Abstract
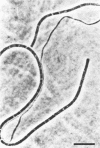
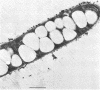
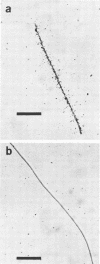
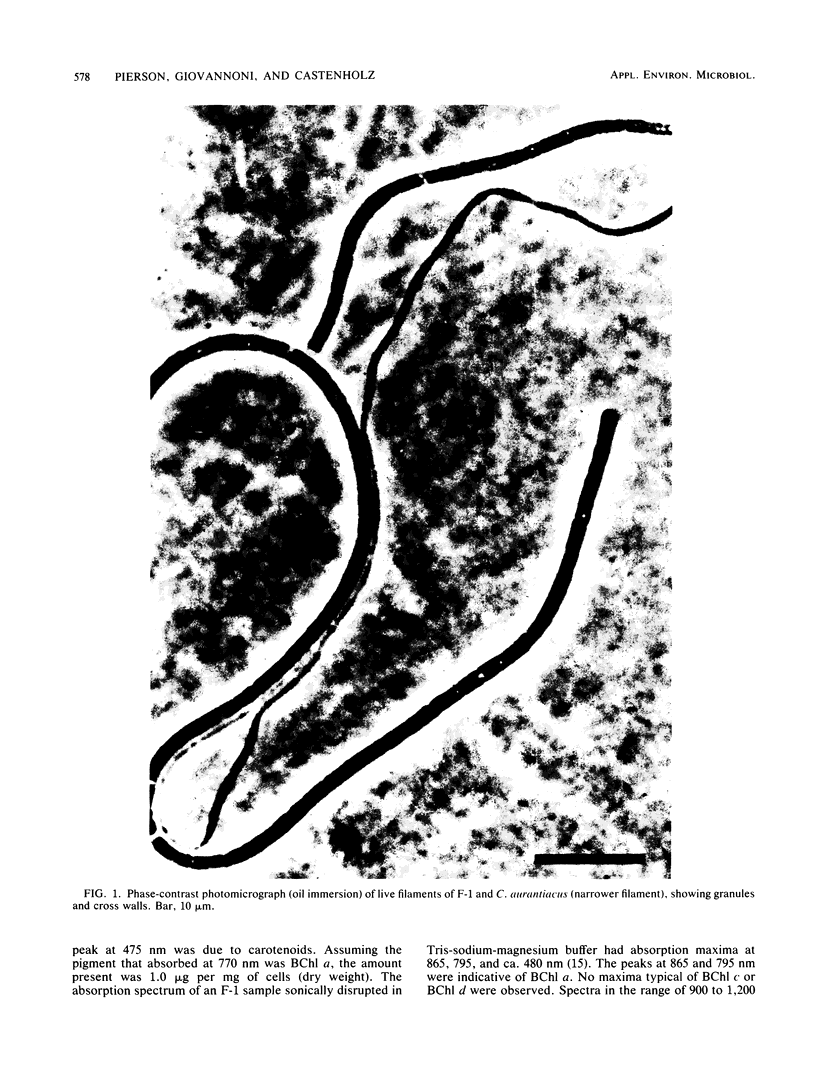
A filamentous, gliding, thermophilic bacterium, found growing abundantly as a surface mat in a limited number of alkaline hot springs in Oregon, is described and designated F-1. The bacteria were studied in the field and in coculture with an aerobic chemoheterotroph. The bacteria are phototrophic and contain bacteriochlorophyll a and several carotenoid pigments. Unlike the other gliding phototrophic bacteria, members of the family Chloroflexaceae, F-1 does not contain chlorosomes or bacteriochlorophyll c or d. The light-dependent uptake of simple organic compounds (acetate and glucose) was demonstrated in field populations. Near-infrared radiation sustained this uptake, which occurred equally well under aerobic or anaerobic conditions and was insensitive to 3-(3,4-dichlorophenyl)-1,1-dimethylurea. The bacteria formed conspicuous dominant mats from about 35 to 56°C, and they covered mats of cyanobacteria in the spring, summer, and autumn months. It appears that they depend on high light intensities to maintain a dense population.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Madigan M. T., Brock T. D. Adaptation by hot spring phototrophs to reduced light intensities. Arch Microbiol. 1977 May 13;113(1-2):111–120. doi: 10.1007/BF00428590. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol. 1974;100(1):5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. Bacteriochlorophylls in gliding filamentous prokaryotes from hot springs. Nat New Biol. 1971 Sep 1;233(35):25–27. doi: 10.1038/newbio233025a0. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R., CLAYTON R. K. STUDIES ON A MUTANT OF RHODOPSEUDOMONAS SPHEROIDES UNABLE TO GROW PHOTOSYNTHETICALLY. Biochim Biophys Acta. 1964 Jul 29;88:61–73. doi: 10.1016/0926-6577(64)90154-8. [DOI] [PubMed] [Google Scholar]
- Sato K. Bacteriochlorophyll formation by facultative methylotrophs, Protaminobacter ruber and Pseudomonas AM 1. FEBS Lett. 1978 Jan 15;85(2):207–210. doi: 10.1016/0014-5793(78)80456-6. [DOI] [PubMed] [Google Scholar]
- Shiba T., Simidu U., Taga N. Distribution of aerobic bacteria which contain bacteriochlorophyll a. Appl Environ Microbiol. 1979 Jul;38(1):43–45. doi: 10.1128/aem.38.1.43-45.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague S. G., Staehelin L. A., Fuller R. C. Semiaerobic induction of bacteriochlorophyll synthesis in the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1981 Sep;147(3):1032–1039. doi: 10.1128/jb.147.3.1032-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- van Gool A. P., Lambert R., Laudelout H. The fine structure of frozen etched nitrobacter cells. Arch Mikrobiol. 1969;69(4):281–293. doi: 10.1007/BF00408570. [DOI] [PubMed] [Google Scholar]