Abstract
The ubiquitylation of membrane proteins destined for the vacuole/lysosome is essential for their recognition by the endosomal sorting machinery and their internalization into vesicles of multivesicular bodies (MVBs). In yeast, this process requires Rsp5p, an essential ubiquitin ligase of the Nedd4 family. We describe here two redundant proteins, Ear1p and Ssh4p, required for the vacuolar targeting of several cargoes originating from the Golgi or the plasma membrane. Ear1p is an endosomal protein that interacts with Rsp5p through its PPxY motifs, and it is required for the ubiquitylation of selected cargoes before their MVB sorting. In-frame fusion of cargo to ubiquitin overcomes the need for Ear1p/Ssh4p, confirming a role for these proteins in cargo ubiquitylation. Interestingly, Ear1p is itself ubiquitylated by Rsp5p and targeted to the vacuole. Finally, Ear1p overexpression leads to Rsp5p accumulation at endosomes, interfering with some of its functions in trafficking. Therefore, Ear1p/Ssh4p recruit Rsp5p and assist it in its function at MVBs by directing the ubiquitylation of specific cargoes.
INTRODUCTION
Ubiquitylation is a reversible posttranslational modification that affects protein stability, activity, interactions, or location. This reaction must therefore be tightly regulated, and its specificity must be ensured.
The conjugation of ubiquitin to a substrate is catalyzed by a series of enzyme activities (E1, E2, and E3; Kerscher et al., 2006). Ubiquitin-protein ligases, or E3, are in charge of interacting with the substrate; consequently, they control the specificity of the ubiquitylation reaction.
Really interesting new gene (RING) domain-containing E3s mediate the E2/substrate interaction, whereas homologous to E6-AP C-terminus (HECT) domain-containing E3s act through an E3-Ub intermediate (Pickart and Eddins, 2004). HECT E3s therefore interact with the substrate and perform the transfer of ubiquitin. The Nedd4 family of HECT E3s illustrates the way in which these proteins cope with this particular requirement (Ingham et al., 2004). Nedd proteins contain a HECT domain responsible for ubiquitin transfer, a lipid interaction domain (C2 domain) and protein–protein interaction modules (WW domains) that bind to proline-rich motifs (such as PPxY motifs; Sudol, 1996), initially identified as involved in substrate recognition (Rotin et al., 2000).
Rsp5p is the only Nedd4-like protein in budding yeast where it is essential for viability. It has been clearly demonstrated to play a key role in the intracellular trafficking of proteins. Indeed, Rsp5p is essential for the endocytic internalization of most plasma membrane proteins (Haguenauer-Tsapis and André, 2004), and for the sorting of lysosome/vacuole-targeted proteins originating from the Golgi into the internal vesicles of late endosomes/multivesicular bodies (MVBs) (Dunn et al., 2004; Katzmann et al., 2004; Morvan et al., 2004)—a key step in the vacuolar protein sorting (VPS) pathway. Surprisingly, among the proteins trafficking in an Rsp5p-dependent manner, only a very limited number bear a PPxY motif, raising questions about their recognition by Rsp5p. Rsp5p has been reported to use “accessory proteins,” which may act as cofactors (Bul1p/Bul2p) or as specificity factors for substrate recognition (reviewed in Shearwin-Whyatt et al., 2006). For example, the PPxY motif-containing membrane proteins Bsd2p and Tre1p/Tre2p interact with Rsp5p and are involved in the ubiquitylation and vacuolar targeting of the manganese transporter Smf1p (Hettema et al., 2004; Stimpson et al., 2006).
However, this limited number of adaptor proteins cannot fully account for the specificity of the trafficking of the large number of proteins passing through the endosomal/vacuolar pathway, and there are many cargoes for which the cognate adaptor protein still remains to be identified. In this report, we describe new Rsp5p adaptors involved in MVB sorting of selected cargoes.
MATERIALS AND METHODS
Plasmid Constructs
The plasmids used in this study are described in Supplemental Table 1. Primer sequences are available upon request. We constructed pSL1 for the C-terminal tagging of proteins, at the locus, with the red fluorescent protein variant mCherry (Shaner et al., 2004). The sequence encoding mCherry was amplified by polymerase chain reaction (PCR) from pRSETB-mCherry (kindly provided by Dr. Roger Tsien, University of California, San Diego, La Jolla, CA) and inserted into pYM35 (Janke et al., 2004) in place of DsRedI at BamHI/BssHII sites. Genes were tagged with this plasmid by using the S2/S3 primers as described previously (Janke et al., 2004). The pEAR1:EAR1-GFP fusion was amplified from ySL083 genomic DNA (see below; Supplemental Table 2), inserted into pCRII-ZeroBlunt (pSL3; Invitrogen, Carlsbad, CA), and subcloned into pRS425 at SpeI/PstI sites (pSL8). The pEAR1:EAR1-HA fusion was amplified from ySL054 genomic DNA, inserted into pCRII-ZeroBlunt (pSL17) and subcloned into pRS425 at SpeI/PstI sites (pSL18). Site-directed mutagenesis was performed on pSL17 to mutate each PPxY motif in AAxY (pSL23 and pSL24, respectively) before insertion into pRS425 (pSL25 and pSL26, respectively). Sequences encoding Bsd2p, Smf1p, and Rsp5p were amplified by PCR from genomic DNA and inserted downstream from the GFP coding sequence into p416-pADH-GFP (Mumberg et al., 1994) (Bsd2p and Smf1p: pSL30 and pSL31, respectively) or p415-pADH-GFP (Mumberg et al., 1994) (Rsp5p: pSL19). The sequence encoding GFP-Rsp5p was digested from pSL19 (SpeI/SalI) and subcloned (SpeI/XhoI) into pRS415-pCYC1 (Mumberg et al., 1994) to give pSL29. Site-directed mutagenesis was performed on pSL29 to delete the C2 domain (residues 1–103), giving pSL37. pSL21 was constructed for the overexpression of proteins tagged at their C terminus with mCherry: mCherry was PCR amplified from pRSETB-mCherry and cloned at ClaI/XhoI sites into pRS415-pGPD (Mumberg et al., 1994) downstream from the polylinker. The sequence encoding Ear1p was then inserted upstream from the mCherry coding sequence in pSL21, at XmaI/ClaI sites (generating pSL22). Site-directed mutagenesis of Ear1p-mCherry was carried out with pSL22 to mutate each PPxY motif in AAxY (pSL34 and pSL35, respectively).
Yeast Strains and Growth Conditions
Strains are described in Supplemental Table 2, and they are derivatives of the BY4741/2 genetic background. Yeast was transformed by the standard lithium acetate/polyethylene glycol procedure. Cells were grown in yeast extract/peptone/glucose (YPD) rich medium, or in synthetic complete (SC) medium (yeast nitrogen base, supplemented with 2%, wt/vol, glucose and Complete synthetic medium; Difco, Detroit, MI) lacking the appropriate nutrient for plasmid selection, as required. Cells were harvested during the early exponential growth phase (A600 = 0.2–0.8).
For galactose induction, cells were precultured in SC glucose medium, and then they were cultured overnight in SC containing 2% raffinose (wt/vol) and 0.02% (wt/vol) glucose to initiate growth. Galactose was then added to a final concentration of 2% (wt/vol) for the indicated time. Chase was started by adding glucose to a final concentration of 2% (wt/vol) and incubating for the indicated time.
Fluorescence Microscopy and N-[3-triethylammoniumpropyl]- 4-[p-diethylaminophenylhexatrienyl] Pyridinium Dibromide (FM 4-64) Labeling
Cells were mounted in medium and observed with an Olympus BY61 fluorescence microscope. Pictures were taken with a Spot 4.05 charge-coupled device camera. Green fluorescent protein (GFP)-tagged proteins were visualized using a Chroma GFP II filter (excitation wavelength 440–470 nm), and mCherry-tagged proteins and FM 4–64 were visualized using an HcRed I filter (excitation wavelength 525–575 nm).
For FM 4-64 labeling, 1 ml of culture was concentrated into 100 μl, placed on ice, and incubated in the presence of 20 μM FM 4-64 (Invitrogen) for 15 min. Cells were then washed twice with 1 ml of cold medium, resuspended in 1 ml of medium (t0), and incubated for the indicated times at room temperature.
Crude Extracts, Immunoprecipitations, and Antibodies
For crude extracts, 0.5–2 OD units of cells were harvested and resuspended in 900 μl of cold water, treated with 50 μl of 2 M NaOH for 10 min on ice, precipitated with 50 μl of 50% trichloroacetic acid, and resuspended in 50–200 μl of sample buffer containing one fifth volume of Tris-base (1 M). The resulting extract was then denatured by heating at 37°C for 10 min.
Sit1p-GFP was immunoprecipitated in denaturing conditions (Figure 3B) from cells grown in the absence of its substrate, as follows. For the Sit1p-GFP galactose induction, cells were cultured as described above and induced by addition of galactose for 45 min. Sit1p-GFP synthesis was stopped by adding 2% glucose (15 min). Cells (40 OD units) were then harvested and washed with cold TNE buffer (100 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 5 mM EDTA). The following steps were carried out at 4°C unless otherwise indicated. Cells were resuspended in lysis buffer (TNE supplemented with a protease inhibitor cocktail [Complete, EDTA-free mixture; Roche Diagnostics, Mannheim, Germany] and 5 mM N-ethylmaleimide [Sigma, Paris, France]) and broken with glass beads. Cell debris and unbroken cells were removed by centrifugation at 3000 × g for 3 min. Proteins were precipitated from the lysate by adding trichloroacetic acid (10% final concentration). The pellet was resuspended in 60 μl of Laemmli sample buffer without β-mercaptoethanol, and the sample was incubated at 37°C for 15 min. We added 600 μl of TNET buffer (TNE supplemented with 1% Triton X-100) to the sample, which was then centrifuged for 30 min at 13,000 × g to eliminate unsolubilized proteins. The supernatant was then incubated with a monoclonal anti-GFP antibody (clones 7.1 and 13.1; Roche Diagnostics) for 1 h at 4°C. Prewashed protein G-Sepharose beads (GammaBind-Sepharose; GE Healthcare, Chalfont St. Giles, United Kingdom) were added to the sample and incubated overnight. Beads were collected by centrifugation at 2500 × g for 1 min and washed with TNET buffer. The immune complex was eluted by adding SDS sample buffer containing 2% β-mercaptoethanol and incubating at 37°C for 15 min. Immunoprecipitated Sit1p-GFP and its ubiquitin conjugates were detected by immunoblotting with a monoclonal anti-ubiquitin antibody coupled to horseradish peroxidase (clone P4D1; Santa Cruz Biotechnology, Santa Cruz, CA), and Sit1p-GFP was detected with rabbit polyclonal anti-GFP (generated by our laboratory). We compensated for the smaller amount of full-length Sit1p-GFP in the wild-type strain (due to vacuolar degradation) compared with the mutants, by loading twice as much sample in the corresponding lane.
Figure 3.
Ear1p/Ssh4p are involved in cargo ubiquitylation at MVBs. (A) Subcellular localization of Ub-GFP-Phm5p wild-type and ear1Δ ssh4Δ cells. Bar, 5 μm. (B) Lysates of wild-type, ear1Δ ssh4Δ, and rsp5 cells expressing Sit1p-GFP were subjected to immunoprecipitation in denaturing conditions with a monoclonal GFP antibody, and then they were immunoblotted with a polyclonal anti-GFP or a monoclonal anti-ubiquitin antibody. Arrowhead, Sit1p-GFP; asterisk, GFP-containing degradation products. (C) Subcellular localization of the PPxY-motif containing proteins Sna3p-GFP and GFP-Bsd2p in wild-type and ear1Δ ssh4Δ cells. Bar, 5 μm.
Coimmunoprecipitations were carried out using 20–30 OD units of cells. Cells were resuspended in immunoprecipitation (IP) lysis buffer (50 mM HEPES-KOH, pH 7.5, 0.25 M NaCl, 0.1% NP-40, 10% glycerol, 1 mM EDTA, and protease inhibitor cocktail) at a concentration of 1 OD unit/10 μl, and then they were disrupted with glass beads at 4°C for 10 min. Cell debris and unbroken cells were removed by a centrifugation for 5 min at 3000 × g, and the supernatant (“Input”) was incubated with GammaBind-Sepharose beads (previously incubated with a monoclonal anti-hemagglutinin (HA) antibody (clone 12CA5; Santa Cruz Biotechnology) for 1 h at 4°C. The unbound fraction was eliminated by a 10-s centrifugation in a mini-centrifuge; beads were then washed three times for 10 min with 1 ml of IP lysis buffer before elution of the immunoprecipitate with SDS sample buffer (1μL/initial OD).
For immunoblotting, we used monoclonal antibodies raised against GFP (clones 7.1 and 13.1; Roche Diagnostics), 3-phosphoglycerate kinase (PGK) (clone 22CS; Invitrogen), and HA (clone 12CA5; Santa Cruz Biotechnology) and polyclonal antibodies against Nedd4 (BD Biosciences, San Jose, CA) and DsRed (Clontech, Mountain View, CA).
His6-Ubiquitin Protein Conjugates Purification
For the purification of ubiquitylated proteins, cells were transformed with a plasmid encoding a copper-inducible His6-tagged ubiquitin (Yep352-His-Ub, kindly provided by Dr. C. Gwizdek, Institut Jacques Monod, Paris, France). Cells (100 OD units) were grown overnight in the presence of 100 μM Cu(SO4)2, harvested, and disrupted with glass beads in a buffer containing 6 M guanidium. The extract was incubated with nickel-nitrilotriacetic acid (Ni-NTA) Sepharose beads (QIAGEN, Hilden, Germany), which were then collected and washed several times with a buffer containing 8 M urea. Bound proteins were eluted in SDS sample buffer by heating for 5 min at 37°C. Importantly, ubiquitylated species of Ear1p were best visualized on commercial precast gels [10% NuPAGE gels with 3-(N-morpholino)propanesulfonic acid (MOPS) buffer; Invitrogen].
Other Methods
Uracil permease activity was measured as described previously (Volland et al., 1994) after adding cycloheximide (100 μg/ml, final concentration) at 37°C to induce endocytosis.
Dephosphorylation was carried out on crude extracts prepared as described above, and proteins were resuspended in SDS sample buffer. Samples were diluted 1:10 with phosphatase buffer and incubated for 15 min at 37°C with or without calf intestinal alkaline phosphatase, as recommended by the supplier (Roche Diagnostics). Proteins were then precipitated with 10% trichloroacetic acid, resuspended in a 2× sample buffer, and analyzed by immunoblotting. Importantly, the phosphorylation of Ear1p was difficult to detect on commercial precast gels (10% NuPAGE gels with MOPS buffer; Invitrogen).
RESULTS
Ear1p Interacts with Rsp5p In Vivo
Many proteins interacting with yeast WW domains have been identified by proteomics (Hesselberth et al., 2006). The protein encoded by the previously uncharacterized open reading frame YMR171C was of particular interest to us because of its endosomal localization, as described in a large-scale study (Huh et al., 2003). The product of the YMR171C gene interacted with the Rsp5p WW3 domain and contains two PPxY motifs. For reasons elaborated throughout this report, we named this gene endosomal adaptor of Rsp5p (EAR1).
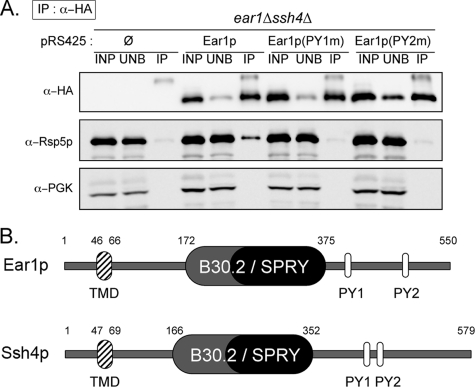
We confirmed this interaction in vivo by coimmunoprecipitation, using a functional version of Ear1p tagged with an HA epitope at its C terminus. Indeed, Rsp5p coimmunoprecipitated with Ear1p-HA (Figure 1A). Using mutant versions of Ear1p, in which the PPxY motifs were mutated into AAxY, we observed that this interaction depended on the integrity of the PPxY motifs (Figure 1A). This observation is consistent with the existence of an interaction with Rsp5p WW domain(s), as suggested in a previous study (Hesselberth et al., 2006).
Figure 1.
Ear1p interacts with Rsp5p, and it is homologous to Ssh4p. (A) Immunoprecipitation of Ear1p-3HA (wild-type or PPxY mutants) expressed in wild-type cells by using an anti-HA antibody. Input fractions (INP), unbound material (UNB), and IPs were immunoblotted with the indicated antibodies. (B) Schematic primary structure of Ear1p and Ssh4p. TMD, transmembrane domain; B30.2/SPRY, B30.2/SPRY domain; PY, PPxY motifs.
Ear1p and Ssh4p Are Redundant Proteins That Are Essential for MVB Sorting of Several VPS Cargoes
We investigated the possible role of EAR1 in protein trafficking, by studying the subcellular distribution of several model cargoes whose trafficking depends on Rsp5p in the ear1Δ deletion strain. However, no defects were observed (data not shown). In a search for homologous genes within the Saccharomyces cerevisiae genome, we identified the gene SSH4 (YKL124W) as a homologue of EAR1 (Figures 1B and Supplemental Figure S1). Ear1p and Ssh4p share 43% similarity (25% identity), and they have a similar architecture, with one predicted transmembrane domain toward the N terminus and a central B30.2 exon/splA and ryanodine receptor domain (B30.2/SPRY: Vernet et al., 1993; Ponting et al., 1997), usually involved in protein interactions (see Woo et al., 2006, and references therein). Both these proteins also have two PPxY motifs, which are known to mediate interactions with WW domains such as those of Rsp5p (Sudol, 1996). The function of SSH4 is unknown, but its overexpression confers resistance to the immunosuppressant leflunomide (Fujimura, 1998) and suppresses shr3Δ defects, in which the exit of most plasma membrane amino acid transporters from the endoplasmic reticulum (ER) is compromised (Kota et al., 2007). The data suggested a role for Ssh4p in protein trafficking, although the ssh4Δ had no obvious trafficking defects (Kota et al., 2007; our unpublished data).
We then hypothesized that Ear1p and Ssh4p might be functionally redundant. We tested this hypothesis by generating the ear1Δ ssh4Δ double mutant, which was viable and showed no growth defects, and studying possible trafficking-related phenotypes. Because Ear1p interacts with Rsp5p (Figure 1A; Hesselberth et al., 2006; Gupta et al., 2007), we focused on cargoes with Rsp5p-dependent trafficking, starting with cargoes following the VPS pathway.
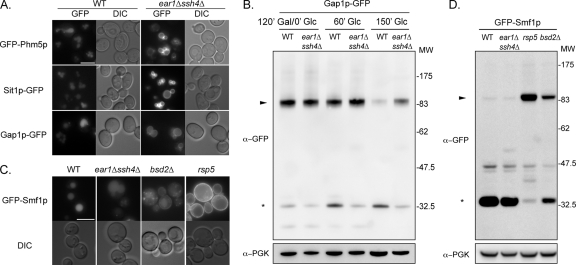
Traditional markers of this pathway include a GFP-tagged version of the vacuolar enzyme Phm5p (polyphosphatase) (Reggiori and Pelham, 2001). As shown in Figure 2A, GFP-Phm5p was targeted to the vacuolar lumen in the wild-type strain, but it was mislocalized to the vacuolar membrane in the ear1Δ ssh4Δ strain (Figure 2A). This demonstrates the existence of a defect in sorting into internal vesicles of MVBs, leading to cargo accumulation at the endosomal membrane and, after fusion with the vacuole, at the vacuolar membrane. A similar phenotype is observed in rsp5 mutants (Dunn et al., 2004; Katzmann et al., 2004; Morvan et al., 2004).
Figure 2.
Ear1p and Ssh4p are required for MVB sorting of specific VPS cargoes. (A) Localization of cargo GFP-fusion proteins in wild-type and ear1Δ ssh4Δ cells. Bar, 5 μm. (B) Crude extracts were prepared from wild-type and ear1Δ ssh4Δ cells expressing GFP-Gap1p, after 120 min of galactose induction and the indicated times of chase. Samples were immunoblotted with anti-GFP or anti-PGK (loading control) antibodies. Arrowhead, GFP-Gap1p; asterisk, GFP-containing degradation product. (C) Localization of GFP-Smf1p in the indicated strains. Bar, 5 μm. (D) Crude extracts were prepared from the indicated strains expressing GFP-Smf1p and immunoblotted with the indicated antibodies. Arrowhead, GFP-Smf1p; asterisk, GFP-containing degradation product.
In defined physiological conditions, several plasma membrane transporters are constitutively targeted from the Golgi to the vacuole for degradation, by following the VPS pathway. These transporters include the general amino acid permease Gap1p, when synthesized in the presence of ammonium (De Craene et al., 2001), and the iron/siderophore transporter Sit1p, when synthesized in the absence of its substrate (Froissard et al., 2007). GFP-tagged versions of these transporters were produced under the control of a galactose-inducible promoter in conditions triggering the vacuolar targeting of these proteins. For both transporters, a pulse/chase-type protocol was used in which expression was induced (addition of galactose) and then repressed (addition of glucose), making it possible to follow the trafficking of newly synthesized transporters over time. Like GFP-Phm5p, Gap1p-GFP and Sit1p-GFP accumulated at the vacuolar membrane in the ear1Δ ssh4Δ double mutant, whereas they were delivered to the vacuolar lumen in wild-type cells (Figure 2A and Supplemental Figure S2A and S2B). It should be noted that deletion of EAR1 or SSH4 alone had no effect on the vacuolar targeting of these transporters (see Figure S2B for Sit1p-GFP; data not shown).
The targeting of GFP-fusion proteins to the vacuolar lumen leads to their degradation by vacuolar proteases. However, degradation of the tightly folded GFP moiety is usually delayed, leading to the transient accumulation of GFP-containing proteolytic fragments of ≈30 kDa, and a sustained luminal vacuolar fluorescence. We used the presence of these fragments as a readout for the vacuolar targeting and degradation of GFP fusion proteins. In wild-type cells, a clear down-regulation of Gap1p-GFP over time was observed, coinciding with the appearance of GFP-containing proteolytic fragments (Figure 2B). These results contrast sharply with those obtained for the ear1Δ ssh4Δ strain, in which Gap1p-GFP degraded more slowly, and little proteolysis was observed, consistent with the fluorescence microscopy data.
Finally, we checked the localization of the manganese transporter Smf1p, which is also targeted to the vacuole for degradation under metal-replete conditions. The sorting of Smf1p in the VPS pathway requires the PPxY motif-containing proteins Bsd2p and Tre1p/Tre2p, which act as adaptors for the ubiquitylation of Smf1p by Rsp5p (Liu and Culotta, 1999; Stimpson et al., 2006; Sullivan et al., 2007). Strikingly, at steady state, GFP-Smf1p reached the vacuolar lumen equally efficiently in the ear1Δ ssh4Δ and the wild-type strains (Figure 2C), whereas in the bsd2Δ mutant, some of the GFP-Smf1p was targeted to the plasma membrane, as reported previously (Liu and Culotta, 1999; Stimpson et al., 2006). Consistent with previous reports, Rsp5p was also found to be essential for the proper sorting of GFP-Smf1p, because this protein was exclusively targeted to the plasma membrane in a hypomorphic rsp5 mutant (Figure 2C). These data were confirmed by assessments of GFP-Smf1p degradation in these strains. GFP-Smf1p was found to be degraded to similar extents in wild-type and ear1Δ ssh4Δ cells, whereas it was partially stabilized in the bsd2Δ mutant, and completely stable in the rsp5 mutant (Figure 2D). Finally, the ear1Δ ssh4Δ strain was insensitive to cadmium (Supplemental Figure S3), which is an indirect measurement of Smf1p activity at the plasma membrane, whereas rsp5 or bsd2Δ mutants displayed enhanced sensitivity, as described previously (Liu et al., 1997; Liu and Culotta, 1999; Stimpson et al., 2006). In conclusion, although the vacuolar targeting of Smf1p requires Rsp5p, it does not depend on Ear1p/Ssh4p.
Altogether, we conclude that Ear1p/Ssh4p are involved in the MVB sorting of several, but not all, MVB cargoes, suggesting that they confer some level of specificity of Rsp5p toward the substrate.
Ear1p/Ssh4p Are Involved in Cargo Ubiquitylation at Late Endosomes
MVB sorting defects like those described above may be due to cargo ubiquitylation defects, because similar phenotypes have been described in rsp5 mutants (Dunn et al., 2004; Katzmann et al., 2004; Morvan et al., 2004).
We investigated whether the trafficking defects displayed by the ear1Δ ssh4Δ mutant were linked to cargo ubiquitylation, by investigating the subcellular distribution of a chimeric construct, Ub-GFP-Phm5p, in which ubiquitin was fused in frame to GFP-Phm5p, therefore mimicking its constitutive ubiquitylation (Reggiori and Pelham, 2001). Ub-GFP-Phm5p was targeted to the lumen of the vacuole in the ear1Δ ssh4Δ double mutant (Figure 3A). Because the linear fusion of ubiquitin to GFP-Phm5p overcomes the need for Ear1p/Ssh4p, we conclude that the mistargeting initially observed in the ear1Δ ssh4Δ mutant was probably due to an ubiquitylation defect of the cargo in this strain.
For confirmation of this finding, we investigated the ubiquitylation status of another cargo, Sit1p-GFP, in the ear1Δ ssh4Δ mutant. Sit1p-GFP expression was induced by adding galactose to the medium, and the vacuolar targeting of newly synthesized transporter molecules was followed over time by fluorescence microscopy. After 45 min of synthesis, when most of the fluorescent signal was detected in endosomes and before it completely reached the vacuole, synthesis was stopped by adding glucose and incubating for 15 min. The cells were then disrupted and Sit1p-GFP was immunoprecipitated in denaturing conditions using a GFP antibody. Immunoblotting of the immunoprecipitate with anti-ubiquitin antibodies revealed that not only was Sit1p-GFP ubiquitylated in wild-type cells but also that this ubiquitylation was strongly affected in the ear1Δ ssh4Δ double mutant, as in the rsp5 mutant (Figure 3B). We therefore conclude that Ear1p/Ss4hp are required for the ubiquitylation of Sit1p during its trafficking to the vacuole in the VPS pathway.
Other cargoes showing Rsp5p-dependent ubiquitylation and vacuolar targeting include PPxY motif-containing membrane proteins, such as Sna3p or Bsd2p, which interact with and are ubiquitylated by Rsp5p (Hettema et al., 2004; Stawiecka-Mirota et al., 2007; Sullivan et al., 2007). An investigation of Sna3p-GFP trafficking in the ear1Δ ssh4Δ mutant revealed that neither its localization, nor its ubiquitylation were affected (Figure 3C and Supplemental Figure S4). Similarly to Sna3p-GFP, GFP-Bsd2p trafficking did not depend on Ear1p/Ssh4p (Figure 3C).
Our results demonstrate that ubiquitylated cargoes (either fused to ubiquitin, or directly ubiquitylated by Rsp5p) are targeted to the vacuole independently of Ear1p/Ssh4p. Furthermore, the absence of Ear1p/Ssh4p affected the MVB sorting and ubiquitylation of Sit1p-GFP. This suggests that Ear1p/Ssh4p play an essential role in the MVB sorting of specific cargoes by affecting their ubiquitylation status.
Ear1p/Ssh4p Are Required for MVB Sorting of the Plasma Membrane Protein Fur4p after Its Endocytosis
Rsp5p is required for endocytosis (Dupré et al., 2004). We therefore investigated the possible involvement of Ear1p/Ssh4p in the endocytosis of a model cargo, the uracil transporter Fur4p, which displays Rsp5p-dependent ubiquitylation and internalization (Galan et al., 1996).
We followed the trafficking of newly synthesized Fur4p-GFP by using a pulse/chase-type protocol, as described above. Fur4p-GFP synthesis was induced by adding galactose, and after 1-h induction, when it had reached the plasma membrane, the induction was stopped by adding glucose, and endocytosis was triggered.
We first investigated the kinetics of Fur4p-GFP internalization in the ear1Δ ssh4Δ mutant by measuring the decrease in uracil transport activity over time during its endocytosis. No difference in Fur4p internalization rate was detected between the wild-type and the ear1Δ ssh4Δ strain (Supplemental Figure S5A). Consistent with this result, the double mutant was not sensitive to 5-fluorouracil, a toxic uracil analogue that is transported by Fur4p (Supplemental Figure S5B). Ear1p/Ssh4p are therefore not required for Fur4p internalization. The ear1Δ ssh4Δ strain displayed no general defect in endocytosis, as shown by the internalization of the fluorescent lipophilic dye FM 4-64 (Supplemental Figure S5C).
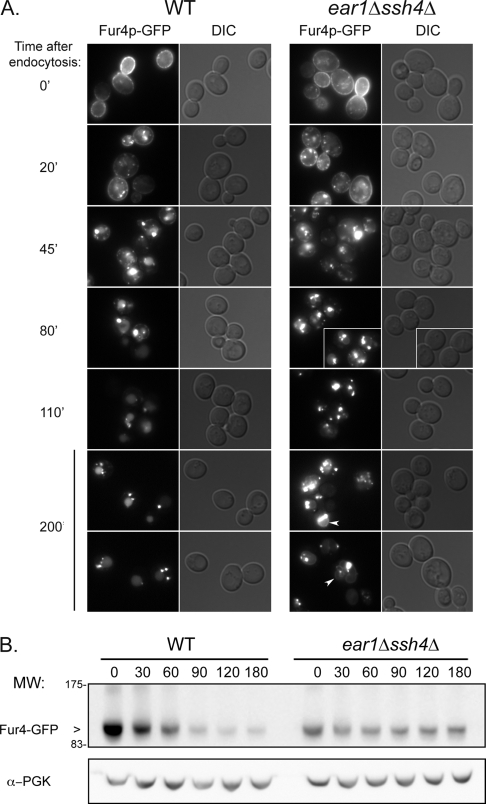
We studied Fur4p-GFP trafficking in more detail by fluorescence microscopy, by using the same pulse/chase-type protocol. Again, Fur4p-GFP was internalized at a similar rate in the ear1Δ ssh4Δ double mutant and in the wild type. However, at later times, Fur4p-GFP accumulated in large, juxtavacuolar structures in the ear1Δ ssh4Δ mutant, eventually reaching the vacuolar membrane, whereas most of the Fur4p-GFP was targeted to the vacuolar lumen in wild-type cells (Figure 4A). This defect in MVB trafficking was confirmed by following the degradation of the protein over time after the triggering of endocytosis (Figure 4B). In wild-type cells, newly synthesized Fur4p-GFP was almost totally degraded within 90 min. This situation contrasts sharply with that in the ear1Δ ssh4Δ mutant, in which little Fur4p-GFP degradation was observed over time.
Figure 4.
Ear1p/Ssh4p are required for MVB sorting of the endocytic cargo Fur4p. Fur4p-GFP expression was induced in wild-type and ear1Δ ssh4Δ cells, and chased before triggering its endocytosis by addition of cycloheximide. (A) Intracellular localization of Fur4p-GFP at the indicated time after endocytosis. Arrowheads indicate vacuolar membrane labeling. Bar, 5 μm. (B) Crude extracts were prepared from cells treated as described in A, and then they were immunoblotted with an anti-GFP or an anti-PGK antibody. For an unknown reason, the amount of Fur4p-GFP synthesized in the double-mutant was lower than in the wild type.
These findings indicate that Ear1p/Ssh4p are involved in Fur4p-GFP endocytosis, at the level of its sorting into MVB vesicles. Ear1p/Ssh4p are therefore required not only for the MVB sorting of VPS cargoes but also for that of proteins originating from the plasma membrane by endocytosis.
Ear1p Is an Endosomal Protein Ubiquitylated by Rsp5p and Targeted to the Vacuole
Based on these results, we hypothesized that Ear1p may act by assisting Rsp5p in its endosomal functions. Interestingly, Ear1p was described as an endosomal protein in one large-scale study (Huh et al., 2003).
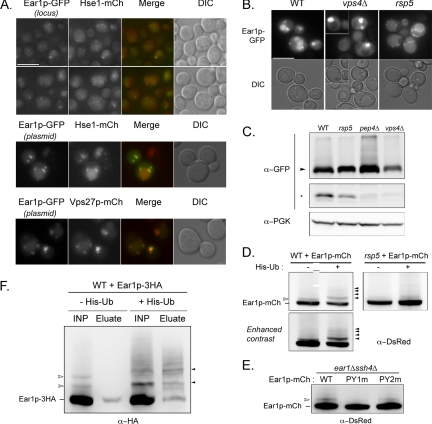
Indeed, a functional version of Ear1p tagged with GFP at its C terminus (inserted at its endogenous locus) was localized to punctate structures and the vacuolar lumen (Figure 5A). These fluorescent structures colocalized with endosomes, as defined by the endosomal markers Hse1-mCherry, Vps27-mCherry, or FM 4-64 (Supplemental Figures 5A and S6A), whereas little or no colocalization was observed with the late-Golgi marker Sec7-mCherry (Supplemental Figure S6B). Mild overexpression of Ear1p-GFP had no major effect on this localization (Figure 5A).
Figure 5.
Ear1p is an endosomal protein that is ubiquitylated and targeted to the vacuole. (A) Colocalization of Ear1p-GFP with endosomal markers (Hse1p-mCherry, top and middle; or Vps27p-mCherry, bottom). Ear1p-GFP expression was driven by its own promoter, either at the endogenous locus (top) or on a multicopy plasmid (middle and bottom). Bar, 5 μm. (B) Localization of Ear1p-GFP (multicopy plasmid) in the indicated strains. Bar, 5 μm. (C) Extracts from the indicated strains expressing Ear1p-GFP (multicopy plasmid) were immunoblotted with anti-GFP and anti-PGK antibodies. Arrowhead, Ear1p-GFP; asterisk, GFP-containing proteolytic fragment. GFP panels are from the same blot, but they represent different areas and exposures. (D) Crude extracts were prepared from the indicated strains expressing Ear1p-mCherry with or without His6-tagged ubiquitin, and they were immunoblotted with anti-DsRed antibody. Empty arrowhead, size of ubiquitin-conjugated Ear1p-mCh; solid arrowheads, size of His6-ubiquitin–conjugated Ear1p-mCh. (E) Crude extracts were prepared from ear1Δssh4Δ expressing wild-type (WT) or PPxY mutants of Ear1p-mCh, and then they were immunoblotted with anti-Ds-Red antibody. Empty arrowhead, size of ubiquitin-conjugated Ear1p-mCh. (F) Crude extracts were prepared from wild-type cells expressing Ear1p-3HA with or without His6-tagged ubiquitin, in denaturing conditions. These extracts (INP) were incubated with Ni-NTA Sepharose beads, and His6-tagged (ubiquitylated) proteins retained on the beads were then eluted with SDS sample buffer (El.). Empty arrow head, size of ubiquitin-conjugated Ear1p-HA; solid arrowheads, size of His6-ubiquitin-conjugated Ear1p-HA.
The detection of fluorescence in the vacuolar lumen of cells expressing Ear1p-GFP suggested that Ear1p might itself be targeted to the vacuole. In the ESCRT mutant vps4Δ (class E of vps mutants, affected in MVB sorting), Ear1p-GFP was detected in enlarged endosomes apposed to the vacuole (class E compartment)—confirming the endosomal localization of Ear1p-GFP—and it was absent from the vacuolar lumen (Figure 5B). In the rsp5 mutant, Ear1p-GFP was detected as punctate staining around the vacuole and at the vacuolar membrane (Figure 5B).
Ear1p-GFP migrated at an apparent molecular mass of 90 kDa (Figure 5C) on SDS-polyacrylamide gel electrophoresis, the band looking diffuse in some cases due to phosphorylation events (Supplemental Figure S7). GFP-containing proteolytic fragments were also detected at a molecular mass of ∼30 kDa, consistent with the degradation of the fusion protein in the vacuole, as demonstrated by the absence of this band in the vacuolar protease mutant pep4Δ (Figure 5C). In agreement with these observations, no degradation of Ear1p-GFP was observed in the vps4Δ mutant, and this degradation was weaker in the rsp5 mutant than in wild type. However, Ear1p was targeted to the vacuole independently of the Rsp5p adaptor Bsd2p (Supplemental Figure S8), unlike the PPxY-containing adaptor protein Tre1p (Stimpson et al., 2006).
Because Ear1p-GFP was found to be targeted to the vacuolar lumen in an Rsp5p- and ESCRT-dependent manner, we investigated whether Ear1p was ubiquitylated in vivo. Immunoblotting of crude extracts of cells expressing Ear1p-mCherry with an anti-DsRed antibody revealed the presence of a higher-molecular-weight band, distinct from the phosphorylation event described above (Supplemental Figure S7) and whose size was in good agreement with the conjugation to ubiquitin (Figure 5D; also see also Supplemental Figure S10). The apparent molecular weight of this top band increased when the cells coexpressed a hexahistine-tagged ubiquitin, indicating that this band represented a ubiquitylated form of Ear1p (Figure 5, D and F). Overexpression of His6-tagged ubiquitin also led to an increase in the overall amount of ubiquitylated Ear1p, and a fraction of Ear1p occurred in these conditions as multimono- or polyubiquitylated (Figure 5, D and F). Interestingly, ubiquitin-modified forms of Ear1p were less abundant in the hypomorphic rsp5 mutant (Figure 5D), and their presence depended on the integrity of the first PPxY motif, whereas mutation of the second PPxY had only little, if any, effect (Figure 5E).
Because the ubiquitylation of a PPxY-containing protein was reported previously to occur on its epitope tag (GFP: Stawiecka-Mirota et al., 2007), we further investigated the ubiquitylation of Ear1p tagged with the 3HA epitope, which is devoid of lysine residues. Denatured extracts of cells coexpressing Ear1p-3HA and His6-tagged ubiquitin were prepared, and we carried out a nickel-based purification of histidine-tagged (ubiquitylated) proteins from these extracts. Indeed, higher-molecular-weight species of Ear1p-3HA were retained on the beads, whose presence was dependent on the expression of His6-ubiquitin (Figure 5F). Altogether, these data suggest that Ear1p is ubiquitylated in vivo in an Rsp5p-dependent manner.
Ear1p Overexpression Affects Rsp5p Localization and Its Function toward Other Cargoes
Given the phenotypes reported for Ssh4p overexpression (Kota et al., 2007), we overexpressed Ear1p-mCherry in wild-type cells to investigate whether this also led to pleiotropic trafficking defects. Ear1p-mCherry was mostly found in the vacuolar lumen, suggesting that the endosomal fraction represents a minor part of the overall pool of Ear1p-mCherry at this level of expression. Ear1p-mCherry overexpression had no effect on sorting of the Ear1p-dependent cargo Sit1p-GFP (Figure 6A). However, these cells were clearly sensitive to cadmium, indicating a defect in Smf1p trafficking (Figure 6B). Thus, Ear1p overexpression had no effect on sorting of cargoes it is in charge of delivering to the vacuole, but may have an effect in trans on cargoes, such as Smf1p, that require other adaptors.
Figure 6.
Ear1p overexpression affects Rsp5p localization and function toward an Ear1p-independent cargo. (A) Subcellular localization of Sit1p-GFP in Ear1p-mCherry–overexpressing cells after induction and the indicated time of chase. Bar, 5 μm. (B) Wild-type cells, rsp5 mutant cells, and wild-type cells overexpressing Ear1p-mCherry (WT or PPxY mutants) were serially diluted and spotted on SC solid medium supplemented or not with cadmium, and grown for 3 d at 30°C. (C) Localization of GFP-Rsp5p and GFP-Rsp5p(ΔC2) was addressed in wild-type cells or in cells overexpressing Ear1p-mCherry. Arrowheads indicate plasma membrane labeling. The asterisk denotes a cell in which Ear1p-mCherry is not expressed, leading to a wild-type localization of GFP-Rsp5p. For additional pictures, see Supplemental Figure S9. Bar, 5 μm.
The overexpression of Ear1p—and probably that of Ssh4p as well—may affect the cellular Rsp5p pool by attenuating its availability toward other adaptors. We therefore studied the localization of a functional GFP-Rsp5p fusion protein in wild-type cells and upon Ear1p-mCherry overexpression. GFP-Rsp5p expression was driven by the constitutive, weak promoter pCYC1 to a level that was comparable to that of endogenous Rsp5p (Supplemental Figure S10). In wild-type cells, GFP-Rsp5p was detected in the cytoplasm, at the plasma membrane, and in punctate structures previously identified as endosomes (Wang et al., 2001; Dunn et al., 2004; Katzmann et al., 2004). Strikingly, strong Ear1p-mCherry overexpression promoted a drastic mislocalization of GFP-Rsp5p, which was found to accumulate mostly in intracellular structures thought to be endosomes due to the presence of Ear1p-mCherry (Figure 6C; see also Supplemental Figure S9 for additional pictures). The vacuolar lumen and vacuolar membrane were also stained, probably because the ectopic accumulation of Rsp5p at endosomes eventually led to its aberrant sorting to the vacuole. However, this had no significant effect on Rsp5p steady-state levels in the cells (Supplemental Figure S10). Although Rsp5p depends on the C2 domain for its plasma membrane and endosomal localization in wild-type cells (Figure 6C; Dunn and Hicke, 2001; Wang et al., 2001; Dunn et al., 2004), its recruitment at endosomes in Ear1p-overexpressing cells did not depend on the C2 domain (Figure 6C), suggesting that endosomal recruitment through lipid binding was not a prerequisite for the interaction with Ear1p when it is overexpressed.
Interestingly, mutation of either of the PPxY motifs in Ear1p-mCherry abolished the cadmium sensitivity that was conferred by overexpression of the wild-type protein (Figure 6B). Therefore, the cadmium sensitivity conferred by Ear1p overexpression requires interaction of Ear1p with Rsp5p.
Altogether, these data demonstrate that Ear1p is able to recruit Rsp5p at endosomes. The overexpression of Ear1p leads to an increased localization of Rsp5p at endosomes, and it may interfere with the function of other adaptors, leading to defects in the sorting of the corresponding cargoes.
DISCUSSION
In this report, we identified Ear1p and Ssh4p as new players in the ubiquitylation of specific cargoes by Rsp5p at multivesicular bodies. Although this modification is essential for MVB sorting, its regulation is poorly documented. So far, two adaptors were documented in yeast (Bsd2p and Tre1p/Tre2p), which are responsible for the sorting of a few cargoes via the recognition of polar transmembrane domains (Hettema et al., 2004; Stimpson et al., 2006; Sullivan et al., 2007).
Instead, the cargoes whose sorting depends on Ear1p/Ssh4p vary greatly in their structure (1–14 transmembrane domains) and nature. The mechanism for cargo selection by Ear1p/Ssh4p is not yet defined, but they clearly act as specificity factors because they are essential for the sorting of certain cargoes. Ear1p/Ssh4p may simply interact with cargoes and recruit Rsp5p in their vicinity. The protein interaction domain in Ear1p/Ssh4p (B30.2/SPRY) may be involved in cargo recognition, either directly or by combination with other adaptors, perhaps leading to a higher selectivity. This is the case for Smf1p trafficking, where “primary” (Bsd2p) and “secondary” (Tre1p/Tre2p) adaptors act in concert. Alternatively, Ear1p/Ssh4p could recruit and concentrate Rsp5p at specific endosomal domains, within which certain cargoes are concentrated and from which others are evicted. Further studies are required to elucidate the molecular mechanisms underlying Ear1p/Ssh4p function in more detail.
Other regulators, such as the cytosolic PY-containing proteins Bul1p/Bul2p, have also been implicated in the Rsp5p-dependent sorting of several cargoes. Bul1p/Bul2p interact with Rsp5p (Yashiroda et al., 1996, 1998), and they are involved in the sorting of Gap1p at Golgi/endosomes (Helliwell et al., 2001). These proteins might regulate Rsp5p activity at endosomes by controlling the length of the ubiquitin chain on the substrate (Helliwell et al., 2001). Bul1p/Bul2p are also required for the correct ubiquitylation and endosomal sorting of the tryptophan permease Tat2p (Umebayashi and Nakano, 2003) and the plasma membrane ATPase mutant Pma1–7 (Pizzirusso and Chang, 2004), and for the ubiquitylation and endocytosis of several transporters (Gap1p, Tat2p, copper transporter Ctr1p: Soetens et al., 2001; Abe and Iida, 2003; Liu et al., 2007). Although Bul1p/Bul2p bind Rsp5p, whether they recruit Rsp5p at specific subcellular locations and whether they interact with cargoes has not been investigated. It therefore remains unclear whether Bul1p/Bul2p contribute to the specificity of Rsp5p activity, or simply act as “regulators” of its activity.
The role of Rsp5p in the MVB sorting of several VPS cargoes is well documented, but it remained unclear whether it played a similar role on endocytic cargoes. Because Rsp5p is required for early stages of endocytosis (internalization), the identification of endosomal adaptors of Rsp5p provided the first opportunity to investigate the involvement of Rsp5p later in the endocytic pathway. We showed that Ear1p/Ssh4p were not required for Fur4p internalization, but they were essential for its vacuolar targeting. Thus, the ubiquitylation of Fur4p at the plasma membrane seems to be insufficient for full vacuolar delivery. Instead, an additional ubiquitylation step, mediated by Rsp5p/Ear1p/Ssh4p, is probably required for MVB sorting. Whether this is preceded by cargo deubiquitylation or not is unknown, but a growing body of evidence suggests that ubiquitylation in the endocytic pathway is a dynamic process (reviewed in Clague and Urbé, 2006). Although this was not the primary focus of this work, and it may be experimentally challenging to prove, we provide a unique tool for its assessment.
Ear1p contains canonical PPxY motifs mediating the interaction with the WW domains of Rsp5p, which were necessary for recruitment of Rsp5p at endosomes when Ear1p was overexpressed. This relocalization did not depend on the C2 domain of Rsp5p, which is known to be important for endosomal localization of this protein through interaction with phosphoinositides (Dunn et al., 2004). A combination of several determinants, including protein–lipid and protein–protein interactions, must therefore be involved in determining Rsp5p localization.
Ssh4p has been identified as a multicopy suppressor of the shr3Δ mutation (Kota et al., 2007). In this mutant, devoid of the ER membrane chaperone Shr3p, many transporters aggregate in the ER and are not secreted (Ljungdahl et al., 1992). The subsequent decrease in the amount of amino acid transporters at the plasma membrane prevents the efficient uptake of amino acids. It has been proposed that overexpression of Ssh4p rescues shr3Δ mutant through an indirect effect on endosomal/vacuolar sorting (Kota et al., 2007). Because Ear1p overexpression caused Rsp5p-related trafficking defects, Ssh4p overexpression might have a similar effect on Rsp5p function, leading to stabilization of the small fraction of amino acid transporters present at the plasma membrane and compensating for the secretion defect of the shr3Δ mutant. This might be due to an impairment in endocytosis and/or MVB sorting followed by recycling (Bugnicourt et al., 2004; Rubio-Texeira and Kaiser, 2006). Overexpression of the adaptor Bsd2p confers resistance to the anticancer drug adriamycin, and this may also be due to indirect effects on Rsp5p function (Takahashi et al., 2005).
In addition to acting as an adaptor, Ear1p is itself ubiquitylated by Rsp5p and targeted to the vacuole in the VPS pathway. However, this ubiquitylation is mostly observed after overexpression of both Ear1p and His-tagged ubiquitin, and it is likely that only a small fraction of Ear1p is ubiquitylated at steady state. Ear1p ubiquitylation was strictly dependent on the first PPxY motif, whereas mutation of the second PPxY motif did not abolish Ear1p ubiquitylation in vivo. This suggests that this mutant still interacts weakly with Rsp5p, although this interaction is too weak for detection by coimmunoprecipitation and it does not confer cadmium sensitivity upon overexpression. During the course of this work, another large-scale study showed Ear1p to be an excellent target for ubiquitylation by Rsp5p in vitro (Gupta et al., 2007), confirming our findings. Indeed, PPxY-containing proteins are often substrates of Rsp5p, as described previously (Bsd2p, Tre1p/Tre2p, Sna3p; also see Gupta et al., 2007). As in other ubiquitin conjugation systems, such as cullin-based ligases, specificity factors are often targeted for degradation (Willems et al., 2004). However, it remains unclear whether this is an essential part of their function.
A key question concerns the targeting of specific cargoes by Nedd4-like proteins in higher eukaryotes. Apart from several ion channels and receptors which contain PPxY motifs, many cargoes devoid of PPxY motifs still depend on Nedd4-like enzymes for their internalization and lysosomal sorting (Staub and Rotin, 2006). A well documented example is that of the insulin-like growth factor I receptor, which is ubiquitylated at the plasma membrane by Nedd4, through association with the adaptor Grb10 (Vecchione et al., 2003; Monami et al., 2008). Other candidate adaptor proteins have been identified in mammals, based on their interaction with Nedd4 proteins (Jolliffe et al., 2000; Murillas et al., 2002). Some are ubiquitylated, and they are found at Golgi/endosomes, where they may recruit Nedd4 enzymes (Harvey et al., 2002; Konstas et al., 2002; Cristillo et al., 2003; Xu et al., 2003; Shearwin-Whyatt et al., 2004), although their target substrates are largely unknown. Interestingly, the Nedd4 protein AIP4/Itch, essential for lysosomal sorting of the chemokine receptor CXCR4 (Marchese et al., 2003), uses the endosomal protein arrestin-2 as an adaptor for this function (Bhandari et al., 2007). The general mechanisms by which Nedd4 proteins are recruited at endosomes, and possibly the mechanism underlying cargo selection, is likely to be conserved in all eukaryotes.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the laboratory for constructs, advice, and helpful discussions, as well as Drs. B. André (Free University of Brussels, Brussels, Belgium), C. Gwizdek (Institut Jacques Monod, Paris, France), H. Pelham (MRC, Cambridge, United Kingdom), and R. Tsien for sharing reagents. This work was supported by the Centre National de la Recherche Scientifique (CNRS), Universities of Paris 6 and 7, and by the Association pour la Recherche sur le Cancer (grant 3298), the CNRS Action Concertée Incitative: Biologie Cellulaire, Moléculaire et Structurale, and the European Union 6th Framework Programme (Role of Ubiquitin and Ubiquitin-like Modifiers in Cellular Regulation: RUBICON NoE, contract LSHG-CT-2005-018683; and Marie Curie research training network: UBIREGULATORS, contract MRTN-CT-2006-034555). S.L. is the recipient of a post-doctoral fellowship from the RUBICON program, and Z.E. is a postdoctoral fellow of the UBIREGULATORS network.
Abbreviations used:
- HECT
Homologous to E6-AP C-Terminus
- MVB
multivesicular body
- SPRY
splA and ryanodine receptor
- VPS
vacuolar protein sorting.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0068) on March 26, 2008.
REFERENCES
- Abe F., Iida H. Pressure-induced differential regulation of the two tryptophan permeases Tat1 and Tat2 by ubiquitin ligase Rsp5 and its binding proteins, Bul1 and Bul2. Mol. Cell. Biol. 2003;23:7566–7584. doi: 10.1128/MCB.23.21.7566-7584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari D., Trejo J., Benovic J. L., Marchese A. Arrestin-2 interacts with the E3 ubiquitin ligase AIP4 and mediates endosomal sorting of the chemokine receptor CXCR4. J. Biol. Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- Bugnicourt A., Froissard M., Sereti K., Ulrich H. D., Haguenauer-Tsapis R., Galan J. M. Antagonistic roles of ESCRT and Vps class C/HOPS complexes in the recycling of yeast membrane proteins. Mol. Biol. Cell. 2004;15:4203–4214. doi: 10.1091/mbc.E04-05-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague M. J., Urbé S. Endocytosis: the DUB version. Trends Cell Biol. 2006;16:551–559. doi: 10.1016/j.tcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Cristillo A. D., Nie L., Macri M. J., Bierer B. E. Cloning and characterization of N4WBP5A, an inducible, cyclosporine-sensitive, Nedd4-binding protein in human T lymphocytes. J. Biol. Chem. 2003;278:34587–34597. doi: 10.1074/jbc.M304723200. [DOI] [PubMed] [Google Scholar]
- De Craene J. O., Soetens O., André B. The Npr1 kinase controls biosynthetic and endocytic sorting of the yeast Gap1 permease. J. Biol. Chem. 2001;276:43939–43948. doi: 10.1074/jbc.M102944200. [DOI] [PubMed] [Google Scholar]
- Dunn R., Hicke L. Domains of the Rsp5 ubiquitin-protein ligase required for receptor-mediated and fluid-phase endocytosis. Mol. Biol. Cell. 2001;12:421–435. doi: 10.1091/mbc.12.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R., Klos D. A., Adler A. S., Hicke L. The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J. Cell Biol. 2004;165:135–144. doi: 10.1083/jcb.200309026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré S., Urban-Grimal D., Haguenauer-Tsapis R. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim. Biophys. Acta. 2004;1695:89–111. doi: 10.1016/j.bbamcr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Froissard M., Belgareh-Touze N., Dias M., Buisson N., Camadro J. M., Haguenauer-Tsapis R., Lesuisse E. Trafficking of siderophore transporters in Saccharomyces cerevisiae and intracellular fate of ferrioxamine B conjugates. Traffic. 2007;8:1601–1616. doi: 10.1111/j.1600-0854.2007.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura H. Molecular cloning of Saccharomyces cerevisiae MLF4/SSH4 gene which confers the immunosuppressant leflunomide resistance. Biochem. Biophys. Res. Commun. 1998;246:378–381. doi: 10.1006/bbrc.1998.8630. [DOI] [PubMed] [Google Scholar]
- Galan J. M., Moreau V., André B., Volland C., Haguenauer-Tsapis R. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J. Biol. Chem. 1996;271:10946–10952. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- Gupta R., Kus B., Fladd C., Wasmuth J., Tonikian R., Sidhu S., Krogan N. J., Parkinson J., Rotin D. Ubiquitination screen using protein microarrays for comprehensive identification of Rsp5 substrates in yeast. Mol. Syst. Biol. 2007;3:116. doi: 10.1038/msb4100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haguenauer-Tsapis R., André B. Membrane trafficking of yeast transporters: mechanisms and physiological control of downregulation. In: Boles E., Krämer R., editors. Molecular Mechanisms Controlling Transmembrane Transport. vol. 9. Berlin/Heidelberg, Germany: Springer; 2004. pp. 273–323. [Google Scholar]
- Harvey K. F., Shearwin-Whyatt L. M., Fotia A., Parton R. G., Kumar S. N4WBP5, a potential target for ubiquitination by the Nedd4 family of proteins, is a novel Golgi-associated protein. J. Biol. Chem. 2002;277:9307–9317. doi: 10.1074/jbc.M110443200. [DOI] [PubMed] [Google Scholar]
- Helliwell S. B., Losko S., Kaiser C. A. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol. 2001;153:649–662. doi: 10.1083/jcb.153.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselberth J. R., Miller J. P., Golob A., Stajich J. E., Michaud G. A., Fields S. Comparative analysis of Saccharomyces cerevisiae WW domains and their interacting proteins. Genome Biol. 2006;7:R30. doi: 10.1186/gb-2006-7-4-r30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema E. H., Valdez-Taubas J., Pelham H. R. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 2004;23:1279–1288. doi: 10.1038/sj.emboj.7600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O'Shea E. K. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Ingham R. J., Gish G., Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- Janke C., et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21:947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- Jolliffe C. N., Harvey K. F., Haines B. P., Parasivam G., Kumar S. Identification of multiple proteins expressed in murine embryos as binding partners for the WW domains of the ubiquitin-protein ligase Nedd4. Biochem. J. 2000;351:557–565. [PMC free article] [PubMed] [Google Scholar]
- Katzmann D. J., Sarkar S., Chu T., Audhya A., Emr S. D. Multivesicular body sorting: ubiquitin ligase Rsp5 is required for the modification and sorting of carboxypeptidase S. Mol. Biol. Cell. 2004;15:468–480. doi: 10.1091/mbc.E03-07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O., Felberbaum R., Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Konstas A. A., Shearwin-Whyatt L. M., Fotia A. B., Degger B., Riccardi D., Cook D. I., Korbmacher C., Kumar S. Regulation of the epithelial sodium channel by N4WBP5A, a novel Nedd4/Nedd4–2-interacting protein. J. Biol. Chem. 2002;277:29406–29416. doi: 10.1074/jbc.M203018200. [DOI] [PubMed] [Google Scholar]
- Kota J., Melin-Larsson M., Ljungdahl P. O., Forsberg H. Ssh4, Rcr2 and Rcr1 affect plasma membrane transporter activity in Saccharomyces cerevisiae. Genetics. 2007;175:1681–1694. doi: 10.1534/genetics.106.069716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Sitaram A., Burd C. G. Regulation of copper-dependent endocytosis and vacuolar degradation of the yeast copper transporter, Ctr1p, by the Rsp5 ubiquitin ligase. Traffic. 2007;8:1375–1384. doi: 10.1111/j.1600-0854.2007.00616.x. [DOI] [PubMed] [Google Scholar]
- Liu X. F., Culotta V. C. Post-translation control of Nramp metal transport in yeast. Role of metal ions and the BSD2 gene. J. Biol. Chem. 1999;274:4863–4868. doi: 10.1074/jbc.274.8.4863. [DOI] [PubMed] [Google Scholar]
- Liu X. F., Supek F., Nelson N., Culotta V. C. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J. Biol. Chem. 1997;272:11763–11769. doi: 10.1074/jbc.272.18.11763. [DOI] [PubMed] [Google Scholar]
- Ljungdahl P. O., Gimeno C. J., Styles C. A., Fink G. R. SHR 3, a novel component of the secretory pathway specifically required for localization of amino acid permeases in yeast. Cell. 1992;71:463–478. doi: 10.1016/0092-8674(92)90515-e. [DOI] [PubMed] [Google Scholar]
- Marchese A., Raiborg C., Santini F., Keen J. H., Stenmark H., Benovic J. L. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev. Cell. 2003;5:709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- Monami G., Emiliozzi V., Morrione A. Grb10/Nedd4-mediated multiubiquitination of the insulin-like growth factor receptor regulates receptor internalization. J. Cell. Physiol. 2008 doi: 10.1002/jcp.21405. (in press), doi: 10.1002/jcp.21405. [DOI] [PubMed] [Google Scholar]
- Morvan J., Froissard M., Haguenauer-Tsapis R., Urban-Grimal D. The ubiquitin ligase Rsp5p is required for modification and sorting of membrane proteins into multivesicular bodies. Traffic. 2004;5:383–392. doi: 10.1111/j.1398-9219.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- Mumberg D., Muller R., Funk M. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillas R., Simms K. S., Hatakeyama S., Weissman A. M., Kuehn M. R. Identification of developmentally expressed proteins that functionally interact with Nedd4 ubiquitin ligase. J. Biol. Chem. 2002;277:2897–2907. doi: 10.1074/jbc.M110047200. [DOI] [PubMed] [Google Scholar]
- Pickart C. M., Eddins M. J. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Pizzirusso M., Chang A. Ubiquitin-mediated targeting of a mutant plasma membrane ATPase, Pma1–7, to the endosomal/vacuolar system in yeast. Mol. Biol. Cell. 2004;15:2401–2409. doi: 10.1091/mbc.E03-10-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting C., Schultz J., Bork P. SPRY domains in ryanodine receptors (Ca(2+)-release channels) Trends Biochem. Sci. 1997;22:193–194. doi: 10.1016/s0968-0004(97)01049-9. [DOI] [PubMed] [Google Scholar]
- Reggiori F., Pelham H. R. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 2001;20:5176–5186. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Staub O., Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 2000;176:1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- Rubio-Texeira M., Kaiser C. A. Amino acids regulate retrieval of the yeast general amino acid permease from the vacuolar targeting pathway. Mol. Biol. Cell. 2006;17:3031–3050. doi: 10.1091/mbc.E05-07-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Shearwin-Whyatt L., Dalton H. E., Foot N., Kumar S. Regulation of functional diversity within the Nedd4 family by accessory and adaptor proteins. Bioessays. 2006;28:617–628. doi: 10.1002/bies.20422. [DOI] [PubMed] [Google Scholar]
- Shearwin-Whyatt L. M., Brown D. L., Wylie F. G., Stow J. L., Kumar S. N4WBP5A (Ndfip2), a Nedd4-interacting protein, localizes to multivesicular bodies and the Golgi, and has a potential role in protein trafficking. J. Cell Sci. 2004;117:3679–3689. doi: 10.1242/jcs.01212. [DOI] [PubMed] [Google Scholar]
- Soetens O., De Craene J. O., Andre B. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J. Biol. Chem. 2001;276:43949–43957. doi: 10.1074/jbc.M102945200. [DOI] [PubMed] [Google Scholar]
- Staub O., Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol. Rev. 2006;86:669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- Stawiecka-Mirota M., Pokrzywa W., Morvan J., Zoladek T., Haguenauer-Tsapis R., Urban-Grimal D., Morsomme P. Targeting of Sna3p to the endosomal pathway depends on its interaction with Rsp5p and multivesicular body sorting on its ubiquitylation. Traffic. 2007;8:1280–1296. doi: 10.1111/j.1600-0854.2007.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson H. E., Lewis M. J., Pelham H. R. Transferrin receptor-like proteins control the degradation of a yeast metal transporter. EMBO J. 2006;25:662–672. doi: 10.1038/sj.emboj.7600984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M. Structure and function of the WW domain. Prog. Biophys. Mol. Biol. 1996;65:113–132. doi: 10.1016/s0079-6107(96)00008-9. [DOI] [PubMed] [Google Scholar]
- Sullivan J. A., Lewis M. J., Nikko E., Pelham H. R. Multiple interactions drive adaptor-mediated recruitment of the ubiquitin ligase Rsp5 to membrane proteins in vivo and in vitro. Mol. Biol. Cell. 2007;18:2429–2440. doi: 10.1091/mbc.E07-01-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Furuchi T., Naganuma A. A novel role for Bsd2 in the resistance of yeast to adriamycin. J. Cell. Physiol. 2005;202:100–104. doi: 10.1002/jcp.20082. [DOI] [PubMed] [Google Scholar]
- Umebayashi K., Nakano A. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J. Cell Biol. 2003;161:1117–1131. doi: 10.1083/jcb.200303088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchione A., Marchese A., Henry P., Rotin D., Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol. Cell. Biol. 2003;23:3363–3372. doi: 10.1128/MCB.23.9.3363-3372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C., Boretto J., Mattei M. G., Takahashi M., Jack L. J., Mather I. H., Rouquier S., Pontarotti P. Evolutionary study of multigenic families mapping close to the human MHC class I region. J. Mol. Evol. 1993;37:600–612. doi: 10.1007/BF00182746. [DOI] [PubMed] [Google Scholar]
- Volland C., Urban-Grimal D., Geraud G., Haguenauer-Tsapis R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J. Biol. Chem. 1994;269:9833–9841. [PubMed] [Google Scholar]
- Wang G., McCaffery J. M., Wendland B., Dupré S., Haguenauer-Tsapis R., Huibregtse J. M. Localization of the Rsp5p ubiquitin-protein ligase at multiple sites within the endocytic pathway. Mol. Cell. Biol. 2001;21:3564–3575. doi: 10.1128/MCB.21.10.3564-3575.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems A. R., Schwab M., Tyers M. A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta. 2004;1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Woo J. S., Suh H. Y., Park S. Y., Oh B. H. Structural basis for protein recognition by B30.2/SPRY domains. Mol. Cell. 2006;24:967–976. doi: 10.1016/j.molcel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Xu L. L., et al. PMEPA1, an androgen-regulated NEDD4-binding protein, exhibits cell growth inhibitory function and decreased expression during prostate cancer progression. Cancer Res. 2003;63:4299–4304. [PubMed] [Google Scholar]
- Yashiroda H., Kaida D., Toh-e A., Kikuchi Y. The PY-motif of Bul1 protein is essential for growth of Saccharomyces cerevisiae under various stress conditions. Gene. 1998;225:39–46. doi: 10.1016/s0378-1119(98)00535-6. [DOI] [PubMed] [Google Scholar]
- Yashiroda H., Oguchi T., Yasuda Y., Toh E. A., Kikuchi Y. Bul1, a new protein that binds to the Rsp5 ubiquitin ligase in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996;16:3255–3263. doi: 10.1128/mcb.16.7.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.