glucose concentrations were compared separately at 4 and 6 p.m. using a repeated measures regression including a period effect. Logistic regression was used to identify factors associated with hypoglycemia and/or treatment for low glucose during exercise. Factors considered in these models included: baseline glucose, age, gender, insulin route (pump vs. injections), HbA1c, BMI, self-reported days with at least 1 hour of exercise during a typical week, and estimated level of cardiovascular fitness. To assess the latter, we predicted the oxygen uptake (ml/kg/min) during the exercise session, at the target heart rate of 140 bpm using the American College of Sports Medicine (ACSM) metabolic equation for graded walking that has been validated for adults (we assume it is approximate for children as well) (8).
Repeated measures regression treating time as a linear continuous factor was used to test for changes in the concentrations of growth hormone, norepinephrine, cortisol and glucagon. Analysis of covariance (ANCOVA) was used to compare changes between subjects whose glucose did vs. did not drop ≤70 mg/dL during exercise adjusting for the baseline concentrations. Logarithm transformation was used for norepinephrine and cortisol and a square root transformation for growth hormone to reduce the skewness of the distributions.
Results
Fifty subjects participated in the study. Their average age was 14.8 ± 1.7 years; 44% female; and 90% Caucasian, 4% African-American, 2% Hispanic, and 4% Asian. Duration of diabetes was 7.0 ± 3.7 years. Fifty-four percent used an insulin pump and 46% used glargine and short acting insulin (26% of those using glargine were also taking NPH in the morning). HbA1c was 7.8 ± 0.8%.
Treadmill Settings and Adherence to Protocol
The median treadmill speed needed to achieve the target heart rate of 140 bpm was 3.5 mph (25th, 75th percentiles= 3.4, 4.0 mph, range 2.7 to 4.0) and the median incline was 6.8 degrees (25th, 75th percentiles= 3.0, 8.0; range 0 to 10.0). Predicted oxygen uptake had a median value of 22.9 ml/kg/min (25th, 75th percentiles= 19.1, 25.9; range 12.9, 33.5).
The full four-cycle exercise session was completed by 46 (92%) of the 50 subjects. Three subjects completed the first three cycles and part of a fourth and the remaining subject completed two cycles fully and two partially. One subject achieved the target heart rate in three of the four exercise cycles and the other 49 subjects achieved target for all four cycles. Median time (25th, 75th percentiles) required to achieve 140 bpm from the start of each cycle was 3 minutes (2, 4) ranging from <1 to 11 minutes.
Glucose Changes during Exercise
Mean baseline glucose concentration at 4 p.m. prior to exercise was 159 ± 61 mg/dL; similar to the 4 p.m. glucose concentrations on the sedentary study day (167 ± 95 mg/dL; p=0.45). Glucose concentrations fell rapidly, beginning in the first 15 minutes of exercise and extending throughout the 75-minute period (Figure 1). The mean glucose concentration at 6 p.m., approximately 45 minutes after completion of the exercise and before dinner, was lower than it was on the sedentary day (112 ± 58 versus 159 ± 78 mg/dL, p<0.001).
Figure 1. Change in Glucose Concentrations during Exercise (N=48).
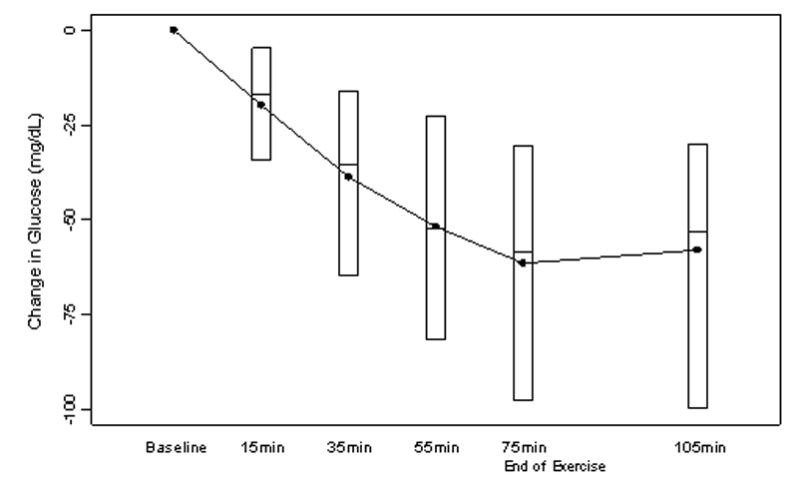
Two subjects excluded because baseline glucose was unavailable (see Methods). Black dots denote mean values and boxes denote median, 25th and 75th percentiles.
The relationship between baseline and nadir glucose concentrations during exercise in individual subjects is shown in Figure 2. Most had a decline in the glucose concentration irrespective of the baseline concentration, with 83% experiencing a drop in the glucose concentration ≥25%. Eleven (23%) subjects became hypoglycemic (laboratory glucose ≤60 mg/dL) either during or immediately following exercise and 4 additional subjects were treated for hypoglycemia due to an Ultra meter glucose ≤60 mg/dL but had central laboratory glucose concentrations between 62 and 68 mg/dL. Eleven subjects had a laboratory glucose concentration between 61-70 mg/dL (overall 26/50, 52% with a concentration ≤70 mg/dL). Only one subject had a meaningful increase in the glucose concentration. Among the 15 subjects who developed and/or were treated for hypoglycemia, hypoglycemia occurred after the first 15-minute cycle in 3, second cycle in 2, third cycle in 5, and fourth cycle in 5.
Figure 2. Nadir Glucose during Exercise vs. Baseline Concentration (N=48).
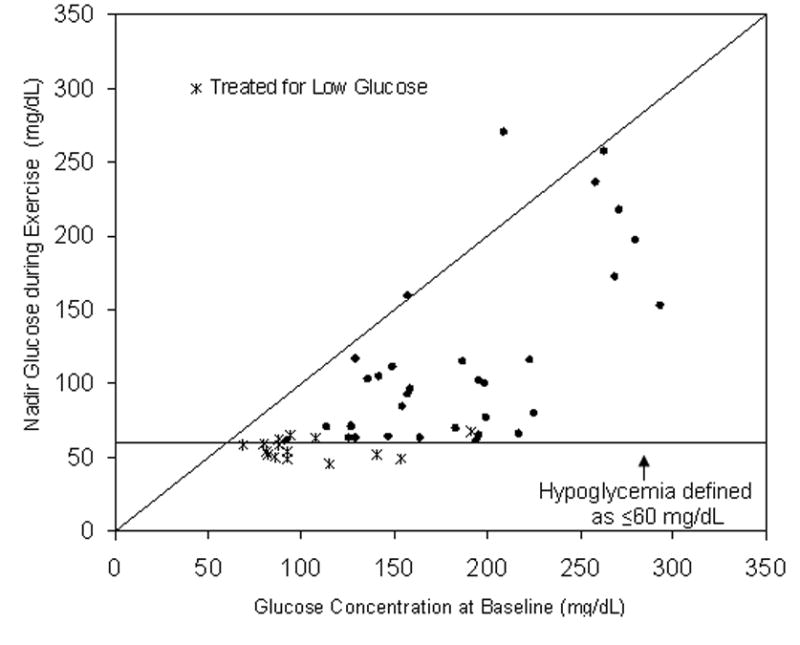
Two subjects excluded because baseline glucose was unavailable (see Methods). Diagonal represents the line of identity.
The drop in glucose from baseline to nadir averaged 34 ± 14 mg/dL when the baseline glucose was <120 mg/dL, 58 ± 29 mg/dL when the baseline was 120-180 mg/dL, and 91 ± 55 mg/dL when the baseline was >180 mg/dL. The incidence of hypoglycemia and/or treatment for low glucose varied significantly by baseline glucose, occurring in 86% vs. 13% vs. 6% of subjects with baseline concentrations <120, 120-180, and >180 mg/dL, respectively (p<0.001). Corresponding percentages for ≤70 mg/dL were 100% vs. 44% vs. 28% (p<0.001). After controlling for baseline glucose, no other factors were associated with the risk of hypoglycemia including age (p=0.71), gender (p=0.64), body mass index (p=0.38), duration of diabetes (p=0.52), HbA1c (p=0.39), insulin route (multiple-daily injections versus pump; p=0.65), treadmill workload required to raise heart rate to 140 bpm (p=0.49), and self-reported days of exercise in a typical week (p=0.91).
Hormone Changes during Exercise
In the group as a whole, exercise induced a sharp rise in circulating concentrations of GH (mean change baseline to peak: 12.0 ng/mL, p<0.001) and norepinephrine (mean change 278 pg/mL, p<0.001), but no statistically significant changes in plasma cortisol (baseline 9 ug/dL vs peak 12 ug/dL, p=0.54) or plasma glucagon (baseline 72 pg/mL vs peak 82 pg/mL, p=0.16). There were trends towards higher GH (p=0.08) and norepinephrine (p=0.04) concentrations in subjects whose glucose dropped ≤70 mg/dL (Figure 3).
Figure 3. Hormone Concentrations during Exercise.
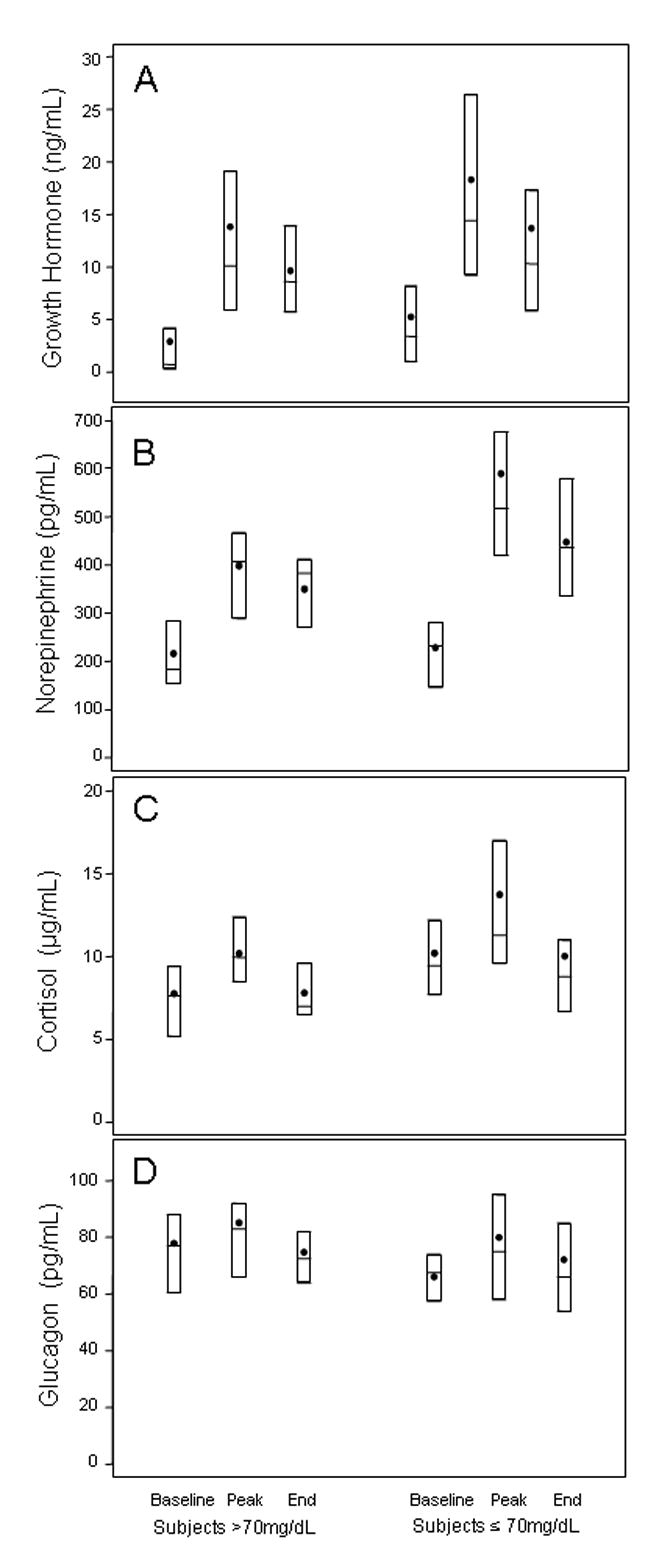
Distributions given for growth hormone (A), norepinephrine (B), cortisol (C) and glucagon (D). Black dots denote mean values and boxes denote median, 25th and 75th percentiles. Hormone data available for N=45 subjects (N=23 whose glucose fell ≤70 mg/dL during exercise vs. N=22 whose glucose stayed >70 mg/dL). Norepinephrine data available for N=30 subjects (16 of whom had their glucose fall ≤70 mg/dL).
Treatment of Hypoglycemia
Based on the Ultra concentrations available at the time, 15 subjects were treated for hypoglycemia (mean 56 ± 5 mg/dL) during exercise. Glucose concentrations increased modestly approximately 10 minutes after 15 grams of carbohydrate (to 77 ± 15 mg/dL, range 57 to 102). Four subjects required a second 15 grams to raise the glucose >70 mg/dL and 2 required a second 15 grams at the end of exercise for recurrent hypoglycemia.
Conclusions
The present study demonstrated that a prolonged period of aerobic exercise at the time of day for after school physical activity and consistent with federal recommendations for health-related physical activity (9) produced an average fall in plasma glucose of approximately 40% of baseline values. Approximately 30% of subjects required treatment for hypoglycemia during exercise. The baseline glucose concentration was a strong predictor of hypoglycemia risk (i.e., plasma glucose ≤60 mg/dL), since the majority of episodes occurred in children with pre-exercise concentrations <120 mg/dL and only one occurred with a baseline glucose >180 mg/dL (192 mg/dL). An American Diabetes Association Work Group recently recommended that hypoglycemia be defined biochemically as a plasma glucose concentration ≤70 mg/dL.(10) By this standard, all of our subjects with baseline concentrations <120 mg/dL became hypoglycemic, 44% when the baseline concentration was 120-180, and 28% when the baseline concentration was >180 mg/dL. These data suggest that it is advisable to achieve a blood glucose level that is at least 120 mg/dL if not higher prior to the start of exercise and that monitoring for hypoglycemia during exercise is important even when the pre-exercise glucose is >180 mg/dL. Other important clinical factors (HbA1c, BMI, age, insulin route) were not associated with hypoglycemia under these experimental conditions.
The reduction in glucose observed in our study following prolonged exercise in the late afternoon was larger than that observed by Guelfi et al.(11) following several short bursts of intense exercise after breakfast but was slightly less than was observed under similar conditions with moderate sustained exercise.(1) These differences emphasize that the type, duration, and timing of exercise, as well as its relation to meals/insulin doses, need to be considered in assessing the metabolic effects of exercise in diabetic children.
Exercise stimulates GH and catecholamine responses in diabetic and non-diabetic children (12) and our subjects showed the expected increases in GH and norepinephrine concentrations. However, these counter-regulatory hormone responses failed to prevent the fall in glucose during exercise in our subjects, indicating that exercise-induced increases in glucose utilization were not being compensated for by appropriate increases in endogenous glucose production. It is noteworthy that the development of hypoglycemia during exercise did not appear to enhance the rise in counterregulatory hormone levels over and above the responses that were stimulated by exercise alone. Reductions in glucose ≤70 mg/dL (a threshold value for stimulation of anti-insulin hormones in non-exercising children) (13) were only marginally associated with larger GH and norepinephrine responses. Guelfi et al. noted significantly higher norepinephrine responses when exercise included several short sprints compared to sustained moderate exercise and similar trends in this direction for epinephrine and GH.(1)
In non-diabetic subjects, the initial response to falling plasma glucose concentrations is a prompt suppression of endogenous insulin secretion. Since we did not adjust the basal rate of insulin during exercise, it is possible that maintenance of basal insulin contributed to the inability of the liver to meet the increased metabolic demands of prolonged physical activity in our subjects. Temporary reduction or suspension of the basal rate can be readily accomplished with CSII. Further studies are needed to carefully determine whether such alterations in basal infusion doses at the start of exercise can prevent hypoglycemia without causing hyperglycemia and metabolic decompensation. Strategies are also needed to prevent nocturnal hypoglycemia on nights following exercise, since subjects in this study had an increased frequency of hypoglycemia during the night after exercise than on the sedentary night.(2)
Because of its complexity, “trial and error” remains the principal method of managing glycemic excursions during exercise in individual patients. Nevertheless, studies such as ours can provide an evidence-based framework to guide the empirical decision-making process. Our findings underscore the importance of checking glucose concentrations prior to exercise to determine whether a pre-exercise snack is required to prevent hypoglycemia.. Ingestion of 15 grams of carbohydrate is a commonly recommended treatment for mild to moderate hypoglycemia in non-exercising children.(14) However, in our subjects, treatment of hypoglycemia with 15 grams of oral glucose resulted in only about a 20 mg/dL rise in glucose concentrations and more than a third of those subjects needed a second 15 grams to complete the exercise regimen. Consequently, 30-45 grams of oral glucose may be a more appropriate amount of carbohydrate to treat hypoglycemia during exercise in children and adolescents with T1D.
Acknowledgments
Support provided by NIH/NICHD Grants: HD041919; HD041915; HD041890; HD041918; HD041908; HD041906 and by Nemours Research Programs. Clinical Centers received funding through GCRC Grant Numbers M01 RR00069; RR00059; RR00125 and RR00070. The Mayo Clinic Laboratory received funding through the General Clinical Research Centers Program, GCRC Grant Number M01 RR00585. LifeScan, Milpitas, CA, provided One Touch® Ultra® Blood Glucose Monitoring Systems and test strips.
Portions of these data were previously published as an abstract and presented at the Scientific Sessions of the ADA in San Diego, CA June 2005.
References
- 1.Guelfi KJ, Jones TW, Fournier PA. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care. 2005;28:1289–1294. doi: 10.2337/diacare.28.6.1289. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Research in Children Network (DirecNet) Study Group. Impact of exercise on overnight glycemic control in children with type 1 diabetes. J Pediatr. 2005 doi: 10.1016/j.jpeds.2005.04.065. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. BMI-for-age charts, 2 to 20 years, LMS parameters and selected smoothed BMI percentiles, by sex and age, 2000. [Accessed February 2003]; Available from: http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm.
- 4.Howley E. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 2001;33:S364–369. doi: 10.1097/00005768-200106001-00005. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Research in Children Network (DirecNet) Study Group. A multicenter study of the accuracy of the OneTouch Ultra home glucose meter in children with type 1 diabetes. Diabetes Technol Ther. 2003;5:933–941. doi: 10.1089/152091503322640971. [DOI] [PubMed] [Google Scholar]
- 6.Neese JW, Duncan P, Bayse D, Robinson M, Cooper T, Stewart C. Development and evaluation of a hexokinase/glucose-6-phosphate dehydrogenase procedure for use as a national glucose reference method. Atlanta, GA: 1976. pp. 77–8330. HEW Publication No. (CDC) [Google Scholar]
- 7.Passey RB, Gillum RL, Fuller JB, Urry FM, Giles ML. Evaluation and comparison of 10 glucose methods and the reference method recommended in the proposed product class standard (1974) Clin Chem. 1977;23:131–139. [PubMed] [Google Scholar]
- 8.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 6. Philadelphia: Lippincott: Williams, and Wilkins; 2000. [Google Scholar]
- 9.PCPFS. Physical activity for children: current patterns and guidelines. President’s Council on Physical Fitness and Sports Research Digest. 2004;5:1–8. [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 11.Guelfi KJ, Jones TW, Fournier PA. Intermittent high-intensity exercise does not increase the risk of early postexercise hypoglycemia in individuals with type 1 diabetes. Diabetes Care. 2005;28:416–418. doi: 10.2337/diacare.28.2.416. [DOI] [PubMed] [Google Scholar]
- 12.Tamborlane WV, Sherwin RS, Koivisto V, Hendler R, Genel M, Felig P. Normalization of the growth hormone and catecholamine response to exercise in juvenile-onset diabetic subjects treated with a portable insulin infusion pump. Diabetes. 1979;28:785–788. doi: 10.2337/diab.28.8.785. [DOI] [PubMed] [Google Scholar]
- 13.Jones TW, Boulware SD, Kraemer DT, Caprio S, Sherwin RS, Tamborlane WV. Independent effects of youth and poor diabetes control on responses to hypoglycemia in children. Diabetes. 1991;40:358–363. doi: 10.2337/diab.40.3.358. [DOI] [PubMed] [Google Scholar]
- 14.Chase HP. Understanding Insulin-Dependent Diabetes. 10. Denver: Children’s Diabetes Foundation; 2002. [Google Scholar]


