Abstract
During spermatid individualization in Drosophila, actin structures (cones) mediate cellular remodeling that separates the syncytial spermatids into individual cells. These actin cones are composed of two structural domains, a front meshwork and a rear region of parallel bundles. We show here that the two domains form separately in time, are regulated by different sets of actin-associated proteins, can be formed independently, and have different roles. Newly forming cones were composed only of bundles, whereas the meshwork formed later, coincident with the onset of cone movement. Polarized distributions of myosin VI, Arp2/3 complex, and the actin-bundling proteins, singed (fascin) and quail (villin), occurred when movement initiated. When the Arp2/3 complex was absent, meshwork formation was compromised, but surprisingly, the cones still moved. Despite the fact that the cones moved, membrane reorganization and cytoplasmic exclusion were abnormal and individualization failed. In contrast, when profilin, a regulator of actin assembly, was absent, bundle formation was greatly reduced. The meshwork still formed, but no movement occurred. Analysis of this actin structure's formation and participation in cellular reorganization provides insight into how the mechanisms used in cell motility are modified to mediate motile processes within specialized cells.
INTRODUCTION
One of the best-studied roles of the actin cytoskeleton is mediating cell and intracellular motility (Carlier et al., 2003; Ridley et al., 2003; Carlier and Pantaloni 2007). Several different types of actin structures are known to contribute to motility. Filopodia, which contain parallel bundles of actin filaments, are thought to be important for exploring the cells' environment and making initial contacts with substrate for movement in a particular direction. Lamellipodial meshwork is proposed to push the leading edge of the cell forward using the force generated by addition of actin monomers at the barbed ends of the filaments, which face the membrane (Pollard and Borisy 2003). Behind the leading edge, a lamellar region consisting primarily of bundles that contain tropomyosin and myosin II plays an important role in cell movement (Gupton et al., 2005). In addition, stress fibers, composed of antiparallel actin filament bundles connected to adhesion complexes, are important for traction and contractile forces that release the cell–substrate attachments so that forward movement can occur.
The different actin structures and domains are regulated by different sets of actin-associated proteins. Much is known about control of assembly of the two main types of actin organizations, meshwork and bundles, from biochemical analysis in vitro and studies of motile cells in vivo. Actin meshwork is nucleated by the Arp2/3 complex, and the branched organization relies on the ability of the complex to bind to the side of an actin filament and nucleate a new filament (Goley and Welch, 2006). Parallel bundles are nucleated by formins, which associate with the barbed ends, but allow monomer addition while remaining bound (Goode and Eck, 2007). For some formins, barbed-end monomer addition requires the presence of profilin (Romero et al., 2004; Kovar et al., 2006), a monomer binding protein. In the case of both meshwork and bundles, other proteins work in conjunction with the nucleators to further regulate organization, polymerization dynamics, attachments, and other aspects important for function.
The mechanisms that control the formation and maintenance of actin structures in specialized cell types are less well understood. Actin structures in differentiating and differentiated cells are important for shape, connections to other cells and the extracellular matrix, and the cell's specialized features that are involved in its physiological roles in the context of the tissue and organism (Revenu et al., 2004). In general, actin structures in differentiated cells appear to be organized based on the same principles as the lamellipodial meshwork, filopodial and lamellar bundles, and stress fibers, but often they are significantly different in size and dynamic properties than those in motile cells (for some examples see Tyska and Mooseker 2002; Tilney and DeRosier 2005; Sekerkova et al., 2006). To understand how the principles and activities that have been characterized using motile cells as models can be applied widely to actin in the context of differentiated cell function, exploring the formation and function of a variety of the specialized actin structures found in a number of different cell types is important.
One model system that presents an interesting case of intracellular motility in the context of a developmental process is Drosophila spermatid individualization. Preceding individualization, the germline precursors of the sperm undergo mitotic and meiotic divisions with incomplete cytokinesis to generate cysts of 64 syncytial spermatids. The cysts elongate and elaborate axonemes. Finally, the syncytial spermatids are separated into individual cells in a process called individualization. Individualization reorganizes the syncytial spermatid membrane and removes most of the cytoplasmic contents (Tokuyasu et al., 1972; Fabrizio et al., 1998; Hicks et al., 1999; Noguchi and Miller 2003). Structures called actin cones mediate this cellular remodeling. Actin cones assemble around the sperm nuclei and travel away from the nuclei along the length of the axoneme, synchronously at constant speed, over a distance of ∼2 mm, taking ∼10 h. The pushing out of the cytoplasm and organelles by the actin cones during individualization results in the formation of a “cystic bulge” of accumulated cell contents ahead of the cones. After individualization is complete, the membrane completely and tightly encloses each nucleus, axoneme, and mitochondrial derivative complex, and most of the cytoplasm and organelles are gone.
We have previously studied the individualization process (Hicks et al., 1999; Rogat and Miller, 2002; Noguchi and Miller, 2003) and determined that the actin cones have two domains, a rear region of parallel bundles and a front region of meshwork (Noguchi et al., 2006). We also showed that myosin VI is important for stabilization of the actin cones as they move (Noguchi et al., 2006). In the present work, we examine how and when each part of the cone is formed and the function of each part during cone movement and cellular reorganization. We find that the two regions form by different polymerization mechanisms, at different times, and play different roles in cone movement and individualization. Surprisingly, the meshwork is not required for movement. Instead, the bundles are important. By analogy to a steam locomotive, the bundles serve as the locomotive and the meshwork serves a structural role, functioning similarly to a “cow catcher,” pushing the cytoplasm and organelles in front of it. This system provides an example of how the basic mechanisms in play in motile cells are modified to achieve a different result in the context of cellular differentiation in vivo.
MATERIALS AND METHODS
Fly Mutants
The arp3 mutant (arp3/Tm6B,Tb) stock and the profilin mutant (chic7886/Cyo) stock were obtained from Lynn Cooley (Yale University) and the Bloomington Drosophila stock center, respectively. Fly stocks were maintained and crosses were performed under standard conditions, except where noted, with Oregon R serving as the wild-type control.
Antibodies
Anti-D. melanogaster-arp3 antibody was a kind gift of Bill Theurkauf (University of Massachusetts Medical Center, Worcester, MA; Stevenson et al., 2002). Anti-quail and anti-singed antibodies were made by Lynn Cooley (Yale University; New Haven, CT) and obtained from the Developmental Studies Hybridoma Bank. Anti-myosin VI mAb (3C7) was described previously (Kellerman and Miller, 1992).
Isolation and Primary Culture of Cysts
Primary culture of individualizing cysts was carried out as previously described (Cross and Shellenbarger, 1979; Noguchi and Miller, 2003). The arp3 mutation causes lethality at a late pupal stage (Hudson and Cooley, 2002), so testes could not be obtained from adults. Therefore, live homozygous arp3 mutant pupae were identified by the lack of the Tb (short body length) marker, which is present on the balancer chromosome, at a late stage (with dark visible wings) when spermatogenic cysts were viable in culture. The pupal arp3 mutant testes were dissected and contained some elongated cysts and some individualizing cysts. Elongated cysts were dissected and cultured.
Immunofluorescence Microscopy
Immunostaining of isolated spermatogenic cysts from various mutants was performed as described previously (Noguchi and Miller, 2003). Specimens were examined using laser scanning microscopy (LSM; Leica, Iyna, Germany) with a 40× lens at 4× digital zoom. For DNA/F-actin staining, DNA was stained with 1 μM DAPI and F-actin was stained using Alexa-568-phalloidin. Specimens were examined with a Nikon inverted microscope equipped with a cooled CCD camera (CoolSNAP ES, Photometrics, Woburn, MA) driven by Metavue software (Universal Imaging, West Chester, PA).
Quantitation of F-actin in actin cones before the onset of individualization was performed by measuring fluorescence intensity of actin cones in isolated cysts. Actin cone bundles associated with sperm nuclei as judged by F-actin/DNA staining were selected for examination. Images were obtained using an Olympus ASW LSM (Melville, NY) with a 10× lens, and the signal intensity of actin cones was measured and processed using ImageJ (http://rsb.info.nih.gov/ij/) and Excel software (Microsoft, Redmond, WA). Quantitation of actin amount in cones that had moved was performed as previously described (Noguchi et al., 2006). Length and width of actin cones that had moved was measured in high-magnification images (40× lens) using ImageJ software. Cones that were not associated with nuclei were selected for examination. Student's t tests were performed to evaluate the significance of differences in measurements between genotypes.
Electron Microscopy
For cross sections of spermatogenic cysts, dissected testes from adult male flies were fixed with 1.5% glutaraldehyde, postfixed in 1% OsO4, and embedded in PolyBed 812 resin (Polysciences, Warrington, PA) using the procedure described previously (Noguchi et al., 2006). Stained sections (60–70 nm) were cut using a Leica UTC ultramicrotome and then examined using a JEOL EM 1010 transmission electron microscope (Peabody, MA).
Myosin Subfragment 1 (S1) Fragment Decoration
Purification of rabbit skeletal myosin II and preparation of S1 subfragment were carried out using conventional methods (Margossian and Lowey, 1982). For myosin II S1 fragment decoration, isolated cysts were permeabilized with 0.1% saponin and treated with 4 mg/ml S1 fragment using the procedure described previously (Noguchi et al., 2006). For this study we used cysts that were classified into three different stages by examination of morphology under a dissection microscope: 1) before cone movement, a very early stage without any signs of cystic bulge formation, 2) an early stage of individualization, with a small cystic bulge near the end of the cyst, and 3) individualizing cysts with the cystic bulge positioned between one-fourth and one-third of the cyst length. To obtain the five samples for which actin cone organization in early cones could be seen that were used in this study, we performed six to seven experiments. In each experiment testes were dissected from ∼50 males, and 60–70 cysts at the right stage were selected. Although the cysts were handled carefully under a dissection microscope, the majority of them were lost during the long procedure. In addition, only a subset had actin cones. In most cases one preparation yielded one to three S1 decorated samples at the end. After sectioning, some samples were not oriented properly to see cones in sections, resulting in only a few cysts that yielded results.
chic7886/chic7886 mutant cysts do not form any cystic bulges, so individualizing cysts cannot be identified. To identify cysts that had cones, actin was stained with Alexa488-phalloidin during extraction with 0.1% saponin. Cysts with actin staining were selected using a fluorescence dissection microscope, and S1 decoration was performed as previously described (Noguchi et al., 2006). S1-decorated cysts were fixed with 1% glutaraldehyde and 0.2% tannic acid, postfixed in 2% OsO4, and embedded in Poly/Bed 812 resin as described previously (Noguchi et al., 2006). Ultrathin sections were cut, stained, and examined as described above.
RESULTS
The Two Domains of Actin Cones Form at Different Times with Different Organizations
We wanted to determine how the actin cones formed and the structural changes that occurred when movement initiated. To examine actin cone structure at the earliest time points, fully elongated cysts with no signs of individualization were selected from cultures (Noguchi and Miller, 2003). Such cysts were fixed and processed for myosin S1 fragment decoration and EM visualization as previously described (Noguchi et al., 2006). Cysts were positioned so that the ends where the nuclei were present were sectioned longitudinally.
We saw two different organizations of actin in cones of early cysts. In most early cysts, the cones contained only bundles (four of five early cysts, 12 cones). In these cones, loose bundles of actin filaments surrounded the nuclei (Figure 1, A–C). On the side of the nuclei away from the direction of cone movement (large arrow in Figure 1A), the space between the syncytial membrane (arrows, B and C) and the nuclei was small, and the whole area was filled with actin bundles that ran parallel to the long axis of the cyst (Figure 1, B–D). Along the sides of the nuclei, the membrane was also close and the area was filled with parallel bundles. In the region in front of and further from the nuclei, where axonemes/mitochondria were observed, there was a slight shift in the actin (Figure 1C, front). Some of the filaments in this region did not lie parallel to the long axis of the cysts, but instead were at an angle (Figure 1, C and E).
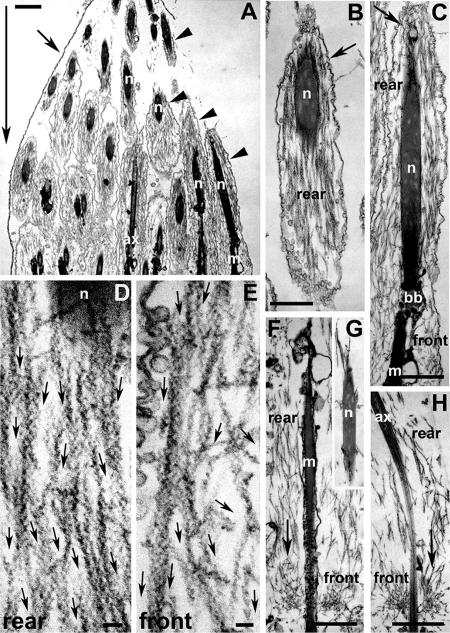
Figure 1.
Ultrastructure of S1 decorated wild-type early actin cones, before the onset of movement. (A) Longitudinal section of a single cyst terminal end (where the condensed spermatid nuclei reside). Large arrow indicates the eventual direction of cone movement. This cyst did not show any signs of cystic bulge formation. The cyst cell membrane (small arrow) can be seen enclosing some actin cones (arrowheads). (B) The rear domain of an early cone was composed of actin bundles near and around the spermatid nucleus. Most of the actin bundles were parallel to the longitudinal axis of the cone. The syncytial membrane (arrow) is indicated. (C) Longitudinal section of a whole early cone. The cone was composed of actin bundles in the rear domain (around nucleus) and short actin filaments in the front domain. Small arrow indicates the syncytial membrane. (D and E) High-magnification images of the early cone rear (D) and front (E) domains. Small arrows indicate the direction of the pointed end of each actin filament, as judged by the “arrowhead” shape generated by the myosin II S1 fragment that was used to decorate the filament. (D) The rear domain was composed of parallel-bundled F-actin with pointed ends facing in the direction of (eventual) movement. (E) Actin filaments in the front domain were less bundled, and some were oriented more perpendicular to the cyst long axis, but the pointed ends still pointed forward. (F–H) In one cyst with cones near the nuclei and no cystic bulge, a small amount of actin meshwork was visible at the front of the cones (small arrows). In the rear domain, actin bundles parallel to the longitudinal axis of the cones are visible. (G) Around a spermatid nucleus located behind an actin cone with a little meshwork, only a few actin filaments were visible; ax, axoneme; bb, basal body; m, mitochondria; n, nucleus. Bar, (A–C and F–H) 1 μm; (D and E) 0.1 μm.
In one of the five early cysts (three cones), even though there were no signs of cystic bulge formation, a very small amount of meshwork was present on cones that were very near the nuclei (Figure 1, F and H). Parallel bundles were present in the region in front of the nuclei, around the basal bodies, and axonemes and formed most of the cone. In this cyst, very few actin filaments were visible in the region surrounding the nuclei (Figure 1G). The meshwork was present only at the front of the cones (relative to the direction of movement). We interpret this as an example of a later stage than the cones with no meshwork, in which the cones had just initiated movement (see below).
We evaluated filament orientation in both of these types of cones. We quantitated filament orientation by classifying filaments in the front and rear domains as parallel or perpendicular to the long axis of the cyst, and noted the direction of the barbed end, as determined by S1 decoration (Figure 2A). We determined that the filaments were primarily oriented with barbed ends facing away from the direction of movement and toward the membrane surrounding the back of the cone in both cones with no meshwork and cones with a small amount of meshwork (Figure 2B). This is similar to our observations of filament orientation in moving cones (Noguchi et al., 2006). These data show that overall orientation of the filaments is the same throughout assembly and movement.
Figure 2.
Quantitation of filament orientation. (A) Schematic diagram showing the criteria used to count the number of actin filaments with different orientations. Large black arrow indicates longitudinal axis of the actin cone pointing in the direction of cone movement. Along this axis, the area was divided into three sections. In cases where the barbed end was in area a, the filament would be counted as parallel facing backward (opposite the direction of movement; for example, black filament). Filaments with their barbed ends in area c were counted as parallel facing forward (in the direction of movement). If the barbed end was in area b, the filament was classified as lying perpendicular to the direction of movement (for example, white filament). The orientation of perpendicular filaments was classified as either barbed end facing the outside edge of the cone where it is surrounded by membrane or the center where the axoneme lies. (B) Graph of filament orientation. For cones with no meshwork (as in Figure 1, A–E), n = 425 and 317 for front and rear, respectively, whereas for cones with a little meshwork (as in Figure 1, F–H), n = 118 and 119 for front and rear, respectively.
When cysts were selected that had even the smallest signs of cystic bulge formation, which indicated that cone movement had begun, we observed that cones always had some meshwork. In cones that were still near the nuclei, a small amount of meshwork was seen (Figure 3A), and as the bulges got bigger and farther away from the nuclei, the amount and extent of meshwork increased. (Figure 3B; Noguchi et al., 2006). Thus, before movement began, the cones had no meshwork and were entirely made up of bundles. The meshwork formed coincident with initiation of movement.
Figure 3.
Ultrastructure of S1 decorated actin cones at various stages of individualization. (A) Actin cones right after onset of movement, from a cyst that had a small cystic bulge near its basal end. These cones had actin meshwork at the front (arrows). (B) Actin cones from a cyst with the cystic bulge positioned one-fourth to one-third of the cyst length from the end of the cyst where the nuclei are located. As the cones moved farther away from the nuclei, the amount and extent of meshwork increased (Figure 3 A and B). The rear domains were composed of actin bundles parallel to the longitudinal axis of the cyst, similar to other stages. Large arrows in A and B indicate the direction of movement. ax, axoneme; m, mitochondria. Bar, 1 μm.
Localization of Actin-regulating Proteins on Cones
Because the two structural domains are formed separately in time and have different organizations, it seemed likely that different actin-regulating proteins would be involved in generating each part. We previously observed that the Arp2/3 complex and myosin VI were strongly enriched at the cone fronts once movement was underway (Rogat and Miller, 2002). If the Arp2/3 complex is important for meshwork formation, we would predict that it should become enriched at the cone front, where the meshwork is formed, upon initiation of movement. We would also predict myosin VI would become enriched at the front coincident with Arp2/3 complex, because myosin VI function is required for Arp2/3 complex accumulation there (Noguchi et al., 2006). We quantitated the distribution of arp3 (representing Arp2/3 complex localization) and myosin VI on cones that had not yet moved and cones that had initiated movement. Cones that had not moved were identified as those with a rectangular shape that were associated with DAPI-stained nuclei. Myosin VI (Figure 4Aa) and arp3 (Figure 4Ab) were present at levels above background in about half of the population of cysts that contained cones with these characteristics (myosin VI: 47 ± 9%, arp3: 54 ± 14%; n = 3 experiments; ∼30 cysts in each experiment). Among these, the vast majority showed uniform staining along the length, with no concentration on the front (myosin VI: 88 ± 6%, arp3: 100%).
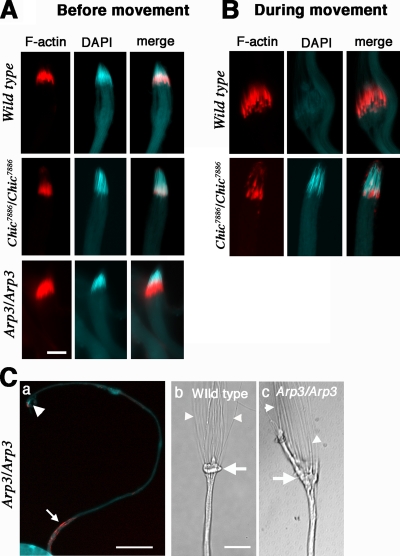
Figure 4.
Localization of various actin-regulating proteins on actin cones. Double staining of actin-regulating proteins and F-actin before (A) and during (B) movement of the cones. Localization of myosin VI (a), Arp3 (b), quail (c), and singed (d) are shown in the left column (green). Alexa-568-phalloidin staining of actin cones is shown in the middle column (red; a′–d′). The merged images are shown in the right column (a″–d″). The intensity of staining before movement begins was generally much lower than during movement. The intensities in the images in A were adjusted to be comparable to that in B. Schematic representations of localization of actin-regulating proteins on actin cones, before (C) and during (D) cone movement. Bar, (A) 10 μm; (B) 5 μm.
During movement, actin cones are triangular in shape with the thicker portion (triangle's base) facing in the direction of movement (forward). Most cysts with triangular-shaped cones had myosin VI (Figure 4Ba; 92 ± 3%, n = 3 experiments, ∼30 cysts in each experiment) and arp3 (Figure 4Bb; 88 ± 17%) at the front. Only a very few showed no concentration at the front with staining evenly spread over the cones (not shown; myosin VI: 3 ± 3%; arp3: 4 ± 4%). The rest showed no concentration above background. The polarized distribution was maintained throughout movement. This tight coupling of polarization of these actin regulators with the onset of movement is consistent with our observations above, that formation of the meshwork, which is likely to be an Arp2/3 complex–nucleated process, shows strict correlation with the start of movement.
Because the rear of the cones is composed of actin bundles, it seemed likely that actin-bundling proteins would be present in this domain. We examined the distribution of two actin-bundling proteins: quail (Mahajan-Miklos and Cooley, 1994) and singed (Cant et al., 1994). Quail is an ortholog of villin that bundles actin filaments, but does not sever in vitro and is important for bundle formation during oogenesis (Mahajan-Miklos and Cooley, 1994), whereas singed is a fascin ortholog that is important for actin bundle formation both during oogenesis and in bristles (Cant et al., 1994). As cones formed and before movement, when the cones contained only bundles, both quail (villin) and singed (fascin) were present all over the cones (Figure 4A, c and d). Quail (villin) is concentrated at levels well above background on the cones at this early stage, whereas singed (fascin) appears to be less enriched. After movement began, both these proteins' distributions changed (Figure 4B, c and d). Both were enriched in the rear region where bundles were present and absent from the front region where the meshwork was present. However, their distributions were not identical. Quail (villin) occupied the middle portion and singed (fascin) localized to the extreme rear of the cones (compare the overlays in Figure 4B, c″ and d″). It is likely that these actin-bundling proteins contribute to formation and stabilization of actin cone bundles. The distributions of these four actin-associated proteins (quail, singed, arp3, and myosin VI) show that the two structural domains have functionally different actin-regulating proteins associated with them. The proteins present in each domain have activities that are consistent with a role in generating and/or stabilizing those domains. These results also reveal that the actin cone structure is complex with at least four distinct regions based on protein composition (see schematic diagrams, Figure 4, C and D).
The Front Meshwork Requires Arp2/3 Complex to Form
Because of the very different organization at the front and rear, we anticipated that they would be built using two different actin polymerization mechanisms. The front meshwork structure is consistent with Arp2/3 complex–mediated branched network formation. We disrupted Arp2/3 complex function using a mutation in the arp3 gene. The arp3 mutation is homozygous lethal at the pupal stage, so we could not examine testes from adults. However, if larvae are grown in optimal conditions, the homozygous mutant animals survive until the very late pupal stage. Late stage pupae were dissected to obtain testes, and cysts were isolated and cultured. Fully elongated cysts were present and individualizing cysts could be observed, but individualization was abnormal (see Supplemental Data, movie of individualization of arp3 mutant cysts, and below). F-actin staining revealed that arp3 mutant cones in individualizing cysts were not normal in shape, but instead appeared very narrow, much more like cones in wild type that had not initiated movement (Figure 5Aa′). Measurement of the width of moving cones in the arp3 mutant supported the idea that they are narrower than normal (arp3 mutant: 1.0 ± 0.3 μm [n = 12]; wild type: 1.8 μm ± 0.3 [n = 23]; p < 0.0001). arp3 mutant moving cones also contained less actin than wild-type cones, as shown by the intensity of actin staining (relative amount of actin staining: arp3/wt = 0.60 ± 0.3; n = 15 cones; p < 0.005). However, before the initiation of movement, arp3 mutant cones had an equivalent amount of actin as wild-type cones (relative intensity of actin in arp3/wt = 0.92 ± 0.36, n = 11; p > 0.5). The fact that the cones form normally before movement initiates when only bundles are present, but have less actin after movement begins when the meshwork normally grows, is similar to myosin VI mutant cones (Noguchi et al., 2006) in which the meshwork does not grow properly. Immunofluorescence localization of actin regulators showed that these distributions were altered in the arp3 mutant. Myosin VI was not concentrated on the cones or localized at the front (Figure 5Aa), quail (villin) was present from the front to approximately the middle (Figure 5Ab), and singed (fascin) occupied the rear half of the cone (Figure 5Ac). Because bundling proteins were observed along the entire length of the cone and cone shape was not triangular, we conclude that the front meshwork was greatly reduced or absent in arp3 mutant cones. Direct observations of filament organization using myosin II S1-decoration at the EM level were not possible in the arp3 mutants, because we could not reliably identify cystic bulges to find moving cones (see the Supplemental Movie for an example). In addition, it was difficult to collect a large number of individualizing cysts from the arp3 mutant, due to the lethality of this genotype.
Figure 5.
Localization of actin-regulating proteins in arp3 and profilin mutants. (A) Immunolocalization of myosin VI (a), quail (b), and singed (c) in arp3 mutant (arp3/arp3). (B) Immunolocalization of myosin VI (d), Arp3 (e), and quail (f) in profilin mutant (chic7886/chic7886) actin cones. Actin stained with Alexa-568-phalloidin (a′–f′) is shown in the middle columns. The merged images are shown in the right columns (a″–f″). (C) Schematic drawings summarize the size and shape of the actin cone and the localization of actin-regulating proteins in wild-type, arp3, and chic (profilin) mutants. Bars, 5 μm.
Rear Bundle Formation Requires the Actin Polymerization Regulator, Profilin
We hypothesized that the rear bundles are formed by formin-mediated nucleation, similar to bundles in other systems. Although we could not examine formin regulation of bundles (see Discussion), formin-mediated actin polymerization sometimes requires profilin in vitro, and the profilin mutant (chic7886) is homozygous viable (Verheyen and Cooley, 1994), so testes could be isolated from the mutant. This mutant has cytokinesis defects during earlier spermatocyte divisions (Giansanti et al., 1998), but these defects do not prevent elongation. Numerous fully elongated cysts with mature sperm nuclei were observed at the basal end of the testis, but almost no actin cones were detected along the length of the testis where they normally can be seen as they move along the axoneme during individualization (see below). chic (profilin) mutant cones were almost always associated with nuclei at the basal end of the cyst. chic (profilin) mutant cones had a very different appearance (Figures 5B and 7A) from wild-type cones. In cysts that correspond to the stage when the cones initially form, chic (profilin) mutant cones were significantly smaller than wild type (see Figure 7A). Quantitative analysis of F-actin in cones at this stage demonstrated that the amount of F-actin is reduced to one-third of wild type (0.32 ± 0.06, p < 0.0001; n = 3 experiments, ∼10 cysts in each experiment). Because during early stages of cone formation, the cones consist of only actin bundles, the small amount of actin present is consistent with the idea that profilin is important for polymerization of the bundles. Some cones associated with nuclei had a short triangular shape with a very wide front (Figures 5B, d′–f′, and 7B). The length of these chic (profilin) mutant actin cones was significantly shorter than wild-type moving cones (actin cone length: wild type, 12.0 ± 1.21 μm, n = 23; chic7886/chic7886, 6.1 ± 1.6 μm, n = 23, p < 0.0001). However, the width was comparable (width: wild type, 1.8 ± 0.3 μm; chic7886/chic7886, 2.0 ± 0.4 μm, p > 0.05). Myosin VI (Figure 5Bd) and arp3 (Figure 5Be) localized normally relative to each other on these triangular-shaped cones. However, arp3 occupied the whole length of the cones (Figure 5B, e–e″). In addition, singed (fascin; not shown) and quail (villin; Figure 5Bf) were barely detectable on the mutant cones. The lack of bundling proteins, the short length, and the distribution of arp3 along the entire length of the cone suggest that the rear region of bundles was greatly reduced in chic (profilin) mutant cones. S1 decoration demonstrated that chic (profilin) mutant cones that were associated with nuclei contained a large amount of meshwork and almost no bundles (Figure 6). In wild-type cysts, cones associated with nuclei were composed of a large number of bundled filaments and rarely had any meshwork at all (see Figure 1). We never observed any cones associated with nuclei with extensive meshwork in wild type, as seen in the chic (profilin) mutant.
Figure 7.
Actin cone movements in profilin (chic7886/chic7886) and arp3 (arp3/arp3) mutants. F-actin and DNA staining of a cyst before (A) and during (B and C) individualization. Actin, DNA staining, and merged images are shown in the left, middle, and right columns, respectively. (C) A merged image of actin and DNA at lower magnification from an arp3 mutant cyst. Before the onset of individualization (A), a bundle of 64 sperm nuclei and actin cones are seen at the basal tip of the cyst associated with DNA. The actin cones in the profilin mutant were significantly smaller than in the other genotypes. During individualization (B, top panels), actin cones have moved away from sperm nuclei in wild type. However, most actin cones have not moved in the profilin mutant (B; bottom row). In the arp3 mutant (C), the actin cones moved a great distance (Ca). The large arrowhead indicates the position of sperm nuclei, and the arrow points to the position of the actin cones (red). In the DIC image of a wild-type cyst, accumulation of cytoplasm is clearly visible (Cb, large arrow), and thin sperm tails are visible behind the bulge (Cb, small arrowheads). However, in the arp3 mutant, there is no clear accumulation of cytoplasm at the cystic bulge position, and the bulge is elongated along the axis of the axoneme, indicating that the cones are not moving in register (Cc, large arrow). In addition, sperm tails visible behind the bulge are somewhat thicker than in wild type (Cc, small arrowheads). Bars, (A) 10 μm; (Ca) 100 μm; (Cb) 50 μm.
Figure 6.
Longitudinal EM sections of the nucleus end of a chic (profilin) mutant cyst. Low- (A) and high- (B) magnification views are shown. Profilin mutant cones with extensive meshwork were present near the sperm nuclei (white letter n in A). The mutant cones consisted mainly of actin meshwork and contained few bundles. Bars, (A) 1 μm; (B) 400 nm.
The Two Structural Domains Have Different Roles during Individualization
The purpose or function of each structural domain of the cones in the mediation of individualization is not clear. However, the analysis of mutants above demonstrated that the two parts were differentially affected in the chic (profilin) and arp3 mutants. Thus, the function of each part should be clear from observing defects in individualization in these mutants. We determined for each mutant: 1) whether the actin cones could move and 2) whether the cones successfully excluded cytoplasm and mediated membrane reorganization.
In cultures of cysts from chic (profilin) mutants, we did not observe any cystic bulge formation or signs of individualization. In addition, we rarely observed cones that had moved away from nuclei, and cones associated with nuclei were often triangular in shape (Figures 5B and 7B), like moving cones in wild type. Because we showed above that profilin mutant cones have a greatly reduced amount of bundles and contain mostly meshwork, we conclude that cones with a deficit of bundles cannot move.
Strikingly, in arp3 mutant cysts, in which cones lack the front meshwork, we observed many cones that had moved a significant distance from the nuclei (Figure 7C). In many cases, cones reached near the end of the axoneme, apparently almost completing the full length of movement. In vitro culture demonstrated that, in fact, cones moved. Weak, misshapen cystic bulges were observed (cf. Figure 7C, b and c; see Supplemental Movie). The individualized portion of the sperm tails appeared thicker than the sperm tails in wild type (Figure 7C, b and c), suggesting that cytoplasm was not effectively eliminated by the cones. In cross sections of the individualized portion of arp3 mutant cysts visualized by EM, elements of cytoplasm could be observed (Figure 8). Individualization was incomplete, often with several axoneme/mitochondrial pairs enclosed in a single membrane. Even in the individualized sperm tails, spaces were observed between axoneme/mitochondria pairs and plasma membrane. The defects observed are similar to those seen in myosin VI mutants, but somewhat more severe, in that the amount of space between the membrane and axoneme/mitochondrial derivatives was larger. In addition to these defects, morphogenesis of the minor mitochondrial derivative is abnormal in arp3 mutants. An abnormally condensed minor mitochondrial derivative is a general feature of individualization mutants, including myosin VI, that leave some space between axoneme/mitochondria and the plasma membrane (Noguchi et al., 2006 and T. Noguchi, unpublished observations).
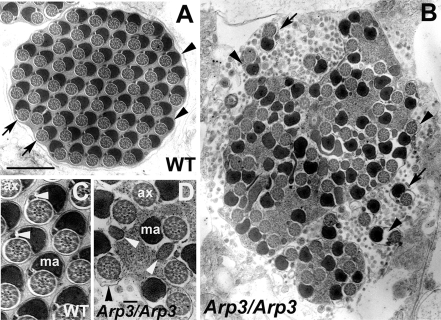
Figure 8.
Electron microscopic analysis of cross sections after individualization. (A) Each wild-type cyst contained 64 pairs of spermatid axonemes (arrows) and mitochondria derivatives (arrowheads) enclosed by a plasma membrane. (B) Individualization defects in the arp3 mutant. The arp3 mutant cyst contained only a few individualized regions (arrows). Most of the axoneme/mitochondrial pairs were not separated by plasma membrane and cytoplasm/membranous organelles were still present. The space between the axoneme/mitochondrial derivative pairs, and the membrane is larger that in wild type and sometimes contained cytoplasm and organelles (arrowheads). (C and D) Sperm tails of wild-type (C) and arp3 mutant (D) at higher magnification. Both axoneme and major mitochondria derivative structure appeared normal, but some cytoplasm remained surrounding the axoneme in arp3 mutant (black arrowhead in D). However, the morphology of the minor mitochondria derivative was abnormal in the arp3 mutant (white arrowheads in D; compare with wild-type); ax, axoneme; ma, major mitochondria derivative. Bar, (A and B) 1 μm; (C and D) 0.1 μm.
Taken together, the observations that cones that lack meshwork can still move, whereas those with a reduced amount of bundles could not and that cytoplasm is not effectively removed when the meshwork is absent suggests that 1) actin bundles are involved in force generation for actin cone movement and 2) the actin meshwork is responsible for effectively pushing out the cytoplasm.
DISCUSSION
In this work we have investigated the formation and function of actin cones that mediate membrane and cytoplasmic reorganization during Drosophila spermatid individualization as an example that illustrates how actin polymerization and structures contribute to differentiated cell processes. Our work revealed that these structures are composed of two distinct actin organizations, which form at different developmental times and are nucleated using different mechanisms. The rear region contains bundles that are stabilized by the actin cross-linkers, quail (villin) and singed (fascin). Formation of the bundle domain relies on profilin activity. The front of the cones is composed of a meshwork that is formed by Arp2/3 complex–mediated actin branch formation. The two domains form relatively independently, because when the formation of one part is compromised using mutations in actin regulators, the other forms relatively normally. Each region also has a specific role. The bundles are required for cone movement. The meshwork is required for normal cytoplasmic exclusion and membrane reorganization.
To our knowledge, this Arp2/3 complex–nucleated meshwork's role in mediating cytoplasm exclusion is novel. This meshwork is dense enough to completely exclude cytoplasm from a compartment of the cell. To form such a dense structure, myosin VI stabilization could be important. Myosin VI mutant cones are not as dense as normal and do not effectively exclude cytoplasm and organelles (Noguchi et al., 2006). Very dense actin meshwork might be used in other cell types and organisms to mediate processes that require cytoplasmic exclusion or might function as a barrier to create cytoplasmic regions with different compositions.
Several aspects of our results seem particularly interesting and perhaps unexpected. First, movement of the cones relies on the bundles, not the meshwork. In other motility systems, such as the leading edge of motile cells and Listeria comet tails, Arp2/3 complex–mediated meshwork formation is thought to be important for motility (Carlier and Pantaloni, 2007). In the case of actin cones, the meshwork forms just as movement initiates. Thus, we expected that the meshwork would be critical for movement. However, cones that lack meshwork (arp3 mutant) can move.
Second, profilin loss of function affects the formation of bundles but not meshwork. Profilin has been implicated in both formin-mediated actin bundle formation and Arp2/3 complex–mediated meshwork formation. Therefore, we might have expected both meshwork and bundle formation to be affected by profilin loss of function. During Arp2/3 complex–dependent Listeria monocytogenes motility in vitro, profilin is not absolutely required, but accelerates the rate of movement (Loisel et al., 1999). Perhaps the slow turnover of filaments in the cones (Noguchi and Miller, 2003) compared with turnover in Listeria comet tails (Theriot et al., 1992) reduces the need for profilin-mediated acceleration of monomer addition. Alternatively, maybe the small amount of activity that remains in this hypomorphic mutant is sufficient to participate in actin cone meshwork formation. Another possible explanation is that a different regulator of assembly, such as capulet (Baum et al., 2000; Baum and Perrimon, 2001), a CAP/SRV2 ortholog, or ciboulot (Boquet et al., 2000), a thymosin β-4 ortholog, has a more important role in regulating monomer availability and addition during actin cone meshwork formation.
Interestingly, bundle formation has a stringent requirement for profilin. Although we have not yet been able to directly implicate a formin in bundle formation (see below), it seems likely that actin cone bundles would be nucleated by a formin, similar to bundles in other cells (Evangelista et al., 2002, 2003; Sagot et al., 2002). The fact that profilin activity is required suggests that the profilin-mediated gating of monomer addition during formin nucleation that has been observed in vitro (Kovar et al., 2003, 2006; Romero et al., 2004) is likely to be critical in vivo. This is similar to the situation in yeast where the formin, Bni1, and profilin are both required for and work together during actin cable assembly (Evangelista et al., 2002; Sagot et al., 2002).
The protein responsible for nucleating bundle formation remains unidentified, although a formin seems the most likely candidate. There are six predicted formin orthologues in Drosophila (Goldstein and Gunawardena, 2000; Higgs and Peterson, 2005). We have examined the diaphanous mutant (Castrillon and Wasserman, 1994), but defects that occur during the spermatocyte divisions make interpretation of phenotypes during individualization difficult (T. Noguchi, unpublished observations). Of the other five predicted formin orthologues in Drosophila, two (CG6807, GC5797) are not yet characterized and mutants are not available at present. Mutations have been identified in the other formins, cappuccino (Manseau and Schupbach, 1989; Emmons et al., 1995), DAAM (Matusek et al., 2006), and formin3 (Tanaka et al., 2004). cappuccino mutations do not appear to affect spermatogenesis, whereas DAAM and formin3 mutations are lethal. Further experiments will be required to determine which nucleator is important here.
In studies of the interplay between actin bundles and meshwork at the leading edge of motile cells, bundled filaments of filopodia were hypothesized to arise via convergence of dendritic arrays of actin in lamellipodial meshwork (Svitkina et al., 2003). However, this idea has been challenged by the demonstration that in both mammalian cells and Dictyostelium, filopodia still form when Arp2/3 complex–mediated meshwork assembly is absent (Steffen et al., 2006). Similarly, in actin cones, bundle formation is not dependant on the meshwork. Bundles form before the meshwork is present and can form even when meshwork never forms, as in the arp3 mutant. In addition, the meshwork does not appear to be a precursor for the bundles during movement, because there is no “flow” of actin from the meshwork into the bundles (Noguchi and Miller, 2003).
An open question is what provides the force for movement. Monomer addition at the barbed ends of the bundles could propel the cone away from the membrane, which would result in movement in the forward (pointed end) direction. Actin turnover (assembly and disassembly) is required for movement (Noguchi and Miller, 2003), but whether this is important for force production remains unclear. If barbed-end elongation of bundles mediates movement, we would expect to see movement of a bleached spot of GFP-actin from the rear to the front of the cone in photobleaching experiments. However, we did not detect any movement of bleached areas, either forward or backward, in FRAP experiments (Noguchi and Miller, 2003). Motors may be involved, although it is unlikely that myosin VI motor activity is required. The cones move at normal speed (at least in the initial stages) when myosin VI function is absent (Noguchi et al., 2006). We cannot eliminate a contribution by another myosin, perhaps binding to the axoneme using its tail and moving in the barbed-end direction, allowing the observed cone movement in the pointed-end direction. The only other myosin that is known to play a role in spermatogenesis is myosin V (Mermall et al., 2005). In myosin V mutant testes, actin cones do not form normally and no normal individualization complexes can be seen moving along the axonemes. These abnormalities preclude drawing conclusions about whether myosin V has a role in cone movement. Alternatively, a microtubule-based motor that uses the axoneme as a track and pulls the cones forward remains a possibility. However, why actin turnover would be important for either microtubule or actin motors to move the cones is unclear. Further study will be required to fully understand all the interesting aspects of this structure's formation, regulation, and motility.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dana Hodge, Julie Morrison, Olga Narbutt, and Michal Swidzinski for technical assistance and Bill Theurkauf for arp3 antibody. Mike Veith provided great support for the EM studies. We thank all the members of the Miller lab for discussions and lab members and Kate Beckingham for critical reading of the manuscript. This work was supported by National Institutes of Health Grant GM 060494 to K.G.M. and by the Regional Fund of Research and Training of Kujawsko-Pomorski Province.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-08-0840) on March 19, 2008.
REFERENCES
- Baum B., Li W., Perrimon N. A cyclase-associated protein regulates actin and cell polarity during Drosophila oogenesis and in yeast. Curr. Biol. 2000;10:964–973. doi: 10.1016/s0960-9822(00)00640-0. [DOI] [PubMed] [Google Scholar]
- Baum B., Perrimon N. Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat. Cell Biol. 2001;3(10):883–890. doi: 10.1038/ncb1001-883. [DOI] [PubMed] [Google Scholar]
- Boquet I., Boujemaa R., Carlier M. F., Preat T. Ciboulot regulates actin assembly during Drosophila brain metamorphosis. Cell. 2000;102(6):797–808. doi: 10.1016/s0092-8674(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Cant K., Knowles B. A., Mooseker M. S., Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J. Cell Biol. 1994;125(2):369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Le Clainche C., Wiesner S., Pantaloni D. Actin-based motility: from molecules to movement. Bioessays. 2003;25(4):336–345. doi: 10.1002/bies.10257. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Control of actin assembly dynamics in cell motility. J. Biol. Chem. 2007;32:23005–23009. doi: 10.1074/jbc.R700020200. [DOI] [PubMed] [Google Scholar]
- Castrillon D. H., Wasserman S. A. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120(12):3367–3377. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- Cross D. P., Shellenbarger D. L. The dynamics of Drosophila melanogaster spermatogenesis in in vitro cultures. J. Embryol. Exp. Morphol. 1979;53:345–351. [PubMed] [Google Scholar]
- Emmons S., Phan H., Calley J., Chen W., James B., Manseau L. Cappuccino, a Drosophila maternal effect gene required for polarity of the egg and embryo, is related to the vertebrate limb deformity locus. Genes Dev. 1995;9(20):2482–2494. doi: 10.1101/gad.9.20.2482. [DOI] [PubMed] [Google Scholar]
- Evangelista M., Pruyne D., Amberg D. C., Boone C., Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 2002;4(3):260–269. doi: 10.1038/ncb770. [DOI] [PubMed] [Google Scholar]
- Evangelista M., Zigmond S., Boone C. Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 2003;116(Pt 13):2603–2611. doi: 10.1242/jcs.00611. [DOI] [PubMed] [Google Scholar]
- Fabrizio J. J., Hime G., Lemmon S. K., Bazinet C. Genetic dissection of sperm individualization in Drosophila melanogaster. Development. 1998;125(10):1833–1843. doi: 10.1242/dev.125.10.1833. [DOI] [PubMed] [Google Scholar]
- Giansanti M. G., Bonaccorsi S., Williams B., Williams E. V., Santolamazza C., Goldberg M. L., Gatti M. Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev. 1998;12(3):396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. S., Gunawardena S. Flying through the Drosophila cytoskeletal genome. J. Cell Biol. 2000;150(2):F63–F68. doi: 10.1083/jcb.150.2.f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley E. D., Welch M. D. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 2006;7(10):713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Goode B. L., Eck M. J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Gupton S. L., et al. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J. Cell Biol. 2005;168(4):619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. L., Deng W. M., Rogat A. D., Miller K. G., Bownes M. Class VI unconventional myosin is required for spermatogenesis in Drosophila. Mol. Biol. Cell. 1999;10(12):4341–4353. doi: 10.1091/mbc.10.12.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs H. N., Peterson K. J. Phylogenetic analysis of the formin homology 2 domain. Mol. Biol. Cell. 2005;16(1):1–13. doi: 10.1091/mbc.E04-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. M., Cooley L. A subset of dynamic actin rearrangements in Drosophila requires the Arp2/3 complex. J. Cell Biol. 2002;156(4):677–687. doi: 10.1083/jcb.200109065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman K. A., Miller K. G. An unconventional myosin heavy chain from drosophila melanogaster. J. Cell Biol. 1992;119(4):823–834. doi: 10.1083/jcb.119.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Harris E. S., Mahaffy R., Higgs H. N., Pollard T. D. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124(2):423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Kovar D. R., Kuhn J. R., Tichy A. L., Pollard T. D. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 2003;161(5):875–887. doi: 10.1083/jcb.200211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel T. P., Boujemaa R., Pantaloni D., Carlier M. F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401(6753):613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- Mahajan-Miklos S., Cooley L. The villin-like protein encoded by the Drosophila quail gene is required for actin bundle assembly during oogenesis. Cell. 1994;78(2):291–301. doi: 10.1016/0092-8674(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Manseau L. J., Schupbach T. cappuccino and spire: two unique maternal-effect loci required for both the anteroposterior and dorsoventral patterns of the Drosophila embryo. Genes Dev. 1989;3(9):437–452. doi: 10.1101/gad.3.9.1437. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Matusek T., Djiane A., Jankovics F., Brunner D., Mlodzik M., Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133(5):957–966. doi: 10.1242/dev.02266. [DOI] [PubMed] [Google Scholar]
- Mermall V., Bonafe N., Jones L., Sellers J. R., Cooley L., Mooseker M. S. Drosophila myosin V is required for larval development and spermatid individualization. Dev. Biol. 2005;286(1):238–2255. doi: 10.1016/j.ydbio.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Lenartowska M., Miller K. G. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol. Biol. Cell. 2006;17(6):2559–2571. doi: 10.1091/mbc.E06-01-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Miller K. G. A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development. 2003;130:i805–816. doi: 10.1242/dev.00406. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Borisy G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Revenu C., Athman R., Robine S., Louvard D. The co-workers of actin filaments: from cell structures to signals. Nat. Rev. Mol. Cell Biol. 2004;5(8):635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rogat A. D., Miller K. G. A role for myosin VI in actin dynamics at sites of membrane remodeling during Drosophila spermatogenesis. J. Cell Sci. 2002;115(Pt 24):4855–4865. doi: 10.1242/jcs.00149. [DOI] [PubMed] [Google Scholar]
- Romero S., Le Clainche C., Didry D., Egile C., Pantaloni D., Carlier M. F. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119(3):419–429. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Sagot I., Rodal A. A., Moseley J., Goode B. L., Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 2002;4(8):626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- Sekerkova G., Zheng L., Loomis P. A., Mugnaini E., Bartles J. R. Espins and the actin cytoskeleton of hair cell stereocilia and sensory cell microvilli. Cell Mol. Life Sci. 2006;63(19–20):2329–2341. doi: 10.1007/s00018-006-6148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A., Faix J., Resch G. P., Linkner J., Wehland J., Small J. V., Rottner K., Stradal T. E. Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol. Biol. Cell. 2006;17(6):2581–2591. doi: 10.1091/mbc.E05-11-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson V., Hudson A., Cooley L., Theurkauf W. E. Arp2/3-dependent pseudocleavage [correction of psuedocleavage] furrow assembly in syncytial Drosophila embryos. Curr. Biol. 2002;12(9):705–711. doi: 10.1016/s0960-9822(02)00807-2. [DOI] [PubMed] [Google Scholar]
- Svitkina T. M., Bulanova E. A., Chaga O. Y., Vignjevic D. M., Kojima S., Vasiliev J. M., Borisy G. G. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 2003;160(3):409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Takasu E., Aigaki T., Kato K., Hayashi S., Nose A. Formin3 is required for assembly of the F-actin structure that mediates tracheal fusion in Drosophila. Dev. Biol. 2004;274(2):413–425. doi: 10.1016/j.ydbio.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357(6375):257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J. How to make a curved Drosophila bristle using straight actin bundles. Proc. Natl. Acad. Sci. USA. 2005;102(52):18785–18792. doi: 10.1073/pnas.0509437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Peacock W. J., Hardy R. W. Dynamics of spermiogenesis in Drosophila melanogaster. I. Individualization process. Z Zellforsch. Mikrosk. Anat. 1972;124(4):479–506. doi: 10.1007/BF00335253. [DOI] [PubMed] [Google Scholar]
- Tyska M. J., Mooseker M. S. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys. J. 2002;82(4):1869–1883. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen E. M., Cooley L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development. 1994;120(4):717–728. doi: 10.1242/dev.120.4.717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.