Abstract
The circadian clock in the suprachiasmatic nucleus (SCN) of the hypothalamus organizes behavioral rhythms, such as the sleep–wake cycle, on a near 24-h time base and synchronizes them to environmental day and night. Light information is transmitted to the SCN by direct retinal projections via the retinohypothalamic tract (RHT). Both glutamate (Glu) and pituitary adenylyl cyclase-activating peptide (PACAP) are localized within the RHT. Whereas Glu is an established mediator of light entrainment, the role of PACAP is unknown. To understand the functional significance of this colocalization, we assessed the effects of nocturnal Glu and PACAP on phasing of the circadian rhythm of neuronal firing in slices of rat SCN. When coadministered, PACAP blocked the phase advance normally induced by Glu during late night. Surprisingly, blocking PACAP neurotransmission, with either PACAP6–38, a specific PACAP receptor antagonist, or anti-PACAP antibodies, augmented the Glu-induced phase advance. Blocking PACAP in vivo also potentiated the light-induced phase advance of the rhythm of hamster wheel-running activity. Conversely, PACAP enhanced the Glu-induced delay in the early night, whereas PACAP6–38 inhibited it. These results reveal that PACAP is a significant component of the Glu-mediated light-entrainment pathway. When Glu activates the system, PACAP receptor-mediated processes can provide gain control that generates graded phase shifts. The relative strengths of the Glu and PACAP signals together may encode the amplitude of adaptive circadian behavioral responses to the natural range of intensities of nocturnal light.
The hypothalamic suprachiasmatic nucleus (SCN), the primary circadian clock, receives an array of distinct neurochemical inputs (1). Among these, the retinohypothalamic tract (RHT) carries light information directly from the retina to the SCN and represents the major nocturnal regulatory pathway (2–4). Under constant darkness, a brief light pulse presented to animals during the subjective day has no effect, whereas during subjective night, light induces characteristic behavioral phase delays in early night and phase advances in late night (5, 6). Converging evidence has established that Glu is the primary neurotransmitter mediating light entrainment (3, 4, 7, 8). Multiple signaling steps, including Glu release, membrane depolarization, NMDA receptor activation, Ca2+ influx, nitric-oxide synthase stimulation, and transcriptional activation (7, 9) at the SCN ultimately are translated into altered behaviors. Thus, light activation of the RHT/Glu pathway interacting with the clock-controlled nocturnal sensitivity of the SCN provides daily synchronization of organisms to the solar cycle (10). This phase-dependent gating of clock sensitivity imposes the environmental period of precisely 24 h upon endogenous circadian processes (11).
The neuromodulator pituitary adenylyl cyclase-activating peptide (PACAP) has emerged as a potential retinal messenger to the SCN (12, 13). Although extracts of the SCN contain the highest PACAP concentration among hypothalamic nuclei (14), PACAP-like immunoreactivity (PACAP-LIR) is localized to terminals of neurons of the visual circadian system innervating the retinorecipient SCN (12). Indeed, recent evidence has revealed that PACAP is costored with Glu in a subpopulation of retinal ganglion cells projecting to the SCN (15). PACAP-LIR within the RHT and in nerve fibers and terminals in the ventrolateral SCN in normal adult rats was largely lost subsequent to enucleation (12), indicating that the retina is the primary source.
The neuromodulatory effects of PACAP can be mediated by three receptor subtypes. The PAC1 receptor is 1,000 times more selective for PACAP than vasoactive intestinal peptide (VIP) and is positively coupled to adenylyl cyclase and phospholipase C (16). VPAC1 and VPAC2 receptors do not discriminate between PACAP and VIP and also couple to adenylyl cyclase (16). Whereas both PAC1 and VPAC1 mRNAs are expressed within the rat SCN, the PAC1 is concentrated in the retinorecipient region (12).
PACAP alters phasing of the circadian rhythm of SCN neuronal firing in a brain slice preparation (12). Robust advances of the SCN clock result from PACAP administration in subjective daytime. During this clock phase, PACAP activates the PAC1 receptor and cAMP-signaling cascade. This relationship between phase of PACAP treatment and the clock-resetting response is fully congruent with the phase-response relationship to agents stimulating the cAMP/protein kinase A (PKA) pathway (17); neither affected clock phase when administered at night when Glu is effective (7). Thus, paradoxically, this sensitivity is in antiphase to the known RHT function mediated by Glu at night. However, the presence of both PACAP and Glu in the RHT and their colocalization in at least some of these terminals raise the possibility that the two transmitters may be coreleased and that light-induced phase shifting could involve interactions of multiple signaling pathways.
Although costoring of a small-molecule neurotransmitter and a peptide molecule is a common phenomenon (18), the potential of PACAP to modulate the light/Glu response on the SCN clock has not yet been evaluated. Peptides usually exert a modulatory effect on the small-molecule neurotransmitters with which they colocalize. This modulatory effect can be diverse and critical in determining the duration, amplitude, and direction of cellular changes induced by the small-molecule transmitter. In the present study, we examined the potential role of PACAP in modulating the effects of Glu in nocturnal phase regulation of the SCN.
Materials and Methods
Electrophysiology.
Brain slice preparation.
SCN were studied in a brain slice to monitor the circadian rhythm of SCN neuronal firing rate. SCN were prepared from 6- to 9-week-old Long-Evans rats inbred in our colony for >35 generations. This exceeds the level of inbreeding for genetic homogeneity and reduces the interexperiment variability and, thus, the number of experiments needed to achieve statistical significance. Coronal brain slices (500 μm) of hypothalamus were made in the daytime from rats housed under 12-h light/12-h dark cycles. The slices were trimmed to ≈0.5 cm wide by 0.5 cm long centered on the paired SCN. Slices were maintained in a large-volume brain slice chamber containing Earle’s Balanced Salt Solution (EBSS; Gibco) that was supplemented with 24.6 mM glucose and 26.2 mM sodium bicarbonate plus gentamicin, at pH 7.4, as described previously (17).
Electrical recording and phase analysis.
The spontaneous activities of single SCN cells were sampled serially by extracellular recording by using glass micropipettes. After a neuron’s mean activity had been determined, the electrode was repositioned to sample throughout a single SCN (17). Mean firing rate of the ensemble of neurons was calculated for sequential 2-h periods with 15-min lags and then plotted with respect to circadian time (CT; CT 0 is the time of “lights on” in the colony). Under these conditions, the ensemble of neuronal activities of the SCN displays a stable, 24-h rhythm over 3 days in vitro with a peak at CT 7, near midsubjective day (17). The time of peak mean activity determined by this recording technique varies little among animals from our inbred line. Thus, time-of-peak in the near 24-h oscillation of the neuronal discharge rate is a reliable marker of clock phase (Fig. 1A).
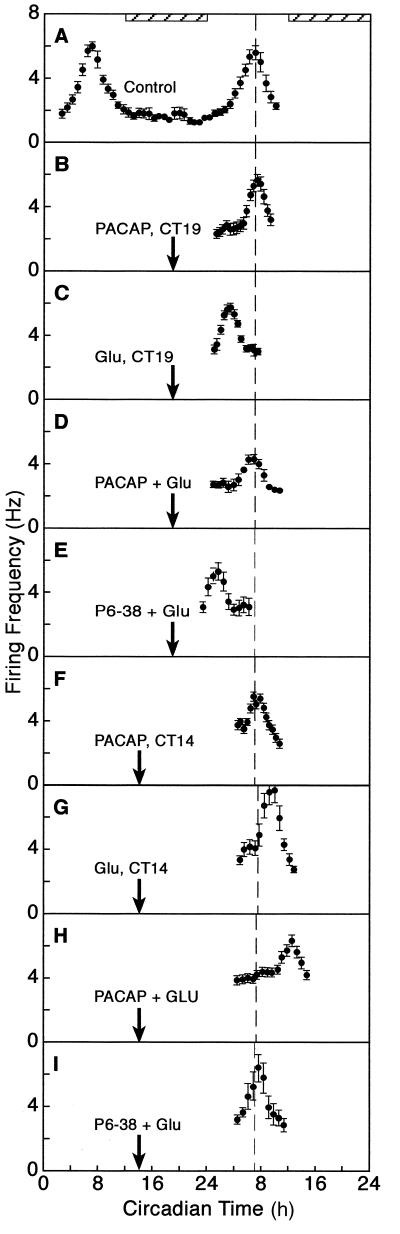
Figure 1.
PACAP modulates the phase-shifting effect of Glu on the rat circadian clock. (A) The mean firing rate of the SCN neuronal ensemble peaked at CT 7 on days 1 and 2 in vitro. Mean peak activity of controls on day 2 is marked by a dashed line for phase reference. (B) PACAP administered at CT 19 did not alter the time of peak. (C) When administered at CT 19, Glu induced a 3.5-h advance in the firing rhythm. (D) When coadministered with Glu at CT 19, PACAP blocked the Glu-induced phase advance. (E) When coadministered with Glu at CT 19, P6–38 potentiated the Glu-induced phase advance to 5.0 h. (F) When administered at CT 14, PACAP did not alter phasing of the neuronal activity rhythm. (G) When applied at CT 14, Glu induced a 3.0-h delay in the rhythm. (H) When coadministered at CT 14, PACAP potentiated the Glu-induced phase delay to 6.0 h. (I) When coadministered with Glu at CT 14, P6–38 blocked the Glu-induced delay. Running averages ± SEM are plotted. Arrows mark the time of drug administration. Subjective night is designated by the horizontal, striped bar.
Drug administration.
Perfusion was stopped temporarily during drug administration. All testing agents were dissolved in fresh medium, the pH was adjusted to 7.2, the medium was reoxygenated, and 1 μl was applied directly to the SCN by micropipette (19). The effective concentration is diluted 10–100 times as the microdrop diffuses into the brain slice. After 10 min, the SCN surface was rinsed with EBSS and perfusion was resumed. For dual treatments, the first reagent was applied 10 min before the time of interest. At the time designated for treatment, the second drug was applied in the continued presence of the first. Experiments were performed at least three times; low interexperiment variation, based on the genetic homogeneity of the inbred rats, permitted a high level of statistical significance with these sample sizes.
Immunocytochemistry.
To assess the effects of long-term incubation of the brain slices in vitro on the level of PACAP immunoreactivity in the SCN, 12 animals were divided equally into short-term (<1-h) and long-term (9-h) slice incubation groups. After incubation, brain slices were fixed with 2% paraformaldehyde and 0.2% picric acid in 0.1 M PBS (pH 7.2) for 12–18 h at 4°C. The slices then were transferred to a cryoprotectant (20% sucrose in PBS) for 24 h before sectioning at 20 μm by cryostat at −20°C; sections were affixed to gelatin-coated microscope slides and stored at −20°C. For PACAP detection, tissue sections were rinsed with PBS and then incubated for 1 h at room temperature with 0.3% Triton X-100 containing 1% heat-inactivated horse serum to permeablize the lipid membrane and to block nonspecific binding sites, respectively. Sections then were incubated for 18–24 h at 4°C with a monoclonal mouse anti-PACAP antibody from cultured cells (MabJHH1) (20) 1:10 in PBS with 0.3% Triton X-100 and 0.5% horse serum. This antibody recognizes both PACAP38 and PACAP27. After PBS washes (3 × 10 min), the sections were incubated at room temperature for 1 h with biotinylated horse anti-mouse antibody. After PBS washes, the signals were amplified by the ABC method (diluted 1:150; Vector Laboratories).
The signal was intensified further by using the tyramide amplification method (21, 22). Briefly, after undergoing the ABC procedure, the sections were incubated with biotinylated tyramide (DuPont/NEN; diluted 1:100) in TSA application buffer. After PBS washes, the sections were incubated with the avidin-peroxidase complex again (diluted 1:150). After washing with 0.05 M Tris buffer (pH 7.6), the sections were incubated with diaminobenzidine (DAB) for 15 min by using glucose oxidase reaction as the peroxide generator to form an insoluble, brown DAB product. The color reaction was stopped by washing in PBS. The sections then were air-dried before dehydration, clearing, and applying coverslips.
Behavioral Analysis.
Wheel-running data acquisition.
Male Syrian hamsters (Mesocricetus auratus) were obtained from Harlan Breeders (Indianapolis). Animals were housed individually in cages equipped with running wheels (5.5-in diameter) and were maintained in a 14-h light/10-h dark (LD) regimen for at least 21 days before being released into constant darkness (DD). Wheel revolutions were counted by using magnets attached to the wheels that activated reed switches with each revolution. Counts were transmitted to a computer running datacol 3 data acquisition software from Minimitter (Sun River, OR). Actograms representing the data were plotted by using the Ratman suite of programs (23). Activity onset, designated CT 12, was used as a reference point for determining treatment times. Phase shifts were measured as the distance between regression lines drawn through at least 5 days immediately before the day of treatment and 5 days after reestablishment of a stable circadian rhythm after treatment (8).
Cannula implantation and injection.
Hamsters weighing 70–110 g at the time of surgery were stereotaxically implanted intracerebroventricularly (ICV) with guide cannulae, as described previously (7). Briefly, the animals were anesthetized with a mixture of ketamine (125 mg/kg), xylazine (20 mg/kg), and acepromazine maleate (2 mg/kg). Guide cannulae (26 gauge; Plastics One, Roanoke, VA) were implanted 1.0 mm anterior to bregma at the midline (upper incisor bar set to 0) to a depth of 2.8 mm below the dural surface. Guide cannulae were fixed to the skull with a jeweler’s screw and cranioplastic cement (Plastics One). Stylets constructed from 33-gauge stainless steel tubing (Small Parts, Miami) were inserted into the guides to maintain patency. After surgery, animals were returned to their home cages and were allowed to recover for at least 7 days before being released into DD.
Animals were allowed to free-run in DD for 10–14 days before the first ICV injection. Under dim red light (≈1 lux), the animal was removed from its cage, the stylet was extracted from the guide cannula, and a 33-gauge injection cannula (Plastics One) attached to a 10-μl Hamilton syringe was inserted into the guide. The injection cannulae were constructed to extend 4.5 mm beyond the tip of the guides to reach the floor of the third ventricle. Antibodies in tissue culture supernatant were supplied lyophilized and diluted 1:5 in PBS for injection. Vehicle controls contained 1% BSA in PBS. Injections (2 μl) were administered over approximately 1 min with the cannula remaining in place for at least 30 sec after the injection. Stylets were replaced after injections. In treatments that were accompanied by a light pulse, the pulse (15 min, 20 lux) (24) was given 15 min after the injection. Subsequent injections were spaced 10–14 days apart to allow recovery of a stable, free-running rhythm. Each animal received up to three injections.
Statistical Analysis.
ANOVA (sigmastat 2.0) compared differences in phase shifts of SCN neuronal activity while factoring for time of day and drug treatment. Where appropriate, posthoc comparisons [Tukey’s HSD (Honestly Significantly Different) or Student’s t test] identified significant differences (P < 0.05).
Results
PACAP Blocks Glu-Induced Phase Advance in Late Night.
The circadian rhythm of the SCN neuronal ensemble peaked normally at CT 7 in untreated and vehicle-treated slices (Fig. 1A). PACAP38 application (1 μM in 1 μl) during late subjective night (CT 19) did not effect clock phasing (Fig. 1B). Application of Glu (10 mM in 1 μl) at this time induced a phase advance of 3.5 ± 0.2 h (Figs. 1C and 2A). However, when PACAP was applied 10 min before as well as during Glu stimulation at CT 19, the mean time of peak (7.0 ± 0.4, Figs. 1D and 2A) was not significantly different from controls or PACAP-treated slices. This revealed that the Glu-induced phase advance was effectively blocked by PACAP. These observations indicate that PACAP can interact with Glu in the late night so as to diminish the phase advance.
Figure 2.
Modulatory effects of PACAP vs. anti-PACAP reagents on the phase-shifting effect of Glu on the neuronal activity rhythm. (A) The differential modulatory effects of PACAP and P6–38 in early vs. late night. (B) Summary of various anti-PACAP reagents on the Glu-induced phase delay at CT 14. n = 3 for all conditions, except for P6–38 + Glu and P6–27 + Glu, where n = 4 and 6, respectively. ∗, P < 0.05; ∗∗, P < 0.01.
PACAP Potentiates Glu-Induced Phase Delay in Early Night.
In the early subjective night (CT 14), Glu induced a 2.5 ± 0.3-h phase delay (Figs. 1G and 2A), whereas PACAP applied alone had no effect on the timing of the peak (Fig. 1F). When the SCN slice was treated with Glu in the presence of PACAP, the time of peak of the SCN neuronal activity occurred at CT 11 (Fig. 1H). This is 4.0 ± 0.5 h later than the normal peak time (Fig. 2A). This 4-h delay is significantly larger (175%, P < 0.05) than the delay induced by Glu alone.
PACAP Antagonists Alter Glu Effects at Both CT 19 and 14.
To test the specificity of the effect of PACAP on the Glu-stimulated shifts, we employed a specific PAC1 receptor antagonist, the peptide fragment PACAP6–38 (P6–38) (25). P6–38 was without effect at CT 19, but when applied to the SCN slice before and during Glu administration, the subsequent peak in neuronal firing occurred at CT 2 (Figs. 1E and 2A). This 5-h advance in peak time is significantly larger than that induced by Glu alone (Fig. 2A, P < 0.01). When the PACAP receptor antagonist was tested at CT 14 instead of CT 19, the opposite effect was observed. That is, when PACAP6–38 was administered with Glu in early night, there was no significant change in phase (Figs. 1I and 2A), indicating that Glu-induced phase delay had been blocked.
To examine the specificity of the interaction of PACAP with Glu, two other reagents were employed. Antibodies selective for PACAP (20) blocked the phase-delaying effect of Glu (Fig. 2B). However, PACAP6–27, a peptide fragment of PACAP27 that interferes with receptor binding of PACAP27 but not PACAP38 (25), did not antagonize the effect of Glu at CT 14. Together, these data implicate PACAP38, rather than PACAP27, as the endogenous PACAP peptide and PAC1 as the receptor mediating the interaction with Glu.
PACAP-LIR in the SCN in Vitro.
Because peptides that block PACAP receptor activation affected responses to exogenous Glu, it appeared likely that PACAP persists in the cut RHT terminals in vitro. Therefore, we examined PACAP-LIR in two groups of SCN slices. For one group, slices were prepared and fixed immediately (<1 h), whereas the other group of slices was incubated in the chamber for >9 h, the same of time length as slices treated at CT 19 in electrophysiological experiments. PACAP-LIR was pronounced in both groups (Fig. 3). The distribution and relative intensities of PACAP-LIR in RHT terminals in the hypothalamic slice were similar to those observed ex vivo (12). This observation supports the notion that PACAP endogenous to the brain slice can modulate the effect of Glu on the SCN clock.
Figure 3.
PACAP persists in the SCN brain slice. (A) PACAP-LIR was detected in the SCN slices after <1 h of incubation. (B) PACAP-LIR was similar in the SCN slices that were incubated for ≈9 h. Results are representative of those from six animals in each group.
PACAP Modulation of Light-Induced Phase Shifts.
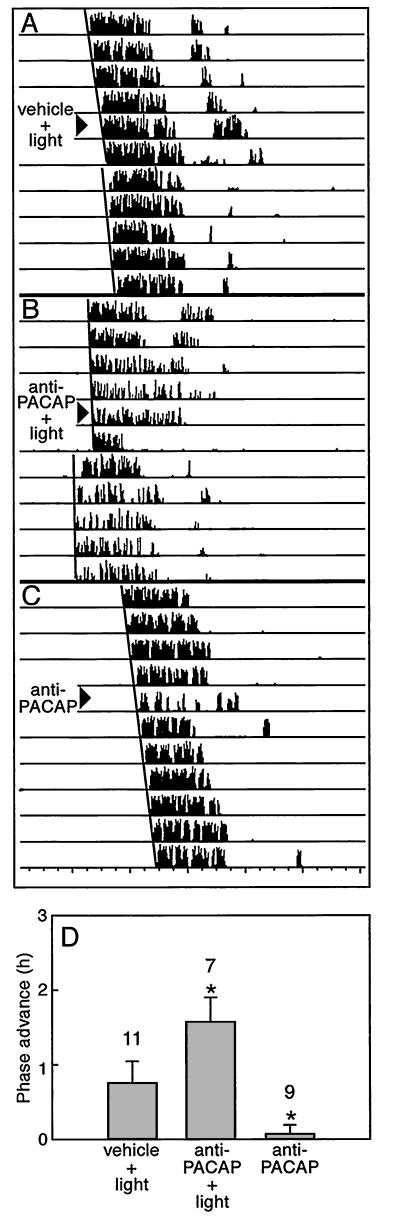
To determine whether PACAP contributes to light-induced phase shifts, we tested the effects of anti-PACAP antibodies in vivo. A nonsaturating light stimulus (20 lux) was employed to evaluate possible potentiating effects of anti-PACAP antibodies on the light response. Under constant darkness, ICV injection of anti-PACAP antibodies before a light pulse at CT 18 (the time of maximal phase advance to light in hamsters) advanced the phase of wheel-running activity by 1.58 ± 0.33 h, compared with phase advances resulting from light alone (0.74 ± 0.29 h) (Fig. 4). Neither vehicle nor antibody alone affected clock phasing. Therefore, in parallel to the effect of anti-PACAP antibody on the Glu-induced phase advance seen in vitro, blocking PACAP action in vivo potentiated the light-induced phase advance (P < 0.05, Fig. 4D).
Figure 4.
Anti-PACAP antibodies potentiate light-induced phase advances of the wheel-running activity rhythm in vivo. Hamsters were maintained under constant conditions in darkness to express free-running circadian rhythms. (A– C) Sequential daily activity records from three hamsters representing the effects of various treatments. Each horizontal bar represents 24 h. (D) The phase advance in the anti-PACAP antibody plus light group was significantly different from that for either light exposure or anti-PACAP antibodies alone. Light exposure (20 lux, 15 min) at CT 18 (6 h after activity onset) after vehicle (2 μl) was administered by ICV injection induced a 0.74 ± 0.29-h phase advance. Anti-PACAP antibodies administered before light exposure significantly potentiated the light-induced phase advance (1.58 ± 0.33 h, P < 0.05). Anti-PACAP antibodies themselves did not induce significant phase shifts (+0.06 ± 0.12 h). n/condition is shown above the bars.
Discussion
PACAP Modulates Glu-Induced Phase Shifts at Night.
The present study demonstrates that PACAP modulates the nocturnal effects of Glu on the clock. Varying PACAP participation, from applying exogenous PACAP to inhibiting endogenous PACAP, produced graded effects on phase-shifting amplitudes. Coapplication of PACAP to the SCN fully inhibited the phase advance normally induced by Glu in the late night. In contrast to blocking the Glu-induced phase advance, PACAP in early night significantly amplified the Glu-induced phase delay. When a specific peptide inhibitor of the PAC1 receptor was coapplied with GLU instead of PACAP, the direction of these responses was reversed. As would be predicted for signals transduced via the PAC1 receptor, this modulation is mediated by the cAMP signal transduction cascade (D.C. and S. A. Tischkau, unpublished observations). Therefore, Glu is necessary to initiate the phase shift, but the level of PACAP participation alters the strength of the Glu signal. These observations are distinct from the recently reported effects of very low concentrations of PACAP, which appear to induce small phase changes via a non-cAMP-mediated mechanism that involves potentiation of the NMDA receptor (26), rather than crosstalk among second messengers. Together, these observations reveal a range of PACAP-Glu interactions that are significant in view of their colocalization in retinal ganglion cells that innervate the SCN (15).
PACAP Is an Endogenous Component in the Light/Glu Pathway.
The effects of the peptide antagonist of the PAC1 receptor on the Glu-induced phase shifts reveal an unanticipated result—they did not simply cause a response equivalent to Glu alone, they actually biased the response to Glu in a direction opposite to PACAP. P6–38 potentiated the phase advance but prevented the phase delay. This indicates that there is a native PACAPergic component to the response stimulated by exogenous Glu. Because neither PACAP nor P6–38 alone altered clock phasing during the nighttime, activation of the Glu-signaling pathway is a permissive event that primes the system for events downstream from PAC1 receptor activation. Therefore, although Glu stimulation is necessary to adjust clock phasing at night, PACAP appears to be an integral, endogenous element that varies the amplitude.
Whether PACAP normally is released in conjunction with Glu in response to a light pulse has not yet been determined. However, because blocking the action of P6–38 at its receptor potentiated light- and Glu-induced phase advances in vivo and in vitro, respectively, PACAP released from the RHT likely contributes normally to the Glu-mediated response to light. The relationship between the level of PACAP stimulation and the amplitude of delay vs. advance is such that decreasing PACAP increases the amplitude of the phase advance but decreases the amplitude of the delay. This suggests that integration of the two pathways may provide a neurochemical reflection of more subtle aspects of the light stimulus, such as stimulus strength. cAMP, the intracellular messenger of PACAP’s action via PAC1 receptors, is a likely mediator of integration between PACAP and Glu-signaling pathways. Furthermore, levels of cAMP may be influenced by different, afferent neurotransmitter systems from other brain sites (1, 27). Thus, cAMP is a potential converging point of multiple signaling pathways, and the light/Glu response likely integrates the state of these modulators, as well. However, in the context of nocturnal phase regulation by light, our data predict that PACAP may be the predominant physiological modulator.
The observation that P6–38 has a robust effect on Glu-induced phase shifts suggests that some endogenous PACAP38 is released from the brain slice. Although this seemed surprising, this interpretation is supported by two types of evidence. First, the PACAP antagonist significantly reduced endogenous cAMP levels of SCN in vitro (data not presented). Second, PACAP-IR persisted in the SCN slice >9 h after incubating the slice in chamber. That P6–27 was ineffective reflects the specificity of the two PACAP antagonists: P6–38 blocks the effect of PACAP38, whereas P6–27 only antagonizes PACAP27 action (25). This strongly supports native PACAP38 as the modulator of Glu effects. Moreover, ICV injection of anti-PACAP antibody before the light pulse amplified the light-induced phase advance in vivo. Thus, PACAP38 could contribute gain control to the light/Glu signal that mediates nocturnal phase regulation of the circadian system.
A Coherent Model: PACAP Acts as a Negative Force at Night.
The modulatory effect of PACAP on light- and Glu-induced nocturnal phase shifts indicates that the signal encoding light at the SCN is more complex than previously thought. Based on our present findings, we have constructed a model in an attempt to reconcile and unify the paradoxical effects of PACAP on the Glu-induced phase delays and advances. The model schematically presents the nocturnal portion of the Glu phase-response curve (Fig. 5). Extrapolated trajectories of the effects of PACAP and PACAP antagonists are superimposed on the Glu-induced shifts. From this model, a consistent pattern of modulation is evident. For both phase delays and advances, PACAP moves the response down along the vertical axis representing the amplitude of the shift, whereas the PACAP antagonist moves the response up. By blocking the PACAP arm of the stimulus, the PACAP antagonist permits the full effect of Glu to be expressed, and this is a positive influence on the response. In sum, greater activation of the PACAP pathway in the presence of Glu negatively influences the phase change induced by Glu throughout the night whereas lesser involvement by PACAP positively biases the response.
Figure 5.
A schematic depiction of the stimulus–response relationships for nocturnal Glu (solid line), PACAP + Glu (small dashes), and P6–38 + Glu (large dashes). The arrows indicate the direction of modulation from the Glu response. PACAP induces opposite modulatory effects on Glu in both early and late night from P6–38, which has antagonist effects on PAC1 receptor activation. Note that PACAP moves the effect of Glu down along the amplitude axis whereas P6–38 moves the effect of Glu upward during both phase delay and advance. Subjective night is designated by the horizontal, striped bar.
Our data provide insights into fundamental aspects of nocturnal phase regulation of the SCN clock by light. Although nocturnal Glu is a critical element, we have identified PACAP as an integral modulatory component. A surprising discovery is that, in addition to activating the state change, Glu signals the opposite clock motion from PACAP. The amplitude of the shift is imparted through the action of coreleased PACAP, a negative influence. Directionality of the shift depends on temporal clock state: the molecular substrates of time keeping (28) must confer delay on the response to light in early night and advance in late night. Integration of the relative strengths of the positive and negative forces encoded in the Glu and PACAP signal transduction cascades then would determine the amplitude of the actual shift within the context of what is possible at that clock state. Such a dual-regulatory system would provide finer control than a single regulator capable of conveying only a monotonic shift: it would permit the clock to assess a richer representation of brain and world state during the decision-making process. We predict that the result would be a clock capable of a continuum of finely tuned alterations that would become manifest as adaptive shifts in circadian behaviors to the range of environmental light intensities.
Acknowledgments
We thank members of the Gillette lab for helpful discussion, Shelley Tischkau for insightful comments on the model and text, and Penny Burgoon for critique of the manuscript. This work was supported by Public Health Service Grant NS22155 (National Institute of Neurological Disorders and Stroke) to M.U.G. and Grant 9702202 from the Danish Medical Research Council to J.H.
Abbreviations
- PACAP
pituitary adenylate cyclase-activating peptide
- PACAP-LIR
PACAP-like immunoreactivity
- RHT
retinohypothalamic tract
- SCN
suprachiasmatic nucleus
- CT
circadian time
- ICV
intracerebroventricularly
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Moore R Y. Prog Brain Res. 1996;111:103–119. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- 2.Moore R Y, Lenn N J. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 3.Pickard G E. J Comp Neurol. 1982;211:65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- 4.Johnson R F, Morin L P, Moore R Y. Brain Res. 1988;462:301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- 5.Daan S, Pittendrigh C S. J Comp Physiol. 1976;106:253–266. [Google Scholar]
- 6.Summer T L, Ferraro J S, McCormack C E. Am J Physiol. 1984;246:R299–R304. doi: 10.1152/ajpregu.1984.246.3.R299. [DOI] [PubMed] [Google Scholar]
- 7.Ding J M, Chen D, Weber E T, Faiman L E, Rea M A, Gillette M U. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 8.Ding J M, Buchanan G F, Tischkau S A, Chen D, Kuriashkina L R, Faiman L E, Alster J M, McPherson P S, Campbell K P, Gillette M U. Nature (London) 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- 9.Amir S. Brain Res. 1992;586:336–339. doi: 10.1016/0006-8993(92)91644-t. [DOI] [PubMed] [Google Scholar]
- 10.Gillette M U. Prog Brain Res. 1996;111:119–130. doi: 10.1016/s0079-6123(08)60404-5. [DOI] [PubMed] [Google Scholar]
- 11.DeCoursey P J. Science. 1960;131:33–35. [Google Scholar]
- 12.Hannibal J, Ding J M, Chen D, Fahrenkrug J, Larsen P, Gillette M U, Mikkelsen J D. J Neurosci. 1997;17:2637–2644. doi: 10.1523/JNEUROSCI.17-07-02637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piggins H D, Stamp J A, Burns J, Rusak B, Semba K. J Comp Neurol. 1996;376:278–294. doi: 10.1002/(SICI)1096-9861(19961209)376:2<278::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, Fujino M. Brain Res. 1993;602:57–63. doi: 10.1016/0006-8993(93)90241-e. [DOI] [PubMed] [Google Scholar]
- 15.Hannibal, J., Moller, M., Ottersen, O. & Fahrenkrug, J. (1999) J. Comp. Neurol., in press. [PubMed]
- 16.Harmar A J, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna J R, Rawlings S R, Robberecht P, Said S I, Sreedharan S P, et al. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- 17.Prosser R A, Gillette M U. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millhorn D E, Hokfelt T. News Physiol Sci. 1988;3:1–5. [Google Scholar]
- 19.Medanic M, Gillette M. J Physiol (London) 1992;450:629–642. doi: 10.1113/jphysiol.1992.sp019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannibal J, Mikkelsen J D, Clausen H, Holst J J, Wulff B S, Fahrenkrug J. Regul Peptides. 1995;55:133–148. doi: 10.1016/0167-0115(94)00099-j. [DOI] [PubMed] [Google Scholar]
- 21.Berghorn K A, Bonnette J H, Hoffman G E. J Histochem Cytochem. 1994;42:1635–1642. doi: 10.1177/42.12.7983364. [DOI] [PubMed] [Google Scholar]
- 22.Fahrenkrug J, Hannibal J. Neuroscience. 1998;83:1261–1272. doi: 10.1016/s0306-4522(97)00474-0. [DOI] [PubMed] [Google Scholar]
- 23.Klemfuss H, Clopton P L. J Interdis Cycle Res. 1993;24:1–16. [Google Scholar]
- 24.Weber E T, Gannon R L, Rea M A. Neurosci Lett. 1995;197:227–230. doi: 10.1016/0304-3940(95)11961-u. [DOI] [PubMed] [Google Scholar]
- 25.Robberecht P, Gourlet P, De Neef P, Woussen-colle M-C, Vandermeers-Piret M-C, Vandermeers A, Christophe J. Eur J Biochem. 1992;207:239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- 26.Harrington M, Hoque S, Hall A, Golombek D, Biello S. J Neurosci. 1999;19:6637–6642. doi: 10.1523/JNEUROSCI.19-15-06637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mons N, Cooper M F. Trends Neurosci. 1995;18:536–542. doi: 10.1016/0166-2236(95)98375-9. [DOI] [PubMed] [Google Scholar]
- 28.Dunlap J C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]