Abstract
Uncontrolled activation of the coagulation cascade after tissue injury has been implicated in both inflammation and tissue fibrosis. Thrombin exerts pluripotent cellular effects via its high-affinity receptor, proteinase-activated receptor-1 (PAR1) and signaling via Gαi/o, Gαq, or Gα12/13. Activation of PAR1 on fibroblasts, a key effector cell in fibrosis, results in the induction of several mediators, including the potent monocyte and fibrocyte chemoattractant CCL2. The aim of this study was to identify the G protein and signaling pathway involved in PAR1-mediated CCL2 production and release. Using a novel PAR1 antagonist that blocks the interaction between PAR1 and Gαq, we report for the first time that PAR1 coupling to Gαq is essential for thrombin-induced CCL2 gene expression and protein release in murine lung fibroblasts. We further demonstrate that these effects are mediated via the cooperation between ERK1/2 and Rho kinase signaling pathways: a calcium-independent protein kinase C (PKC), c-Raf, and ERK1/2 pathway was found to mediate PAR1-induced CCL2 gene transcription, whereas a phospholipase C, calcium-dependent PKC, and Rho kinase pathway influences CCL2 protein release. We propose that targeting the interaction between PAR1 and Gαq may allow us to selectively interfere with PAR1 proinflammatory and profibrotic signaling, while preserving the essential role of other PAR1-mediated cellular responses.
INTRODUCTION
Inflammation and the subsequent fibroproliferative response are critical components of tissue repair after injury. However, if uncontrolled, these processes can lead to the development of tissue remodeling and fibrosis of the skin, vasculature, and internal organs, including the lung. Previously, the fibroblast was considered to be a passive participant in tissue repair through its end-stage contribution of extracellular matrix synthesis. However, emerging evidence now points to a more active role for fibroblasts in the response to tissue injury by releasing a host of mediators, including the CC-chemokine: CCL2 (MCP-1/CCL2/JE; Hogaboam et al., 1998). Although primarily considered a potent chemoattractant for monocytes, T-cells, and natural killer cells, CCL2 is also involved in the direct activation of fibroblasts leading to extracellular matrix generation via the induction of the potent profibrotic mediator, transforming growth factor β1 (TGF-β1; Gharaee-Kermani et al., 1996). Recent evidence further suggests that CCL2 may also contribute to excessive collagen deposition via the recruitment of fibrocytes (Moore et al., 2005), which are believed to represent a source of fibroblasts and myofibroblasts during the fibroproliferative response to tissue damage (Phillips et al., 2004).
One of the earliest responses to tissue injury involves the highly coordinated activation of the coagulation cascade with the resultant generation of thrombin. In addition to its central role in hemostasis, thrombin exerts a number of cellular effects that initiate and influence subsequent inflammatory and tissue repair responses (Chambers, 2003). Thrombin exerts potent profibrotic effects by influencing fibroblast function and has also been shown to up-regulate CCL2 expression by several cell types, including monocytes (Colotta et al., 1994), endothelial cells (Colotta et al., 1994; Marin et al., 2001), smooth muscle cells (Brandes et al., 2001), and dermal fibroblasts (Bachli et al., 2003). The cellular effects of thrombin are largely, but not exclusively, mediated via the activation of a unique family of cell surface receptors termed, proteinase-activated receptors (PARs). To date, four PARs have been described, of which three (PAR1, PAR3, and PAR4) are activated by thrombin. These receptors belong to the seven transmembrane domain G protein–coupled receptor (GPCR) superfamily, but are activated by a unique mechanism involving limited proteolytic cleavage of the N-terminal extracellular domain leading to the unmasking of a tethered ligand that in turn activates the receptor by intramolecular binding (Vu et al., 1991).
PAR1 is the high-affinity thrombin receptor and the major receptor responsible for mediating many of the proinflammatory and profibrotic effects of thrombin via the induction and activation of a host of secondary mediators(Chambers, 2003). PAR1 exhibits the ability to couple to multiple G protein family subunits, including Gαi/o, Gαq, or Gα12/13 within the same cell type. In general, the Gαi/o pathway inhibits adenylate cyclase and the generation of cyclic adenosine monophosphate (cAMP); the Gαq pathway involves phospholipase C-β (PLC-β) activation and concomitant calcium mobilization and protein kinase C (PKC) activation, whereas the Gα12/13 pathway activates Rho kinase and regulates actin remodeling (Coughlin, 2000).
Studies employing PAR1 antagonists and PAR1-deficient mice have provided strong evidence that PAR1 signaling plays an important role in inflammation and tissue remodeling in a number of tissues, including the vasculature (Cheung et al., 1999), the kidney (Cunningham et al., 2000), the liver (Fiorucci et al., 2004), and the lung (Howell et al., 2005). In the context of lung injury, we have recently reported that protection from bleomycin-induced lung inflammation and fibrosis in PAR1-deficient mice is accompanied by a marked attenuation of the characteristic increase in CCL2 lung levels (Howell et al., 2005). In human fibroproliferative lung diseases, CCL2 levels in bronchoalveolar lavage fluid (BALF) correlate with the severity of lung injury in acute respiratory distress syndrome (ARDS; Goodman et al., 1996). CCL2 lung levels are also increased in patients with interstitial lung disease (ILD) and in patients with the fatal chronic fibrotic lung condition, idiopathic pulmonary fibrosis (IPF), where CCL2 may serve as a useful biomarker for the clinical course of the disease (Suga et al., 1999). In patients with fibrotic lung disease and in animal models, fibroblast numbers are dramatically increased, and numerous cells are strongly immunoreactive for both CCL2 and PAR1 (Mercer, Johns, Scotton, Krupiczojc, Koenigshoff, Howell, McAnulty, Das, Eickelberg, and Chambers, unpublished data). However, the signaling pathways involved in PAR1-mediated CCL2 production remain poorly understood. The aim of this study was therefore to begin to delineate the signaling pathways by which thrombin induces CCL2 production by lung fibroblasts. We report for the first time that thrombin induces fibroblast CCL2 production and release via coupling of PAR1 to Gαq and the cooperation between ERK1/2 and Rho kinase signaling pathways. We further provide evidence that the Ca2+-independent PKC, c-Raf, and ERK1/2 pathway is responsible for thrombin-induced CCL2 gene expression, whereas the second PLC, Ca2+, Ca2+-dependent PKC, Rho kinase pathway influences CCL2 protein release via a posttranscriptional mechanism. These data demonstrate a central role for Gαq in thrombin-induced CCL2 signaling. In this report, we further demonstrate the effectiveness of blocking this response using a recently developed novel small-molecule PAR1 antagonist, which blocks PAR1 at the intracellular interaction site with Gαq (Caden Biosciences, Madison, WI). This antagonist may allow more selective targeting of some, but not all, PAR1-mediated cellular responses and may hold promise for interfering with deleterious PAR1 signaling in a number of inflammatory and fibrotic conditions associated with uncontrolled activation of the coagulation cascade.
MATERIALS AND METHODS
Materials
Human thrombin, Ro-318425, GF109203X, Gö6976, U0126, SB 203580, Y- 27632, H-1152, U73122, BAPTA-AM, and c-Raf inhibitor were purchased from Calbiochem (Merck Biosciences, Nottingham, United Kingdom). Tumor necrosis factor α (TNF-α) was purchased from Peprotech (London, United Kingdom). Anti-phospho-p38, anti-p38, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-c-Raf, anti-c-Raf, and anti-MLC antibodies were purchased from New England Biolabs (Hitchin, United Kingdom). Anti-phospho-MLC antibody was a kind and generous gift from Dr. James M. Staddon (Eisai London Research Laboratories, United Kingdom). Anti-CCL2 antibody was obtained from R&D Systems (Abingdon, United Kingdom). Wild-type MEK1 (wt-MEK1), dominant negative MEK1 (dn-MEK1), and constitutively active MEK1 (ca-MEK1) cloned to pBABEpuro eukaryotic expression vectors were generous gifts from Professor Chris Marshall (Cancer Research UK, London, United Kingdom). Selective PAR1 agonists, corresponding to the sequence TFLLR-NH2 (TF) and the reverse control peptide RLLFT-NH2 (RL), were obtained from Dr. Robert P. Mecham (University of Washington Medical School, St. Louis, MO). pRev Tet-on vector was purchased from BD Biosciences (San Jose, CA). The pRevTRE2-dEGFP (a control vector encoding enhanced green fluorescent protein [EGFP]), pRevTRE2-Gαq, pRevTRE2-Gα12, pRevTRE-Gα13, and pRevTRE-Gαi/o minigenes and the PAR1 antagonist Q94 were developed by Dr. Annette Gilchrist (Caden Biosciences). These C-terminal G alpha minigenes encode 11 amino acid C-terminal sequences of Gαq, Gα12, Gα13, and Gαi, which act as highly specific competitive inhibitors for each isoform (Gilchrist et al., 2001, 2002). The novel PAR1 antagonist, Q94, is a small molecule (MW <500) that meets the Lipinski rule of five and was identified during an ELISA screen for competition of a high-affinity peptide that mimics the C-terminus of Gαq using a commercially available library (ChemDiv). Further details of the Q94 selection criteria and binding affinity for PAR1 are provided as supplementary data (see Supplementary Data Table S1 and Figure 1). A patent application for this compound has been filed (patent application EFS ID 2841714; application number 61027665) This compound will be made freely available to qualified investigators upon application to Dr. A. Gilchrist (Caden Biosciences).
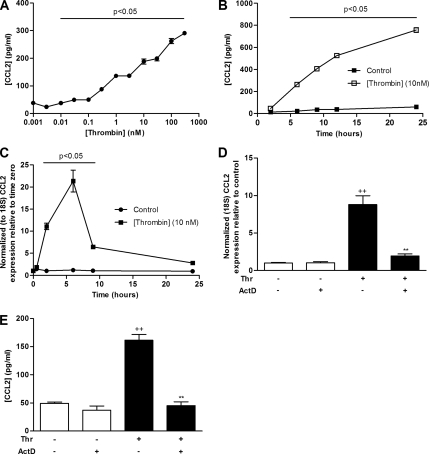
Figure 1.
Thrombin stimulates fibroblast CCL2 gene expression and protein production. (A and B) Dose–response (A) and time-course (B) data for the effect of thrombin on MLF CCL2 protein release. MLFs were exposed to thrombin (0.001–300 nM) for 6 h or to 10 nM thrombin for varying durations (2–24 h). Supernatants from cell cultures after incubation were analyzed for CCL2 protein secretion by ELISA. (C) Time-course data for the effect of thrombin on CCL2 mRNA levels. MLFs were exposed to serum-free control medium (DMEM) or thrombin (10 nM) for incubation times from 0.5 to 24 h. CCL2 mRNA levels at each time point were assessed by quantitative real time RT-PCR. Data are expressed as fold change relative to time zero for each time point (mean ± SEM from triplicates) after normalization to 18S RNA. (D) The effect ActD on thrombin-induced CCL2 mRNA levels. MLFs were exposed to thrombin for 2 h with or without preincubation with ActD (1 μg/ml) for 30 min. CCL2 mRNA levels were determined as in C. (E) The effect of ActD on thrombin-induced CCL2 protein release. MLFs were preincubated with ActD (1 μg/ml) for 30 min before exposure to thrombin for 6 h. CCL2 release into cell culture were analyzed by ELISA as in A. Data are expressed as the mean ± SEM from triplicates. p < 0.05, comparison with unstimulated cells or time point-matched media control cells; ++ p < 0.01, comparison with medium control; ** p < 0.01, comparison with thrombin alone.
Fibroblast Culture
Mouse lung fibroblasts (MLFs) from PAR1 knockout (PAR1 KO) and corresponding wild-type MLFs were a kind gift from Professor Shaun Coughlin (University of California, San Francisco, CA) and have been described previously (Trejo et al., 1996). Cells were maintained in DMEM supplemented with penicillin (100 U/ml), glutamine (100 U/ml), streptomycin (100 U/ml), and 10% (vol/vol) FCS (DMEM, 10% FCS), in a humidified atmosphere containing 10% CO2. Cells were routinely passaged every 5–6 d. There were no noticeable effects on the parameters measured for cells used between passages 8 and 20. Cells were routinely tested and found negative for mycoplasma infection. For all experiments, cells were grown to confluence and 0.01% serum-starved for 24 h before stimulation.
Detection of CCL2 by ELISA
Cells were seeded in 96-well plates (Nunc, Naperville, IL). For each condition there were three biological replicates, and after the specified period, supernatants from each replicate were evaluated for CCL2 levels in duplicate by sandwich ELISA according to the manufacturer's instructions (BD Biosciences, Bath, United Kingdom). The lowest detection limit of the assay was 15.6 pg ml−1, and the standard curve was linear up to 1000 pg ml−1.
Western Blotting of Phosphorylated Kinases and Proteins
MLFs monolayers from six-well plates were washed twice with ice-cold phosphate-buffered saline (PBS), and cells were lysed by adding 100 μl Laemmli sample buffer directly to the monolayer, followed by scraping with a cell scraper. The cell lysate was passed through a 2-gauge needle several times to shear DNA and heated for 10 min at 85°C. Proteins from each lysate were separated by electrophoresis on a 10% or 12.5% SDS-polyacrylamide gel with a 7% stacking gel. Separated proteins were transferred onto Hybond-ECL nylon membranes (GE Healthcare, Waukesha, WI). The membranes were incubated with various primary antibodies: anti-phospho-p38 (1/1000), anti-p38 (1/2500), anti-phospho-ERK (1/2000), anti-ERK (1/2500), anti-phospho-Raf (1/2500), anti-Raf (1/2500), anti-phospho-MLC (1/500), and anti-MLC (1/1000) overnight at 4°C. A horseradish peroxidase–conjugated anti-rabbit IgG (DAKO, Cambridge, United Kingdom) was added at a 1:5000 dilution for 1 h at room temperature. Immunoreactive bands were visualized by standard enhanced chemiluminescence detection (Amersham Pharmacia Biotech, Piscataway, NJ).
Quantitative Real-Time RT-PCR Analysis of CCL2 mRNA Levels
Total RNA from cell cultures was isolated with TRIzol reagent per the manufacturer's protocol. RNA was DNase-treated using a DNAfree kit (Ambion, Europe, Ltd, Huntingdon, United Kingdom). Random hexamers were used as the primer for reverse transcription (RT) of 1 μg of total RNA in a reaction volume of 20 μl using the Applied Biosystems Geneamp RNA PCR core kit (Applied Biosystems, United Kingdom) following the manufacturer's instructions. Real-time RT-PCR was conducted using the Platinum SYBR Green qPCR SuperMix UDG (Invitrogen, Paisley, United Kingdom) on a LightCycler 1.5 Real-Time Detection System (Roche, Lewes, United Kingdom) and analyzed using LightCycler Real-time PCR Detection System (software version 3.5). Cycling conditions were as follows: one cycle of 50°C (2 min) and 95°C (2 min) and 45 cycles of 95°C (5 s), 55°C (5 s), and 72°C (15 s). The specificity of the PCR product was confirmed by melting curve analysis and gel electrophoresis. Relative quantitation was performed using the 2−ΔΔCp method, with 18S as the reference gene. The CCL2 primers were as follows: 5′-AGCTCTCTCTTCCTCCACCAC-3′ and 3′-CGTTAACTGCATCTGGCTGA-5′.
Retroviral Transduction with Mutant MEK1 Constructs
Ecotropic Phoenix packaging cells were transiently transfected with either wild-type MEK1, dominant negative MEK1, or constitutively active MEK1 using a calcium phosphate precipitation transfection protocol. Virus containing supernatants were harvested and used to infect target MLFs as previously described (Janes and Watt, 2004). Stably transduced MLFs were grown in the presence of puromycin (2.5 μg/ml; Calbiochem, Nottingham, United Kingdom).
Retroviral Transduction with G Protein Minigenes
Ecotropic Phoenix packaging cells were transiently transfected with either pRevTet-On vector or pRevTRE2 minigenes (pRevTRE2-EGFP and pRevTRE2-Gαq) according to the instructions from the manufacturer (CLONTECH, Palo Alto, CA), and virus-containing supernatants were used to infect and transducer target MLFs. Transduced Tet-On-MLFs were selected in medium containing 400 μg/ml G418. Tet-On-MLFs were subsequently infected with pRevTRE2-EGFP, pRevTRE2-Gαq, pRevTRE2-Gα12, pRevTRE2-Gα13, or pRevTRE2-Gαi minigenes containing viral supernatants. The Tet-On-MLFs transduced with EGFP or G protein minigenes were selected by culture in medium containing 400 μg/ml G418 and 500 μg/ml hygromycin. Expression of EGFP or minigenes was induced by incubation with 2 μg/ml doxycycline. Subsequent experiments were performed 48 h after doxycycline addition. Transduction efficiency was ∼90% as determined by assessing the expression obtained with the EGFP encoding RevTRE2-EGFP vector.
Immunocytofluoresence
MLFs were plated on eight-well chamber glass slides. After stimulation, cells were rinsed with PBS and then fixed for 10 min with 4% formaldehyde in PBS and permeabilized with 0.2% Triton X-100 at room temperature. To avoid nonspecific binding, the cells were incubated with 4% rabbit normal serum for 1 h at room temperature and then washed three times with PBS. Primary antibody (goat polyclonal anti-CCL2; R&D Systems, Europe, Ltd, Abingdon, United Kingdom) was incubated for 1 h at room temperature after three washes with PBS. Secondary antibody (FITC-conjugated rabbit anti-goat IgG) was incubated in the dark for 1 h at room temperature, and subsequently slides were washed three times with PBS. Immunofluoresence images were acquired by confocal microscopy using the Bio-Rad MRC 1024 confocal system (Hercules, CA), and the images were analyzed for the pixel intensity of the fluorescence using the Bio-Rad LaserPix image analysis software.
Statistical Analysis
Data were analyzed by two-tailed Student's t test for single and by one-way analysis of variance with the Newman-Keuls post hoc analysis for multiple group comparisons. Differences were considered significant at p < 0.05.
RESULTS
Thrombin Induces CCL2 Production and CCL2 mRNA Accumulation in Murine Lung Fibroblasts (MLFs)
To determine the effect of thrombin on MLF CCL2 production and release, MLFs were exposed to various concentrations of thrombin, and CCL2 protein levels in culture supernatants were assessed by ELISA. Figure 1, A and B, shows that thrombin stimulates CCL2 protein release in a time- and dose-dependent manner from 0.03 nM onward. CCL2 production continued to increase at all concentrations examined, and the effect did not plateau at the highest concentration of thrombin (300 nM) examined. Time-course experiments (Figure 1B) with thrombin at a physiologically relevant concentration (10 nM) showed that the sharpest increase in CCL2 release occurs over the first 12 h.
To determine whether thrombin influences CCL2 gene expression, the effect of thrombin on CCL2 mRNA levels was assessed by quantitative real-time RT-PCR. Figure 1C shows that thrombin increases CCL2 mRNA levels within 30 min, with a maximal increase (21 ± 3-fold relative to control) observed at 6 h (p < 0.01). Thrombin-induced CCL2 protein release is completely blocked by actinomycin D (ActD; Figure 1E) at concentrations at which this transcriptional inhibitor also blocked the increase in thrombin-induced mRNA levels (Figure 1D). Thrombin-induced CCL2 protein release is therefore not due to the release of prestored CCL2.
The Stimulatory Effects of Thrombin on Fibroblast CCL2 Gene Expression and Protein Production Are Mediated via PAR1 Coupling to Gαq
To begin to unravel the mechanisms by which thrombin exerts its stimulatory effects on CCL2 mRNA and protein levels, we first examined the potential involvement of the high-affinity thrombin receptor PAR1. Wild-type and PAR1 knockout (KO) MLFs were exposed to thrombin (10 nM) and the specific PAR1 agonist peptide TFLLR (200 μM) for 6 h (Figure 2A). Wild-type MLFs responded to thrombin and TFLLR, whereas PAR1 KO MLFs were completely unresponsive (Figure 2A). The inactive reverse peptide RLLFT had no effect on either wild-type or PAR1 KO MLFs. Experiments were also performed with TNF-α (10 ng/ml) as a positive control known to induce CCL2 independent of PAR signaling. The results show that both wild-type and PAR1 KO MLFs respond normally to TNF-α (Figure 2A). Taken together, these data show that thrombin exerts its effects on CCL2 protein production and release via PAR1 at this concentration of the proteinase.
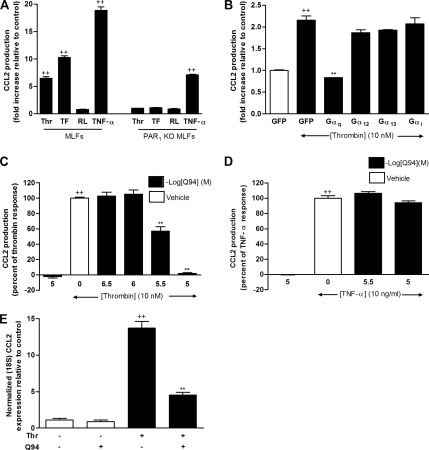
Figure 2.
PAR1 coupling to Gαq is necessary and sufficient for thrombin-induced CCL2 mRNA levels and protein release. (A) The effects of thrombin, PAR1 agonist TFLLR-NH2, the reverse peptide RLLFT-NH2, and TNF-α on CCL2 protein release in MLFs and PAR1 KO fibroblasts. Cells were exposed to thrombin (Thr, 10 nM), TFLLR-NH2 (TF, 200 μM), RLLFT- NH2 (RL, 200 μM), or TNF-α (10 ng/ml) for 6 h. CCL2 levels in culture supernatants were measured by ELISA. Data are presented as fold-increase relative to media control. (B) The effects of pRevTRE2-EGFP, pRevTRE2-Gαq, pRevTRE2-Gαi, pRevTRE2-Gα12, or pRevTRE2-Gα13 on thrombin-induced CCL2 protein release. MLFs transduced with the pRevTRE2-EGFP or the C-terminal Gα minigenes were exposed to thrombin (10 nM) for 6 h, and CCL2 levels in culture supernatant were measured by ELISA. Data are presented as fold change over control. (C and D) The effect of antagonist Q94 (targeting PAR1 coupling to Gαq) on thrombin- and TNF-α–induced CCL2 protein release. Data are presented as a percentage of the maximal response obtained with thrombin and drug vehicle alone (0.1% DMSO in DMEM). Cells were treated with increasing concentrations of Q94 for 3 h before exposure to thrombin for 6 h. Final concentrations of DMSO were kept constant for all treatment conditions. The first bar represents the highest dose of Q94 used and shows that this compound has no effect on basal CCL2 production. Negative log of the concentrations of Q94 are presented. (E) The effect of Q94 on thrombin-induced CCL2 mRNA levels. MLFs were exposed to thrombin for 2 h with or without preincubation with Q94 (10 μM) for 3 h. Data represent the mean ± SEM of triplicates. ++ p < 0.01, comparison with medium control; and ** p < 0.01, comparison with thrombin alone.
PAR1 exerts its pluripotent cellular effects via the ability to interact with multiple downstream G proteins, including Gαi/o, Gαq/11, and Gα12/13. To identify the G protein involved in mediating PAR1 activation–induced CCL2 release, we used minigene vectors that encode 11 unique carboxyl-terminal amino acid residues of the Gαq, Gαi, Gα12, and Gα13 subunits. These minigenes have previously been shown to effectively inhibit G protein signaling, including thrombin-mediated cellular effects (Ellis et al., 1999; Gilchrist et al., 2001; Vanhauwe et al., 2002). Retroviral plasmids encoding EGFP or the C-terminal Gα minigenes were transduced into MLFs in parallel culture. Transduction efficiency was monitored by visualization of EGFP expression. Approximately 90% of EGFP-transduced cells were EGFP positive (data not shown). Figure 2B shows that the Gαq C-terminal antagonist encoding minigene completely abolished thrombin-induced CCL2 release. In contrast, transduction with a control minigene encoding EGFP, or C-terminal Gαi, Gα12, or Gα13 had no effect on this response.
To further confirm the role of Gαq in this response, we examined the effect of a novel small molecule antagonist, Q94, which specifically targets PAR1 coupling to Gαq. Q94 has been shown to compete for binding at the carboxy terminus of activated PAR1 with a high-affinity peptide mimic of carboxy terminal Gαq, with an IC50 of 916 nM (see Supplementary Data Figure S1, left panel). Additionally, this compound has been shown to inhibit thrombin receptor activating peptide (TRAP) induced calcium transients in a concentration-dependent manner (see Supplementary Data Figure S1, right panel). Figure 2C shows that Q94 blocked PAR1-mediated CCL2 production in a dose-dependent manner with complete inhibition obtained at 10 μM. Experiments performed with TNF-α as the stimulus showed that Q94 had no effect on TNF-α–stimulated CCL2 production (Figure 2D), indicating that the antagonist was specific for PAR1 in blocking this response. We also examined the role of this antagonist on thrombin-induced CCL2 mRNA levels. Figure 2E shows that Q94 (10 μM) blocked thrombin-induced CCL2 mRNA levels by ∼70% (p < 0.01). Taken together, these data show that Gαq plays a central role in PAR1-induced CCL2 protein release and gene expression.
ERK1/2 Is Required for Thrombin-induced CCL2 Gene Expression and Protein Production
It has previously been reported that activation of Gαq by PAR1 leads to the activation of ERK1/2 in MLFs (Trejo et al., 1996). However, in terms of thrombin-induced CCL2 release by other cell types, the p38 MAPK pathway has been shown to play an important role (Brandes et al., 2001; Marin et al., 2001). Thus, we next performed experiments to determine the relative roles of the p38 MAPK and ERK1/2 pathways in PAR1-induced CCL2 expression. Figure 3A shows that inhibition of p38 MAPK with SB203580 had no effect on thrombin-induced CCL2 protein release at concentrations at which this compound was effective at blocking thrombin-induced p38 phosphorylation (Figure 3B). It is worth pointing out that in these experiments, the inactive control compound SB202474 at the highest dose used significantly inhibited thrombin-induced CCL2 release by ∼50% (data not shown), suggesting that at high concentrations these compounds may exert off-target effects. We therefore conclude that although thrombin activates p38 MAPK in MLFs, p38 is not involved in mediating PAR1-induced CCL2 release. In contrast, preincubation of MLFs with the MEK1/2 inhibitor U0126 blocked this response in a dose-dependent manner from 0.3 μM onward (Figure 3C). The IC50 of U0126 was determined to be 1.3 μM for this response. U0126 also inhibited thrombin-induced phosphorylation of the MEK1/2 substrate, ERK1/2, in a dose-dependent manner (Figure 3D). We next determined the role of ERK1/2 in thrombin-induced CCL2 mRNA levels. Figure 3E shows that U0126 (1 μM) blocked the effect of thrombin on CCL2 mRNA levels by ∼70% (p < 0.01).
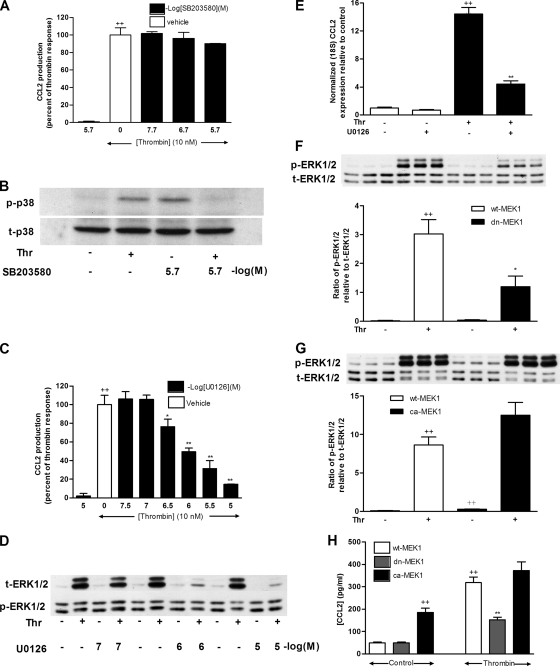
Figure 3.
ERK1/2 is necessary and sufficient for thrombin-induced CCL2 mRNA levels and protein release. (A) The effect of SB203580 on CCL2 protein release in response to thrombin. Data are presented as a percentage of the maximal response obtained with thrombin and drug vehicle alone (0.2% DMSO in DMEM). Cells were treated with increasing concentrations of SB203580 for 30 min before exposure to thrombin (10 nM) for 6 h. Final concentrations of DMSO were kept constant for all treatment conditions. The first bar represents the highest dose of SB203580 examined and shows that this compound has no effect on basal CCL2 production. Negative log of the concentration of SB203580 is presented. (B) The effect of SB203580 (2 μM) on thrombin-induced p38 phosphorylation. Cells were treated with or without SB203580 for 30 min before exposure to thrombin for 2 min. p38 phosphorylation was assessed by Western blotting of cell lysates using an anti-phospho-p38 antibody (B, p-p38). Protein loading was verified by blotting with an anti-p38 antibody (B, t-p38). The blot is representative of three separate experiments performed. (C) The effect of U0126 on CCL2 protein release in response to thrombin. Data were analyzed as in A. (D) Dose–response data for the effect of U0126 on thrombin-induced ERK1/2 phosphorylation. Cells were treated with increasing concentrations of U0126 for 30 min before exposure to thrombin for 2 min. ERK1/2 phosphorylation was assessed by Western blotting of cell lysates as in B. (E) The effect of U0126 on thrombin-induced CCL2 mRNA levels. MLFs were exposed to thrombin (10 nM) for 2 h with or without preincubation with U0126 (1 μM) for 30 min. CCL2 mRNA levels were determined by quantitative RT-PCR. (F and G) The effects of wt-MEK1, dn-MEK1, or ca-MEK1 on thrombin-induced ERK1/2 phosphorylation. MLFs transduced with MEK1-pBabePuro constructs expressing the wt-MEK1, dn-MEK1 (F) or ca-MEK1 (G) were exposed to thrombin (10 nM) for 2 min. ERK1/2 phosphorylation was assessed by Western blotting of cell lysates using an anti-phospho-ERK1/2 antibody (F and G, p-ERK1/2). Protein loading was verified by blotting with an anti-ERK1/2 antibody (F and G, t-ERK1/2). The blot is representative of three separate experiments performed. The column graphs represent the densitometry analysis of the blots as a result of phospho-ERK1/2 relative to total ERK1/2 and are representative of three separate Figure 3 (cont). experiments performed with three replicates per sample. (H) The effects of wt-MEK1, dn-MEK1, or ca-MEK1 on thrombin-induced CCL2 protein release. MLFs transduced with MEK1-pBabePuro constructs expressing the wt-MEK1, dn-MEK1, or ca-MEK1 were exposed to thrombin (10 nM) for 6 h, and subsequent CCL2 protein levels in culture supernatants were measured by ELISA. ++ p < 0.01, comparison with medium control; * p < 0.05 and ** p < 0.01, comparison with thrombin alone.
To further examine the role of the ERK1/2 pathway in thrombin-induced CCL2 production, a genetic approach was used. Wildtype-(wt-MEK1), dominant-negative (dn-MEK1), or constitutively active (ca-MEK1) constructs were transduced into MLFs (Cowley et al., 1994). Figure 3F shows that thrombin-induced ERK1/2 phosphorylation was blocked by up to 70% in cells transduced with dn-MEK1. Figure 3G shows that ca-MEK1 significantly increased basal ERK1/2 phosphorylation, but had no additive effect on thrombin-induced ERK1/2 phosphorylation. Transduction with dn-MEK1 also significantly reduced thrombin-induced CCL2 production, whereas transduction with ca-MEK1 significantly increased basal CCL2 production by about fourfold (p < 0.01), but had no additive effect on thrombin-stimulated CCL2 production (Figure 3H). These results provide strong evidence that the MEK1-ERK1/2 pathway rather than the p38 MAPK pathway is both necessary and sufficient for PAR1-mediated CCL2 production in MLFs.
We next examined the role of PKC and c-Raf in thrombin-induced ERK1/2 activation and CCL2 expression. Both broad spectrum PKC inhibitors Ro-318425 (Figure 4A) and the c-Raf inhibitor (Figure 4B) blocked thrombin-induced ERK1/2 phosphorylation in a dose-dependent manner from 1 μM onward for Ro-318425 and 0.3 μM onward for c-Raf inhibitor. To determine whether PKC is upstream of c-Raf, cells were preincubated with Ro-318425, and c-Raf phosphorylation was assessed by Western blotting. As shown in Figure 4C, Ro-318425 (1 μM) completely blocked c-Raf phosphorylation induced by thrombin.
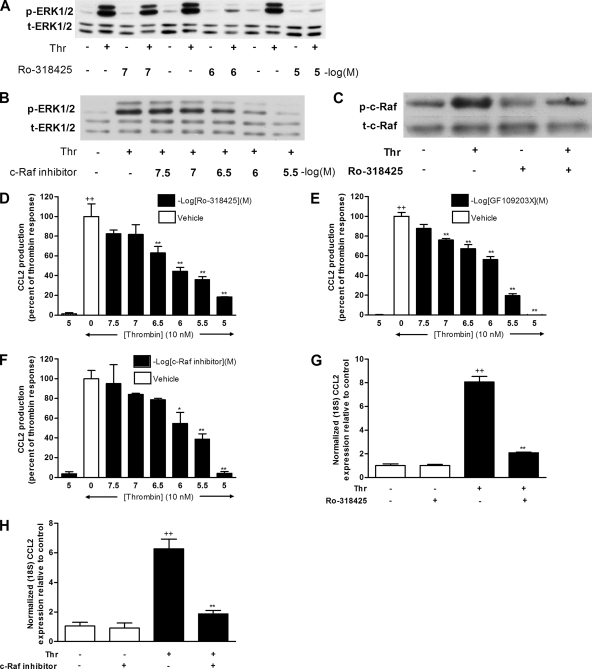
Figure 4.
Inhibition of c-Raf kinase and PKC attenuates thrombin-induced ERK1/2 phosphorylation, CCL2 protein production, and CCL2 mRNA levels. (A and B) Dose–response data for the effects of Ro-318425 (A) and c-Raf inhibitor (B) on thrombin-induced ERK1/2 phosphorylation. Cells were treated with increasing concentrations of inhibitors for 30 min before exposure to thrombin (10 nM) for 2 min. ERK1/2 phosphorylation was assessed by Western blotting of cell lysates using an anti-phospho-ERK1/2 antibody (A and B, p-ERK1/2). Protein loading control was verified by blotting with an anti-ERK1/2 antibody (A and B, t-ERK). The blots are representative of three separate experiments performed. (C) The effect of Ro-318425 on thrombin-induced c-Raf phosphorylation. Cells were treated with Ro-318425 (1 μM) for 30 min before stimulation with thrombin (10 nM) for 10 min. c-Raf phosphorylation was assessed by Western blotting of cell lysates using an anti-phospho-c-Raf antibody (C, p-c-Raf). Protein loading was verified by blotting with an anti-c-Raf antibody (C, p-c-Raf). The blot is representative of three separate experiments performed. (D–F) The effects of Ro-318425 (D), GF109203X (E), and c-Raf inhibitor (F) on CCL2 protein production in response to thrombin. Data are presented as a percentage of the maximal response obtained with thrombin (10 nM) and drug vehicle alone (0.1% DMSO in DMEM). Cells were treated with increasing concentrations of Ro-318425, GF109203X, or c-Raf kinase inhibitor for 30 min before exposure to thrombin for 6 h. Final concentrations of DMSO were kept constant for all treatment conditions. The first bar in each graph represents the highest doses of inhibitors examined and shows that these compounds have no effect on basal CCL2 production. Negative log of the concentrations of inhibitors are presented. (G and H) The effects of Ro-318425 (G) and c-Raf inhibitor (H) on thrombin-induced CCL2 mRNA levels. MLFs were exposed to thrombin (10 nM) for 2 h with or without preincubation with c-Raf inhibitor (3 μM) or Ro-318425 (1 μM) for 30 min. CCL2 mRNA levels were determined by quantitative RT-PCR. Final concentrations of DMSO were kept constant for all treatment conditions (0.01% DMSO in DMEM). Data represent the mean ± SEM from triplicates. ++ p < 0.01, comparison with medium control; ** p < 0.01, comparison with thrombin alone.
We next determined whether PKC and c-Raf are involved in thrombin-induced CCL2 production and gene expression. Figure 4, D–F, shows that both PKC broad spectrum inhibitors Ro-318425 and GF109203X, and the c-Raf inhibitor inhibited thrombin-induced CCL2 release in a dose-dependent manner. At concentrations of Ro-318425 and c-Raf inhibitor that blocked thrombin-induced ERK1/2 phosphorylation, thrombin-induced CCL2 production was blocked by 56 ± 4% (p < 0.01) with Ro-318425 (1 μM) and by 61 ± 5% (p < 0.01) with the c-Raf inhibitor (3 μM). At these concentrations, these inhibitors inhibited thrombin-induced CCL2 mRNA accumulation by 75 and 81%, respectively (Figure 4, F and G). Taken together, these data show that PKC, c-Raf, and ERK1/2 are in a linear pathway for thrombin-induced CCL2 gene expression and protein production.
The Rho Kinase Pathway Mediates Thrombin-induced CCL2 Protein Release via a Nontranscriptional Mechanism
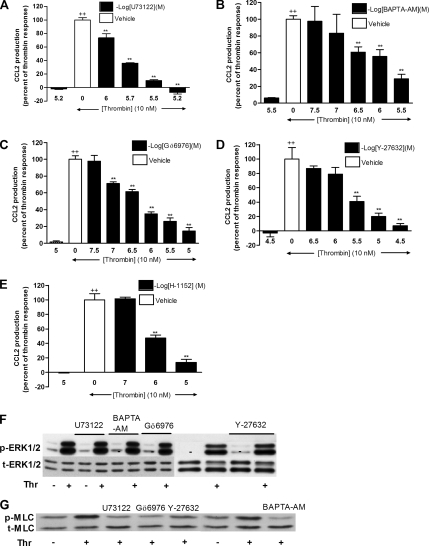
The data obtained so far point to a central role for Gαq and ERK1/2 in mediating the effects of PAR1 activation on CCL2 production. Although, Gα12/13 is generally considered as a major activator of Rho kinase, there is emerging evidence that Gαq is also able to signal via Rho kinase by activating the PLC-Ca2+ pathway (Singh et al., 2007). Moreover, thrombin is known to mediate Ca2+ signaling via PAR1 in fibroblasts, and this can be inhibited by BAPTA (Trejo et al., 1996; Tanaka et al., 2004). We therefore next examined the role of the PLC-Ca2+ pathway and Rho kinase in PAR1-mediated CCL2 release. Cells were treated with increasing concentrations of U73122 (PLC inhibitor, Figure 5A), BAPTA-AM (Ca2+ chelator, Figure 5B), Gö6976 (Ca2+-dependent PKC inhibitor, Figure 5C), or Y-27632 and H-1152 (Rho kinase inhibitors, Figure 5, D and E). The data obtained show that all these inhibitors blocked PAR1-mediated CCL2 production in a dose-dependent manner. The IC50 of each inhibitor was determined to be 1.5 μM for U73122, 1 μM for BAPTA-AM, 0.5 μM for Gö6976, 3 μM for Y-27632, and 2.7 μM for H-1152, for this response. In contrast, the inactive control compound for U73122, U73343, was found to have no effect on PAR1-mediated CCL2 production at the highest concentrations used (data not shown).
Figure 5.
Inhibition of PLC (U73122), Ca2+ (BAPTA-AM), Ca2+-dependent PKC (Gö6976), and Rho kinase (Y-27632 and H-1152) attenuate thrombin-induced CCL2 production in an ERK1/2-independent mechanism. (A–E) The effects of U73122 (A), BAPTA-AM (B), Gö6976 (C), Y-27632 (D), and H-1152 (E) on thrombin-induced CCL2 protein release. Cells were preincubated with increasing concentrations of each inhibitor for 30 min before exposure to thrombin (10 nM) for 6 h. Data are presented as a percentage of the maximal response obtained with thrombin and drug vehicle alone (0.1% DMSO in DMEM). Final concentrations of DMSO were kept constant for all treatment conditions. The first bar in each graph represents the highest dose of each inhibitor examined and shows that these compounds have no significant effect on basal CCL2 production. Negative log of the concentrations of each inhibitor are presented. (F) The effect of U73122, BAPTA-AM, Gö6976, and Y-27632 on thrombin-induced ERK1/2 phosphorylation. MLFs were treated with U73122 (2 μM), BAPTA-AM (2 μM), Gö6976 (1 μM), or Y-27632 (3 μM) for 30 min before exposure to thrombin (10 nM) for 2 min. ERK1/2 phosphorylation was assessed by Western blotting of cell lysates using an anti-phospho-ERK1/2 antibody (F, p-ERK1/2). Protein loading was verified by blotting with an anti-ERK1/2 antibody (F, t-ERK1/2). The blot is representative of three separate experiments performed. (G) The effect of U73122, BAPTA-AM, Gö6976, and Y-27632 on thrombin-induced MLC phosphorylation. MLFs were treated with U73122 (2 μM), BAPTA- AM (2 μM), Gö6976 (1 μM), or Y-27632 (3 μM) for 30 min before stimulation with thrombin (10 nM) for 10 min. MLC phosphorylation was assessed by Western blotting of cell lysates using an anti-phospho-MLC antibody (G, p-MLC). Protein loading was verified by blotting with an anti-MLC antibody (G, t-MLC). The blot is representative of three separate experiments performed. Data represent the mean ± SEM from triplicates. ++ p < 0.01, comparison with medium control; ** p < 0.01, comparison with thrombin alone.
To determine whether these kinases are upstream of the ERK1/2 pathway, we examined the effects of these inhibitors at their respective IC50 concentrations on thrombin-induced ERK1/2 phosphorylation. Figure 5F shows that none of the four inhibitors interfered with thrombin-induced ERK1/2 phosphorylation, indicating that PLC, Ca2+, Ca2+-dependent PKC, and Rho kinase mediate their effects on thrombin-induced CCL2 protein release in an ERK1/2-independent manner.
To determine whether PLC, Ca2+, and Ca2+-dependent PKC signal via Rho kinase, we examined the effects of the above inhibitors on the phosphorylation of myosin light chain (MLC), a downstream substrate of Rho kinase (Amano et al., 1996). Figure 5G shows that PAR1 activation induces MLC phosphorylation within 10 min of stimulation and that this response is blocked by U73122, BAPTA-AM, Gö679, and Y-27632. These data indicate that PLC, Ca2+, and Ca2+-dependent PKC signals to Rho kinase rather than ERK1/2 to mediate the effects of thrombin on CCL2 release.
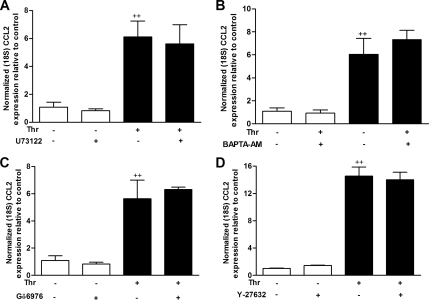
We also examined the effect of PLC-Rho kinase pathway on thrombin-induced CCL2 mRNA levels. As shown in Figure 6, A–D, none of the four inhibitors blocked PAR1-stimulated CCL2 mRNA levels. Taken together the data obtained suggest that the PLC-Rho kinase pathway influences CCL2 release via a posttranscriptional mechanism.
Figure 6.
Inhibition of PLC, Ca2+, Ca2+-dependent PKC or Rho kinase does not interfere with thrombin-induced CCL2 mRNA levels. (A–D) The effects of U73122 (A), BAPTA-AM (B), Gö6976 (C), and Y-27632 (D) on thrombin-induced CCL2 mRNA levels. MLFs were exposed to thrombin (10 nM) for 2 h with or without preincubation with U73122 (2 μM), BAPTA-AM (2 μM), Gö6976 (1 μM), or Y-27632 (3 μM) for 30 min. CCL2 mRNA levels were determined by quantitative RT-PCR. Final concentrations of DMSO were kept constant for all treatment conditions (0.01% DMSO in DMEM). Data represent mean ± SEM from triplicates at each condition. ++ p < 0.01, comparison with untreated cells.
Inhibition of Rho Kinase by Y-27632 Has No Effect on Intracellular Protein Production
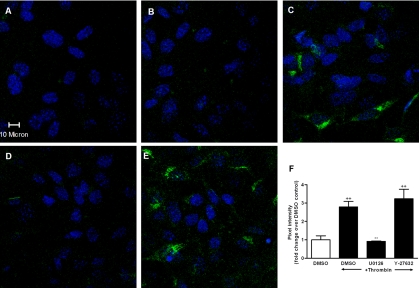
The results obtained thus far demonstrate an essential role for ERK1/2 in PAR1 activation-induced CCL2 production by influencing CCL2 mRNA levels, whereas the Rho kinase pathway acts via a posttranscriptional mechanism. To begin to elucidate the mechanism by which the Rho kinase pathway influences this response, we used immunocytofluorescence to examine thrombin-induced intracellular CCL2 protein production. The isotype-matched control antibody panels are completely negative, indicating that the signal obtained with the CCL2 antibody was specific (Figure 7A). Unstimulated cells also yielded no immunostaining or a very weak signal for CCL2 (Figure 7B). In contrast, up to 60% cells were positive for intracellular CCL2 staining for both thrombin alone and for Y-27632 pretreated cultures, whereas only 5–10% of cells were positive for thrombin-treated cells in the presence of U0126. The pixel intensity of each treatment group was also analyzed (Figure 7F). The mean pixel intensity of three randomly chosen fields of view was calculated for each group, and the data are presented as fold change over control. Thrombin induced a 2.8 ± 0.1-fold increase in fluorescent signal over control, and this was significantly inhibited by U0126. In contrast, pretreatment of cells with Y-27632 did not block thrombin-induced intracellular CCL2 accumulation (3.2 ± 0.5-fold increase over control). We therefore conclude that blockade of ERK1/2 signaling results in near total blockade of thrombin-induced CCL2 production. In contrast, blockade of Rho kinase signaling is not essential for CCL2 protein production but likely affects the subsequent release of CCL2 from the cell, because thrombin-induced CCL2 levels in cell culture supernatants are inhibited by Y-27632 (Figure 5D).
Figure 7.
Immunocytofluorescence demonstrates that CCL2 intracellular protein production is blocked by MEK1/2 inhibition (U0126) but not by Rho kinase inhibition (Y-27632). (A) Isotype control; (B) untreated cells; (C) the effect of thrombin on CCL2 intracellular protein production; (D) the effect of U0126 on CCL2 intracellular protein production induced by thrombin; (E) the effect of Y-27632 on CCL2 intracellular protein production induced by thrombin. Cells were preincubated with or without U0126 (10 μM) or Y-27632 (10 μM) for 30 min before stimulation with thrombin (10 nM) for 3 h. At the end of incubation, cells were immunostained with normal goat IgG (A) or anti-CCL2 antibody (B–E) followed by DAPI staining. Areas of CCL2 localization are shown in green, and nuclei appear blue. (F) The mean pixel intensity of three randomly chosen fields of view, expressed as fold increase over DMSO control, for each group. ++ p < 0.01, comparison with untreated cells. ** p < 0.01, comparison with thrombin alone.
The ERK1/2 and Rho Kinase Pathways Are Both Downstream of PAR1 Coupling to Gαq
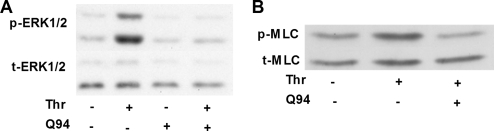
To determine whether ERK1/2 and Rho kinase pathways are both downstream of PAR1-coupling to Gαq, we evaluated the effect of the Gαq selective PAR1 antagonist Q94 on thrombin-induced ERK1/2 and MLC phosphorylation. Figure 8 shows that Q94 completely blocked thrombin-induced ERK1/2 (Figure 8A) and MLC phosphorylation (Figure 8B). These data confirm that ERK1/2 and Rho kinase pathways are downstream of PAR1-coupling to Gαq and act in cooperation to mediate the effects of PAR1 activation on CCL2 gene expression, protein production and release.
Figure 8.
ERK1/2 and MLC pathways are downstream of PAR1 coupling to Gαq. (A and B) The effect of Q94 on thrombin-induced ERK1/2 (A) and MLC (B) phosphorylation. Cells were treated with Q94 (10 μM) for 3 h before exposure to thrombin (2 min for ERK1/2, 10 min for MLC). Phosphorylation of ERK1/2 or MLC was assessed by Western blotting of cell lysates using an anti-phospho-ERK1/2 antibody (A, p-ERK1/2) or an anti-phospho-MLC antibody (B, p-MLC). Protein loading was verified by blotting with an anti-ERK1/2 antibody (A, t-ERK1/2) or an anti-MLC antibody (B, t-MLC). The blots are representative of three separate experiments.
DISCUSSION
The activation of the coagulation cascade leading to the generation of thrombin is one of the earliest events after tissue injury. PAR1, the main high-affinity thrombin signaling receptor, contributes to excessive inflammation and tissue remodeling in a number of disease settings, including atherosclerosis (Major et al., 2003), vascular neointima formation (Cheung et al., 1999), glomerulonephritis (Cunningham et al., 2000), liver fibrosis (Fiorucci et al., 2004), inflammatory bowel disease (Vergnolle et al., 2004), acute lung injury (Jenkins et al., 2006), and fibrotic lung disease (Howell et al., 2005). In this setting, we have recently shown that the dramatic attenuation of the acute inflammatory and late fibrotic response in PAR1-deficient mice after bleomycin-induced lung injury is associated with a marked reduction in pulmonary levels of CCL2 (Howell et al., 2005). However, the signal transduction pathways involved in the induction of CCL2 after PAR1 activation are currently poorly understood. This study sheds important light on both the G protein pathway and subsequent signaling cascades involved and identifies a central role for Gαq, ERK1/2, and Rho signaling pathways in this response.
Thrombin Stimulates CCL2 Protein Production via CCL2 Gene Transcription
In this report, we show that thrombin induces CCL2 protein production in a dose-dependent manner from 0.03 nM onward. Strikingly, this response did not reach a plateau at 300 nM, the highest concentration examined. In normal human plasma, the zymogen prothrombin is present at concentrations around 1.4 μM, but concentrations of around 130 nM have been calculated to represent the maximum concentration of active thrombin generated during blood clotting in vivo (Walz et al., 1985). We further show that the stimulatory effects of thrombin at the protein level were accompanied by a rapid and ActD-sensitive increase in CCL2 mRNA levels, indicating that the observed increase in CCL2 release is not due to the release of prestored CCL2. These data are consistent with previous reports of thrombin-induced CCL2 production in endothelial cells and monocytes (Colotta et al., 1994).
Thrombin Exerts Its Stimulatory Effects on CCL2 Expression via PAR1 Coupling to Gαq
Thrombin exerts most of its cellular effects via activation of at least three PARs (PAR1, PAR3, and PAR4) by limited proteolytic cleavage of the N-terminus. We and others have shown that PAR1 is the major receptor involved in mediating the mitogenic and profibrotic effects of thrombin in fibroblasts (reviewed in Chambers, 2003). In the present study, the involvement of PAR1 in mediating CCL2 production was demonstrated in experiments employing the highly selective PAR1 peptide agonist, TFLLR-NH2. This agonist activates PAR1 independently of receptor cleavage, and unlike the commonly used peptide agonists, based on the tethered ligand sequence of PAR1 (SFLLRN), does not activate PAR2 in human mesenchymal cells (Hollenberg et al., 1997). The necessity for PAR1 in mediating the effects of thrombin at 10 nM was confirmed in experiments demonstrating that CCL2 production was not up-regulated in fibroblasts derived from PAR1 KO mice.
Although many of the resultant biological consequences of PAR1 activation in fibroblasts are known, less is known about which G proteins mediate these events. The C-terminal region of Gα subunits has been shown to be critical in determining the specificity of GPCR–G protein interactions (Hamm and Gilchrist, 1996; Hamm, 1998). This interaction is highly specific. For example, substituting a single amino acid has been shown to annul the ability of Gαi to bind the A1 adenosine receptor (Gilchrist et al., 1998). Dominant-negative constructs of the α subunit of G proteins (termed G protein minigenes), encoding the C-terminal sequence for each family of Gα subunits, have been developed as powerful tools for identifying the G protein that mediates a given physiological function after thrombin activation (Gilchrist et al., 2001). In experiments employing such a Gα subunit minigene approach, we were able to show that only Gαq is necessary for PAR1-mediated CCL2 protein release, and that Gα12, Gα13, and Gαi play no role. The critical involvement of Gαq was further confirmed with a recently developed novel PAR1 selective Gαq protein signaling antagonist (Q94, Caden Biosciences), which blocked PAR1-mediated increases in both CCL2 mRNA and protein levels in a dose-dependent manner. It is worth mentioning that in these experiments, the CCL2 response obtained was usually greater for TFLLR-NH2 over thrombin. This observation might be explained by recent evidence that there are differences between thrombin and peptide agonists in terms of the subsequent ability of PAR1 to activate different G proteins (McLaughlin et al., 2005). PAR1 activation studies in endothelial cells showed that peptide activation altered receptor/G protein binding to favor Gαq activation over Gα12/13. It is tempting to speculate that such functional selectivity may also occur in fibroblasts and may similarly explain the finding that TFLLR-NH2 induces a greater CCL2 response compared with the physiological activator thrombin in our experiments.
PAR1 Activation Induces CCL2 Release via a Ca2+-dependent PKC/ERK1/2 Pathway and Increased CCL2 Gene Expression
Gαq has been shown to be necessary for mediating thrombin-induced ERK1/2 phosphorylation in MLFs (Trejo et al., 1996). We therefore examined the possibility that the ERK1/2 pathway may be downstream of PAR1 coupling to Gαq and therefore may be involved in thrombin-induced CCL2 gene expression and protein production. Using either the pharmacological inhibitor, U0126, or dn-MEK1 gene constructs, we show that the ERK1/2 pathway is necessary for CCL2 protein production and release mediated by PAR1 coupling to Gαq. Gαq was further found to be necessary for mediating thrombin-induced ERK1/2 phosphorylation because the PAR1 selective Gαq antagonist Q94 blocked this response.
In this study, we exclude a role for the p38 MAPK pathway since although thrombin was found to activate the p38 MAPK pathway in our cells, the p38 MAPK inhibitor, SB 203580, had no effect on thrombin-induced CCL2 protein production This latter finding is not in agreement with previous reports demonstrating a role for p38 MAPK in thrombin-induced CCL2 production using comparable doses of SB203580 in other cell types, including human endothelial cells (Marin et al., 2001) and human smooth muscle cells (Brandes et al., 2001). The discrepancy between our findings in MLFs and others may have several explanations, including important differences in both the species and the cell type examined, as well as differences in the concentrations of thrombin used in our (10 nM is equivalent to 0.5 U/ml) and other studies (8 U/ml; Marin et al., 2001). Moreover, from our experience with PAR1 KO fibroblasts, we are aware that thrombin can induce CCL2 release at higher concentrations via a non-PAR mediated mechanism because only PAR1 and PAR4 are expressed, and PAR4 agonist peptides fail to induce CCL2 release in PAR1 KO fibroblasts (data not shown). As thrombin is a proteinase, we propose it is likely that at higher concentrations, CCL2 protein production may be mediated via the release of other stimulatory mediators bound to the pericellular matrix. In this regard, thrombin has previously been shown to release TGF-β bound to the pericellular matrix of fibroblasts (Taipale et al., 1992). It is therefore possible that non-PAR1–dependent pathways lead to activation of the p38 MAPK pathway to mediate thrombin-induced CCL2 release at high concentrations of the proteinase. Although concentrations around 130 nM may be generated after intravascular coagulation and are therefore relevant in the context of thrombin signaling in vascular cell types, thrombin concentrations in extravascular compartments and hence in the context of fibroblast signaling responses are likely to be much lower.
The downstream signaling molecules that mediate the actions of G proteins on ERK1/2 include ras, PKC, and c-Raf kinase (Blumer and Johnson, 1994), which can activate MEK (Cobb et al., 1994). Indeed, our data show that both c-Raf and PKC are involved in PAR1-mediated ERK1/2 phosphorylation as previously reported (Trejo et al., 1996). We also examined which class of PKC isozymes are involved in this response. PKC isozymes can be generally divided into two groups: Ca2+-dependent (conventional PKC) and Ca2+-independent (novel and atypical isozymes). Experiments using the specific Ca2+-dependent PKC inhibitor, Gö6976, showed that Ca2+-dependent PKC is not involved in the activation of ERK1/2 after PAR1 activation. Our data further show that the Ca2+-independent PKC-ERK1/2 pathway mediates CCL2 protein production by influencing CCL2 gene transcription because inhibition of ERK1/2, c-Raf, and PKC using the broad-spectrum inhibitor, Ro-318425, abolished PAR1-induced CCL2 mRNA accumulation and protein production, whereas inhibition of Ca2+-dependent PKC by Gö6976 had no effect on PAR1-induced CCL2 mRNA accumulation but did inhibit CCL2 release into cell culture supernatants.
The Ca2+-dependent PKC Rho Pathway Mediates the Effects of PAR1 Activation on CCL2 Release via a Posttranscriptional Mechanism
Ca2+-dependent PKC is a well-recognized downstream effector of Gαq and is activated in response to a rise in intracellular Ca2+ concentration. Although the α-subunit of Gαq is well-known to trigger an increase in intracellular Ca2+ concentration by stimulating PLC-β activity and Gα12/13 activation preferentially induces Rho kinase activation, a recent study by Singh et al. (2007) demonstrated that Gαq can lead to Rho kinase activation via PLC-β-Ca2+–dependent PKC to mediate thrombin-induced endothelial cell contraction. In the present study, our data similarly suggest that Rho kinase activation is mediated by a Gαq-PLC-Ca2+–dependent PKC pathway to mediate thrombin-induced CCL2 release. First, we show that inhibitors of PLC, Ca2+, and Ca2+-dependent PKC all block thrombin-induced phosphorylation of MLC, one of the major downstream substrates of Rho kinase (Amano et al., 1996). Second, a direct link between this pathway and Gαq coupling to PAR1 was demonstrated by showing that the novel PAR1 selective Gαq antagonist (Q94) also abolishes thrombin-induced MLC phosphorylation.
Our findings and those reported by Singh et al. (2007) contrast with a previous report demonstrating that Gαq-mediated Rho kinase activation occurs independently of PLCβ in mouse embryonic fibroblasts (MEFs; Vogt et al., 2003). The activation of Rho A requires its dissociation from the Rho kinase/GDP/GDI-1 complex followed by GTP exchange mediated by guanine nucleotide exchange factors (GEFs). In terms of Rho kinase activation by GPCRs, the RhoGEF (Rho guanine nucleotide exchange factor) proteins, p115RhoGEF, PDZ-RhoGEF, and leukemia-associated Rho guanine-nucleotide exchange factor (LARG), have been shown to stimulate Rho kinase activity (Hart et al., 1998; Kozasa et al., 1998; Fukuhara et al., 2001; Suzuki et al., 2003). Singh and colleagues provided evidence that Gαq-mediated Rho kinase activation is p115RhoGEF dependent after PLC-Ca2+–dependent PKC activation, whereas in studies by Vogt and colleagues in MEFs, thrombin-induced Rho kinase activation was shown to be mediated by the RhoGEF, LARG. Taken together, our findings are consistent with a PLC-Ca2+–dependent PKC-dependent Rho kinase activation mechanism rather than a LARG-mediated mechanism.
Although Rho kinase was first characterized as a major regulator of actin dynamics, it is now well recognized that Rho family proteins influence a range of other cellular processes, including gene transcription and protein secretion (reviewed in Etienne-Manneville and Hall, 2002). In the present study, we provide evidence that the Rho kinase pathway is involved in PAR1-mediated CCL2 protein release posttranscriptionally, likely by influencing protein secretion. This is based on two observations. First, inhibition of Rho kinase only blocks thrombin-induced CCL2 protein release but not CCL2 mRNA levels. Second, immunocytofluorescence experiments to elucidate the mechanism by which the Rho kinase pathway influences CCL2 release, demonstrate that intracellular protein production is blocked with the MEK1/2 inhibitor, U0126, but is unaffected by the Rho kinase inhibitor, Y-27632. Taken together, these data indicate that blockade of Rho kinase signaling is not essential for PAR1-mediated CCL2 gene expression and protein production. Given that Y-27632 blocks PAR1-mediated increases in CCL2 levels measured in cell culture supernatants, we propose that this pathway most likely affects CCL2 protein secretion.
Specificity of Pharmacological Inhibitors Used
In this study we used a range of small cell-permeable inhibitors of protein kinases to dissect the signaling pathways involved in PAR1-mediated CCL2 release. These inhibitors exhibit a relatively high degree of specificity for the target kinase, but these compounds are not always totally specific and need to be used with caution. In this study, all experiments involving such inhibitors were designed according to expert recommendations (Davies et al., 2000; Bain et al., 2003). First, the role of most of the targeting kinases was verified by more than one structurally unrelated inhibitor. For instance, the involvement of ERK1/2 was confirmed by both U0126 and PD98059 (data no shown). Similarly for PKC, we used the broad-spectrum PKC inhibitors, Ro- 318425 and GF109203X. For Rho kinase, we used two compounds: Y-27632 and H-1152. Second, wherever available, inactive control compounds (e.g., SB202474 as a negative control for SB203580 and U73343 as a negative control for U73122) for each inhibitor were tested in parallel cultures. This was particularly important when high concentrations of the active compounds were used. Third, genetic approaches were used to strengthen the results obtained with MEK1/2 inhibition and to identify the G protein involved in coupling to PAR1. Finally, we demonstrated that most inhibitors blocked CCL2 release at the same concentrations that prevented the phosphorylation of an authentic physiological substrate of the protein kinase. For example, ERK1/2, p38 MAPK, and MLC phosphorylation was assessed to identify the lowest concentration of respective inhibitors required for subsequent CCL2 release experiments.
Integration of the Flow of Information
In this study, we provide evidence that thrombin-induced CCL2 release is mediated via coupling of PAR1 to Gαq and the cooperation between ERK1/2 and Rho kinase signaling pathways. This raises the question as to how the flow of information to the two pathways, one directed at the nucleus and the other at a specific secretory apparatus, is regulated? Signal transduction leading to cellular responses is a complex processes initiated by protein–protein interactions between ligands, receptors, and kinases. Recent hypotheses including the formation of lipid rafts and “transduceosomes” have shed light on how these multiple components work in harmony. Lipid rafts are specialized structures on the plasma membrane that have an altered lipid composition as well as links to the cytoskeleton. Recent studies indicate that some GPCRs, G proteins, and their effectors localize to lipid rafts or dynamically move in and out of microdomains (Simons and Toomre, 2000). A recent study has shown that PAR1 is present in endothelial cell plasma membrane rafts and caveolae and that the localization of PAR1 specifically to rafts serves as an important mechanism for the regulation of thrombin-induced cytoskeletal changes in endothelial cells. Of particular interest to the current work, these authors further demonstrate a role for lipid rafts in mediating PAR1 activation of both RhoA/Rho kinase signaling and MLC phosphorylation (Carlile-Klusacek and Rizzo, 2007). Because PAR1 coupling to Gαq mediates thrombin-induced CCL2 secretion via the RhoA/Rho kinase pathway, we propose that it is likely that the components for secretion in this pathway might integrate on lipid rafts. In contrast, the role of lipid rafts in MAPK signaling remains controversial. For example, Gαq/11 has been shown to activate p38 MAPK via lipid rafts, whereas two studies have shown that Gαq does not mediate ERK1/2 phosphorylation via such rafts (Hiol et al., 2003; Sugawara et al., 2007). In this study, we report that PAR1 coupling to Gαq mediates thrombin-induced CCL2 production via ERK1/2 rather than p38 signaling so that current evidence would lead us to propose that the components involved in the transcriptional response may not float together on lipid rafts and would therefore be segregated from the components involved in CCL2 secretion. It is also possible that these pathways involve the assembly of a transduceosome that would act as a relay to assemble and integrate the signals derived from the two pathways in order to optimize the amplitude of downstream signaling. Transduceosomes are most well described in terms of the essential role of the scaffold and anchoring protein, A-kinase anchor proteins (AKAP) in cAMP signaling (Feliciello et al., 2001). cAMP-dependent protein kinase is targeted to discrete subcellular locations by the AKAPs. Localization recruits protein kinase A (PKA) holoenzyme close to its substrate/effector proteins, directing and amplifying the biological effects of cAMP signaling. Although AKAPs were identified on the basis of their interaction with PKA, AKAPs bind other signaling molecules, principally phosphatases and kinases, including notably PKC (Feliciello et al., 2001). It is therefore tempting to speculate and propose a role for both lipid rafts and transduceosomes to integrate and assemble the signaling components leading from PAR1 activation of Gαq to CCL2 gene transcription via the ERK1/2 pathway and CCL2 release via the RhoA/RhoA pathway.
Summary and Therapeutic Implications
We provide compelling evidence that thrombin mediates its potent stimulatory effects on fibroblast CCL2 production and release by coupling to Gαq and the cooperation between ERK1/2 and Rho kinase signaling pathways (Figure 9). Inhibition of the Ca2+-independent PKC-ERK1/2 pathway not only inhibits CCL2 protein levels, but also inhibits intracellular CCL2 protein production and CCL2 mRNA levels, suggesting that this pathway mediates thrombin-induced CCL2 release by influencing CCL2 gene transcription. Inhibition of the PLC-Rho kinase pathway inhibits CCL2 protein release, but has no effect on thrombin-induced intracellular CCL2 protein production and CCL2 mRNA levels. The RhoA/Rho kinases are well known for their regulatory role in stress fiber formation and secretory vesicle trafficking, suggesting that this pathway mediates thrombin-induced CCL2 secretion. Blockade of PAR1 coupling to Gαq was found to inhibit both ERK1/2 and MLC phosphorylation induced by thrombin, indicating that these two pathways both lie downstream of PAR1 coupling to Gαq.
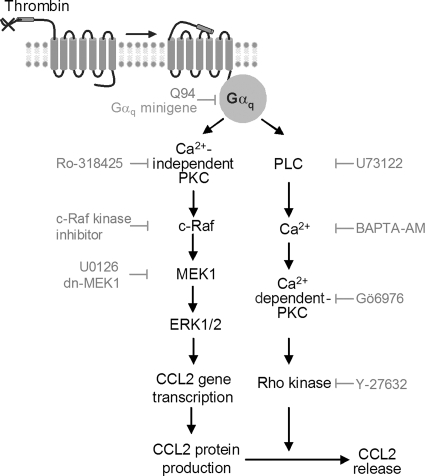
Figure 9.
Model for thrombin-induced CCL2 production and release. Thrombin ligation of PAR1 stimulates Gαq. Activation of Ca2+-independent PKC leads to the sequential activation of c-Raf, MEK1 and ERK1/2 to stimulate CCL2 gene transcription. Gαq also leads to the activation of PLC, Ca2+, and Ca2+-dependent PKC to activate Rho kinase (likely via p115RhoGEF, Singh et al., 2007) to mediate CCL2 protein release.
To the best of our knowledge, this report represents the first demonstration of the cooperation between these two pathways in mediating the stimulatory effects of thrombin, or indeed any other extracellular stimulus, on the induction and release of the potent chemoattractant, CCL2. In this report, we further demonstrate the effectiveness of blocking this response using the recently developed novel PAR1 antagonist, Q94, which blocks PAR1 at the intracellular interaction site with Gαq (Caden Biosciences) and therefore allows more selective targeting of some, but not all, PAR1-mediated cellular responses. We propose that such an antagonist approach may hold promise for selectively interfering with deleterious thrombin signaling in the context of tissue inflammation and fibroproliferative disease, while preserving other essential PAR1-mediatedcellular responses.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the following for kindly providing essential research reagents: Professor Shaun Coughlin (University of California, San Francisco, CA) for WT and PAR1 KO murine lung fibroblasts; Professor Chris Marshall (Cancer Research UK) for MEK1 mutant constructs, and Dr. James M. Staddon (Eisai London Research Laboratories) for the anti-phospho MLC antibody. The authors gratefully acknowledge grant support from the British Lung Foundation (Project Grant P03/8) and the Rosetrees Trust for P.F.M. and from the Wellcome Trust (Programme Grant GR071124MA) for C.J.S. X.D. was supported by a Dorothy Hodgkin Postgraduate Award from the Medical Research Council UK and Hutchinson Whampoa.
Abbreviations used:
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- ca-MEK1
constitutively active MEK1
- dn-MEK1
dominant negative-MEK1
- EGFP
enhanced green fluorescent protein
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- KO
knockout
- MLF
mouse lung fibroblast
- PAR
proteinase-activated receptor
- wt-MEK1
wild-type MEK1.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0720) on March 19, 2008.
REFERENCES
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Bachli E. B., Pech C. M., Johnson K. M., Johnson D. J., Tuddenham E. G., McVey J. H. Factor Xa and thrombin, but not factor VIIa, elicit specific cellular responses in dermal fibroblasts. J. Thromb. Haemost. 2003;1:1935–1944. doi: 10.1046/j.1538-7836.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- Bain J., McLauchlan H., Elliott M., Cohen P. The specificities of protein kinase inhibitors: an update. Biochem. J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer K. J., Johnson G. L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem. Sci. 1994;19:236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Brandes R. P., Viedt C., Nguyen K., Beer S., Kreuzer J., Busse R., Gorlach A. Thrombin-induced MCP-1 expression involves activation of the p22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb. Haemost. 2001;85:1104–1110. [PubMed] [Google Scholar]
- Carlile-Klusacek M., Rizzo V. Endothelial cytoskeletal reorganization in response to PAR1 stimulation is mediated by membrane rafts but not caveolae. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H366–H375. doi: 10.1152/ajpheart.01044.2006. [DOI] [PubMed] [Google Scholar]
- Chambers R. C. Role of coagulation cascade proteases in lung repair and fibrosis. Eur. Respir. J. Suppl. 2003;44:33s–35s. doi: 10.1183/09031936.03.00001003. [DOI] [PubMed] [Google Scholar]
- Cheung W. M., D'Andrea M. R., Andrade-Gordon P., Damiano B. P. Altered vascular injury responses in mice deficient in protease-activated receptor-1. Arterioscler. Thromb. Vasc. Biol. 1999;19:3014–3024. doi: 10.1161/01.atv.19.12.3014. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Xu S., Hepler J. E., Hutchison M., Frost J., Robbins D. J. Regulation of the MAP kinase cascade. Cell. Mol. Biol. Res. 1994;40:253–256. [PubMed] [Google Scholar]
- Colotta F., Sciacca F. L., Sironi M., Luini W., Rabiet M. J., Mantovani A. Expression of monocyte chemotactic protein-1 by monocytes and endothelial cells exposed to thrombin. Am. J. Pathol. 1994;144:975. [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Cowley S., Paterson H., Kemp P., Marshall C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Cunningham M. A., Rondeau E., Chen X., Coughlin S. R., Holdsworth S. R., Tipping P. G. Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J. Exp. Med. 2000;191:455–462. doi: 10.1084/jem.191.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C. A., Malik A. B., Gilchrist A., Hamm H., Sandoval R., Voyno-Yasenetskaya T., Tiruppathi C. Thrombin induces proteinase-activated receptor-1 gene expression in endothelial cells via activation of Gi-linked Ras/mitogen-activated protein kinase pathway. J. Biol. Chem. 1999;274:13718–13727. doi: 10.1074/jbc.274.19.13718. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Feliciello A., Gottesman M. E., Avvedimento E. V. The biological functions of A-kinase anchor proteins. J. Mol. Biol. 2001;308:99–114. doi: 10.1006/jmbi.2001.4585. [DOI] [PubMed] [Google Scholar]
- Fiorucci S., Antonelli E., Distrutti E., Severino B., Fiorentina R., Baldoni M., Caliendo G., Santagada V., Morelli A., Cirino G. PAR1 antagonism protects against experimental liver fibrosis. Role of proteinase receptors in stellate cell activation. Hepatology. 2004;39:365–375. doi: 10.1002/hep.20054. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Chikumi H., Gutkind J. S. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene. 2001;20:1661–1668. doi: 10.1038/sj.onc.1204182. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M., Denholm E. M., Phan S. H. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J. Biol. Chem. 1996;271:17779–17784. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- Gilchrist A., Li A., Hamm H. E. Design and use of C-terminal minigene vectors for studying role of heterotrimeric G proteins. Methods Enzymol. 2002;344:58–69. doi: 10.1016/s0076-6879(02)44705-2. [DOI] [PubMed] [Google Scholar]
- Gilchrist A., Mazzoni M. R., Dineen B., Dice A., Linden J., Proctor W. R., Lupica C. R., Dunwiddie T. V., Hamm H. E. Antagonists of the receptor-G protein interface block Gi-coupled signal transduction. J. Biol. Chem. 1998;273:14912–14919. doi: 10.1074/jbc.273.24.14912. [DOI] [PubMed] [Google Scholar]
- Gilchrist A., Vanhauwe J. F., Li A., Thomas T. O., Voyno-Yasenetskaya T., Hamm H. E. G alpha minigenes expressing C-terminal peptides serve as specific inhibitors of thrombin-mediated endothelial activation. J. Biol. Chem. 2001;276:25672–25679. doi: 10.1074/jbc.M100914200. [DOI] [PubMed] [Google Scholar]
- Goodman R. B., Strieter R. M., Martin D. P., Steinberg K. P., Milberg J. A., Maunder R. J., Kunkel S. L., Walz A., Hudson L. D., Martin T. R. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- Hamm H. E. The many faces of G protein signaling. J. Biol. Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- Hamm H. E., Gilchrist A. Heterotrimeric G proteins. Curr. Opin. Cell Biol. 1996;8:189–196. doi: 10.1016/s0955-0674(96)80065-2. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Jiang X., Kozasa T., Roscoe W., Singer W. D., Gilman A. G., Sternweis P. C., Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Hiol A., Davey P. C., Osterhout J. L., Waheed A. A., Fischer E. R., Chen C. K., Milligan G., Druey K. M., Jones T. L. Palmitoylation regulates regulators of G-protein signaling (RGS) 16 function. I. Mutation of amino-terminal cysteine residues on RGS16 prevents its targeting to lipid rafts and palmitoylation of an internal cysteine residue. J. Biol. Chem. 2003;278:19301–19308. doi: 10.1074/jbc.M210123200. [DOI] [PubMed] [Google Scholar]
- Hogaboam C. M., Steinhauser M. L., Chensue S. W., Kunkel S. L. Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney Int. 1998;54:2152–2159. doi: 10.1046/j.1523-1755.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Saifeddine M., al-Ani B., Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can. J. Physiol. Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- Howell D. C., Johns R. H., Lasky J. A., Shan B., Scotton C. J., Laurent G. J., Chambers R. C. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am. J. Pathol. 2005;166:1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes S. M., Watt F. M. Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J. Cell Biol. 2004;166:419–431. doi: 10.1083/jcb.200312074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. G., Su X., Su G., Scotton C. J., Camerer E., Laurent G. J., Davis G. E., Chambers R. C., Matthay M. A., Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J. Clin. Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T., Jiang X., Hart M. J., Sternweis P. M., Singer W. D., Gilman A. G., Bollag G., Sternweis P. C. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Major C. D., Santulli R. J., Derian C. K., Andrade-Gordon P. Extracellular mediators in atherosclerosis and thrombosis: lessons from thrombin receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2003;23:931–939. doi: 10.1161/01.ATV.0000070100.47907.26. [DOI] [PubMed] [Google Scholar]
- Marin V., Farnarier C., Gres S., Kaplanski S., Su M. S., Dinarello C. A., Kaplanski G. The p38 mitogen-activated protein kinase pathway plays a critical role in thrombin-induced endothelial chemokine production and leukocyte recruitment. Blood. 2001;98:667–673. doi: 10.1182/blood.v98.3.667. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. N., Shen L., Holinstat M., Brooks J. D., Dibenedetto E., Hamm H. E. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J. Biol. Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- Moore B. B., Kolodsick J. E., Thannickal V. J., Cooke K., Moore T. A., Hogaboam C., Wilke C. A., Toews G. B. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am. J. Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. J., Burdick M. D., Hong K., Lutz M. A., Murray L. A., Xue Y. Y., Belperio J. A., Keane M. P., Strieter R. M. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Singh I., Knezevic N., Ahmmed G. U., Kini V., Malik A. B., Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J. Biol. Chem. 2007;282:7833–7843. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- Suga M., Iyonaga K., Ichiyasu H., Saita N., Yamasaki H., Ando M. Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur. Respir. J. 1999;14:376–382. doi: 10.1034/j.1399-3003.1999.14b23.x. [DOI] [PubMed] [Google Scholar]
- Sugawara Y., Nishii H., Takahashi T., Yamauchi J., Mizuno N., Tago K., Itoh H. The lipid raft proteins flotillins/reggies interact with Galphaq and are involved in Gq-mediated p38 mitogen-activated protein kinase activation through tyrosine kinase. Cell Signal. 2007;19:1301–1308. doi: 10.1016/j.cellsig.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Nakamura S., Mano H., Kozasa T. Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc. Natl. Acad. Sci. USA. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J., Koli K., Keski-Oja J. Release of transforming growth factor-beta 1 from the pericellular matrix of cultured fibroblasts and fibrosarcoma cells by plasmin and thrombin. J. Biol. Chem. 1992;267:25378–25384. [PubMed] [Google Scholar]
- Tanaka N., Morita T., Nezu A., Tanimura A., Mizoguchi I., Tojyo Y. Signaling mechanisms involved in protease-activated receptor-1-mediated interleukin-6 production by human gingival fibroblasts. J. Pharmacol. Exp. Ther. 2004;311:778–786. doi: 10.1124/jpet.104.068569. [DOI] [PubMed] [Google Scholar]
- Trejo J., Connolly A. J., Coughlin S. R. The cloned thrombin receptor is necessary and sufficient for activation of mitogen-activated protein kinase and mitogenesis in mouse lung fibroblasts. Loss of responses in fibroblasts from receptor knockout mice. J. Biol. Chem. 1996;271:21536–21541. doi: 10.1074/jbc.271.35.21536. [DOI] [PubMed] [Google Scholar]
- Vanhauwe J. F., Thomas T. O., Minshall R. D., Tiruppathi C., Li A., Gilchrist A., Yoon E. J., Malik A. B., Hamm H. E. Thrombin receptors activate G (o) proteins in endothelial cells to regulate intracellular calcium and cell shape changes. J. Biol. Chem. 2002;277:34143–34149. doi: 10.1074/jbc.M204477200. [DOI] [PubMed] [Google Scholar]
- Vergnolle N., et al. A role for proteinase-activated receptor-1 in inflammatory bowel diseases. J. Clin. Invest. 2004;114:1444–1456. doi: 10.1172/JCI21689. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vogt S., Grosse R., Schultz G., Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J. Biol. Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Walz D. A., Anderson G. F., Ciaglowski R. E., Aiken M., Fenton J. W., 2nd. Thrombin-elicited contractile responses of aortic smooth muscle. Proc. Soc. Exp. Biol. Med. 1985;180:518–526. doi: 10.3181/00379727-180-42211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.