Abstract
The spindle pole body (SPB) of Schizosaccharomyces pombe is required for assembly of the forespore membrane (FSM) during meiosis. Before de novo biogenesis of the FSM, the meiotic SPB forms outer plaques, an event referred to as SPB modification. A constitutive SPB component, Spo15, plays an indispensable role in SPB modification and sporulation. Here, we analyzed two sporulation-specific genes, spo13+ and spo2+, which are not required for progression of meiotic nuclear divisions, but are essential for sporulation. Spo13 is a 16-kDa coiled-coil protein, and Spo2 is a 15-kDa nonconserved protein. Both Spo13 and Spo2 specifically associated with the meiotic SPB. The respective deletion mutants are viable, but defective in SPB modification and in the onset of FSM formation. Spo13 and Spo2 localized on the cytoplasmic side of the SPB in close contact with the nascent FSM. Localization of Spo13 to the SPB was dependent on Spo15 and Spo2; that of Spo2 depended only on Spo15, suggesting that their recruitment to the SPB is strictly controlled. Spo2 physically associated with both Spo15 and Spo13, but Spo13 and Spo15 did not interact directly. Taken together, these observations indicate that Spo2 is recruited to the SPB during meiosis and then assists in the localization of Spo13 to the outer surface of the SPB.
INTRODUCTION
Gametogenesis in higher eukaryotes is an important life cycle step. This process is comprised of haploidization by meiotic nuclear divisions and cellular specialization suitable for fertilization. Although fundamental mechanisms of meiosis are shared by eukaryotes from yeast to humans, the events involved in gametic differentiation vary widely among species.
Sporulation in the fission yeast Schizosaccharomyces pombe is equivalent to gametogenesis, in that this morphogenetic process accompanies meiosis and a cell specialization process culminating in formation of ascospores (Shimoda and Nakamura, 2003; Shimoda, 2004). Ascospores are characterized by their dormancy, a high degree of resistance to environmental stress, and increased genetic diversity. In addition, a marked feature of yeast sporulation is de novo biogenesis of a double unit membrane, called the forespore membrane (FSM), within the cytoplasm of the diploid mother cell (Yoo et al., 1973; Hirata and Tanaka, 1982; Tanaka and Hirata, 1982; Nakamura et al., 2001). The FSM expands by fusion of membranous vesicles encapsulating a haploid nucleus. Spore wall material is then deposited between inner and outer layers of the FSM (Yoo et al., 1973; Hirata and Tanaka, 1982). The inner layer of the FSM becomes the plasma membrane of newborn spores. The outer membrane eventually degrades during sporulation. The mechanisms coordinating the meiotic nuclear divisions with assembly of the FSM are not well understood.
Assembly of the FSM begins at the spindle pole body (SPB), equivalent to the centrosome of animal cells, during meiosis II. It is possible that the SPB links formation of the FSM with meiosis. The outer plaque of the SPB is markedly enlarged in the budding yeast Saccharomyces cerevisiae (Byers, 1981), and multiple outer plaques are newly formed in the fission yeast S. pombe (Hirata and Tanaka, 1982). These morphological alterations of the SPB are referred to as “SPB modification.” SPB modification was also detected by fluorescent immunostaining with an anti-Sad1 antibody as a change in shape from a dot to a crescent (Hagan and Yanagida, 1995). The mitotic outer plaque component Spc72 is replaced by meiosis-specific components, Mpc54, Mpc70/Spo21, and Spo74, before FSM formation in S. cerevisiae (Knop and Strasser, 2000; Bajgier et al., 2001; Rabitsch et al., 2001). These components construct a specialized outer plaque termed meiotic plaque (Knop and Strasser, 2000). The outer plaque components of the meiotic SPB in S. pombe are totally unknown. We reported that one SPB component protein, Spo15, is dispensable for growth, but essential for meiosis-specific SPB modification and for spore formation. Like Mpc54 and Mpc70/Spo21, Spo15 is a coiled-coil protein of 220 kDa, but has no homology with these S. cerevisiae SPB proteins (Ikemoto et al., 2000). The spo15 deletion mutant fails to initiate FSM formation (Nakamura, unpublished data), indicating that Spo15 plays an essential role in the meiotic SPB for assembly of the FSM. Although disruption of spo15+ caused no detectable defects in vegetative growth, Spo15 is a constitutive component of the SPB. We presume that as-yet unknown SPB components may be involved in meiosis-specific functions.
In the present study, we analyzed two novel SPB components, Spo13 and Spo2, that are only expressed during meiosis. The spo13 and spo2 deletion mutants displayed normal vegetative growth and completed meiosis, but were defective in the onset of FSM assembly. The SPB outer plaque formation during the second meiotic division was severely impaired in spo13Δ as in spo2Δ. Spo13 was recruited to the outermost layer of the meiotic SPB, dependent on both Spo2 and Spo15, and then associated closely with the nascent FSM. Our study illustrates construction of the meiotic plaque of the SPB in S. pombe, which serves as a platform for FSM assembly.
MATERIALS AND METHODS
Yeast Strains and Media
The S. pombe strains and plasmids used in this study are listed in Tables 1 and 2, respectively. Standard methods were used for growth, transformation, and genetic manipulation (Moreno et al., 1991). S. pombe cells were grown in YE, MM, and SD media and sporulated in ME, SSA, and SSL-N media (Egel and Egel-Mitani, 1974; Gutz et al., 1974). Incubation temperatures were 30°C for growth and 28°C for mating and sporulation, unless stated otherwise. Synchronous meiosis was induced by a temperature shift using strains carrying the pat1-114 allele as described (Iino et al., 1995).
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| B82 | h90 spo13-B82 ade6-M210 | Bresch et al. (1968) |
| B317 | h90 spo2-B317 ade6-M210 | Bresch et al. (1968) |
| MK13-2BL (FY7071)a | h90 spo13-B82 leu1 | This study |
| MK18-2CL (FY7111)a | h90 spo2-B317 leu1 | This study |
| TN8 (FY7132)a | h90 leu1-32 | Nakamura et al. (2001) |
| TN29 (FY7816)a | h90 leu1-32 ura4-D18 | Ikemoto et al. (2000) |
| TN104 (FY7273)a | h90 ade6-M210 leu1-32 | |
| JZ670 (FY7051)a | h−/h−pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1-32 | M. Yamamoto (unpublished data) |
| AB4 (FY7476)a | h−/h−pat1-114/pat1-114 mei4::ura4+/mei4::ura4+ade6-M210/ade6-M216 leu1-32/leu1-32 | Abe and Shimoda (2000) |
| SI52 (FY7434)a | h90 spo15::ura4+ura4-D18 | Ikemoto et al. (2000) |
| YN12 (FY7813)a | h90 | Nakase et al. (2001) |
| YN47 (FY12275)a | h90 spo15::ura4+leu1≪GFP-psy1 ade6-M216 ura4-D18 | This study |
| YN48 (FY12276)a | h90 spo13::ura4+ura4-D18 leu1-32 | This study |
| YN63 | h90 spo13::ura4+ura4-D18 leu1≪spo13-HA | This study |
| YN66 | h90 spo13::ura4+ura4-D18 leu1≪GFP-psy1 | This study |
| YN67 (FY12295)a | h90 spo15::ura4+ura4-D18 leu1≪GFP-psy1 | This study |
| YN68 (FY12296)a | h90 leu1≪GFP-psy1 | Nakase et al. (2004) |
| YN77 (FY12305)a | h90 spo15-HA ≪LEU2 leu1-32 | This study |
| YN78 | h90 spo13::ura4+spo15-HA≪LEU2 leu1-32 ura4-D18 | This study |
| YN90 | h90 spo13::ura4+ura4-D18 spo15-GFP≪LEU2 leu1-32 | This study |
| YN98 | h−/h−pat1-114/pat1-114 ade6-M210/ade6-M216 leu1/leu1≪spo13-HA | This study |
| YN114 | h90 ade6≪spo13-HA leu1≪GFP-psy1 | This study |
| YN310 | h−leu1≪GFP-psy1 ade6-M210 | This study |
| YN312 | h90 spo13::ura4+leu1≪GFP-psy1 ade6-M210 ura4-D18 | This study |
| YN388 | h90 spo15::ura4 leu1≪spo2-GFP ura4-D18 | This study |
| YN389 | h90 spo2::ura4+spo15-GFP≪LEU2 leu1-32 ura4-D18 | This study |
| YN391 | h90 spo13::ura4+leu1≪spo2-GFP ura4-D18 | This study |
| YN433 | h90 spo2::ura4+leu1≪spo2-GFP ura4-D18 | This study |
| YN442 | h90 spo2::ura4+ura4-D18 | This study |
| YN457 | h90 spo2::ura4+leu1≪GFP-Psy1 ura4-D18 | This study |
| YN459 | h90 spo2::ura4+leu1≪spo13-GFP ade6-M216 ura4-D18 | This study |
| YN463 | h−/h−pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1≪spo2-HA | This study |
| YN471 (FY12275)a | h90 spo15::ura4+leu1≪spo13-GFP ura4-D18 | This study |
| YND2 | h90/h90 spo2::ura4+/spo2-B317 leu1-32/leu1+ura4+/ura4-D18 | This study |
| YND13 | h90/h90 spo13::ura4+/spo13-B82 leu1-32/leu1+ura4+/ura4-D18 | This study |
The S. pombe strains constructed in this study will be deposited at the YGRC/NBRP.
a This strain was obtained from the Yeast Genetic Resource Center of Japan supported by the National BioResource Project (YGRC/NBRP) (http://yeast.lab.nig.ac.jp/nig/).
Table 2.
Plasmids used in this study
| Plasmid | Characteristics | Source |
|---|---|---|
| pDB248′ | 2 μm origin, LEU2-based vector | Beach and Nurse (1981) |
| pREP1 | ars1, LEU2-based expression vector carrying nmt1+ promoter | Maundrell (1993) |
| pREP2 | ars1, ura4+-based expression vector carrying nmt1+ promoter | Maundrell (1993) |
| pREP41 | ars1, LEU2-based expression vector carrying nmt41+ promoter | Maundrell (1993) |
| pREP42 | ars1, ura4+-based expression vector carrying nmt41+ promoter | Maundrell (1993) |
| pREP1A | derivative of pREP1 that contains ade6+ as selectable marker | T. Tamai (unpublished data) |
| pREP1 (mei4) | pREP1, mei4+-coding region | Abe and Shimoda (2000) |
| pBR (leu1) (FYP1248)a | leu1+ in pBR322 | Nakamura-Kubo et al. (2003) |
| pIL-HA | LEU2-based integration vector, HA-epitope-coding region | This study |
| pAL-KS | ars1, LEU2-based multicopy shuttle vector | Tanaka et al. (2001) |
| pAU-KS | ars1, URA3-based multicopy shuttle vector | Tanaka et al. (2001) |
| pTN143 (FYP410)a | pAL-KS, GFP and nmt1+ terminator | Ikemoto et al. (2000) |
| pREP41 (GFP) | pREP41, GFP | This study |
| pAL (spo13-GFP) | pTN143, spo13-GFP | This study |
| pAL (spo13-HA) | pTN144, spo13-HA | This study |
| pAL (spo13B82-GFP) | pTN143, spo13B82-GFP | This study |
| pREP1A (spo2) | pREP1A, spo2-containing PCR fragment | This study |
| pREP1A (spo15) | pREP1A, spo15, spo13-containing PCR fragment | This study |
| pREP41 (spo13-GFP) | pREP41, spo13-GFP | This study |
| pREP41 (spo2-GFP) | pREP41, spo2-GFP | This study |
| pREP42 (GST-spo2) | pREP42, GST-spo2 | This study |
| pREP42 (GST) | pREP42, Glutathione-S-transferase (GST) | This study |
| pAU (spo2-GFP) | pAU, spo2-GFP | This study |
| pBR (leu1) (spo13-HA) | pBR(leu1), spo13-HA | This study |
| pBR (leu1) (spo2-HA) | pBR(leu1), spo2-HA | This study |
| pBR (leu1) (spo2-GFP) | pBR(leu1), spo2-GFP | This study |
The plasmids constructed in this study will be deposited at the YGRC/NBRP.
a This plasmid was obtained from the Yeast Genetic Resource Center of Japan supported by the National BioResource Project (YGRC/NBRP) (http://yeast.lab.nig.ac.jp/nig/).
Cloning of spo13+ and spo2+
Sporulation-deficient strains were transformed with an S. pombe genomic library containing partial Sau3AI DNA fragments (a gift from Dr. Y. Watanabe) constructed in a multicopy plasmid, pDB248′ (Beach and Nurse, 1981). About 105 independent Leu+ transformants were obtained. These transformants were allowed to sporulate on selective SSA plates and were then treated with 30% ethanol for 30 min to kill nonsporulating vegetative cells (Gutz et al., 1974). Cells were spread on SSA sporulation plates and incubated for several days at 28°C. Colonies were exposed to iodine vapor (Gutz et al., 1974) to screen for sporulation-proficient clones. Iodine-positive (brown) colonies were removed and inspected for recovery of sporulation proficiency. Plasmid DNA was transferred from some of the Leu+ Spo+ colonies to Escherichia coli DH5α.
Strain MK13–2BL harboring spo13-B82 was used to clone spo13+. One isolated plasmid, designated pDB(spo13), contained a 2.7-kb DNA insert (Supplementary Figure 1A). Partial DNA sequencing of this insert revealed that it was derived from a region of chromosome III that had been sequenced by the S. pombe genome project (cosmid SPCC1183; EMBL/GenBank/DDBJ accession no. AL031740). Subcloning defined a 2.2-kb ClaI fragment (pYN70) able to rescue the spo13 mutation. The S. pombe genome project does not annotate an open reading frame (ORF) in this fragment, likely due to the presence of an intron. We analyzed the corresponding cDNA sequence by 5′-RACE (rapid amplification of cDNA ends) using a commercial kit (Clontech, Palo Alto, CA; Chenchik et al., 1996). The 5′-RACE fragment was amplified using the spo13 primer, 5′-CCCGAGCTC(SacI)CGTACGTCCAGGAATTCC-3′, as well as an adaptor primer from the kit. Underlined sequences indicate the restriction enzyme sites. Nucleotide sequencing of the RACE fragments indicated one complete ORF split by a single 51-base pair intron (Supplementary Figure 1B). The sequence data implies that spo13+ potentially encodes a 15-kDa protein composed of 138 amino acids (Supplementary Figure 1B). This ORF represents the spo13+ gene itself, but not the multicopy suppressor, as described below.
Our previous genetic analysis suggested that the spo2 and spo18 genes were closely linked on chromosome II (Kishida and Shimoda, 1986). Reexamination of possible allelism indicated that spo2 was allelic to spo18 (data not shown). spo18+ was subsequently designated spo2+. The spo2+ gene was isolated using strain MK18-2CL harboring spo2-B317. A plasmid, pDB(spo2), which complemented this mutation, was isolated (Supplementary Figure 1D), and the terminal sequences of the insert (∼3 kb) were determined. The DNA sequence matched that from a cosmid SPBC16C6 (Accession no. AL021767) derived from chromosome II. This region was annotated by the genome project as the 3′ terminus of a large ORF, presumed to contain 5 exons, SPBC16C6.02c (vps1302), encoding a 354-kDa Vps13 family protein. Because the fourth intron contained an in-frame termination codon, we speculated that the downstream sequence that was formerly thought to be the fifth exon encoded the spo2+ ORF with no intronic sequence. To confirm this possibility, we obtained a full-length cDNA of spo2+. Sequencing of this cDNA clearly indicated that the spo2+ gene was transcribed independently of vps1302 (Supplementary Figure 1E).
Disruption of spo13+ and spo2+
The plasmids used for disruption of spo13+ were constructed as follows. A 2.2-kb XhoI-NotI fragment containing the spo13+ ORF was cloned into the corresponding site of pBluescript II-KS+ (Stratagene, La Jolla, CA). A 0.6-kb HindIII fragment of the resulting plasmid was replaced by ura4+, yielding pYN71 (Supplementary Figure 1A). A 3.2-kb ClaI fragment containing the deleted spo13 allele (spo13::ura4+) was used to transform the strain TN29. Disruptions were confirmed by genomic Southern hybridization (data not shown).
The plasmids used for disruption of spo2+ were constructed as follows. A 3.2-kb XhoI-NotI fragment containing the spo2+ ORF was cloned into the corresponding site of pBluescript II-KS+. A 0.5-kb NdeI-EcoRI fragment of the resulting plasmid was replaced by ura4+, yielding pYN187 (Supplementary Figure 1D). A BstEII fragment of 4.5 kb containing the deleted spo2 allele (spo2::ura4+) was used to transform strain TN29. Disruption was confirmed by genomic Southern hybridization (data not shown).
Southern and Northern Analysis
Genomic DNA was restricted, fractionated in a 1.0% agarose gel, and then transferred onto nylon membranes (Biodyne A, Nihon Pall, Tokyo, Japan). Total RNA was prepared from S. pombe cultures (Jensen et al., 1983) and fractionated on a 1.0% gel containing 3.7% formaldehyde as previously reported (Thomas, 1980).
Nucleotide Sequence Analysis of Mutant Alleles of spo13 and spo2
The entire spo13+ ORF was amplified by PCR using genomic DNA from strain MK13–2BL harboring spo13-B82 as a template and was then cloned into pGEM-T Easy Vector (Promega, Madison, WI). The nucleotide sequences of three clones derived from independent PCR amplifications were determined in their entirety. Comparison of the nucleotide sequences of spo13-B82 with spo13+ revealed a single nucleotide change, from C to T, which resulted in replacement of the fifty-third glutamine codon with an opal nonsense codon in the spo13-B82 allele (Supplementary Figure 2A).
The spo2-B317 mutant allele was also sequenced. A 3.2-kb region spanning the spo2+-coding sequence was amplified by PCR using genomic DNA from strain MK18–2CL harboring spo2-B317. Direct sequencing of the resulting fragment revealed that a single T residue had inserted into the thirty-second codon resulting in a frameshift mutation.
Western Blotting
Plasmid pBR(leu1)(spo13-HA) was constructed by inserting the 3.2-kb ApaI-SacI fragment, which contained the spo13+ promoter region, the ORF, a 3× hemagglutinin (HA) epitope and nmt1 terminator region of pAL(spo13-HA), into pBR(leu1) (Nakamura-Kubo et al., 2003). This plasmid was linearized by restriction with NruI near the center of the leu1+ sequences and was introduced into strain YN48. Several Leu+ transformants were tested for sporulation ability. Because the chromosomal integrant strain (YN63) sporulated, a single copy of spo13-HA proved to be functional. Similarly, the spo2-HA fusion gene was cloned into the integration vector pBR(leu1). The spo13-HA or spo2-HA gene was then integrated at the leu1 locus of the diploid JZ670. The resulting strains YN98 (spo13-HA) and YN463 (spo2-HA) were cultured in MM-N at 24°C for 18 h, and the temperature was then shifted to 34°C to induce synchronous meiosis. At specific intervals, portions of the culture were sampled, and crude cell extracts were prepared as described (Masai et al., 1995). Polypeptides were resolved by SDS-PAGE on 15% gels and then transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). Filters were probed with mouse anti-HA antibody 12CA5 (Roche Diagnostics, Mannheim, Germany) at a 1:1000 dilution. Blots were also probed with the anti-α-tubulin antibody, TAT-1 (Woods et al., 1989), to normalize protein loading. Immunoreactive bands were visualized by chemiluminescence (NEN Life Sciences, Boston, MA) with horseradish peroxidase–conjugated goat anti-mouse IgG (Promega).
In Vivo Protein Interaction Assay
To assay binding of Spo13 and Spo2, the wild-type strain TN29 was cotransformed with plasmids pREP41(spo13-GFP) and pREP42(GST-spo2). pREP42(GST) was used as control plasmid pREP42(GST-spo2). Transformants were precultured in liquid minimal medium (MM) containing 20 mM thiamine. Cells were then transferred to MM without thiamine and grown to midlog phase. The cells were harvested, resuspended in extraction buffer (50 mM Tris-HCl, pH 7.5, 10 mM EDTA, 2 mM EGTA, 200 mM NaCl, 1% Triton X-100, 1 mM PMSF), and then ruptured with glass beads. The lysate was centrifuged at 13,000 × g for 20 min to prepare a soluble fraction. The homogenates were incubated with glutathione Sepharose (GE Healthcare, Little Chalfont, United Kingdom). After further incubation at 4°C for 1 h, the solution was centrifuged at 800 × g for 5 min. The pellet was washed three times with extraction buffer and resuspended in sample buffer. The target proteins were detected by Western blotting using goat anti-glutathione S-transferase (GST; GE Healthcare, Waukesha, WI) or mouse anti-green fluorescent protein (GFP) antibodies (Roche Diagnostics).
In vivo interaction between Spo15 and Spo2 was examined by a similar assay. Strain TN8 was transformed with pREP41(GFP) or pREP41(spo2-GFP), and the resultant transformants were grown in MM medium. The cell-free homogenates were incubated with mouse anti-GFP antibody (Roche Diagnostics) at 4°C for 1 h and then mixed with protein G Sepharose (GE Healthcare). The target proteins were detected by Western blot analysis using rat anti-GFP (a generous gift of S. Fujita) or rabbit anti-Spo15 (Itadani, Nakamura, and Shimoda, unpublished data) antibody.
Immunofluorescence Microscopy
Cells were fixed with glutaraldehyde and paraformaldehyde as described (Hagan and Hyams, 1988). Spo13-HA was visualized by indirect immunofluorescence microscopy using rat anti-HA antibody 3F10 (Roche Diagnostics) and Alexa 594–conjugated goat anti-rat IgG (Molecular Probes, Eugene, OR). The SPB was visualized by indirect immunofluorescence microscopy using rabbit anti-Sad1 antibody (a generous gift from Dr. O. Niwa) and Alexa 546-conjugated goat anti-rabbit IgG (Molecular Probes). For microtubule staining, mouse anti-α-tubulin antibody TAT-1 (Woods et al., 1989) and Cy3-conjugated secondary antibody (Sigma Chemical, St. Louis, MO) were used. To visualize the nuclear chromatin region, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml. Stained cells were observed under a fluorescence microscope (model BX50; Olympus, Tokyo, Japan) equipped with a charge-coupled device camera (Cool-SNAP; Roper Scientific, San Diego, CA).
Electron Microscopy
Samples for electron microscopy were prepared as described (Ye et al., 2007), and sections were viewed on an electron microscope (H-7600, Hitachi, Tokyo, Japan) at 100 kV.
RESULTS
Spo13 and Spo2 Are Sporulation-specific Proteins
Among a set of sporulation-specific genes (designated spo), eight genes (spo3+, spo4+, spo5+/mug12+, spo6+, spo9+, spo14+, spo15+, and spo20+) have been isolated and analyzed (Nakamura et al., 2000, 2001, 2002; Ikemoto et al., 2000; Nakase et al., 2001; Nakamura-Kubo et al., 2003; Kasama et al., 2006; Ye et al., 2007). In addition, here we characterized spo2+ and spo13+. The spo13-B82 mutant is defective in ascospore formation (Bresch et al., 1968; Hirata and Shimoda, 1992). Using this mutant, spo13+ was cloned from an S. pombe genomic library by functional complementation (see Materials and Methods; Supplementary Figure 1A). Taken together with the 5′ RACE analysis, the spo13+ ORF was defined to be split by one short intron (Supplementary Figure 1A). The spo13+ gene potentially encodes a 16-kDa protein composed of 138 amino acid residues (Supplementary Figure 1B). Spo13 is predicted to be rich in coiled-coil regions (Lupas et al., 1991; http://www.ch.embnet.org/software/COILS_form.html), otherwise it contains no recognized functional motifs (Supplementary Figure 1B). It shares no homology with any reported proteins in public databases.
The spo13 deletion mutant did not differ from the wild-type strain in growth rate, cell size, or shape in complete medium, indicating that spo13+ is not essential for normal growth. The first and second meiotic divisions in the spo13 deletion mutant proceeded at a rate similar to that observed in an isogenic wild-type strain (data not shown). As expected, the deletion mutant was asporogenous like the original spo13-B82 mutant. These results indicate that the spo13Δ mutant undergoes a normal meiosis but is defective in ascospore formation. A diploid strain (YND13) that is heteroallelic at the spo13 locus, spo13::ura4+/spo13-B82, was unable to sporulate, confirming that the cloned gene is spo13+ itself.
The spo2+ gene was cloned and sequenced similarly (Supplementary Figure 1D). The spo2+ gene encodes a 15-kDa protein composed of 133 amino acid residues (Supplementary Figure 1E). The Spo2 protein has neither apparent functional motifs nor significant homology with any proteins in public databases.
The original spo2-B317 mutant carries a frame-shift mutation, resulting in truncation of the protein. Neither the spo2-B317 mutant nor the spo2 deletion mutant was defective for mitotic growth or for meiosis but both were asporogenous (data not shown). As expected, a heteroallelic diploid YND2 (spo2-B317/spo2::ura4+) was unable to sporulate, indicating that the cloned gene is genetically defined spo2+ itself.
Meiosis-specific Expression of spo13+ and spo2+
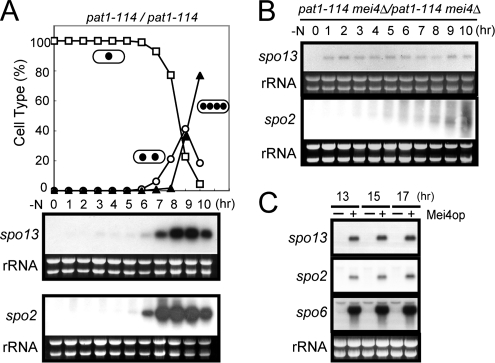
Transcription of spo13+ and spo2+ was analyzed in homothallic haploid strains cultured in nitrogen-free sporulation medium by Northern blotting. No spo13+ or spo2+ transcripts could be detected in vegetative cells, but were observed to accumulate abruptly after shifting to nitrogen-free medium (data not shown). The exact timing of transcriptional induction during meiosis was further explored using the temperature-sensitive pat1-114 mutation to induce synchronous meiosis (Iino et al., 1995). The transcription of spo13+ was induced at 6 h after the temperature shift (a trigger for the onset of meiosis), and peaked at 8–9 h, when cells were in meiosis I (Figure 1A). Meiotic induction of spo2+ transcription was also confirmed, although the timing was slightly earlier than for spo13+ (Figure 1A).
Figure 1.
Transcription of the spo13+ and spo2+ genes. Northern analysis of spo13+ and spo2+ transcripts in pat1-driven meiosis. Synchronous meiosis was initiated in diploid strains homozygous for pat1-114 (JZ670) (A) and pat1-114 mei4Δ (AB4) (B). At hourly intervals, total RNA was prepared and analyzed by Northern blot hybridization. Meiotic nuclear division was monitored by counting the number of nuclei per cell. □, mononucleate; ○, binucleate; ▴, tri- or tetranucleate cells. (C) Effect of ectopic expression of mei4+ on spo13+ and spo2+ transcription. Wild-type cells (TN8) carrying either pREP1 or pREP1(mei4) were incubated in MM+N at 30°C for 13, 15, and 17 h. The approximate quantity of RNA was checked by staining gels with ethidium bromide. A Mei4-dependent gene spo6+ was included as a positive control.
We found FLEX-like cis elements in the 5′ upstream region of the spo13+ and spo2+ genes (Supplementary Figure 1, C and E). The FLEX element containing a core heptamer (5′-GTAAACA-3′) has been identified as a recognition site of the meiosis-specific transcription factor Mei4, which has a forkhead DNA-binding domain (Horie et al., 1998). It is probable that spo13+ and spo2+ are induced during meiosis in a Mei4-dependent manner. To test this hypothesis, we examined induction of spo13+ and spo2+ in a mei4Δ strain. As shown in Figure 1B, no gross accumulation of mRNA was observed after induction of meiosis. A faint band due to the spo13-specific probe was discerned, suggesting that a low level of Mei4-independent transcription may occur. Overexpression of mei4+ leads to ectopic accumulation of the spo13+ and spo2+ mRNA in vegetative cells (Figure 1C). These observations suggest that transcription of spo13+ and spo2+ is induced during meiosis in a largely Mei4-dependent manner.
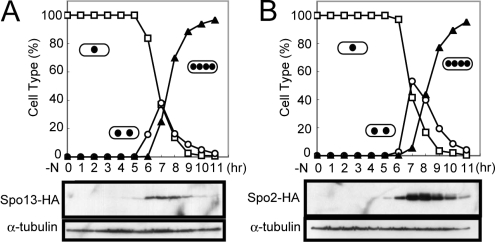
To assess abundance of Spo13 and Spo2 proteins during meiosis, epitope-tagged alleles (spo13-HA and spo2-HA) were chromosomally integrated in a homozygous pat1-114 diploid strain JZ670. The strain YN98 carrying both spo13-HA and pat1-114 was grown at 34°C to induce synchronous meiosis. Western blotting revealed that Spo13-HA was not detectable in vegetative cells (at 0 h) and appeared as a 18-kDa band when meiosis I began (Figure 2A). This apparent molecular mass is consistent with that deduced from the sequence data. Spo13 became more abundant when cells proceeded to meiosis II (6–8 h). Once meiosis was completed, Spo13-HA levels decreased.
Figure 2.
Changes in Spo13 and Spo2 abundance during meiosis. (A) Cells carrying spo13-HA (YN98) (A) or spo2-HA (YN463) (B) were allowed to proceed through synchronous meiosis. Aliquots were removed at hourly intervals and protein extracts were subjected to Western blot analysis with the mouse anti-HA antibody 12CA5 and with anti-α-tubulin antibody TAT-1 as the loading control. Meiotic nuclear division was monitored by counting the number of nuclei per cell. □, mononucleate; ○, binucleate; ▴, tri- or tetranucleate cells.
Similar experiments were carried out with the Spo2-HA strain, YN463 (Figure 2B). Spo2-HA was detected slightly before the first meiotic division. Spo2-HA levels reached a peak during meiosis II and then slowly declined. We conclude that both Spo13 and Spo2 are transiently enriched in meiotic cells. Our experiments clearly show that the spo2+ and spo13+ genes are transcribed during meiosis by the forkhead transcription factor Mei4, and that protein levels rapidly increased during meiosis and then declined.
Spo13 and Spo2 Are Localized to the Cytoplasmic Side of the SPB
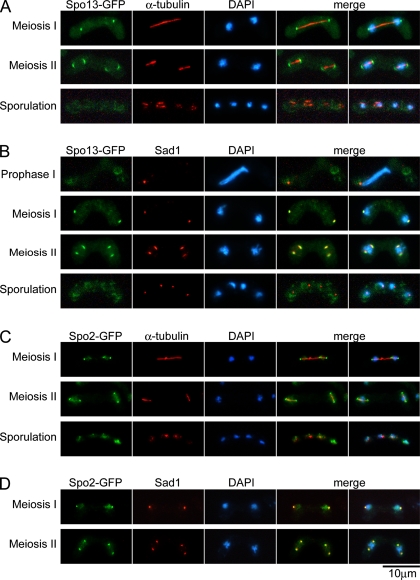
To obtain more information about the potential functions of Spo13 and Spo2, their intracellular localization was determined. A single copy of the spo13-GFP allele in the deletion mutant background was found to be fully functional. In some experiments, an h90 spo13+ strain (TN8) transformed with a multicopy plasmid pAL(spo13-GFP) was also used. The plasmid-bearing cells also displayed normal sporulation ability. Cells expressing Spo13-GFP were transferred to sporulation medium, SSL-N, in order to induce meiosis and sporulation. At hourly intervals, cells were fixed and counterstained with DAPI to monitor the meiotic stage of the cells. In accordance with the Western analysis (cf. Figure 2A), a Spo13-GFP signal was not detectable in vegetative cells (data not shown) nor in cells at meiotic prophase I (Figure 3B). During meiosis I, the Spo13-GFP fluorescent signal first appeared as two dots at both ends of the meiotic spindle, as revealed by the anti-α-tubulin antibody TAT-1 (Woods et al., 1989; Figure 3A). The S. pombe sad1+ gene encodes a constitutive component of the SPB essential for normal bipolar spindle formation (Hagan and Yanagida, 1995). Immunostaining of the SPB with the anti-Sad1 antibody showed that Spo13 was colocalized with Sad1 (Figure 3B), indicating that Spo13 is localized to the meiotic SPBs. We previously reported that Spo13-GFP also colocalized with Sad1 in aberrant forms of the SPB in spo20 mutant cells (Nakase et al., 2004). The Spo13-GFP signal disappeared upon completion of meiosis (Figure 3, A and B). We also performed the localization study of Spo13-GFP using an integrant strain and obtained results similar to those described above.
Figure 3.
Localization of Spo13 and Spo2 during meiosis and sporulation. (A and B) Homothallic haploid wild-type cells (TN8) carrying pAL(spo13-GFP) were cultured in SSL-N to induce meiosis. Fixed cells at different stages of meiosis were examined by DAPI and GFP and with the anti-α-tubulin antibody TAT-1 (A) or with the anti-Sad1 antibody (B). (C and D) The homothallic haploid strain YN433 harboring chromosomally integrated Spo2-GFP was cultured in SSL-N to induce meiosis. Fixed cells at different stages of meiosis were examined by DAPI and GFP and with the anti-α-tubulin antibody TAT-1 (C) or with the anti-Sad1 antibody (D).
The fluorescent signal for Spo2-GFP in meiotic cells was also analyzed. The spo2-GFP fusion gene was able to complement the spo2 deletion mutation, indicating that Spo2-GFP is functional (data not shown). Like Spo13-GFP, the Spo2 signal was absent in vegetative cells, but was detected when cells entered meiosis. In these cells, the fluorescent signal was observed as a dot at both ends of the meiotic spindles (Figure 3C). Immunostaining using anti-Sad1 antibody clearly indicated that Spo2-GFP localized to the meiotic SPB (Figure 3D). Colocalization of Spo13 and Spo2 at the meiotic SPBs is shown directly in Figure 4A.
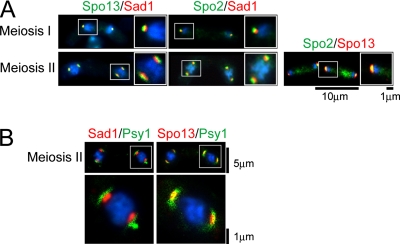
Figure 4.
Magnified and merged images of Spo13 and Spo2 at the SPB. (A) Double staining of SPB components. Strain TN8 carrying pAL(spo13-GFP) (left) and strain YN433 (middle) were induced to undergo meiosis. Cells were fixed and stained with DAPI. Fluorescent images created by use of DAPI, GFP, and the anti-Sad1 antibody were observed under a fluorescence microscope. Blue, DAPI; red, Sad1; green, GFP signal. Right, strain TN29 carrying pAL(Spo13-HA) and pAU(Spo2-GFP) was examined as described above. (B) Double staining of SPB components and the FSM. Left, magnified and merged images of wild-type (YN68) cells harboring integrated GFP-Psy1 during formation of the FSM. Blue, DAPI; red, Sad1; green, GFP-Psy1. Right, magnified and merged images of wild-type (YN114) cells harboring integrated GFP-Psy1 and Spo13-HA. Images of YN114 cells were obtained as described for YN68. Blue, DAPI; red, Spo13-HA; green, GFP-Psy1.
We previously reported that Spo15-GFP and Sad1 fully overlapped by fluorescence microscopy (Ikemoto et al., 2000). However, magnified fluorescence images of the double staining with Spo13-GFP and Sad1 revealed that such was not the case, but rather that Spo13 was present outside of the SPB relative to its core protein, Sad1 (Figure 4A). Similarly, Spo13 was present outside of Spo15 (data not shown). Like Spo13-GFP, Spo2-GFP also exhibited positioning in the SPB relative to Sad1 (Figure 4A). However, Spo13 and Spo2 completely overlapped (Figure 4A). Together, we conclude that Spo13 and Spo2 localize to the cytoplasmic side of the meiotic SPB.
As we have reported, the FSM, a precursor of the spore plasma membrane, is first assembled on the SPB (Hirata and Shimoda, 1994; Nakamura et al., 2001). Spo13 is possibly positioned close to the nascent FSM, because this protein localizes at the cytoplasmic side of the SPB (Figure 4A). The S. pombe Psy1 is a homologue of budding yeast syntaxins Sso1 and Sso2 (Aalto et al., 1993) and human syntaxin-1A (Bennett et al., 1992). In vegetative cells, Psy1 localizes to the plasma membrane where it functions as a t-SNARE protein. We have reported that GFP-tagged Psy1 is a good marker for tracing development of the FSM (Nakamura et al., 2001). Spatial arrangement of the FSM relative to the meiotic SPB marked by Sad1 or Spo13 was examined by fluorescence microscopy. The nascent FSM visualized by GFP-Psy1 overlapped with Spo13-HA (Figure 4B-right), whereas the GFP signal was found outside of the Sad1 signals (Figure 4B-left). These observations confirm Spo13 localization at the cytoplasmic side of the SPB and its close spatial relationship with de novo synthesized FSMs.
The spo13-B82 mutant exhibited a strict sporulation-deficient phenotype like the spo13 deletion mutant. As noted earlier, the spo13-B82 allele contained an opal nonsense codon at the 53rd glutamine residue (Supplementary Figure 2A). Thus, this allele likely produces a truncated protein missing amino acid residues downstream of the lesion (C-terminal two-thirds of the protein). Accordingly, we have designated this mutant protein Spo1352aa. The GFP-fused Spo1352aa protein was found to be rather dispersed in the nucleus and not localized to the SPB (Supplementary Figure 2B). This observation suggests that the N-terminal 52-amino acid residues of Spo13 are insufficient for its normal SPB localization and function.
Morphological Changes of the Meiotic SPB in spo13Δ and spo2Δ Mutants
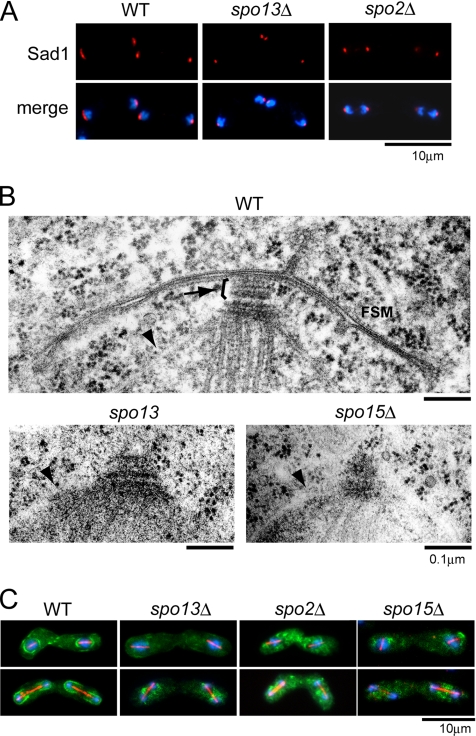
Because spo13 and spo2 deletion mutants produced virtually no spores, but underwent meiosis with normal kinetics (data not shown), we analyzed their defects in spore morphogenesis in greater detail. Electron microscopy revealed that a few outer plaques are added to the SPB at meiosis II, a structural alteration termed SPB modification (Hirata and Shimoda, 1994; see Figure 5B, WT). The SPB visualized by the anti-Sad1 antibody undergoes a structural alteration from a compact dot to a crescent form at a similar stage of meiosis (Hagan and Yanagida, 1995; see Figure 5A, WT). Nonetheless, the relationship between morphological changes observed by electron microscopy and those seen by fluorescence microscopy is still unclear. In any case, this SPB modification was presumed to be indispensable for spore formation (Hirata and Shimoda, 1994). Spo15, another SPB component necessary for sporulation, is essential for the SPB modification and thus sporulation (Ikemoto et al., 2000). Because Spo13 is associated with the SPB before spore formation, we presumed that the modification of the SPB was impaired by the spo13Δ mutation. Immunofluorescence analysis by use of the anti-Sad1 antibody showed that the modified crescent-shaped SPBs were not observed in spo13Δ during the second meiotic division (Figure 5A). Fine structures of the meiotic SPB in spo13Δ were studied and compared with the SPBs of the wild-type and spo15Δ strains at the same stage. spo15Δ cells formed no apparent outer plaques, whereas the spo13 mutant was also impaired in outer plaque formation relative to wild-type cells (Figure 5B).
Figure 5.
Morphological changes in the SPBs and formation of the FSM in spo13Δ and spo2Δ. (A) Morphological changes in SPBs in spo13Δ and spo2Δ mutants. Wild-type (YN12), spo13Δ (YN48), and spo2Δ (YN442) strains were cultured in SSL-N sporulation medium and doubly stained with the anti-Sad1 antibody and DAPI. Blue, DAPI; red, Sad1. (B) Fine structures of SPBs in wild-type, spo13Δ, and spo15Δ strains. Wild-type (YN12), spo13 (MK13–2BL), and spo15Δ (SI52) cells cultured on SSA medium at 30°C for 1 d were observed by electron microscopy. Arrow and arrowheads indicate the meiotic outer plaque of SPB and nuclear envelopes, respectively. (C) FSM formation in spo13Δ, spo2Δ, and spo15Δ mutants. Wild-type (YN68), spo13Δ (YN66), spo2Δ (YN457), and spo15Δ (YN67) strains harboring integrated GFP-Psy1 were cultured in SSL-N sporulation medium and doubly stained with the anti-α-tubulin antibody TAT-1 and DAPI. Blue, DAPI; red, α-tubulin; green, GFP-Psy1.
Modification of SPBs in the spo2 deletion mutant was also analyzed by fluorescence microscopy. As Figure 5A indicates, the SPBs failed to undergo sporulation-specific alterations similar to the spo13Δ mutant. Our previous electron microscopic study indicated that no SPB modifications could be observed in spo2 mutant cells (Hirata and Shimoda, 1994). These observations clearly indicate that both Spo13 and Spo2 associate with the meiotic SPBs and play indispensable roles in normal modification of the SPB.
The FSM Is Not Formed in spo13Δ and spo2Δ Mutants
We examined assembly of the FSM visualized by GFP-Psy1 in spo2Δ, spo13Δ, and spo15Δ single mutants. Progression of meiosis was monitored simultaneously by elongation of spindle microtubules. Although the GFP-Psy1 protein appeared at the developing FSM in the wild-type strain, the FSM was not constructed in either of the three deletion mutants (Figure 5C). The inability of these mutants to form the FSM was corroborated by fluorescence microscopic observation of GFP-tagged Spo3, which is another intrinsic FSM protein (data not shown). Furthermore, the electron micrographs showed that no FSM was observed near the SPB in meiosis II (Figure 5B). These results suggest that two newly found SPB components, Spo2 and Spo13, are indispensable for initiating FSM formation, similar to Spo15.
Hierarchy of Spo15, Spo2, and Spo13 Recruitment to the SPB
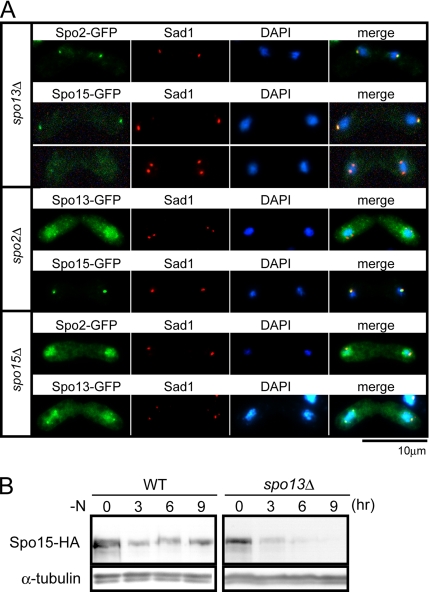
Three sporulation-specific proteins, Spo2, Spo13, and Spo15, are localized to the SPB (Figures 4 and 5; Ikemoto et al., 2000). Accurate recruitment of these proteins to the SPB during meiosis may be a prerequisite for SPB modification and for formation of the FSM. Spo15 is a constitutive component of the SPB, but Spo2 and Spo13 are synthesized de novo during meiosis. We asked whether there was a dependency hierarchy in their recruitment to the meiotic SPB. To answer this question, localization of the GFP-fused proteins was examined in the spo2Δ, spo13Δ, and spo15Δ mutants (Figure 6A). In spo13Δ cells, Spo2 and Spo15 were localized normally to the SPB. In spo2Δ cells, Spo15 preferentially localized at the SPB, whereas a faint Spo13-GFP signal was observed at the SPBs, but mostly in the nucleus. In spo15Δ cells, both Spo13 and Spo2 failed to localize to the SPB and accumulated in the nucleus. We conclude that recruitment of these proteins to the SPB was not interdependent, but hierarchic in the order: Spo15, Spo2, and Spo13. We speculate that after induction of meiosis, Spo2 is initially recruited to the SPB, depending on pre-existing Spo15. Next, Spo13 is recruited to the SPB, dependent on Spo2 or on both Spo2 and Spo15.
Figure 6.
Hierarchic recruitment of Spo13, Spo2, and Spo15 to the SPB. (A) Localization of Spo13, Spo2, and Spo15. Top, localization of Spo2 and Spo15 in the spo13Δ mutant. spo13Δ cells harboring integrated spo2-GFP (YN391) or spo15-GFP (YN90) were cultured in SSL-N sporulation medium. Middle, Spo13 and Spo15 localization in spo2Δ. spo2Δ cells harboring integrated spo13-GFP (YN459) or spo15-GFP (YN389) were cultured in SSL-N sporulation medium. Bottom, Spo2 and Spo13 localization in the spo15Δ mutant. spo15Δ cells harboring integrated spo2-GFP (YN388) or spo13-GFP (YN471) were cultured in SSL-N sporulation medium. Fixed cells at different stages of meiosis were examined after staining by DAPI and GFP and with the anti-Sad1 antibody. (B) Time course of Spo15 levels during meiosis. Wild-type cells harboring integrated spo15-HA (YN77) and spo13Δ cells harboring integrated spo15-HA (YN78) were precultured overnight in SSL+N medium and then transferred to SSL-N sporulation medium. Cells entered meiosis II at 6 h. Protein extracts were prepared at intervals and subjected to Western blot analysis with the anti-HA antibody and with the anti-α-tubulin antibody as the loading control.
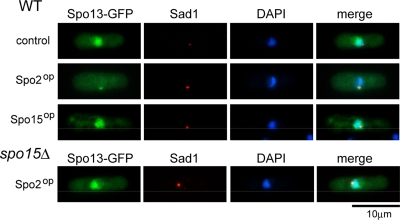
To confirm the dependency of Spo13 localization on Spo2, both proteins were expressed ectopically in mitotically growing cells. First, Spo13-GFP was overexpressed under the control of the nmt1 promoter. As shown in Figure 7, Spo13-GFP did not localize to the SPB, but accumulated in the nucleus. Simultaneous overexpression of Spo15 did not stimulate localization of Spo13-GFP to the SPB (Figure 7). Ectopically expressed Spo2-GFP localized to the SPB in vegetative cells (data not shown). Interestingly, when Spo13-GFP was coexpressed with Spo2 in vegetative cells, Spo13-GFP localized to the SPB (Figure 7). This targeted localization was not observed in spo15Δ cells (Figure 7). These results verify that recruitment of Spo13 to the SPB is dependent on Spo2 in the presence of Spo15.
Figure 7.
Localization of ectopically expressed Spo13 and Spo2 during vegetative growth. TN104 cells harboring pREP41(spo13-GFP) together with either pREP1A [control], pREP1A(spo2) [Spo2op], or pREP1A(spo15) [Spo15op] were incubated in MM+N medium at 28°C for 16 h. YN47 (spo15Δ) cells harboring pREP41(spo13-GFP) and pREP1A(spo2) were incubated similarly. Fixed cells were examined after staining by DAPI and GFP, and with the anti-Sad1 antibody.
Spo13 and Spo2 are transiently expressed during meiosis, primarily due to transcriptional activation of spo13+ and spo2+. In contrast, Spo15 is retained in wild-type cells during vegetative growth and is relatively stable during meiosis (Ikemoto et al., 2000). We noticed that the Spo15-GFP fluorescent signal became faint after meiosis II in spo13Δ (Figure 6A), suggesting that levels of Spo15 diminish after meiosis II in the absence of Spo13. The abundance of Spo15 was assayed by Western blotting. Spo15 levels in the spo13Δ mutant immediately decreased when cells proceeded to meiosis II (6 h), whereas levels were retained in wild-type cells (Figure 6B). We conclude that Spo13 localization to the SPB is dependent on pre-existing Spo15 and that the presence of Spo13 in turn stabilizes Spo15.
Physical Interaction of Three Sporulation-specific SPB Component Proteins
Because localization experiments and mutant phenotypes suggested that Spo2, Spo13, and Spo15 might function collaboratively in FSM assembly, we tested for physical interactions among these proteins by use of the budding yeast two-hybrid assay. The spo2-, spo13-, and spo15-coding regions were fused with the GAL4 activation domain or with the binding domain on appropriate plasmids (Figure 8A). The plasmids were introduced into the S. cerevisiae tester strain (AH109) so that transcriptional activation could be assessed by growth on histidine-free selection plates. Physical interactions between Spo15 and Spo2 and between Spo13 and Spo2 were positive, whereas an interaction between Spo13 and Spo15 appeared to be negative (Figure 8A). These two-hybrid results are consistent with an indirect interaction between Spo13 and Spo15 mediated by Spo2.
Figure 8.
Physical interactions among Spo15, Spo13, and Spo2. (A) Yeast two-hybrid analysis. spo15, spo13, and spo2 ORFs were fused with a GAL4-activation domain (AD) or a GAL4-DNA–binding domain (BD). The plasmids carrying these fusion constructs were introduced into S. cerevisiae tester strain (AH109). Transformants were assayed for growth on SD-histidine medium. (B) Top, GST or GST-Spo2 was coexpressed with GFP-tagged Spo13 in strain TN29. Whole cell lysates (input) and proteins bound to glutathione beads (GSH) were analyzed by Western blotting using anti-GFP and anti-GST antibodies (α-GFP and α-GST, respectively). Asterisks indicate nonspecific bands. Bottom, GFP or Spo2-GFP was expressed in strain TN8. Whole cell lysates (input) were subjected to immunoprecipitation with the anti-GFP antibody (IP). Precipitates were analyzed by Western blotting using the anti-Spo15 and the anti-GFP antibodies (α-Spo15 and α-GFP, respectively).
These results led to analysis of possible in vivo physical interactions in S. pombe between Spo2 and Spo13, and Spo2 and Spo15. To facilitate these studies, strains expressing GST- or GFP-tagged versions of Spo2, Spo13, and Spo15 were constructed (see Materials and Methods). Because these polypeptides could not be detected by Western blotting in lysates prepared from sporulating cells, cell-free extracts were prepared from vegetative cells. Initially, GST-fused Spo2 and Spo13-GFP were ectopically expressed under the control of nmt41 or nmt42 promoters. When GST-Spo2 was pulled down using glutathione beads, Spo13-GFP was copurified, but not with the GST-only control (Figure 8B, top panel). Spo15 was found to coprecipitate with Spo2-GFP but not with the GFP-only control, suggesting that these proteins bound in vivo (Figure 8B, bottom panel). In contrast to these combinations of proteins, no interaction was detected between Spo13 and Spo15 (data not shown). We conclude that Spo2 can physically interact with both Spo13 and Spo15 in S. pombe.
DISCUSSION
In the present study, we isolated and analyzed two sporulation-specific genes, spo13+ and spo2+. The encoded products are expressed during meiosis and are localized to the SPB. Both SPB modification and initiation of the FSM formation are severely impaired in the corresponding deletion mutants.
Both spo13+ and spo2+ are transcriptionally induced during meiosis I, in contrast to spo15+ that is transcribed constitutively. Like several other sporulation-specific genes, transcriptional activation of spo13+ and spo2+ is under the control of a forkhead transcription factor Mei4. Consistent with this, we found the Mei4-binding motif, called FLEX, at the 5′ promoter-like region. Two FLEX motifs found in spo2+ and one FLEX in spo13+ are canonical, in that all are composed of a core heptamer, GTAAACA, flanked by AT-rich stretches (Horie et al., 1998; Abe and Shimoda, 2000).
The SPB plays two different roles during meiosis; one is for microtubule assembly and the other for construction of the FSM. The latter function is accompanied by meiotic SPB modification, the addition of outer plaques to the mitotic SPB. In the present study, fluorescence microscopy revealed that Spo13 and Spo2 are localized to the cytoplasmic side of the SPB, where the FSM initiates. Recruitment of Spo13 and Spo2 is dependent on Spo15, a large coiled-coil protein that colocalizes with several core SPB components (Ikemoto et al., 2000). Spo15 may serve as a structural scaffold for the meiosis-specific outer layer of the SPB, likely mostly composed of Spo13 and Spo2, and specialized for FSM assembly. In S. cerevisiae, the outer plaque fully develops by recruiting novel components, Mpc54, Mpc70/Spo21, Spo74, and Ady4 (Knop and Strasser 2000; Nickas et al., 2003). This modified outer plaque has been referred to as meiotic plaque. Here, we propose that S. pombe outer plaques should also be called “meiotic plaques.” Except for Mpc54 and Mpc70/Spo21 that are coiled-coil proteins like Spo15 and Spo13, there is no sequence similarity between meiotic plaque components in S. pombe and S. cerevisiae. The leading edge of the FSM plays a critical roles in FSM morphogenesis. The leading edge is coated by several proteins including Don1, Ssp1, and Ady3 in S. cerevisiae (Knop and Strasser 2000; Nickas and Neiman, 2002). These proteins first accumulate at the cytoplasmic face of the meiotic outer plaque. Among them, Ady3 is responsible for association of the FSM with the meiotic plaque (Nickas and Neiman, 2002). Although S. pombe has no homologue for Ady3, Meu14 has been identified as an S. pombe leading edge protein (Okuzaki et al., 2003). It is possible that Meu14 is involved in the association of the FSM with components of the meiotic plaque, such as Spo13.
Spo13 and Spo2 are recruited to the SPB at meiosis I, dependent on pre-existing Spo15, whereas multilayered plaques are built at an early stage of meiosis II. Therefore, the recruitment of these sporulation-specific components per se cannot determine the timing of SPB modification. It is possible that posttranslational modification of these SPB components and/or other unknown SPB-associated proteins are responsible for triggering SPB modification.
In the absence of Spo15, Spo13 and Spo2 are unable to localize to the SPB, but do accumulate in the nucleus. Furthermore, localization of Spo13 to the SPB is dependent on Spo2, and Spo13 again accumulates in the nucleus in the absence of Spo2. We speculate that an unknown transport mechanism ferries Spo13 and Spo2 to the SPB through the nucleus.
Both Spo15 and Spo13 are proteins rich in coiled-coil domains. However, no direct interaction between Spo15 and Spo13 was detected by the yeast two-hybrid assay or by in vivo pulldown and immunoprecipitation assays. In contrast, Spo2 can physically interact with both Spo15 and Spo13. Therefore it is possible that Spo2 bridges Spo15 and Spo13. It is also possible that Spo2 performs additional functions in formation of the FSM unrelated to connecting Spo13 with Spo15. We conclude that the SPB proteins necessary for sporulation accumulate at the SPB in a hierarchic manner. In spite of the hierarchy of recruitment, Spo15 becomes unstable in the absence of Spo13 during meiosis II. This suggests that these three proteins form a tight complex in the meiotic SPB that may be protected from the elevated proteolytic activity found in sporulating cells.
Normal development of the FSM leading to a nucleated compartment likely requires continuous association with the SPB. After the FSM fully extends and its leading edge closes, the FSM is released from the SPB (T. Nakamura, unpublished data). Timing of this dissociation may possibly be determined by disappearance of Spo13. In fact, after completion of meiosis II, Spo13 disappeared from the SPB (Figure 3A and B) and could not be detected by Western analysis (Figure 2A). These observations suggest that breakdown of Spo13 may trigger disconnection of the FSM from the SPB. Whether Spo13 is actively eliminated by an unknown mechanism remains to be determined.
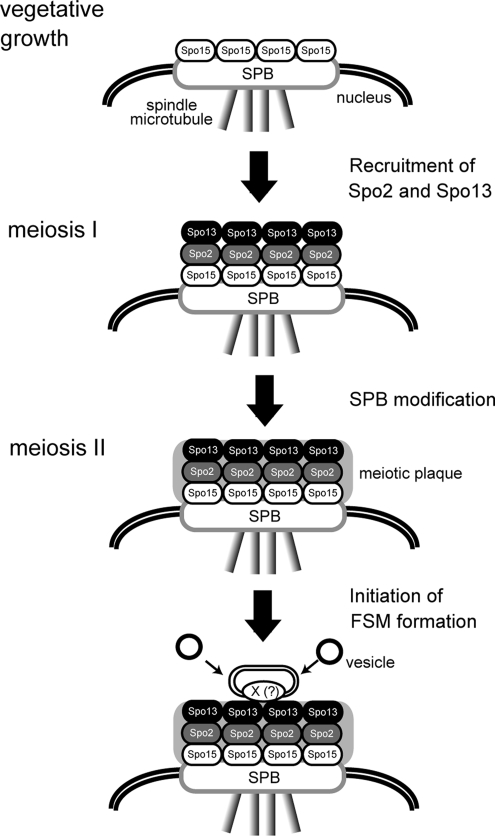
On the basis of these data, we summarize the process of SPB modification followed by FSM formation in fission yeast as depicted in Figure 9. During mitosis, the coiled-coil protein Spo15 is a component of a single plaque SPB. During meiosis I, two sporulation-specific proteins, Spo2 and Spo13, are newly produced and are recruited to the cytoplasmic side of the SPB dependent on pre-existing Spo15. When cells enter meiosis II, meiotic outer plaques composed of Spo2 and Spo13 are differentiated (SPB modification). The meiotic plaque anchors the FSM through Spo13 or an as-yet unknown protein X. The FSM that is continuously associated with the meiotic plaque expands by fusion with membrane vesicles and eventually forms a nucleated compartment called the prespore, a precursor form of spores.
Figure 9.
A model for initiation of FSM formation at the SPB.
Supplementary Material
ACKNOWLEDGMENTS
We thank Y. Watanabe of University of Tokyo for an S. pombe genomic library; O. Niwa of Kazusa DNA Research Institute for affinity-purified antibodies against Sad1; K. Gull of the University of Manchester for anti-α-tubulin antibody, TAT-1; A. Itadani of Osaka City University for anti-Spo15 antibody, and S. Fujita, Mitsubishi Kagaku Institute of Life for anti-GFP antibody. We also thank M. Yamamoto of University of Tokyo and the Yeast Genetic Resource Center of Japan supported by the National BioResource Project (NBRP/YGRC) for S. pombe strains. This study was supported by a Sasakawa Scientific Research Grant to Y.N., the Japan Securities Scholarship Foundation to Y.N., Grant-in-Aid for Scientific Research on Priority Areas “Genome Biology” to C.S., “Cell Cycle Control” and “Life of Proteins” to T.N. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and Novartis Foundation (Japan) for the Promotion of Science. M.N-K. is a recipient of the Research Fellowship for Young Scientist from the Japan Society for the Promotion of Science (JSPS).
Abbreviations used:
- FSM
forespore membrane
- GFP
green fluorescent protein
- SNARE
soluble NSF attachment protein receptor
- SPB
spindle pole body.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-02-0118) on March 26, 2008.
REFERENCES
- Aalto M. K., Ronne H., Keranen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H., Shimoda C. Autoregulated expression of Schizosaccharomyces pombe meiosis-specific transcription factor Mei4 and a genome-wide search for its target genes. Genetics. 2000;154:1497–1508. doi: 10.1093/genetics/154.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgier B. K., Malzone M., Nickas M., Neiman A. M. SPO21 is required for meiosis-specific modification of the spindle pole body in yeast. Mol. Biol. Cell. 2001;12:1611–1621. doi: 10.1091/mbc.12.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D., Nurse P. High efficiency transformation of the fission yeast Schizosaccharomyces pombe. Nature. 1981;292:140–142. doi: 10.1038/290140a0. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin; a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bresch C., Muller G., Egel R. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 1968;102:301–306. doi: 10.1007/BF00433721. [DOI] [PubMed] [Google Scholar]
- Byers B. Cytology of the yeast life cycle. The Molecular Biology of the Yeast Saccharomyces cerevisiae: Life Cycle and Inheritance. In: Strathern J. N., Jones E. W., Broach J. R., editors. New York: Cold Spring Harbor Laboratory; 1981. pp. 59–96. [Google Scholar]
- Chenchik A., Moqadam F., Siebert P. A new method for full-length cDNA cloning by PCR. In: Krieg P. A., editor. A Laboratory Guide to RNA: Isolation, Analysis, and Synthesis. New York: Wiley-Liss; 1996. pp. 273–321. [Google Scholar]
- Egel R., Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp. Cell Res. 1974;88:127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- Gutz H., Heslot H., Leupold U., Loprieno N. Handbook of Genetics. Vol. 1. New York: Plenum Press; 1974. Schizosaccharomyces pombe; pp. 395–446. [Google Scholar]
- Hagan I. M., Hyams J. S. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hagan I., Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J. Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A., Shimoda C. Electron microscopic examination of sporulation-deficient mutants of the fission yeast Schizosaccharomyces pombe. Arch. Microbiol. 1992;158:249–255. doi: 10.1007/BF00245240. [DOI] [PubMed] [Google Scholar]
- Hirata A., Shimoda C. Structural modification of spindle pole bodies during meiosis II is essential for normal formation of ascospores in Schizosaccharomyces pombe: ultrastructural analysis of spo mutants. Yeast. 1994;10:173–183. doi: 10.1002/yea.320100205. [DOI] [PubMed] [Google Scholar]
- Hirata A., Tanaka K. Nuclear behavior during conjugation and meiosis in the fission yeast Schizosaccharomyces pombe. J. Gen. Appl. Microbiol. 1982;28:263–274. [Google Scholar]
- Horie S., Watanabe Y., Tanaka K., Nishiwaki S., Fujioka H., Abe H., Yamamoto M., Shimoda C. The Schizosaccharomyces pombe mei4+ gene encodes a meiosis-specific transcriptional factor containing a forkhead DNA-binding domain. Mol. Cell. Biol. 1998;18:2118–2129. doi: 10.1128/mcb.18.4.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y., Hiramine Y., Yamamoto M. The role of cdc2 and other genes in meiosis in Schizosaccharomyces pombe. Genetics. 1995;140:1235–1245. doi: 10.1093/genetics/140.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S., Nakamura T., Kubo M., Shimoda C. S. pombe sporulation-specific coiled-coil protein Spo15p is localized to the spindle pole body and essential for its modification. J. Cell Sci. 2000;113:545–554. doi: 10.1242/jcs.113.3.545. [DOI] [PubMed] [Google Scholar]
- Jensen R., Sprague G. F., Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc. Natl. Acad. Sci. USA. 1983;80:3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasama T., Shigehisa A., Hirata A., Saito T. T., Tougan T., Okuzaki D., Nojima H. Spo5/Mug12, a putative meiosis-specific RNA-binding protein, is essential for meiotic progression and forms Mei2 dot-like nuclear foci. Eukaryot. Cell. 2006;5:1301–1313. doi: 10.1128/EC.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida M., Shimoda C. Genetic mapping of eleven spo genes essential for ascospore formation in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 1986;10:443–447. doi: 10.1007/BF00419871. [DOI] [PubMed] [Google Scholar]
- Knop M., Strasser K. Role of the spindle pole body of yeast in mediating assembly of the prospore membrane during meiosis. EMBO J. 2000;19:3657–3667. doi: 10.1093/emboj/19.14.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting colied-coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Masai H., Miyake T., Arai K. hsk1+, a Schizosaccharomyces pombe gene related to Saccharomyces cerevisiae CDC7, is required for chromosomal replication. EMBO J. 1995;14:3094–3104. doi: 10.1002/j.1460-2075.1995.tb07312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. 1993 doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:793–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Kishida M., Shimoda C. The Schizosaccharomyces pombe spo6+ gene encoding a nuclear protein with sequence similarity to budding yeast Dbf4 is required for meiotic second division and sporulation. Genes Cells. 2000;5:463–479. doi: 10.1046/j.1365-2443.2000.00343.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nakamura-Kubo M., Hirata A., Shimoda C. The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1+ encoding syntaxin-like protein. Mol. Biol. Cell. 2001;12:3955–3972. doi: 10.1091/mbc.12.12.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Nakamura-Kubo M., Nakamura T., Shimoda C. Novel fission yeast Cdc7-Dbf4-like kinase complex required for the initiation and progression of meiotic second division. Mol. Cell. Biol. 2002;22:309–320. doi: 10.1128/MCB.22.1.309-320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Kubo M., Nakamura T., Hirata A., Shimoda C. The fission yeast spo14+ gene encoding a functional homologue of budding yeast Sec12 is required for the development of forespore membranes. Mol. Biol. Cell. 2003;14:1109–1124. doi: 10.1091/mbc.E02-08-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y., Nakamura T., Hirata A., Routt S. M., Skinner H. B., Bankaitis V. A., Shimoda C. The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in FSM formation. Mol. Biol. Cell. 2001;12:901–917. doi: 10.1091/mbc.12.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y., Nakamura T., Okazaki K., Hirata A., Shimoda C. The Sec14 family glycerophospholipid-transfer protein is required for structural integrity of the spindle pole body during meiosis in fission yeast. Genes Cells. 2004;9:1275–1286. doi: 10.1111/j.1365-2443.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- Nickas M. E., Neiman A. M. Ady3p links spindle pole body function to spore wall synthesis in Saccharomyces cerevisiae. Genetics. 2002;160:1439–1450. doi: 10.1093/genetics/160.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickas M. E., Schwartz C., Neiman A. M. Ady4p and Spo74p are components of the meiotic spindle pole body that promote growth of the prospore membrane in Saccharomyces cerevisiae. Eukaryot. Cell. 2003;2:431–445. doi: 10.1128/EC.2.3.431-445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuzaki D., Satake W., Hirata A., Nojima H. Fission yeast meu14+ is required for proper nuclear division and accurate forespore membrane formation during meiosis II. J. Cell Sci. 2003;116:2721–2735. doi: 10.1242/jcs.00496. [DOI] [PubMed] [Google Scholar]
- Rabitsch K. P., et al. A screen for genes required for meiosis and spore formation based on whole-genome expression. Curr. Biol. 2001;11:1001–1009. doi: 10.1016/s0960-9822(01)00274-3. [DOI] [PubMed] [Google Scholar]
- Shimoda C. Forespore membrane assembly in yeast: coordinating SPBs and membrane trafficking. J. Cell Sci. 2004;117:389–396. doi: 10.1242/jcs.00980. [DOI] [PubMed] [Google Scholar]
- Shimoda C., Nakamura T. Control of late meiosis and ascospore formation. In: Egel R., editor. Molecular Biology of Schizosaccharomyces pombe. Berlin: Springer; 2003. pp. 311–327. [Google Scholar]
- Tanaka K., Hirata A. Ascospore development in the fission yeasts Schizosaccharomyces pombe and S. japonicus. J. Cell Sci. 1982;56:263–279. doi: 10.1242/jcs.56.1.263. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yonekawa T., Kawasaki Y., Kai M., Furuya K., Iwasaki M., Murakami H., Yanagida M., Okayama H. Fission yeast Eso1p is required for establishing sister chromatid cohesion during S phase. Mol. Cell. Biol. 2000;20:3459–3469. doi: 10.1128/mcb.20.10.3459-3469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc. Natl. Acad. Sci. USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Sherwin T., Sasse R., MacRae T. H., Baines A. J., Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- Ye Y., Fujii M., Hirata A., Kawamukai M., Shimoda C., Nakamura T. Geranylgeranyl diphosphate synthase in fission yeast is a heteromer of farnesyl diphosphate synthase (FPS), Fps1 and an FPS-like protein, Spo9, essential for sporulation. Mol. Biol. Cell. 2007;18:3568–3581. doi: 10.1091/mbc.E07-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B. Y., Calleja G. B., Johnson B. F. Ultrastructural changes of the fission yeast (Schizosaccharomyces pombe) during ascospore formation. Arch. Mikrobiol. 1973;91:1–10. doi: 10.1007/BF00409533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.