Abstract
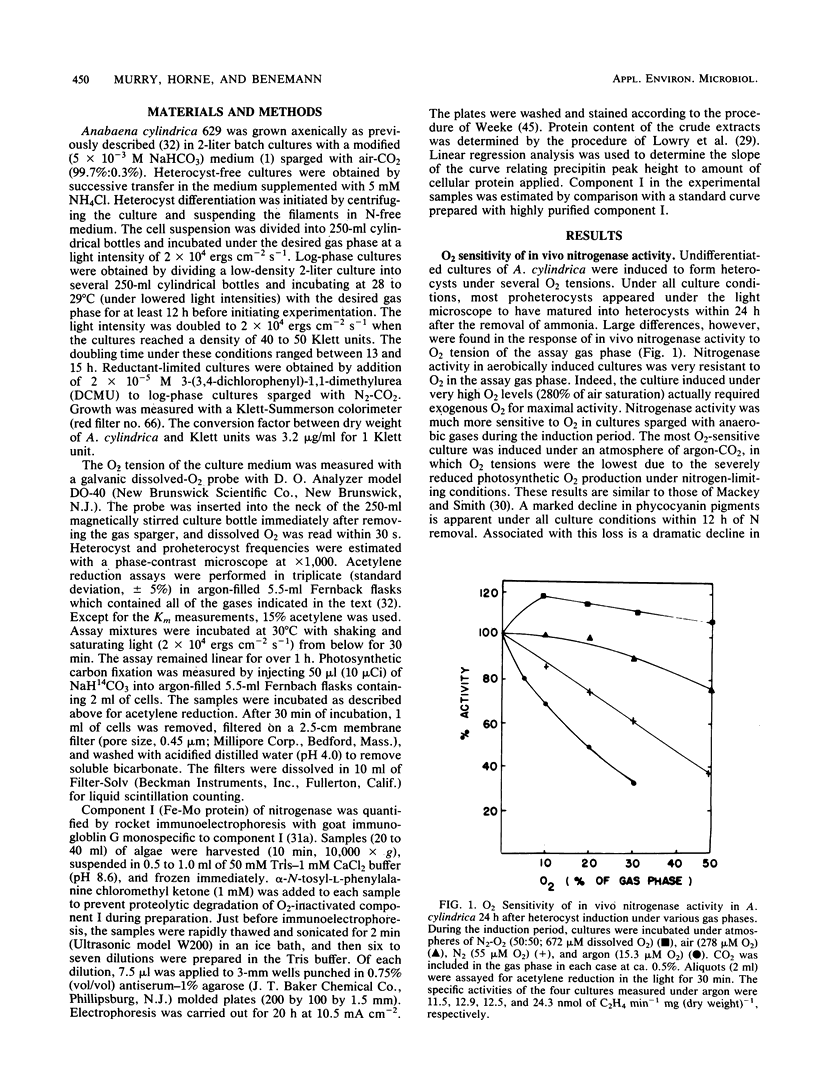
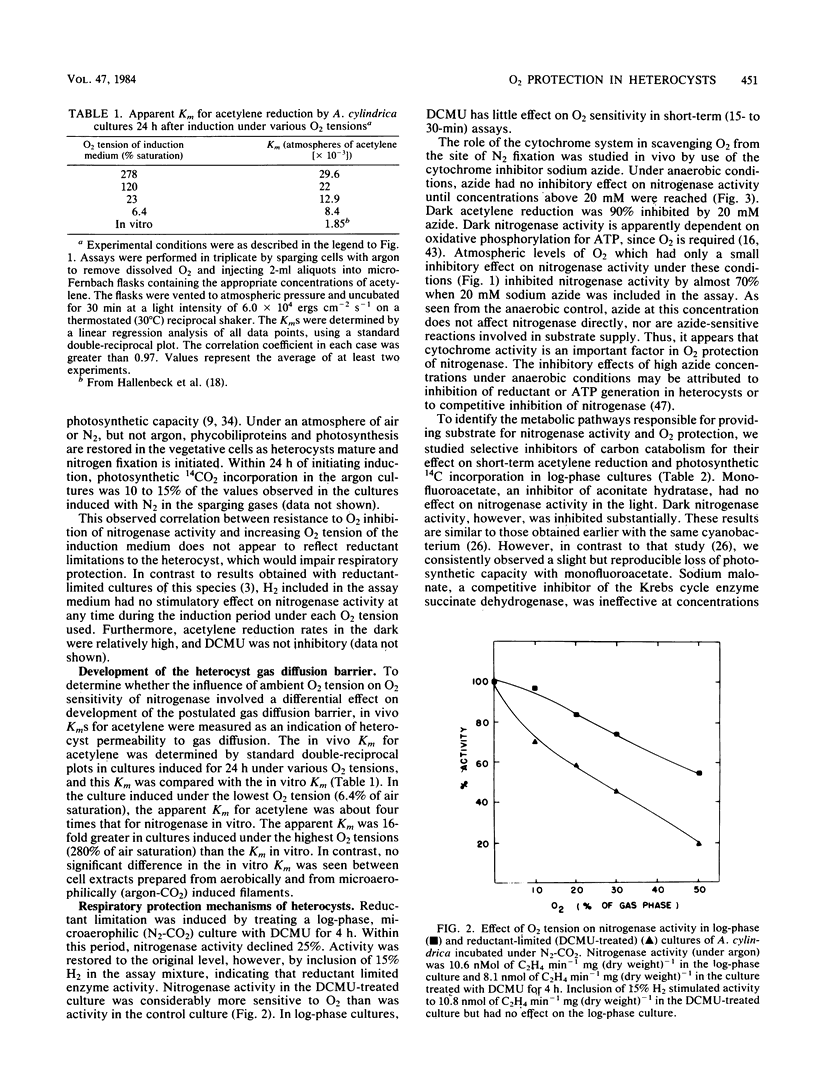
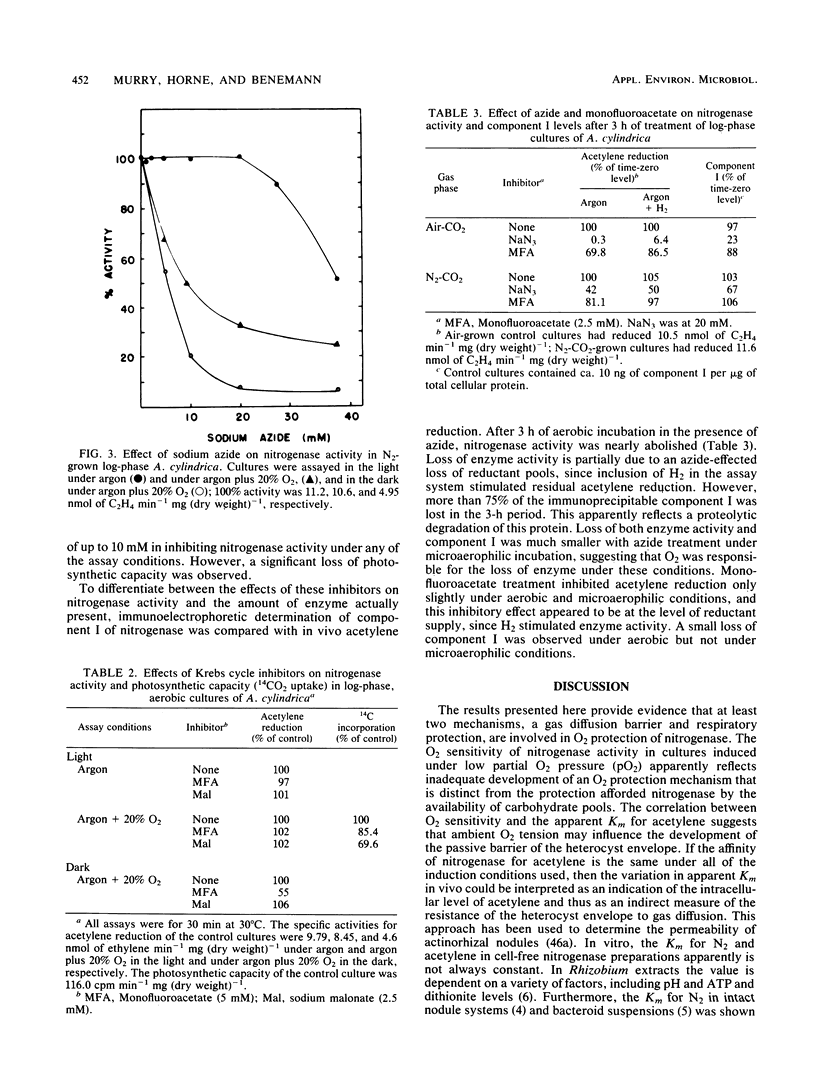
The mechanism of O2 protection of nitrogenase in the heterocysts of Anabaena cylindrica was studied in vivo. Resistance to O2 inhibition of nitrogenase activity correlated with the O2 tension of the medium in which heterocyst formation was induced. O2 resistance also correlated with the apparent Km for acetylene, indicating that O2 tension may influence the development of a gas diffusion barrier in the heterocysts. The role of respiratory activity in protecting nitrogenase from O2 that diffuses into the heterocyst was studied using inhibitors of carbon metabolism. Reductant limitation induced by 3-(3,4-dichlorophenyl)-1, 1-dimethylurea increased the O2 sensitivity of in vivo acetylene reduction. Azide, at concentrations (30 mM) sufficient to completely inhibit dark nitrogenase activity (a process dependent on oxidative phosphorylation for its ATP supply), severely inhibited short-term light-dependent acetylene reduction in the presence of O2 but not in its absence. After 3 h of aerobic incubation in the presence of 20 mM azide, 75% of cross-reactive component I (Fe-Mo protein) in nitrogenase was lost; less than 35% was lost under microaerophilic conditions. Sodium malonate and monofluoroacetate, inhibitors of Krebs cycle activity, had only small inhibitory effects on nitrogenase activity in the light and on cross-reactive material. The results suggest that oxygen protection is dependent on both an O2 diffusion barrier and active respiration by the heterocyst.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almon H., Böhme H. Components and activity of the photosynthetic electron transport system of intact heterocysts isolated from the blue-green alga Nostoc muscorum. Biochim Biophys Acta. 1980 Aug 5;592(1):113–120. doi: 10.1016/0005-2728(80)90118-8. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. Kinetic studies of nitrogenase from soya-bean root-nodule bacteroids. Biochem J. 1973 Jan;131(1):61–75. doi: 10.1042/bj1310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. M., Fay P. Special aspects of nitrogen fixation by blue-green algae. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):357–366. doi: 10.1098/rspb.1969.0026. [DOI] [PubMed] [Google Scholar]
- Daday A., Platz R. A., Smith G. D. Anaerobic and aerobic hydrogen gas formation by the blue-green alga Anabaena cylindrica. Appl Environ Microbiol. 1977 Nov;34(5):478–483. doi: 10.1128/aem.34.5.478-483.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Donze M., Haveman J., Schiereck P. Absence of photosystem 2 in heterocysts of the blue-green alga Anabaena. Biochim Biophys Acta. 1972 Jan 21;256(1):157–161. doi: 10.1016/0005-2728(72)90170-3. [DOI] [PubMed] [Google Scholar]
- Drozd J., Postgate J. R. Effects of oxygen on acetylene reduction, cytochrome content and respiratory activity of Azotobacter chroococcum. J Gen Microbiol. 1970 Sep;63(1):63–73. doi: 10.1099/00221287-63-1-63. [DOI] [PubMed] [Google Scholar]
- Fay P. Factors influencing dark nitrogen fixation in a blue-green alga. Appl Environ Microbiol. 1976 Mar;31(3):376–379. doi: 10.1128/aem.31.3.376-379.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P., Walsby A. E. Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature. 1966 Jan 1;209(5018):94–95. doi: 10.1038/209094a0. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Kostel P. J., Benemann Purification and properties of nitrogenase from the cyanobacterium, Anabaena cylindrica. Eur J Biochem. 1979 Jul;98(1):275–284. doi: 10.1111/j.1432-1033.1979.tb13186.x. [DOI] [PubMed] [Google Scholar]
- Haury J. F., Wolk C. P. Classes of Anabaena variabilis mutants with oxygen-sensitive nitrogenase activity. J Bacteriol. 1978 Nov;136(2):688–692. doi: 10.1128/jb.136.2.688-692.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead A., Robinson R., Stewart W. D. Nitrogenase activity in extracts of heterocystous and non-heterocystous blue-green algae. Arch Mikrobiol. 1970;74(3):235–243. doi: 10.1007/BF00408884. [DOI] [PubMed] [Google Scholar]
- Kellar P. E., Paerl H. W. Physiological adaptations in response to environmental stress during an n(2)-fixing anabaena bloom. Appl Environ Microbiol. 1980 Sep;40(3):587–595. doi: 10.1128/aem.40.3.587-595.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasooriya S. A., Lang N. J., Fay P. The heterocysts of blue-green algae. 3. Differentiation and nitrogenase activity. Proc R Soc Lond B Biol Sci. 1972 Jun 6;181(1063):199–209. doi: 10.1098/rspb.1972.0046. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambein F., Wolk C. P. Structural studies on the glycolipids from the envelope of the heterocyst of Anabaena cylindrica. Biochemistry. 1973 Feb 27;12(5):791–798. doi: 10.1021/bi00729a002. [DOI] [PubMed] [Google Scholar]
- Lockau W., Peterson R. B., Wolk C. P., Burris R. H. Modes of reduction of nitrogen in heterocysts isolated from Anabaena species. Biochim Biophys Acta. 1978 May 10;502(2):298–308. doi: 10.1016/0005-2728(78)90051-8. [DOI] [PubMed] [Google Scholar]
- Neilson A., Rippka R., Kunisawa R. Heterocyst formation and nitrogenase synthesis in Anabaena sp. A kinetic study. Arch Mikrobiol. 1971;76(2):139–150. doi: 10.1007/BF00411788. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Burris R. H. Properties of heterocysts isolated with colloidal silica. Arch Microbiol. 1976 May 3;108(1):35–40. doi: 10.1007/BF00425090. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. Localization of an uptake hydrogenase in anabaena. Plant Physiol. 1978 Apr;61(4):688–691. doi: 10.1104/pp.61.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Bodmer S., Tabita F. R. Oxygen inactivation and recovery of nitrogenase activity in cyanobacteria. J Bacteriol. 1983 Jan;153(1):182–190. doi: 10.1128/jb.153.1.182-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Stewart W. D. Photosynthetic electron transport, ATP synthesis and nitrogenase activity in isolated heterocysts of Anabaena cylindrica. Biochim Biophys Acta. 1976 Feb 16;423(2):189–195. doi: 10.1016/0005-2728(76)90177-8. [DOI] [PubMed] [Google Scholar]
- Thomas J. Absence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. Nature. 1970 Oct 10;228(5267):181–183. doi: 10.1038/228181b0. [DOI] [PubMed] [Google Scholar]
- Weare N. M., Benemann J. R. Nitrogen fixation by Anabaena cylindrica. II. Nitrogenase activity during induction and aging of batch cultures. Arch Mikrobiol. 1973 Oct 19;93(2):101–112. [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Winkenbach F., Wolk C. P. Activities of enzymes of the oxidative and the reductive pentose phosphate pathways in heterocysts of a blue-green alga. Plant Physiol. 1973 Nov;52(5):480–483. doi: 10.1104/pp.52.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. P., Burris R. H. Nature of oxygen inhibition of nitrogenase from Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1972 Mar;69(3):672–675. doi: 10.1073/pnas.69.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. G. Control of respiration and nitrogen fixation by oxygen and adenine nucleotides in N2-grown Azotobacter chroococcum. J Gen Microbiol. 1970 Mar;60(3):393–401. doi: 10.1099/00221287-60-3-393. [DOI] [PubMed] [Google Scholar]



