Abstract
The R-Spondin (RSpo) family of secreted proteins is implicated in the activation of the Wnt signaling pathway. Despite the high structural homology between the four members, expression patterns and phenotypes in knockout mice have demonstrated striking differences. Here we dissected and compared the molecular and cellular function of all RSpo family members. Although all four RSpo proteins activate the canonical Wnt pathway, RSpo2 and 3 are more potent than RSpo1, whereas RSpo4 is relatively inactive. All RSpo members require Wnt ligands and LRP6 for activity and amplify signaling of Wnt3A, Wnt1, and Wnt7A, suggesting that RSpo proteins are general regulators of canonical Wnt signaling. Like RSpo1, RSpo2-4 antagonize DKK1 activity by interfering with DKK1 mediated LRP6 and Kremen association. Analysis of RSpo deletion mutants indicates that the cysteine-rich furin domains are sufficient and essential for the amplification of Wnt signaling and inhibition of DKK1, suggesting that Wnt amplification by RSpo proteins may be a direct consequence of DKK1 inhibition. Together, these findings indicate that RSpo proteins modulate the Wnt pathway by a common mechanism and suggest that coexpression with specific Wnt ligands and DKK1 may determine their biological specificity in vivo.
INTRODUCTION
The R-Spondin (RSpo) family of secreted proteins is implicated in the activation and regulation of Wnt signaling pathway. The RSpo family is comprised of 4 members (RSpo1-4) that share overall ∼60% sequence homology and similar domain organization (Kim et al., 2006). All RSpo proteins contain two furin-like cysteine-rich domains at the N-terminus followed by a thrombospondin domain and a basic charged C-terminal tail. Numerous studies have implicated RSpo proteins in the canonical Wnt signaling pathway. We have previously demonstrated that all four RSpo members induce proliferation of crypt epithelial cells resulting in enlargement of mouse gastrointestinal tract (Kim et al., 2005, 2006). Moreover, RSpo proteins can stabilize the levels of cytosolic β-catenin and dramatically synergize with Wnt3A, suggesting a potential role for RSpo proteins in modulating the Wnt signaling pathway (Kazanskaya et al., 2004; Binnerts et al., 2007; Wei et al., 2007).
The Wnt signaling pathway plays a pivotal role in diverse biological processes during embryonic development, adult homeostasis and disease pathogenesis (Logan and Nusse, 2004; Moon et al., 2004; Nusse, 2005). Wnt ligands are a family of secreted proteins that regulate the β-catenin turnover through interactions with Frizzled receptors and LRP5/6 co-receptors (Davidson et al., 2005; He et al., 2004; Tamai et al., 2004; Zeng et al., 2005). In the absence of Wnt ligand, cytosolic β-catenin is targeted for proteosomal degradation by the APC/Axin/GSK destruction complex (Behrens et al., 1998; Salic et al., 2000). On engagement with the receptors, Wnt ligands trigger a series of events resulting in the stabilization and subsequent translocation of cytosolic β-catenin into the nucleus and transactivation of Wnt target genes through interaction with the transcription factor T-cell factor (TCF; Bejsovec, 2005; Gordon and Nusse, 2006).
Expression of RSpo proteins overlaps with expression of Wnt proteins during mouse development implying a possible link of RSpo proteins with the Wnt signaling pathway (Parr et al., 1993; Echelard et al., 1994; Yoshida et al., 1997; Nam et al., 2006b). Consistent with this, RSpo1 expression was detected in the developing dorsal neural tube and was reduced in Wnt1 and Wnt 3A double knockout mice (Kamata et al., 2004). Likewise, in Xenopus, mRNA expression of RSpo proteins overlapped with expression of Wnt ligands during embryonic development (Kazanskaya et al., 2004). Recently, an important role of RSpo1 in development of the reproductive system was described in humans with a loss of function mutation of RSpo1 (Parma et al., 2006). Similar to phenotypes observed in Wnt-4 knockout mice, a homozygous null mutation of RSpo1 in members of a family with a recessive syndrome was characterized by XX sex reversal. These findings indicate a key role for RSpo1 in Wnt signaling during the development of reproductive organs.
Although RSpo proteins are linked to the Wnt pathway, the precise mechanism by which each member regulates the Wnt pathway is poorly understood. Previously, it was shown that RSpo proteins interact with FZD8 and LRP6 receptors (Nam et al., 2006a; Wei et al., 2007). However, these studies did not demonstrate whether these interactions are required for the activation of the Wnt signaling pathway by RSpo proteins. Recently, we have demonstrated that RSpo1 activity is dependent on the presence of Wnt ligands and that RSpo1 regulates the Wnt signaling by antagonizing Dickkopf (DKK)1-mediated LRP6 internalization (Binnerts et al., 2007). To further explore and compare the mechanism of action of different RSPo proteins, we generated and tested purified recombinant proteins of all RSpo family members. Here we show that all four members regulate the Wnt pathway by a common mechanism. In addition, we provide data that indicates that the furin domain of RSpo protein is sufficient to synergize with Wnt3A and antagonize DKK1 function.
MATERIALS AND METHODS
Purification of RSpo Proteins
HEK293 cells were transfected with V5-His6-tagged RSpo expression constructs, and stable cells were selected using a medium containing 500 μg/ml geneticin (Invitrogen, Carlsbad, CA). Stable clones were suspension adapted to grow in serum-free 293 free-style media (GIBCO, Rockville, MD). The media containing the secreted individual RSpo proteins were harvested for further purification. In brief, the media were filtered and concentrated using a PES filter (Corning Costar, Acton, MA) and a TFF system (Pall Filtron, Northborough, MA), respectively, followed by a HiTrap Ni-chelating affinity column (Amersham Pharmacia, Piscataway, NJ). The Ni column was washed with 20 mM imidazole for 10 column volume (CV) and protein was eluted with a gradient of 20 to 300 mM imidazole. RSpo proteins isolated through the Ni column were loaded onto a SP cation exchange column that had been equilibrated with 20 mM sodium phosphate, 0.3 M arginine, pH 7. The column was washed with 8 CV of 0.1 M NaCl and eluted with a gradient of 0.1 to 1 M NaCl over 30 CV. Fractions containing V5-His tagged RSpo proteins were pooled to yield a protein solution that was between 85 and 98% pure.
Cell Culture and Transfection
HEK293 cells were purchased from ATCC (Manassas, VA) and maintained in DMEM containing 10% fetal bovine serum. To generate a HEK293 clone that stably expresses a 16TCF luciferase reporter construct, cells were transfected with a pTA16TCF-Luc construct using Fugene-6 (Roche, Indianapolis, IN) transfection reagent, and stable clones were selected using a culture medium containing 1 mg/ml geneticin (Invitrogen). A HEK 293 stable clone expressing mLRP6-FLAG was generated in a similar way using a pcDNA3.1/Intron mLRP6-FLAG expression construct.
Plasmids and DNA Constructs
A 16TCF luciferase reporter construct was generated as described (Binnerts et al., 2007). Open reading frame (ORF) nucleotide (nt) sequences for hKremen2 (BF312414, ATCC, Manassas, VA) and mLRP6 (BC060704, Open Biosystems, Huntsville, AL) were amplified from indicated cDNA clones and inserted into the pcDNA3.1/Intron plasmid. mLRP6ΔC was generated by deleting the LRP6 cytoplasmic domain as described (Binnerts et al., 2007). LRP6-FLAG was generated by in-frame fusion of the LRP6 ORF sequence with a FLAG epitope tag (DYKDDDDK) and Kremen2-HA was generated with a HA epitope tag (YPYDVPDYA) encoding sequence. The expression constructs of RSpo chimeric molecules in which the furin domain of RSpo1 is replaced by furin domains of RSpo2, 3, or 4 were generated by a stitching PCR method using following primers: a universal forward primer, GTC GTC GAC ACG TGT GAT CAG ATA; RSpo2 reverse primer, TTC ACA TCC TTC CAC ACA TTC CAT; RSpo3 reverse primer, CTC ACA GTG CAC AAT ACT GAC ACA; RSpo4 reverse primer, TTC ACA CTC CCC CTG GCACT, a forward primer for RSpo1 C-terminal with thrombospondin domain, ATG AGC GAG TGG TCT CCG T; a reverse primer for RSpo1 C-terminal with thrombospondin domain, TGG ACA AAC CAC AAC TAG AAT GCA G. PCR amplified furin domains of RSpo2, 3, or 4 were ligated with the thrombospondin domain containing the C-terminal half of the RSpo1 molecule followed by PCR amplification of chimeric molecules using a forward primer, GTC GTC GAC ACG TGT GAT CAG ATA, and a reverse primer, TGG ACA AAC CAC AAC TAG AAT GCA G. The PCR-amplified products of three chimeric molecules were confirmed by sequencing and digested with EcoRI/XbaI to clone into the pcDNA/Intron vector.
Luciferase Reporter Assays Small Interfering RNA Experiment
A 16TCF luciferase reporter construct was generated by cloning 16 repeats of a TCF consensus site (AGATCAAAGG) into the pTA-Luc vector (Clontech, Mountain View, CA) as reported (Binnerts et al., 2007). A stable HEK293 clone, A6 was selected using a geneticin selection marker, and the selected clone demonstrated minimal basal reporter activity. Stable HEK293 cells were seeded in 96-well plates in DMEM containing 1% FBS (Invitrogen) the day before treatment. The stable cells were treated in triplicate for 20 h with indicated concentrations of proteins, and reporter activity was determined using BrightGlo reagent (Promega, Madison, WI) by a Veritas Luminometer (Turner Biosystems, Sunnyvale, CA). Recombinant hDKK1. mWnt3A proteins were purchased from R&D systems (Minneapolis, MN), and recombinant RSpo1, 2, 3, and 4 were purified from conditioned media derived from stable HEK293 cells by standard column chromatography and filtration. Wntless (WLS) gene-specific and nontargeting control small interfering RNA (siRNA) duplexes were purchased from Dharmacon (Chicago, IL); WLS: GCGUCAGUCCAAGUGAA (Banziger et al., 2006). HEK-293 A6 reporter cells were transfected in triplicate in 96-well plates with indicated siRNA duplexes by reverse transfection with Neofect (Ambion, Austin, TX) according to manufacturer's instructions. Cells were treated as indicated 48 h after siRNA transfection and analyzed for reporter activity as described above.
Western Blotting and Coimmunoprecipitation
β-Catenin stabilization was performed as previously described. In brief, the cell lysate was prepared from HEK 293 cells using hypotonic lysis buffer. The cytosolic fraction was prepared by ultracentrifugation of the cell lysates (100,000 × g) for 30 min, resolved by SDS-PAGE, and transferred to nitrocellulose membrane. Immunoblotting was performed using anti- β-catenin antibodies (Cell Signaling). β-Actin was used as a protein-loading control. To measure LRP6 phosphorylation either HEK 293 cells or stable 293 cells expressing FLAG-tagged LRP6 were seeded in six-well dishes and treated with indicated proteins for 4 h. Cells were placed on ice, rinsed with ice-cold PBS, and lysed in cell lysis buffer containing phosphatase inhibitors (Cell Signaling). Lysates were resolved on 3–8% SDS-PAGE gels and LRP6 phosphorylation was analyzed using a phospho-Ser1490–specific anti-LRP6 polyclonal antibody (Cell Signaling). To determine the interaction of Kremen and LRP6, HEK293 cells, stably expressing LRP6-C-FLAG, were transiently transfected with a Kremen2-C-HA expression construct using Fugen-6 transfection reagent (Roche). The total cell lysates were prepared after indicated treatments, and the immunoprecipitation was performed by incubating the cell lysates with anti-HA antibodies (Abcam, Cambridge, MA) overnight at 4°C. The immunoprecipitated molecules were precipitation using protein G Dynabeads (Invitrogen). After stringent washing with lysis buffer, the protein complex was eluted from the beads by boiling in sample buffer with DTT (Pierce) and resolved in 3–8% Tris-acetate gels (Invitrogen) for immunoblotting with anti-FLAG, anti-hemagglutinin (HA; Abcam), or anti-DKK1 (R&D Systems) antibodies.
RESULTS
RSpo Family Proteins Activate the Wnt Signaling Pathway
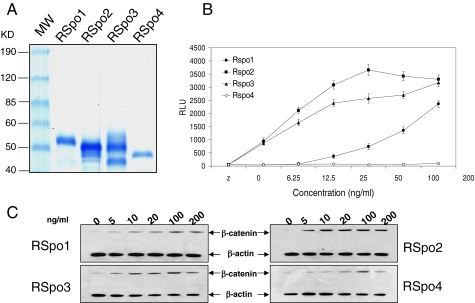
We and others have previously shown that RSpo1 stimulates the accumulation of cytosolic β-catenin and TCF luciferase reporter activity in HEK293 cells (Kim et al., 2005, 2006; Binnerts et al., 2007). Although genetic studies with the various RSpo proteins indicated a wide range of phenotypes, it is unclear whether all RSpo proteins activate the Wnt pathway through a common mechanism. To further understand the biochemical and cellular activities of all RSpo family proteins, we generated recombinant proteins of four RSpo members (Figure 1A) and compared the TCF reporter activity between RSpo1-4 (Figure 1B). As we previously demonstrated, RSpo1 induced TCF reporter activity in a dose-dependent manner. Interestingly, RSpo2 and RSpo3 were more potent than RSpo1, particularly at a lower concentration (<100 ng/ml), whereas RSpo4 was relatively inactive. The relative activities of RSpo family proteins in Wnt/β-catenin signaling were further confirmed by measuring the accumulation of cytosolic β-catenin (Figure 1C). Consistent with the activities observed in the TCF-mediated reporter assay, RSpo2 and 3 induced stabilization of cytosolic β-catenin in HEK293 cells at lower concentrations than RSpo1, whereas RSpo4 had a minimal effect on cytosolic β-catenin levels.
Figure 1.
RSpo family proteins activate β-catenin signaling. (A) Coomassie-stained RSpo 1, 2, 3, and 4 proteins. Purified Rspo proteins were resolved on a 4–20% SDS-PAGE gel under reducing conditions and were visualized by Coomassie blue staining. Each RSpo lane contains 500 ng of the purified protein. The first lane contains markers with molecular masses as indicated at the left. Estimated purities for RSpo1, 2, 3, and 4 are 98, 90, 93, and 85%, respectively, based on immunoreactive bands in Western blotting using antiV5 antibodies. (B) Activities of RSpo proteins were measured by TCF reporter assay. HEK293 cells stably transfected with a 16-TCF-luciferase reporter construct were treated with increasing concentrations of RSpo proteins for 20 h, and reporter activity was measured. Data represent relative light units (RLU). Assays were done in triplicate and the results are mean ± SD. (C) β-Catenin stabilization was measured by Western blotting using an anti-β-catenin antibody. HEK293 cells were treated with various concentrations of RSpo proteins for 3 h, and the cytosolic fractions were prepared for immunoblotting for β-catenin. β-Actin was used as a loading control.
RSpo Proteins Amplify the Activity of Wnt Proteins
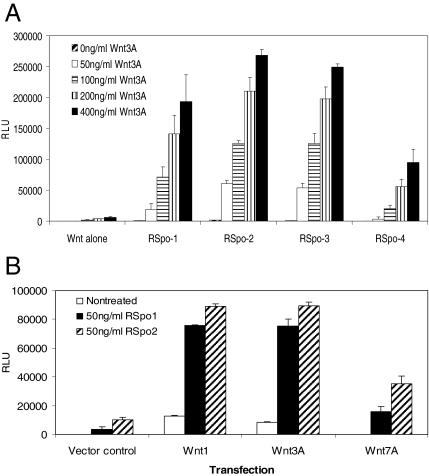
We and others have previously shown that RSpo proteins act synergistically with Wnt3A to stimulate the accumulation of β-catenin and TCF-mediated transcription activation (Kazanskaya et al., 2004; Kim et al., 2005; Binnerts et al., 2007; Wei et al., 2007). To further examine the amplification of Wnt3A activity by RSpo proteins, a HEK293 cell line, stably expressing a TCF reporter gene, was treated with increasing amounts of Wnt3A proteins in the presence of a suboptimal concentration of purified RSpo1-4 (20 ng/ml). Although treating cells with either varying amounts of Wnt3A or RSpo1-4 alone (at 20 ng/ml) was not sufficient to trigger a robust response, a substantial increase of Wnt3A-induced TCF-mediated transcriptional activation (up to ∼260-fold enhancement) was detected when cells were treated in combination with each of four members of RSpo proteins (Figure 2A). Interestingly, although cells treated with RSpo4 alone were virtually nonresponsive, cotreatment with Wnt3A significantly increased TCF reporter activity (∼150-fold). As observed in cells treated with RSpo alone (Figure 1), RSpo2 and RSpo3 were slightly more potent than RSpo1 in amplifying the Wnt signaling. Furthermore, RSpo-mediated enhancement of TCF reporter activity was not limited to Wnt3A. A suboptimal dose of RSpo1 and RSpo2 (50 ng/ml) also substantially amplified Wnt1 or Wnt7A activities (Figure 2B), suggesting that RSpo proteins can modulate general canonical Wnt signaling.
Figure 2.
RSpo proteins synergize with various Wnt proteins in TCF-mediated transcriptional activation. (A) HEK293 cells stably transfected with a 16-TCF-luciferase reporter construct were treated with increasing concentrations of Wnt3A protein in the absence or presence of 20 ng/ml recombinant RSpo proteins. After a 20-h incubation, reporter activity was measured using a luminometer. (B) Stable 293 TCF reporter cells were transfected with Wnt1, Wnt3A, or Wnt7A expression constructs in 96-well (50 ng per transfection). Twenty four hours after the transfection, cells were treated with either RSpo1 or RSpo2 at 50 ng/ml and TCF reporter activity was measured 20 h after the incubation. Empty vector was used as a transfection control.
RSpo Proteins Require Endogenously Secreted Wnt Ligands for Their Activities
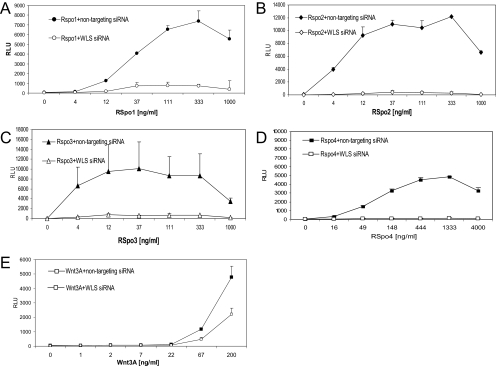
Previously we have shown that activity of RSpo1 is dependent on Wnt proteins to activate Wnt signaling. To examine the implication of Wnt ligands in the activities of other RSpo family members, we used Wntless (WLS) siRNA to block secretion of endogenous Wnt proteins. Knock down of WLS significantly reduced activities of all 4 RSpo proteins (Figure 3), while having a marginal effect on cells that were treated with exogenously Wnt3A protein. These findings indicate that Wnt ligands are required for activities of RSpo proteins.
Figure 3.
Activities of RSpo proteins are dependent on an endogenous pool of Wnt ligands. Stable HEK-293 reporter cells were transfected with either nontargeting control siRNA or WLS siRNA duplexes (10 nM), treated with indicated amounts of RSpo1 (A), RSpo2 (B), RSpo3 (C), RSpo4 (D), or Wnt3A (E) proteins. Reporter activity was analyzed as described above.
LRP6 Is Implicated in RSpo-mediated Signaling
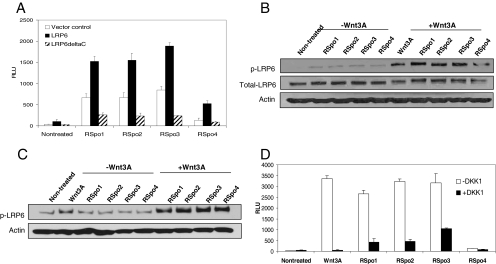
To further characterize the role of RSpo proteins in the Wnt signaling pathway, we examined whether activities of RSpo proteins are regulated by the canonical Wnt coreceptor LRP6. Overexpression of full-length LRP6 in HEK293 cells enhanced RSpo protein-induced TCF reporter activity, whereas expression of a dominant negative C-terminal truncated LRP6 (LRP6ΔC) reduced the activity of all four RSpo members (Figure 4A). These data imply that all RSpo proteins regulate the Wnt signaling pathway at the level of LRP6.
Figure 4.
LRP6 is implicated in RSpo-mediated β-catenin signaling. (A) HEK293 cells stably expressing 16-TCF reporter were transfected with either wt LRP6 or LRP6ΔC expression constructs, treated with indicated RSpo proteins (200 ng/ml), and the luciferase reporter activity was determined 20 h after the incubation. Empty vector was used as a transfection control. (B) Regular HEK293 cells were treated with RSpo proteins (200 ng/ml), Wnt3A (200 ng/ml), or Wnt3A (200 ng/ml), and individual RSpo protein (200 ng/ml) together for 4 h. Total cell lysates were prepared and resolved by SDS-PAGE. Phosphorylation of endogenous LRP6 was detected by immunoblotting using anti-phospho-LRP6 antibodies. (C) HEK293 cells were stably transfected with a LRP6-FLAG expression construct and treated with Wnt3A (200 ng/ml), RSpo proteins alone (200 ng/ml) or Wnt3A and RSpo together for 4 h. The total cell lysates were prepared and resolved by SDS-PAGE followed by immunoblotting using anti-phospho-LRP6 antibodies. (D) Stable HEK293 A6 cells were treated with Wnt3A (250 ng/ml) or RSpo protein alone (125 ng/ml) or in combination with recombinant DKK1 protein (400 ng/ml). The luciferase reporter activity was determined 20 h after incubation.
To explore the link between RSpo signaling and the LRP6 receptor, we next evaluated the phosphorylation of LRP6 using a phospho-LRP6 specific antibody. Although all RSpo proteins marginally increased LRP6 phosphorylation (Figure 4B), cotreatment of each RSpo protein with Wnt3A resulted in a significant increase in phosphorylation of both endogenous as well as overexpressed LRP6 receptor (Figure 4, B and C, respectively). This result is in agreement with the amplification of Wnt-dependent TCF activity by RSpo proteins and suggests that amplification of the Wnt pathway by RSpo proteins may occur at the level of LRP6 phosphorylation.
To further explore the link between LRP6 and RSpo proteins, we next examined the effect of LRP6 inhibitor DKK1 on RSpo activity. Previously, we demonstrated that RSpo1 can antagonize DKK1 activity to regulate the cell surface levels of LRP6 (Binnerts et al., 2007). Similar to the observation seen with RSpo1, DKK1 inhibited activity of RSpo2–4 proteins (Figure 4D) but to a lesser extent compared to the inhibition of Wnt3A protein activity. These data further confirm the implication of LRP6 in RSpo-mediated Wnt signaling.
RSpo Proteins Antagonize DKK-1–induced Interaction of Kremen and LRP6
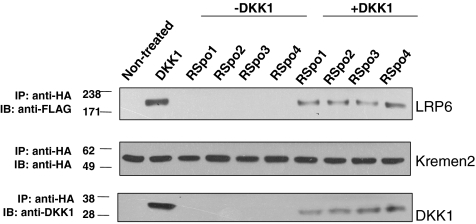
It was previously shown that a direct interaction between DKK1 and LRP6/Kremen is required for the regulation of cell surface levels of LRP6 (Semenov et al., 2001; Mao et al., 2002). DKK1 interacts with Kremen1 or 2 and subsequently triggers the internalization of LRP6 (Mao et al., 2007). We have previously demonstrated that RSpo1 can block DKK1 function and can interact with Kremen expressing cells (Binnerts et al., 2007). We therefore investigated whether all RSpo1-4 can also antagonize DKK1 mediated LRP6 and Kremen interaction. We analyzed the formation of a Kremen2 and LRP6 ternary complex in cells treated either with DKK1 alone or in combination with RSpo proteins. As shown in Figure 5, although LRP6 was coimmunoprecipitated with Kremen2 in cells treated with DKK1, cotreatment with RSpo proteins reduced DKK-1–mediated Kremen2 and LRP6 complex formation. Interaction of Kremen and LRP6 was not altered in cells treated with RSpo proteins alone. Moreover, all RSpo proteins interfered to a similar extent with the association of DKK1 with the Kremen/LRP6 complex. These results suggest that all RSpo proteins can interfere with DKK1 function by disrupting Kremen and LRP6 interaction and subsequent internalization of the receptor complex. Together, these data suggest a common role for RSpo proteins in regulating the Wnt pathway by antagonizing DKK1 activity in LRP6-mediated signaling.
Figure 5.
RSpo proteins antagonize DKK1-mediated interaction of Kremen2 and LRP6. HEK-293 cells, stably expressing LRP6-FLAG, were transfected with Kremen2-HA and treated with DKK-1 (100 ng/ml) in the presence or absence of indicated RSpo proteins (200 ng/ml) for 30 min. The total cell lysates were immunoprecipitated with anti-HA antibody for Kremen2 and coimmunoprecipitated LRP6, or DKK1 proteins were immunoblotted with anti-FLAG or anti-DKK1 antibodies, respectively. DKK1 induced an interaction of Kremen2 and LRP6 proteins and RSpo proteins reduced the DKK1-mediated Kremen2 and LRP6 interaction.
Furin Domains Specify the Activity of RSpo Proteins
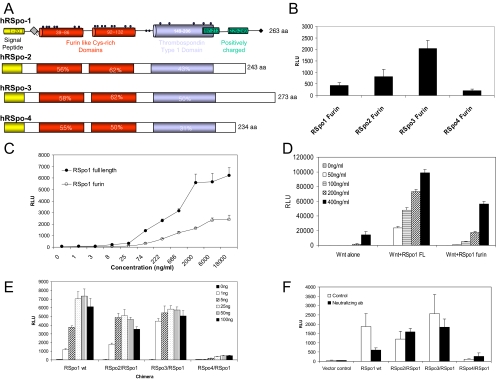
The RSpo family proteins contain two cysteine-rich furin-like domains that share ∼60% sequence homology among family members (Figure 6A). The furin-like domains were shown to be sufficient and indispensable for RSpo proteins activity (Kazanskaya et al., 2004). To further map the minimal domain required for RSpo activity, we next generated series of RSpo deletion mutants and tested their activities in the TCF reporter assay. As previously shown (Kazanskaya et al., 2004), two intact furin domains were required and sufficient to activate Wnt signaling (data not shown). Consistent with the TCF reporter activities of full-length proteins, the furin domains of RSpo2 and 3 proteins were more active than RSpo1, whereas RSpo4 was the least active (Figure 6B). Although the furin domain is required and sufficient for the RSpo activity, it retained only ∼50% of the activity seen with full-length RSpo protein (Figure 6C), suggesting the possible regulatory activity of other domains. Like RSpo wild-type (wt) proteins, the RSpo1 furin domain also amplified Wnt3A activity indicating that the ability of RSpo proteins to amplify Wnt signaling resides in the furin domain (Figure 6D).
Figure 6.
Furin domains confer the relative activity of RSpo proteins. (A) Domain diagrams of RSpo family members. (B) The furin domain of each RSpo family member was cloned and transfected into HEK293 cells stably transfected with TCF reporter construct. Activation of luciferase reporter was determined by a luminometer. (C) Activities of purified full-length and the furin domain of RSpo1 protein were tested by TCF reporter assay. Stable HEK293 TCF reporter cells were treated with increasing concentrations of each protein for 20 h, and TCF transcriptional activity was measured. (D) The furin domain amplifies Wnt3A activity. Stable HEK293 cells were treated with varying concentrations of Wnt3A in the presence of 20 ng/ml RSpo1 full-length protein or furin domain. (E) To examine whether furin domains are responsible for the relative activities of RSpo proteins, various chimeras were generated, in which the furin domain of RSpo1 gene was replaced with those of RSpo2, 3, or 4. Each chimeric construct was transfected into stable HEK293 TCF reporter cells with indicated amounts of DNA in 96-well plates, and TCF reporter activity was measured 48 h after transfection. (F) Specific activities of RSpo chimeras were tested using an RSpo1-specific neutralizing mAb. Cells were treated with 1 μg/ml neutralizing antibody after transfection of stable reporter cells with each chimeric construct, and reporter activity was measured.
To further examine the role of the furin domains, we generated chimeric constructs in which the furin domain of RSpo1 was replaced with the furin domains of RSpo2, 3, or 4. The stable HEK293 cells were transfected with indicated amounts of RSpo chimeric constructs, and TCF reporter activity was measured. As shown in Figure 5E, transfection of RSpo1 wt activated the TCF reporter activity in a dose-dependent manner, reaching maximal activity at 25 ng of transfected DNA construct (Figure 6E). When cells were transfected with RSpo2/1 or 3/1 chimeras, there was a significant increase in TCF reporter activity at lower doses (1 and 5 ng per transfection) in comparison to the RSpo1 wt construct. Furthermore, RSpo1-induced TCF reporter activity was dramatically reduced when its furin domain was replaced with that of RSpo4, indicating that the furin domain is responsible for the relative activities of RSpo proteins. Specificity of these chimeras was further tested using an RSpo1-specific neutralizing antibody that binds the furin domain. As shown in Figure 6F, a neutralizing antibody for RSpo1 inhibited activity of RSpo1, whereas it had no inhibitory activity on the chimeras of RSpo2/RSpo1, RSpo3/RSpo1, and RSpo4/RSpo1. These data suggest that the relative activity of RSpo family members is specified by the furin domain.
Furin Domains Are Essential and Sufficient for Inhibition of DKK1 Activity
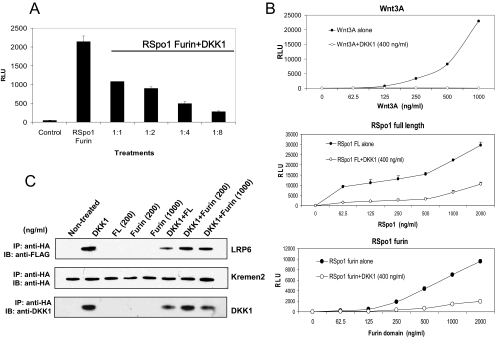
To address whether the furin domain can also interfere with DKK1 activity, purified RSpo1 furin domain was tested in the TCF reporter assay in the presence of increasing concentrations of DKK1. DKK1 inhibited the TCF reporter activity of purified RSpo1 furin domain in a dose-dependent manner (Figure 7A). However, similar to the activity of full-length RSpo1 protein, the DKK1-mediated inhibition was reversed (20–40%) by increasing concentrations of RSpo1 furin domain (at higher doses, >500 ng/ml; Figure 7B, middle and bottom), indicating that the furin domain is responsible for the ability of RSpo proteins to interfere with DKK1 function. In contrast, the DKK1-mediated inhibition of Wnt3A activity was not reversed by increasing amounts of Wnt3A protein (up to 1000 ng/ml; Figure 7B, top).
Figure 7.
The furin domain mediates RSpo-dependent inhibition of DKK1 activity. (A) Stable 293 TCF reporter cells were treated with purified RSpo1 furin domain (100 ng/ml) alone or in combination with increasing concentrations of DKK1 protein (ratios of amounts of RSpo furin domain: DKK1 are shown). TCF reporter activity was measured 20 h after the incubation. (B) Stable 293 TCF reporter cells were treated with DKK1 protein (400 ng/ml) in the presence of increasing amounts of Wnt3A (top panel), RSpo1 full-length (middle panel) or RSpo furin domain (bottom panel) proteins, and TCF reporter activity was determined. (C) To examine the effect of the furin domain on LRP6 and Kremen interaction, coimmunoprecipitation was performed. HEK293 cells stably transfected with a FLAG-tagged LRP6 expression construct, were transfected with HA-tagged hKremen2. Cells were treated as indicated, and total cell lysates were used to immunoprecipitate Kremen2 using anti-HA antibodies. Coimmunoprecipitated LRP6 or DKK1 were detected using anti-FLAG or anti-DKK1 antibodies, respectively. The representative blots are shown.
To extend this finding, we next analyzed whether the RSpo1 furin domain can disrupt DKK1-mediated interaction of LRP6 and Kremen2. Cells were treated with the purified furin domain derived from RSpo1 in the presence or absence of DKK1 followed by immunoprecipitation of Kremen. As seen with purified RSpo1 wt protein, purified RSpo1 furin domain was sufficient to disrupt DKK1-mediated association of LRP6 and Kremen2. Although LRP6 was coimmunoprecipitated with Kremen when cells were incubated with DKK1, a reduced amount of LRP6 was coimmunoprecipitated with Kremen when cells were cotreated with the RSpo1 furin domain and DKK1 (Figure 7C). Together, these data indicate that the furin domain is sufficient and necessary for both Wnt amplification and DKK1 antagonism by RSpo proteins, suggesting that both functions are linked together.
DISCUSSION
The mammalian family of RSpo proteins share 40–60% amino acid sequence identity, and they are predicted to have substantial structural homology (Kim et al., 2006). However, expression of the four RSpo genes during mouse development demonstrated striking differences (Nam et al., 2006b), suggesting unique biological activities of individual members during development. Interestingly, however, when injected into adult mice, all purified recombinant RSpo proteins led to a similar dramatic increase of crypt depth and epithelial cell proliferation in the gastrointestinal tract (Kim et al., 2005, 2006), suggesting a common mechanism of action for all RSpo proteins.
Previous studies have suggested a possible implication of the Wnt coreceptor LRP6 in RSpo1-mediated activation of β-catenin signaling (Binnerts et al., 2007; Wei et al., 2007). Consistent with our recent study with RSpo1 (Binnerts et al., 2007), we demonstrated that LRP6 is required for signaling by all RSpo proteins; first, overexpression of full-length LRP6 enhanced TCF activation by all four RSpo proteins, whereas a C-terminal truncated, dominant negative LRP6 (LRP6ΔC) reduced their activity. Second, although RSpo proteins marginally increased LRP6 phosphorylation, they induced a substantial increase of phosphorylation when combined with Wnt3A. These results further suggest that all RSpo proteins regulate Wnt signaling at the level of the LRP6 receptor.
LRP6 is tightly regulated by the soluble inhibitor DKK1 (Mao et al., 2001, 2002; Semenov et al., 2001). DKK1 is a member of a multigene family and is shown to bind to and antagonize LRP5/6. Kremen is a transmembrane protein and a coreceptor for DKK1, which functionally cooperates with DKK1 to induce endocytosis of LRP6 and down-regulate LRP6 from the cell surface. Recently, we have shown that RSpo1 inhibits DKK1-mediated LRP6 internalization and DKK1-dependent association of LRP6 and Kremen1 (Binnerts et al., 2007). In the present study, we compared the activities of all four RSpo family members and demonstrated that all RSpo proteins can antagonize DKK1-dependent inhibition of the canonical Wnt signaling and disrupt DKK1-dependent association of LRP6 and Kremen.
Our data indicate that RSpo proteins modulate the Wnt pathway through a common biochemical mechanism by competing with DKK1 and as shown previously to reduce internalization of LRP6 and subsequently increasing the levels of LRP6 on the cell surface (Binnerts et al., 2007).
Although our data strongly suggest that RSpo proteins amplify the Wnt pathway and antagonize DKK1 function, it is currently unclear whether these two activities are causally linked each other. Therefore, to map the domain responsible for amplification of Wnt signaling and DKK1 inhibition, we analyzed several RSpo deletion mutants. Previously, the functional domain of RSpo proteins required for Wnt activation was mapped to a furin-like cysteine-rich domain (Kazanskaya et al., 2004). Interestingly, the furin domains derived from all four RSpo members were sufficient for induction of TCF reporter activity and their relative activity was consistent with the activity seen with full-length protein. This observation was further validated by analyzing chimeric RSpo proteins in which the furin domain of RSpo1 was replaced with the furin domain of RSpo2–4, which demonstrated that the furin domains dictate the relative potency of RSpo proteins. Moreover, the furin domains were also shown to be essential and sufficient for RSpo1-mediated competition with DKK1 activity and inhibition of DKK1-induced complex formation between LRP6 and Kremen. Although these data suggest that both Wnt amplification and DKK1 interference are mediated by the same functional furin domain, it will remain to be elucidated whether Wnt amplification is a direct consequence of inhibition of DKK1-mediated LRP6 internalization. It is tempting to speculate that RSpo proteins play a pivotal role by uncoupling Kremen from LRP6 and subsequently permitting a favorable interaction with Wnt and the Frizzled receptor complex.
It is conceivable that the in vivo biological activity of RSpo proteins is dictated by expression of Wnt ligands in target tissues. Consistent with this notion, recent findings showed that loss of function mutation of RSpo1 (Parma et al., 2006), 2 (Bell et al., 2003), 3 (Aoki et al., 2006), and 4 (Blaydon et al., 2006) or knockout of RSpo2 gene (Nam et al., 2007) resulted in similar phenotypes as seen for Wnt or Fzd receptor mutations (Johnson and Tabin, 1997; Vainio et al., 1999; Ishikawa et al., 2001). For example, disruption of the RSpo1 gene was shown to cause a recessive XX sex reversal in an affected patient population, which, similar to the phenotype observed in wnt4+ mice, suggests the cooperative interaction of Wnt and RSpo proteins.
Our current data, in conjunction with previous reports, demonstrate a novel mechanism of action for all four RSpo proteins. We have shown that all RSpo proteins activate Wnt signaling through LRP6 by antagonizing DKK1 function. It is most likely that the specific activities of RSpo proteins reported in vivo are achieved by tissue-specific coexpression of different Wnt ligands, as seen during development and in various mouse (Nam et al., 2007) and human genetic studies (Bell et al., 2003; Aoki et al., 2006; Blaydon et al., 2006; Parma et al., 2006). It remains to be elucidated how RSpo proteins trigger Wnt signaling in a given specific microenvironment in which multiple Wnt signaling components contribute to the differential Wnt signaling activity. RSpo proteins may play a pivotal role in setting the threshold of Wnt activation by antagonizing DKK1 and amplifying signaling by Wnt ligands. Furthermore, as a key modulator of the Wnt pathway, RSpo proteins may prove to be valuable therapeutic agents in various disease conditions where Wnt signaling activity is required for tissue repair such as inflammatory bowel disease (Bouma and Strober, 2003; Radtke and Clevers, 2005; Lim and Hanauer, 2004) and in bone diseases characterized by elevated DKK1 levels, such as bone osteolytic lesions (Tian et al., 2003; Colla et al., 2007; Diarra et al., 2007; Yaccoby et al., 2007).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-02-0187) on April 9, 2008.
REFERENCES
- Aoki M., Mieda M., Ikeda T., Hamada Y., Nakamura H., Okamoto H. R-spondin3 is required for mouse placental development. Dev. Biol. 2006;301:218–226. doi: 10.1016/j.ydbio.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Banziger C., Soldini D., Schutt C., Zipperlen P., Hausmann G., Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Behrens J., Jerchow B. A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kuhl M., Wedlich D., Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280(5363):596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Bejsovec A. Wnt pathway activation: new relations and locations. Cell. 2005;120:11–14. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Bell S. M., Schreiner C. M., Hess K. A., Anderson K. P., Scott W. J. Asymmetric limb malformations in a new transgene insertional mutant, footless. Mech. Dev. 2003;120:597–605. doi: 10.1016/s0925-4773(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Binnerts M., et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl. Acad. Sci. USA. 2007;104(37):14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon D. C., et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat. Genet. 2006;38:1245–1247. doi: 10.1038/ng1883. [DOI] [PubMed] [Google Scholar]
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Colla S., Zhan F., Xiong W, Wu X., Xu H., Stephens O., Yaccoby S., Epstein J., Barlogie B., Shaughnessy J. D. The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood. 2007;109:4470–4477. doi: 10.1182/blood-2006-11-056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G., Wu W., Shen J., Bilic J., Fenger U., Stannek P., Glinka A., Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Diarra D., et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Echelard Y., Vassileva G., McMahon A. P. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- Gordon M. D., Nusse R. Wnt signaling: multiple pathways, multiple receptors and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- He X., Semenov M., Tamai K., Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Tamai Y., Zorn A. M., Yoshida H., Seldin M. F., Nishikawa S., Taketo M. M. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Tabin C. J. Molecular models for vertebrate review limb development. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. [DOI] [PubMed] [Google Scholar]
- Kamata T., Katsube K., Michikawa M., Yamada M., Takada S., Mizusawa H. R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim. Biophys. Acta. 2004;1676:51–62. doi: 10.1016/j.bbaexp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O., Glinka A., del B.B.I., Stannek P., Niehrs C., Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev. Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kim K. A., Zhao J., Andarmani S., Kakitani M., Oshima T., Binnerts M. E., Abo A., Tomizuka K., Funk W. D. R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle. 2006;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- Kim K. A., et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Lim W. C., Hanauer S. B. Emerging biologic therapies in inflammatory bowel disease. Rev. Gastroenterol. Disord. 2004;4:66–85. [PubMed] [Google Scholar]
- Logan C. Y., Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Mao B., et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Kohn A. D., De Ferrari G. V., Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Nam J. S., Turcotte T. J., Smith P. F., Choi S., Yoon J. K. Mouse Cristin/R-spondin family proteins are novel ligands for the frizzled 8 and LRP6 receptors and activate β-catenin-dependent gene expression. J. Biol. Chem. 2006a;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- Nam J. S., Turcotte T. J., Yoon J. K. Dynamic expression of R-spondin family genes in mouse development Gene Expr. Patterns. 2006b;7:306–312. doi: 10.1016/j.modgep.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Nam J. S., Park E., Turcotte T. J, Palencia S., Zhan X., Lee J., Yun K., Funk W. D., Yoon J. K. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hind limb. Dev. Biol. 2007;311:124–135. doi: 10.1016/j.ydbio.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15(1):28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Parma P., Radi O., Vidal V., Chaboissier M. C., Dellambra E., Valentini S., Guerra L., Schedl A., Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1233–1244. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Parr B. A., Shea M. J., Vassileva G., McMahon A. P. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Radtke F., Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Salic A., Lee E., Mayer L., Kirschner M. W. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5(3):523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Semenov M. V., Tamai K., Brott B. K., Kuhl M., Sokol S., He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. A mechanism for Wnt coreceptor activation. Mol. Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tian E., Zhan F., Walker R., Rasmussen E., Ma Y., Barlogie B., Shaughnessy J. D. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Vainio S., Heikkila M., Kispert A., Chin N., McMahon A. P. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Wei Q., Yokota C., Semenov M. V., Doble B., Woodgett J., He X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and β-catenin signaling. J. Biol. Chem. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- Yaccoby S., Ling W., Zhan F., Walker R., Barlogie B., Shaughnessy J. D. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Suda Y., Matsuo I., Miyamoto N., Takeda N., Kuratani S., Aizawa S. Emx1 and Emx2 functions in development of dorsal telencephalon. Development. 1997;124:101–111. doi: 10.1242/dev.124.1.101. [DOI] [PubMed] [Google Scholar]
- Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]