Abstract
Objective
This study assesses the epidemiological pattern of lung cancer cell-types in Ireland, with identification of any underlying gender variations.
Methods
Lung cancer incidence data, including the major cell-types: squamous-cell-carcinoma (SCC), adenocarcinoma (AC), small-cell-lung-carcinoma (SCLC) and large-cell-carcinoma (LCC) were obtained from the national cancer registry (1994–2000), together with individual characteristics, such as age, gender, smoking status, and the year of diagnosis. Age-standardised incidence rates (ASIR), male-to-female (M: F) rate ratios (RR) of ASIR for SCC and AC, as well as RR of AC: SCC according to smoking status for both sexes, were estimated. Estimated-annual-percent-changes for each of the cell-types were calculated.
Results
AC incidence in females is rising annually (8.5%, p=0.008) from 1994 to 2000, while SCC is declining (−5.4%, p=0.01) in males. M: F ratios of ASIR are consistently greater than ‘one’, but converging recently. RR of AC: SCC is also approaching ‘unity’ across both sexes, irrespective of the smoking status
Conclusions
An apparent increase in lung AC incidence in females was observed in Ireland that might indicate some local environmental risk factors, in addition to changing smoking habits. The study findings do not support the hypothesis that females in general are at higher risk for lung cancer development, but tobacco and histologic-specific susceptibility cannot be ruled out.
Keywords: Histology, Incidence, Lung cancer, Ireland, Smoking
INTRODUCTION
Lung cancer occurs in multiple histological cell-types. The four major cell-types include squamous cell carcinoma (SCC), adenocarcinoma (AC), large cell carcinoma (LCC), and small cell carcinoma (SCLC). Together, these four major cell-types account for >90% of lung cancer cases in the United States (US)1. Despite extensive research, the mechanisms leading to these different types of lung cancer remain uncertain. Over recent decades there have been both geographical and temporal changes in the distribution of lung cancer cell-types2–4. Knowledge of these modifications may help to recognize any underlying new aetiological and pathological mechanisms of lung cancer.
Lung adenocarcinoma has become the leading cell-type in North America2, Europe3 and Asia4.This increase may partly be artefactual and involve several biases, or may be a real change5. Geographical and temporal trends also differ in males and females. A recent birth-cohort study in the US concluded that males and females may be ‘equally’ susceptible to developing lung cancer from a given amount of cigarette smoking, rather than supporting the hypothesis that females are more susceptible to developing lung cancer6. This was reinforced in a recent prospective study7. Nonetheless, the gender susceptibility to developing lung cancer is debatable, and is still speculated to be associated with gender differences in their background risk profiles8.
To date, no such temporal variations in lung cancer incidence by major cell-types have been identified in the Republic of Ireland. Therefore, the overall aim of this study is to assess the epidemiological pattern of lung cancer cell-types in Ireland, with identification of any underlying gender and/or temporal variations.
METHODS
Source of lung cancer incidence data
The Irish National Cancer Registry Board based in Cork has been registering lung cancer incident cases from January 1st, 19949. More than 90% of cancer cases are histopathologically verified, and the Registry has a centralised system of uniform data collection and quality assessment9. However, for lung cancer cases only 75% could be verified histo-pathologically9. At the time of this study, all lung cancer incident cases (on an individual basis) registered from 1994 to 2000 were obtained from the Registry. Specific individual covariates, such as age, gender, year of diagnosis, and smoking status (smokers, non-smoker or former smokers) were also collected for further analyses. Based on the morphology codes of the WHO International Histological Classification of Tumors10, invasive carcinomas of lung [ICD Codes: 9 (162) and 10 (C34)] were categorized into four major cell-types. They are: SCC (ICD-O: 8051–52, 8070–76), SCLC (ICD-O: 8041–45), LCC (ICD-O: 8011–12, 8020–21, 8030–33), and AC (ICD-O: 8050, 8140–246, 8260–571).
Estimation of age-standardised incidence rates (ASIR)
Incidence rates for total lung cancer cases in both sexes, together with the major cell-types, were age-standardised to the European Standard Population for better comparison. The estimated-annual-percent-changes (EAPC) in rates for each cell-type were calculated, using generalised log-linear regression model. The annual rates are adjusted for the gender and age-specific annual smoking prevalence of the Irish population for the year 1994, as the baseline year. The annual gender and age-specific smoking prevalence for the year 1994 was obtained from the publication of Lee and colleagues11.
Estimation of age-standardised incidence rate ratios (RR)
Annual male-to-female RR (with 95% confidence intervals: CI) from 1994 to 2000 was estimated for SCC, AC, and for total lung cancer cases. The male-to-female ratios (with 95% CI) were calculated using a spreadsheet (quick-calc) developed by Rothman12. Ratios more than one would generally indicate that males have higher lung cancer rates, thereby the less likelihood of supporting the hypothesis that females are more susceptible to developing lung cancer. Likewise, annual rate ratios of AC: SCC for both sexes was calculated according to their smoking status.
RESULTS
In total, 10,514 lung cancer incident cases (6,823 in males, 3,691 in females) were registered in the Republic of Ireland from 1994 to 2000. Of these, SCC was the most frequent cell-type in both males (34%) and females (22%), while AC was relatively high among female populations across all the periods studied (18% vs. 14% in males). The frequency of SCLC was also high in females (17% vs. 12% in males); LCC was the least frequent cell-type across both sexes (3%). In all our analyses where appropriate, we have combined both former and current smokers as ever-smokers for better estimates.
The overall ASIR across all the periods studied was higher in males (on an average 500 cases / 10,000 smokers) than in females (on an average 300 cases / 10,000 smokers). Total lung cancer incidence is significantly increasing annually (2%, p=0.001) in females, while males show an annual decline (−2.4%, p=0.058). In females, there is a significant annual rise (8.5%, p=0.008) in AC incidence, which translates into an absolute increase from 30 cases/10,000 smokers in the year 1994 to 45 cases/10,000 smokers in the year 2000 (figure 1).
Fig 1.
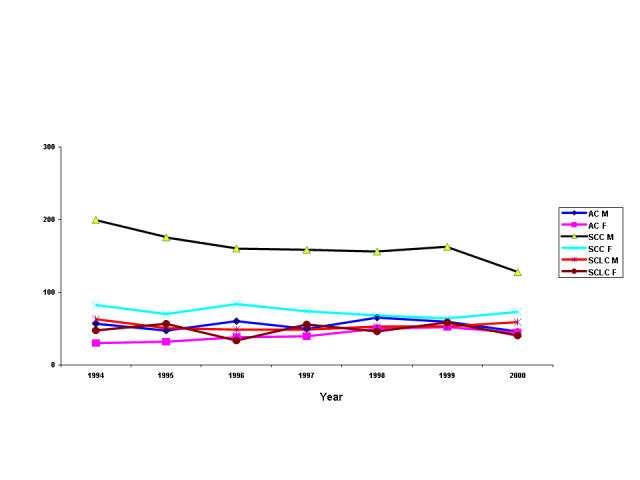
Age-standardised annual incidence rates of lung cancer cell-types in the Republic of Ireland from 1994 to 2000 per 10,000 smokers
AC M = Lung adenocarcinoma in males; AC F = Lung adenocarcinoma in females; SCC M = Squamous cell carcinoma in males; SCC F = Squamous cell carcinoma in females; SCLC M = Small cell lung carcinoma in males; SCLC F = Small cell lung carcinoma in females
Table I shows the annual male-to-female (M: F) age-adjusted population-standardised incidence rate ratios (RR) for AC, SCC and total lung cancer cases. Statistically significant higher RR was observed among ever-smokers. Also, there is gradual convergence in RR in the most recent periods, suggesting an increasing trend among the females. The ratios were relatively low among never-smokers, with very wide confidence intervals and unstable estimates, probably due to small numbers.
Table I.
M: F Standardized Incidence Rate Ratios (age-adjusted) in ever (former and current combined) and never-smokers for total lung cancer cases, Lung Adenocarcinoma (AC) and Squamous cell carcinoma (SCC) cases.
| All cases | AC | SCC | ||
|---|---|---|---|---|
| Year | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Ever-Smokers | ||||
| 1994 | 1.94 (1.78, 2.12) | 1.87 (1.42, 2.43) | 2.41 (2.09, 2.78) | |
| 1995 | 1.70 (1.55, 1.86) | 1.47 (1.07, 1.93) | 2.50 (2.15, 2.90) | |
| 1996 | 1.66 (1.52, 1.82) | 1.57 (1.19, 2.01) | 1.91 (1.62, 2.23) | |
| 1997 | 1.55 (1.40, 1.70) | 1.26 (0.93, 1.66) | 2.14 (1.81, 2.49) | |
| 1998 | 1.62 (1.47, 1.77) | 1.30 (1.00, 1.65) | 2.28 (1.94, 2.67) | |
| 1999 | 1.54 (1.40, 1.68) | 1.14 (0.87, 1.47) | 2.54 (2.16, 2.96) | |
| 2000 | 1.40 (1.27, 1.54) | 1.04 (0.76, 1.38) | 1.74 (1.46, 2.08) | |
| Never-Smokers | ||||
| 1994 | 1.02 (0.51, 1.72) | 1.43 (0.44, 3.15) | 1.63 (0.51, 3.65) | |
| 1995 | 1.42 (0.74, 2.39) | 1.47 (0.33, 4.61) | 2.20 (0.41, 5.84) | |
| 1996 | 1.69 (0.86, 2.91) | 0.71 (0.12, 3.44) | 3.18 (0.99, 9.31) | |
| 1997 | 0.95 (0.42, 1.73) | 0.39 (0.01, 3.10) | 1.37 (0.33, 4.61) | |
| 1998 | 1.53 (0.83, 2.55) | 0.74 (0.08, 2.33) | 6.00 (1.56, NC*) | |
| 1999 | 1.14 (0.60, 2.04) | 1.39 (0.13, 4.01) | 4.00 (1.09, NC*) | |
| 2000 | 1.22 (0.68, 2.10) | 1.03 (0.19, 2.74) | 2.06 (0.39, 5.48) | |
NC: Could not be calculated because of extreme values
In table II, almost all the age-standardised incidence rate ratios (RR) of AC: SCC are less than ‘one’, especially in smokers of both sexes, suggesting that SCC incidence is still high among the Irish ever-smokers. However, the more recent rate ratios of AC: SCC is approaching ‘unity’ in female ever-smokers, indicating a recent annual rise in AC incidence in females (table I). Such ratios are also relatively high in female never-smokers, and are not statistically significant.
Table II.
Age-Standardised Incidence Rate Ratios (RR) of Lung Adenocarcinoma: Squamous cell carcinoma (AC: SCC) in ever (former and current combined) and never-smokers by gender distribution.
| Ever-Smokers | Never-Smokers | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Year | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95%CI) |
| 1994 | 0.3 (0.2, 0.4) | 0.4 (0.3, 0.5) | 1.0 (0.4, 2.0) | 1.2 (0.3, 3.2) |
| 1995 | 0.3 (0.2, 0.3) | 0.5 (0.3, 0.6) | 0.8 (0.2, 2.3) | 1.3 (0.2, 4.8) |
| 1996 | 0.4 (0.3, 0.5) | 0.5 (0.3, 0.6) | 0.4 (0.1, 1.8) | 1.9 (0.2, 6.6) |
| 1997 | 0.3 (0.2, 0.4) | 0.5 (0.4, 0.7) | 0.3 (0.02, 1.8) | 0.9 (0.1, 3.8) |
| 1998 | 0.4 (0.3, 0.5) | 0.7 (0.6, 0.9) | 0.5 (0.08, 1.5) | 4.4 (0.9, NC*) |
| 1999 | 0.4 (0.3, 0.5) | 0.8 (0.6, 1.0) | 0.6 (0.90, 1.6) | 1.8 (0.2, 7.2) |
| 2000 | 0.4 (0.3, 0.5) | 0.6 (0.5, 0.7) | 1.0 (0.2, 2.3) | 2.0 (0.4, 5.5) |
NC: Could not be calculated because of extreme values
DISCUSSION
Our study has two important findings. Firstly, our findings may indicate a real increase in lung AC incidence in females from 1994 to 2000 in Ireland, consistent with other industrialised nations.2–4 The gradual convergence in ASIR (table I), with approaching ‘unity’ ratios between AC and SCC (table II), also suggests that the observed increase in AC incidence is less likely due to the proportionate declining SCC incidence. Secondly, all estimates (rates and ratios) indicate that females in general are unlikely to have a greater susceptibility to developing overall lung cancer, although the ratios are changing recently. This is consistent with a few of the recent observations6,7.
The main strength of our study is the analysis of lung cancer incidence data rather than lung cancer mortality data, although the trend analysis was apparently short. Our study did show that the total lung cancer incidence was significantly increasing in females, but the fact that only 75% of lung cancer cases were histologically verified using the Irish Cancer Registry Data could have had an impact on the study findings. Another weakness is the lack of comprehensive smoking data for the individual patients analysed. However, the population smoking data used in our study for the estimation of the proportions of smokers and never-smokers in Ireland for the baseline year, 1994 for analysing time-trends was from Lee and colleagues' publication11, and the quality of such smoking data has recently been reviewed13.
The increase in lung AC incidence is also less likely due to changing diagnostic techniques or better diagnostic facilities, because the period studied was relatively short. Secondly, evidence suggests that a rise in AC incidence could be antecedent to diagnostic interventions14. Thirdly, the histopathological criteria for diagnosis and classification have not changed during the study period. Fourthly, the WHO's re-classification of LCC in 1999 is unlikely to influence the AC trend10, because our data suggest an opposite trend in AC incidence between the sexes from 1994 to 2000 (figure 1).
In Ireland, the overall survival rate in lung cancer has not improved significantly (from 8.2% in 1994 to 9.0% in 2001)9. However, evidence suggests that females with non-small cell lung carcinoma can have better survival, following both surgery and chemotherapy15,16. This emphasises that females may respond differently to tobacco-specific carcinogens for certain cell-types17,18. Several molecular studies have also suggested that sex-differences in lung cancer biology do exist. Examples include, females having higher DNA adduct levels19, an increased CYP1A1 expression19, a decreased DNA repair capacity20 and an increased incidence of K-ras gene mutations21. A novel oestrogen receptor β was also detected in lung tumours22, although both exogenous and endogenous oestrogens might be involved in lung AC development23. All these indicate that oestrogen signalling could have a biological role in lung carcinogenesis.
Unlike the earlier notions of lung AC being more common among never-smokers, recent evidence suggests a stronger association with smoking, especially in former smokers24. Because only 50% of the cigarettes in the late 1960s were ‘filter-tipped’ in Ireland11, any underlying change in female smoking habits is less likely to contribute to the recent lung AC incidence increase, similar to a recent study25. Despite small effects, potential environmental risk factor such as air quality can have some role26. High residential radon levels have also been reported in Ireland27. Lung AC is strongly associated with asbestos exposure levels28, which also coincides with the increased mesothelioma incidence in Ireland29. In summary, rapid urbanization coupled with recent lifestyle changes can potentially explain the changing lung AC incidence patterns30,31.
In March 2004, the Republic of Ireland introduced a comprehensive workplace smoking ban32, with Northern Ireland being the latest to follow suit33. If lung AC is indeed strongly associated with smoking exposure levels, then a dramatic fall in lung AC incidence over the next few years post-ban will certainly confirm the apparent increase seen in Ireland. In addition to tobacco-specific carcinogen susceptibility and gender variations in nicotine addiction levels33, local environmental factors potentially contributing to such an apparent increase need to be identified, integrating traditional epidemiological approaches with modern molecular techniques32. To conclude, our study findings do not support the hypothesis that females are at a greater risk of developing lung cancer, but histologic-specific lung cancer susceptibility cannot be ruled out.
Acknowledgments
The Royal City of Dublin Hospital Research Trust funded Dr Zubair Kabir (ZK) to undertake this study. Currently, ZK is on a cancer research fellowship to the Harvard School of Public Health jointly funded by the US National Cancer Institute and the Health Research Board of Ireland. We thank Dr Harry Comber of the National Cancer Registry (Cork) for providing us the cancer incidence data.
The authors have no conflict of interest.
REFERENCES
- 1.Churg A. Lung cancer cell type and occupation exposure. In: Samet JM, editor. Epidemiology of lung cancer. New York: Marcel Dekker; 1994. pp. 413–36. [Google Scholar]
- 2.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(1 Suppl):191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Levi F, Franceschi S, La Vecchia C, Randimbison L, Te VC. Lung carcinoma trends by histologic type in Vaud and Neuchatel, Switzerland, 1974–1994. Cancer. 1997;79(5):906–14. doi: 10.1002/(sici)1097-0142(19970301)79:5<906::aid-cncr6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Morita T. A statistical study of lung cancer in the annual of pathological autopsy cases in Japan from 1958 to 1997 with reference to time trends of lung cancer in the world. Jpn J Cancer Res. 2002;93(1):15–23. doi: 10.1111/j.1349-7006.2002.tb01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charloux A, Quoix E, Wolkove N, Small D, Pauli G, Kreisman H. The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol. 1997;26(1):14–23. doi: 10.1093/ije/26.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Travis WD, Tarone RE, Travis L, Devesa SS. Lung cancer rates convergence in young men and women in the United States: analysis by birth cohort and histologic type. Int J Cancer. 2003;105(1):101–7. doi: 10.1002/ijc.11020. [DOI] [PubMed] [Google Scholar]
- 7.Bain C, Feskanich D, Speizer FE, Thun M, Hertzmark E, Rosner BA, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst. 2004;96(11):826–34. doi: 10.1093/jnci/djh143. [DOI] [PubMed] [Google Scholar]
- 8.Pauk N, Kubik A, Zatloukal P, Krepela E. Lung cancer in women. Lung Cancer. 2005;48(1):1–9. doi: 10.1016/j.lungcan.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Walsh PM, Comber H. Patterns of care and survival of cancer patients in Ireland 1994 to 2001: time trends and regional variation for breast, colorectal, lung and prostrate cancer. Cork, Ireland: National Cancer Registry Board; 2006. Available from: http://www.ncri.ie/pubs/pubs.shtml. [Google Scholar]
- 10.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. WHO. New York: Springer; 1999. Histological typing of lung and pleural tumours, International Histological Classification of Tumors. 3rd ed. [Google Scholar]
- 11.Forey B, Hamling J, Lee P, Wald N. International Smoking Statistics: a collection of historical data from 30 economically developed countries (Second Edition) London: Oxford University Press; 2002. [Google Scholar]
- 12.Rothman K. http://members.aol.com./krothman/episheet.xls. (Accessed June 2004)
- 13.Lawlor DA. A book and website all IJE readers should know about. Int J Epidemiol. 2004;33(2):433–4. [Google Scholar]
- 14.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89(21):1580–6. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 15.Alexiou C, Onyeaka CV, Beggs D, Akar R, Beggs L, Salama FD. Do women live longer following lung resection for carcinoma? Eur J Cardiothorac Sur. 2002;21(2):319–25. doi: 10.1016/s1010-7940(01)01114-9. [DOI] [PubMed] [Google Scholar]
- 16.Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomised phase II trial of gefitinib for previously treated patients with advanced non-small cell lung cancer. (The IDEAL 1 Trial) J Clin Oncol. 2003;21(12):2237–46. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB, et al. Are female smokers at higher risk for lung cancer than male smokers? A case-control study by histologic type. Am J Epidemiol. 1993;138(5):281–93. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- 18.Haugen A. Women who smoke: are women more susceptible to tobacco-induced lung cancer? Carcinogenesis. 2002;23(2):227–9. doi: 10.1093/carcin/23.2.227. [DOI] [PubMed] [Google Scholar]
- 19.Mollerup S, Ryberg D, Hewer A, Phillips DH, Haugen A. Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Res. 1999;59(14):3317–20. [PubMed] [Google Scholar]
- 20.Wei Q, Cheng L, Amos CI, Wang LE, Guo Z, Hong WK, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92(21):1764–72. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 21.Nelson HH, Christiani DC, Mark EJ, Wiencke JK, Wain JC, Kelsey KT. Implications and prognostic value of K-ras mutation for early-stage lung cancer in women. J Natl Cancer Inst. 1999;91(23):2032–8. doi: 10.1093/jnci/91.23.2032. [DOI] [PubMed] [Google Scholar]
- 22.Omoto Y, Kobayashi Y, Nishida K, Tisuchiiya E, Eguchi H, Nakagawa K, et al. Expression, function and clinical implications of the estrogen receptor beta in human lung cancers. Biochem Biophys Res Commun. 2001;285(2):340–7. doi: 10.1006/bbrc.2001.5158. [DOI] [PubMed] [Google Scholar]
- 23.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86(11):869–70. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 24.Yang P, Cerhan JR, Vierkant RA, Olson JE, Vachon CM, Limburg PJ, et al. Adenocarcinoma of the lung is strongly associated with cigarette smoking: further evidence from a prospective study of women. Am J Epidemiol. 2002;156(12):1114–22. doi: 10.1093/aje/kwf153. [DOI] [PubMed] [Google Scholar]
- 25.Melikian AA, Djordjevic MV, Hosey J, Zhang J, Chen S, Muscat J, et al. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res. 2007;9(3):377–387. doi: 10.1080/14622200701188836. [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Bina WF, Cole P. Declining incidence rate of lung adenocarcinoma in the United States. Chest. 2007;131(4):1000–5. doi: 10.1378/chest.06-1695. [DOI] [PubMed] [Google Scholar]
- 27.McAulay IR, McLaughlin JP. Indoor natural radiation levels in Ireland. Sci Total Environ. 1985;45:319–25. doi: 10.1016/0048-9697(85)90233-5. [DOI] [PubMed] [Google Scholar]
- 28.Whitwell F, Newhouse ML, Bennet DR. A study of the histological cell types of lung cancer in workers suffering from asbestosis in the United Kingdom. Br J Ind Med. 1974;31(4):298–303. doi: 10.1136/oem.31.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabir Z, Clancy L. Mesothelioma trends in the Republic of Ireland: an epidemiologic study in the context of the causal pathway. Ir Med J. 2003;96:299–302. [PubMed] [Google Scholar]
- 30.Jeffreys M, Smith GD, Martin RM, Frankel S, Gunnell D. Childhood body mass index and later cancer risk: a 50-year follow-up of the Boyd Orr study. Int J Cancer. 2004;112(2):348–51. doi: 10.1002/ijc.20423. [DOI] [PubMed] [Google Scholar]
- 31.Kabir Z, Clancy L. Global trends in adenocarcinomas and obesity: an epidemiologic link? Int J Epidemiol. 2003;32(4):661–2. doi: 10.1093/ije/dyg223. [DOI] [PubMed] [Google Scholar]
- 32.Kabir Z, Clancy L. Preventive oncology. Lancet. 2004;363(9422):1737. doi: 10.1016/S0140-6736(04)16273-9. [DOI] [PubMed] [Google Scholar]
- 33.Morrison PJ. The decline in lung cancer and the genetics of nicotine addiction. Ulster Med J. 2007;76(2):65. [PMC free article] [PubMed] [Google Scholar]


