Abstract
The members of the MyoD family of basic helix-loop-helix (bHLH) transcription factors are critical regulators of skeletal muscle differentiation that function as heterodimers with ubiquitously expressed E-protein bHLH transcription factors. These heterodimers must compete successfully with homodimers of E12 and other E-proteins to enable myogenesis. Here, we show that E12 mutants resistant to Ca2+-loaded calmodulin (CaM) inhibit MyoD-initiated myogenic conversion of transfected fibroblasts. Ca2+ channel blockers reduce, and Ca2+ stimulation increases, transcription by coexpressed MyoD and wild-type E12 but not CaM-resistant mutant E12. Furthermore, CaM-resistant E12 gives lower MyoD binding and higher E12 binding to a MyoD-responsive promoter in vivo and cannot rescue myogenic differentiation that has been inhibited by siRNA against E12 and E47. Our data support the concept that Ca2+-loaded CaM enables myogenesis by inhibiting DNA binding of E-protein homodimers, thereby promoting occupancy of myogenic bHLH protein/E-protein heterodimers on promoters of myogenic target genes.
INTRODUCTION
The members of the MyoD family of basic helix-loop-helix (bHLH) transcription factors are essential transcriptional regulators of skeletal muscle differentiation. These myogenic transcription factors are controlled by cellular signaling, resulting in repression of their activity in proliferating myoblasts and in increases in their activity during myogenic differentiation (Puri and Sartorelli, 2000; Sabourin and Rudnicki, 2000). The MyoD family has four members: MyoD, Myf5, myogenin, and MRF4. MyoD and Myf5 are required for commitment to the myogenic lineage, whereas myogenin plays a critical role in the expression of the terminal muscle phenotype previously established by MyoD and Myf5, and MRF4 partly subserves both roles (Berkes and Tapscott, 2005). The “commitment” or “specification” factors MyoD and Myf5 display a certain redundancy but they have a preferential role in specification of hypaxial versus epaxial muscle lineages, respectively (Berkes and Tapscott, 2005). DNA binding and transcriptional activation by the myogenic bHLH transcription factors requires heterodimer formation with one of the ubiquitously expressed E-protein bHLH transcription factors (Lassar et al., 1991; Massari and Murre, 2000), encoded by three genes in mammals, E2A, E2-2 (also denoted SEF2-1), and HEB (Massari and Murre, 2000). Two alternatively spliced protein products, E12 and E47, are produced from the E2A gene (Massari and Murre, 2000). In developing skeletal muscle, E2A expression increases during the early stages of muscle differentiation and returns to low or undetectable levels in fully differentiated muscle fibers (Rutherford and LeBrun, 1998). E2A proteins are primarily localized in the nucleus in both C2C12 myoblasts and myotubes and the cellular abundance of E2A is relatively stable during this differentiation (Sun et al., 2007). HEB is also expressed in developing muscle (Conway et al., 2004). The HEBβ isoform is up-regulated during terminal differentiation of myoblasts, and siRNA against HEBβ in myoblasts blocks the differentiation (Parker et al., 2006), arguing that HEB plays a role in myogenesis, at least during the later stages. Less is known about E2-2/SEF2-1 during myogenesis. However, no obvious skeletal muscle phenotype was reported for mice with knockout of either E2A, HEB, or E2-2/SEF2-1 (Bain et al., 1994; Zhuang et al., 1994, 1996), arguing that there is a substantial functional overlap between the E proteins as heterodimer partners of the MyoD family proteins in myogenesis.
E proteins can bind to E-box sequences, the DNA recognition sequences of MyoD responsive promoters of muscle-specific genes, as homodimers, but these are poor activators of expression from a MyoD responsive reporter. In contrast to overexpression of MyoD, overexpression of E12, E47, or HEB does not result in conversion of fibroblasts to muscle cells (Davis and Weintraub, 1992). MyoD heterodimers with E-protein must therefore compete successfully with E-protein homodimers to enable muscle differentiation. Activity of MyoD, and most likely also Myf-5, leads to enhanced heterodimerization ability and increased expression of the protein through positive feedback loops (Yun and Wold, 1996; Cole et al., 2004). However, no mechanism has been described that enables initially expressed MyoD (or Myf-5) to successfully compete with E-protein homodimers at the critical promoters before the positive feedback mechanisms.
Various reports have demonstrated the importance of Ca2+ signaling and the Ca2+ sensor protein calmodulin (CaM) in activation of myogenic transcription factors and myogenesis (Delling et al., 2000; McKinsey et al., 2002; Porter et al., 2002; Berger et al., 2003; Friday et al., 2003). E-protein homodimers have been shown to bind to Ca2+-loaded calmodulin (Ca2+/CaM) through their bHLH domain, resulting in inhibition of their DNA binding (Corneliussen et al., 1994). We have also reported that transcriptional activation by E-proteins, but not by MyoD, can be inhibited by Ca2+/CaM through a direct physical interaction with the basic DNA-binding sequence in their bHLH domain, leading to inhibition of their in vivo DNA binding (Saarikettu et al., 2004). The in vitro DNA binding of homodimers of the E-protein E12, but not MyoD heterodimers with E12, is inhibited by Ca2+/CaM (Corneliussen et al., 1994). Ca2+ signaling has therefore the potential to enable activity of MyoD heterodimers with E-proteins in myogenesis through Ca2+/CaM inhibition of DNA binding of competing E-protein homodimers. In this report, we show that CaM-resistant mutants of E12 are inhibitory to MyoD-initiated myogenic conversion of transfected fibroblasts. Our data support the notion that Ca2+ signaling through CaM enables myogenic differentiation by inhibiting DNA binding of E-protein homodimers, thereby selectively promoting DNA binding of myogenic bHLH protein/E-protein heterodimers.
MATERIALS AND METHODS
Cell Culture and Transfections
NIH-3T3 fibroblasts were maintained in growth medium, Dulbecco's MEM supplemented with antibiotics and 10% fetal bovine serum. The cells were generally grown on six-well plates and transiently transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. For myogenic conversion, the medium was replaced 24 h after the start of transfection with differentiation medium, Dulbecco's MEM supplemented with antibiotics and 2% horse serum, and the cells were allowed to differentiate for 4 d with one change of medium at day 2 of differentiation. The Ca2+ channel blockers nifedipine and verapamil or the Ca2+ stimulators ionomycin and thapsigargin (all from Calbiochem, La Jolla, CA) were included in the differentiation medium where indicated. DG75 B-cells were maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum and antibiotics and transiently transfected using electroporation as described previously (Hughes et al., 2002). Luciferase and β-galactosidase activities of transiently transfected cells were determined as described (Hughes et al., 2002).
Plasmids
The cDNAs coding for murine E12, basic sequence mutants m4, m5, and m847 of E12 and murine MyoD, all inserted in the mammalian expression vector pcDNAI/amp (Invitrogen), have been described previously (Saarikettu et al., 2004). The E12 basic sequence mutants m8H47, m8N47, m8S47, and m8T47 inserted in pcDNAI/amp were generated by standard PCR mutagenesis as previously described (Saarikettu et al., 2004). The SEF2-1 expression plasmid has been described previously (Saarikettu et al., 2004). The MyoD and E-protein responsive luciferase reporters, 4x(MEF1)-luciferase and 6x(μE5 + μE2)-luciferase, respectively, and the hCMV-β-gal plasmid for normalization of transfections have been described previously (Corneliussen et al., 1994). The cDNAs coding for rat Id1, Id2, and Id3, all inserted in pcDNA3 (Invitrogen), were kindly provided by Dr. Michael D. Walker (Weizmann Institute of Science, Rehovot, Israel). MyoD–E12 and MyoD–m847 “tethered” dimers in pcDNAI/amp were generated by fusing the C-terminus of MyoD at the restriction site TthIII1 to the N-terminus of either E12 or m847 mutant at the restriction site BsmI, using oligonucleotides encoding a previously described linker peptide (peptide number 2 of Neuhold and Wold, 1993).
Immunocytochemistry and Myogenesis Assays
NIH-3T3 fibroblasts grown on glass coverslips were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature and immunostained essentially as described previously (Hughes et al., 2001). CaM immunostaining was performed using a rabbit polyclonal antibody against CaM (FL-149; Santa Cruz Biotechnology, Santa Cruz, CA) and fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG as secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). In myogenesis analyses, cells were immunostained using rabbit anti-MyoD antibody (M-318; Santa Cruz Biotechnology) and mouse anti-skeletal muscle myosin antibody (MF-20 concentrate; Developmental Studies Hybridoma Bank at the University of Iowa). Secondary antibodies were FITC-conjugated donkey anti-rabbit IgG and TRITC-conjugated donkey anti-mouse IgG (both from Jackson ImmunoResearch Laboratories). All antibodies were used at a dilution of 1:50. CaM immunostaining was analyzed by confocal microscopy using a Leica SP2 confocal imager system (Deerfield, IL). For myogenesis analyses, numbers of myosin-positive cells and the proportion of myosin-stained cells in the population of MyoD-stained cells were determined by visual observation using epifluorescence microscopy. For analyses of rescue of inhibition by small interfering RNA (siRNA) against E2A, NIH-3T3 fibroblasts were cotransfected with appropriate plasmid DNA and 10 pmol/105 cells of murine E2A siRNA (sc-35246; Santa Cruz Biotechnology) using Lipofectamine 2000, according to the manufacturer's recommendations.
Western Blot and Electrophoretic Mobility Shift Assays
Western blot was performed using the WesternBreeze immunodetection system (Invitrogen) according to the manufacturer's instructions, using antibodies to E12 (H-208), to MyoD (M-318; both from Santa Cruz Biotechnology) and to α-tubulin (clone B-5-1-2; Sigma-Aldrich, St. Louis, MO).
For electrophoretic mobility shift assays (EMSA), NIH-3T3 cells were transiently transfected with 2 μg of expression vector for E12 or E12 mutant and 2 μg of expression vector for MyoD. Twenty-four hours later, nuclear extracts were prepared as previously described (Saarikettu et al., 2004) and used in EMSA with a MEF1 E-box sequence probe as previously described (Onions et al., 1997).
Chromatin Immunoprecipitation
Transfected NIH-3T3 fibroblasts were cross-linked with 1% formaldehyde for 10 min at room temperature after 2 d in differentiation medium. Chromatin immunoprecipitation was performed as previously described (Saarikettu et al., 2004) using 5 μg/ml anti-MyoD (M-318; Santa Cruz Biotechnology) antibody or 5 μg/ml anti-E47 (N-649; Santa Cruz Biotechnology) antibody, which also recognizes E12. The immunoprecipitated DNA was used as template to amplify a DNA sequence of the MyoD promoter containing two MyoD-binding E-boxes (Zingg et al., 1994). The semiquantitative PCR amplification was with the primer pair 5′-GGTCAGTACAGGCTGGAGGA-3′ and 5′-GTGTAGTAGGGCGGAGCTTG-3′ and was resolved on a 1.5% agarose gel. The quantitative real-time PCR analysis of the chromatin immunoprecipitation was with the primer pair 5′-ACAGGCTGGAGGAGTAGAC-3′ and 5′-GCCAATAGGAGTGTAGGG-3′ and was normalized to the levels of immunoprecipitated glyceraldehyde-3-phosphate dehydrogenase (GAPDH) DNA.
Real-Time PCR
Total RNA was extracted using Trizol Reagent (Invitrogen) according to the instruction manual. First-strand cDNA was synthesized from 1 μg of total RNA using a cDNA synthesis kit with random hexamers (Fermentas, Hanover, MD) according to the manufacturer's instructions. Real-time PCR analysis was performed in triplicate in 25-μl reaction volumes using iTaq reaction mix (Bio-Rad, Richmond, CA) and the iCycler iQ Real-Time PCR Detection System (Bio-Rad). Primer sets were designed using Beacon Designer software (Bio-Rad). GAPDH was used as an internal control. Real-time PCR (RT-PCR) values were determined by Comparative Quantification (ABI Prism 7700 Sequence Detection System User Bulletin 2 (2001)). The GAPDH primers used were GCTTGTCATCAACGGGAAG and TTGTCATATTTCTCGTGGTTCA, and the TaqMan probe for GAPDH was 6-FAM-TCACCATCTTCCAGGAGCGAGACC-TAMRA. The primer specific for endogenous MyoD mRNA was TCTGACAGGACAGGACAGG, and the primer specific for exogenous MyoD mRNA was CTAGAGAACCCACTGCTTACT. The reverse primer was GCTCCATATCCCAGTTCCTG and the TaqMan probe was 6-FAM-CAACCCAAGCCGTGAGAGTCGTC-TAMRA for both the endogenous and the exogenous MyoD mRNA.
RESULTS
CaM Is Preferentially Nuclear under Myogenic Differentiation Conditions
Previous studies have indicated that Ca2+ and nuclear CaM have a role in regulation of myogenesis and the activity of myogenic transcription factors (Delling et al., 2000; McKinsey et al., 2002; Porter et al., 2002; Berger et al., 2003; Friday et al., 2003). These data suggest that differentiation conditions, i.e., low serum, for in vitro differentiation of cells could correlate with CaM in the nucleus that could modulate the activity of transcription factors. Thus, we analyzed the localization of CaM in a cell type that can convert to muscle cells, NIH-3T3 fibroblasts, grown to confluency in a medium promoting cell proliferation (10% fetal bovine serum; growth medium), and in confluent fibroblasts cultured for 2 d in medium that promotes differentiation of transfected cells (2% horse serum; differentiation medium). As shown in Figure 1A, CaM was localized diffusely, both in the cytoplasm and the nucleus, in fibroblasts maintained in growth medium, whereas cells subjected to 2 d in differentiation medium showed a preferential nuclear localization of CaM. This preferential nuclear localization of CaM under conditions promoting differentiation supports the idea that CaM has a nuclear role in regulation of transcription during myogenesis. The location of CaM in the nucleus is compatible with a model of CaM functioning as a Ca2+-regulated inhibitor of E-protein homodimers that could otherwise repress myogenic differentiation by competing with myogenic MyoD/E-protein heterodimers in binding to their DNA targets (Figure 1B).
Figure 1.
Nuclear calmodulin (CaM) in myogenic differentiation. (A) CaM is preferentially nuclear in NIH-3T3 cells in differentiation medium. NIH-3T3 fibroblasts were grown to confluency in Dulbecco's MEM supplemented with 10% fetal bovine serum (growth medium) or grown to confluency in growth medium and further cultured for 2 d in Dulbecco's MEM supplemented with 2% horse serum (differentiation medium). They were immunostained with CaM-specific antibody and analyzed by confocal microscopy. (B) Model for Ca2+/CaM activation of bHLH transcription factor responsive gene expression. Transcription of genes that are responsive to CaM-resistant heterodimers between an E-protein (E) such as E12 or E47, and a tissue-specific bHLH protein such as MyoD, is hypothesized to be activated by Ca2+/CaM-mediated exclusion of competing inhibitory CaM-sensitive E-protein homodimers from the promoters.
CaM-resistant Mutants of E12 Inhibit Myogenic Conversion of MyoD-transfected NIH-3T3 Fibroblasts
We have previously generated a series of mutants of the E-protein E12 that are resistant to CaM to different extents (Saarikettu et al., 2004). These mutants were generated by amino acid substitutions in the basic DNA and CaM binding sequence of the bHLH domain of E12 and were analyzed for their ability to bind DNA and for their sensitivity to inhibition by CaM both in vitro and in vivo. In the present work, we used three of these mutants, including the most CaM-resistant mutant, m847, together with four novel E12 basic sequence mutants denoted m8H47, m8N47, m8S47, and m8T47 (Figure 2A), to study the effect of their coexpression with MyoD on myogenic conversion of NIH-3T3 fibroblasts. The DNA-binding abilities of the four novel E12 mutants were verified by EMSA, as previously done (Saarikettu et al., 2004) for m847 and the other mutants of E12 (see also below). For myogenesis analysis, the fibroblasts were transiently transfected with the expression vectors encoding the E12 proteins and allowed to differentiate for 4 d. They were then stained for MyoD to show successfully transfected MyoD expressing cells, and for myosin as a marker of myogenic differentiation. The percentage of myosin-positive cells in the population of MyoD expressing cells was counted in each transfection to quantitate the effects of E12 and E12 mutants on the myogenic conversion by MyoD. As shown in Figure 2B, ∼80% of the MyoD-expressing cells expressed myosin, and coexpression of wild-type E12 with MyoD at a ratio of 1:1 did not significantly affect myogenic conversion compared with expression of MyoD alone. In contrast to wild-type E12, all E12 basic domain mutants tested inhibited MyoD from inducing myogenic conversion (Figure 2B). The E12 mutants m4 and m5, which have only slightly decreased sensitivity to CaM (Saarikettu et al., 2004), reduced myogenic conversion with MyoD only slightly, whereas mutant m847 and also mutants m8N47, m8S47, and m8T47 had the effect of reducing myogenic conversion to between 42 and 50% (Figure 2B). We also analyzed the effect of decreasing the ratio of the amount of MyoD expression vector to the amount of wild-type or mutant E12 expression vector. Mutant m847 was selected for this experiment, as this mutant was previously shown to be the most resistant to CaM in the series of E12 basic sequence mutants generated (Saarikettu et al., 2004), and mutant m8N47 was selected because of its similar Id inhibition sensitivity when compared with wild-type E12 (see below). Reduction of the ratio of MyoD expression plasmid to expression plasmid for wild-type E12 from 1:1–1:4 resulted in a small decrease in the myogenic index from an average of 77% to 62% (Figure 2, B and C). The corresponding myogenesis index for mutant m847 decreased from 42 to 17% and for mutant m8N47 from 50 to 36% (Figure 2, B and C). As a control, reducing the ratio of the expression vector for MyoD to the empty E-protein expression vector did not have any significant effect on myogenic conversion of the MyoD-expressing cells (Figure 2, B and C). We conclude that overexpression of wild-type E12 with MyoD interferes only slightly with myogenic conversion of NIH-3T3 cells, whereas E12 mutants that are deficient in inhibition by Ca2+/CaM function as dose-dependent inhibitors of MyoD-mediated myogenic conversion. A very substantial decrease in the total number of myosin-positive cells was also observed in these transfections (Figure 2D). This decrease was more than threefold for the m8N47 mutant and approximately 10-fold for mutant m847. The much larger decrease in the total number of myosin-positive cells (Figure 2D) than in myogenic index (Figure 2C) implies that the number of cells classified as MyoD-positive was also reduced in cells cotransfected with MyoD and CaM-resistant mutants of E12 as shown in Figure 2E. When interpreting that result, it is important to note that the sensitivity level of the immunostaining assay classifying cells into either MyoD+ or MyoD− is relatively low, meaning that only cells with very high MyoD level become sufficiently fluorescent to be classified as MyoD+. The decrease in MyoD+ cells indicates therefore that CaM resistance of E12 interferes with the positive-feedback loop of MyoD to transcriptionally activate its own expression (Zingg et al., 1994), which would otherwise result in activation of the expression of endogenous MyoD by ectopically expressed active MyoD in differentiation. The positive feedback would result in more cells getting sufficiently fluorescent to become classified as MyoD+, and the fewer MyoD+ cells when cotransfecting with CaM-resistant E12 indicates that CaM-resistance interferes with the feedback loop. To directly analyze this possibility, we followed the ratio between endogenous and exogenous MyoD mRNA by quantitative RT-PCR during 4 d of myogenic differentiation. The endogenous MyoD mRNA increased successively during the differentiation of the cells transfected with MyoD together with either wild-type E12 or vector control (Figure 2F), confirming the existence of a positive-feedback loop in MyoD expression and showing that overexpression of wild-type E12 does not disturb the feedback. The increase in the ratio between endogenous and exogenous MyoD mRNA was ∼10-fold by day 4 of differentiation (Figure 2F). In contrast, the ratio between endogenous and exogenous MyoD mRNA remained almost unchanged during 4 d in differentiation medium for the MyoD-transfected cells cotransfected with the m847 mutant of E12, and there was only a relatively small increase in this ratio on cotransfection with the m8N47 mutant (Figure 2F). Thus, the positive-feedback loop of MyoD to transcriptionally activate its own expression, which still functions when wild-type E12 is overexpressed, does not function in cells expressing a CaM-resistant mutant of E12 (see also below).
Figure 2.
CaM-resistant mutants of E12 are deficient in promoting MyoD-induced myogenic conversion of transfected NIH-3T3 fibroblasts. (A) Basic sequence mutants of E12 used in this study. The basic sequences of E12 and the E12 mutants are shown with amino acid substitutions in bold. Mutants m4, m5, and m847 have been described previously (Saarikettu, et al., 2004). (B) NIH-3T3 fibroblasts were transiently transfected with 2 μg expression vector for MyoD together with 2 μg empty expression vector (vec.) or expression vector for E12 or various E12 basic sequence mutants as indicated. Transfected cells were allowed to differentiate for 4 d and were immunostained for MyoD and myosin. The myogenesis index indicates the percentage of cells stained for myosin in the population of cells stained for MyoD. Bars, mean values of myogenesis indices, ±SD from three independent experiments (two experiments for mutants m4 and m5). (C) NIH-3T3 cells were transiently transfected as in B, except that the plasmid ratio of MyoD to E-protein was 1:4 (0.8 μg MyoD plasmid and 3.2 μg E12 plasmid). Bars, myogenesis indices, ±SD from three independent experiments. (D) Counts of myosin-positive cells from transfections of C. (E) Counts of MyoD-positive cells from transfections of C. The number of myosin-positive cells in D and MyoD-positive cells in E from transfection of MyoD together with empty expression vector (vec.) was set at 100%. Bars, the mean relative number of myosin-positive and MyoD-positive cells, respectively, ±SD from at least three independent experiments. (F) Defective positive feedback of MyoD transcription when expressing CaM-resistant mutants of E12. The ratio between endogenous and exogenous MyoD mRNA during 4 d of myogenic differentiation of NIH-3T3 fibroblasts was measured by quantitative RT-PCR. The cells were cotransfected with MyoD expression plasmid (0.8 μg) and either wild-type E12, the m847 mutant, or the m8N47 of E12, or with vector control (3.2 μg). Levels of mRNA were determined by quantitative RT-PCR, and the ratio between endogenous and exogenous MyoD mRNA the day after transfection when the cells were still in growth medium (day 0) was set as 1. (G and H) Increased in vivo binding of a CaM-resistant mutant of E12 to a MyoD-regulated promoter. NIH-3T3 fibroblasts transfected with 2 μg of the expression vector for MyoD together with 8 μg of the expression vector for E12 or the CaM-resistant m8N47 mutant of E12 were cultured for 2 d in differentiation medium and harvested for chromatin immunoprecipitation with antibodies recognizing MyoD or E12 or, as a control, with no antibody added. Immunoprecipitated DNA was PCR-amplified using a primer pair flanking the two MyoD-binding E-boxes in the promoter of MyoD. (G) Immunoprecipitated and PCR-amplified MyoD promoter DNA was resolved on a 1.5% agarose gel. (H) The amounts of immunoprecipitated MyoD promoter DNA determined by quantitative real-time PCR and normalized using the levels of immunoprecipitated GAPDH DNA. The levels are expressed as ‰ of total MyoD promoter DNA before immunoprecipitation. Results are mean ±SD (n = 3).
Increased Binding of Mutant E12 to a MyoD-regulated Promoter
To analyze in vivo DNA-binding of wild-type E12 and a CaM-resistant mutant of E12 on a promoter of a MyoD target gene, we performed chromatin immunoprecipitation (ChIP) analysis of transfected NIH-3T3 fibroblasts. The cells were transfected with MyoD and either wild-type E12 or m8N47 mutant. After 2 d in differentiation medium, the cells were subjected to ChIP to analyze the ratio of MyoD and E12 or m8N47 bound to the promoter of the endogenous MyoD gene, which is a target for autoregulation by MyoD (Zingg et al., 1994). A semiquantitative agarose gel analysis of a representative ChIP experiment is shown in Figure 2G, and the results of quantitative real-time PCR of the ChIP experiments are summarized in Figure 2H. In cells transfected with m8N47 and MyoD, less MyoD and more of the mutant E12 protein was present on the promoter compared with transfection with wild-type E12 and MyoD, suggesting that the m8N47 mutant preferentially binds to the promoter as a homodimer instead of the myogenic heterodimer with MyoD (Figure 2, G and H).
Correlation between CaM Resistance and Myogenesis Inhibition Properties of the E12 Basic Sequence Mutants
Analysis of the sensitivity of the m847 CaM-resistant mutant of E12 to the inhibitory HLH proteins Id1, Id2, and Id3 (Ruzinova and Benezra, 2003) showed that this mutant is partially resistant to Id proteins (Figure 3A), possibly due to a reduced ability to dimerize with them. Because the sensitivity of E12 mutants to inhibition by Id proteins could affect their cooperation with MyoD in induction of myogenesis, CaM-resistant mutants of E12 that showed an Id sensitivity similar to that of wild-type E12 were essential to distinguish CaM and Id protein effects in our myogenesis analyses. Thus, the four E12 basic sequence mutants m8H47, m8N47, m8S47, and m8T47 (Figure 2A) were generated, and their sensitivities to inhibition by CaM and Id overexpression were determined and compared. As shown in Figure 3A, the four mutants were more sensitive than mutant m847 to inhibition by all Id proteins, but to different extents.
Figure 3.
Correlation between CaM sensitivity and induction of myogenesis for wild-type and basic sequence mutants of E12. (A) Expression vectors for E12, basic sequence mutants of E12, or empty expression vector (vec.), all 2.5 μg, were used to transiently transfect DG75 B-cells together with 1 μg of expression vector for the indicated Id protein or empty Id vector (−), 2.5 μg of the E-protein responsive luciferase reporter 6x(μE5 + μE2)-luciferase, and 2.5 μg of hCMV-β-gal for normalization of transfections. The cells were harvested 24 h later and analyzed for luciferase and β-galactosidase activity. The mean luciferase activities are shown normalized to β-galactosidase, ±SD from three independent experiments. The activity of wild-type E12 together with empty Id vector was set at 100%. (B) Expression vectors for either wild-type E12 or the indicated E12 mutant, 2.5 μg, were used to transiently transfect DG75 B-cells together with 2.5 μg each of expression vector for CaM, 6x(μE5 + μE2)-luciferase reporter and hCMV-β-gal expression vector for normalization of transfections. The transcriptional activity of E12 and E12 mutants cotransfected with the CaM expression vector is expressed as percentage of their activities when cotransfected with empty pcDNAI/amp vector. Bars, mean transcription in the presence of CaM, ±SD from three independent experiments. (C) Relative inhibitory effect of Id protein overexpression on transcriptional activation by wild-type (wt) E12 and basic sequence mutants of E12 from A, expressed as a function of the respective myogenesis index from Figure 2B. (D) CaM inhibition of transcriptional activation by wild-type E12 and basic sequence mutants of E12 from B, expressed as a function of the myogenesis index from Figure 2B. (E) Amounts of MyoD/E-protein DNA complex (indicated with arrow) in electrophoretic mobility shift assay (EMSA). Two micrograms of expression vector for wild-type E12 or the indicated E12 mutant was transiently transfected with 2 μg expression vector for MyoD in NIH-3T3 cells. Transfection with 4 μg of empty expression vector (vec.) vas used as control. Twenty-four hours later, nuclear extracts were prepared and used in EMSA with an MEF1 E-box sequence probe. No extract was added in the first lane.
Mutant m8S47 was more potent than wild-type E12 in activation of transcription of the E-box luciferase reporter, whereas the other mutants were slightly less potent activators than wild-type E12. All mutants were quite resistant to CaM inhibition, with the exception of m8S47, which showed an inhibition by CaM overexpression that was intermediate between that shown by the other mutants and wild-type E12 (Figure 3B). To determine a possible relationship between the effects of the E12 mutants on myogenic conversion and their sensitivity to Id proteins or CaM, these properties were compared (Figure 3, C and D). No linear relationship between the sensitivities of the E12 mutants to any of the Id proteins and their effect on myogenic conversion was found (Figure 3C). In contrast, the effect of CaM on transcriptional activation by the E12 mutants correlated well with their effects on myogenic conversion (Figure 3D). Mutant m8S47, which was highly inhibitory for myogenesis but only partially resistant to CaM, might be considered an exception (Figure 3D). However, because of the higher transcriptional activity of m8S47 relative to the other mutants and wild-type E12 (Figure 3A), this mutant would be expected to possess a level of activity in the presence of CaM overexpression that is comparable to or higher than that of the more CaM-resistant mutants, and this mutant could therefore function as an inhibitor of myogenesis in spite of its intermediate CaM resistance.
An alternative explanation to the results presented above would be that heterodimerization with MyoD to bind DNA is intrinsically poor in the mutants. We analyzed therefore the ability of the mutants to dimerize with MyoD to bind DNA. The analysis was made by EMSA in the absence of Ca2+ using nuclear extracts of transfected NIH-3T3 cells under growth conditions (Figure 3E). The appearance of the MyoD-E12-DNA complex was dependent on transfection with both the MyoD and the E12 expression plasmid (lane 3 and data not shown). All mutants were found to form DNA-binding heterodimers with MyoD with as high efficiency as wild-type E12 (Figure 3E). Thus, the inhibition of myogenesis by the CaM-resistant mutants is not through a deficient intrinsic ability of these mutants to efficiently heterodimerize with MyoD to bind DNA.
CaM-resistant Mutants of E12 Show Reduced Activation of Transcription upon Coexpression with MyoD
We then studied the effects of the E12 basic sequence mutants on transcriptional activation of a MyoD-responsive luciferase reporter by MyoD. This reporter contains four tandem MyoD-binding E-boxes and a basal promoter to drive luciferase expression (Corneliussen et al., 1994). NIH-3T3 cells were transiently transfected and were allowed to differentiate for 4 d before analysis of the reporter activity. This activity was sevenfold higher in transfections of MyoD compared with transfections of E12, and as expected, coexpression of wild-type E12 with MyoD further activated the reporter activity synergistically by a factor of 7 (Figure 4A). All CaM-resistant mutants of E12 were less efficient than wild-type E12 in promoting transcriptional activation of the MyoD reporter by MyoD. The most CaM-resistant mutant, m847, was also the most deficient mutant in cooperating with MyoD to activate the reporter. Even mutant m8S47, which exhibited increased activation of transcription from the E-protein homodimer driven luciferase reporter in the transfected B-cell line (Figure 3A), was deficient in cooperation with MyoD to activate the MyoD reporter (Figure 4A). Thus, the results of the mutant analyses are compatible with our model (Figure 1B) where CaM functions as a Ca2+-regulated inhibitor of E-protein homodimers that could otherwise repress myogenic differentiation by competing with myogenic MyoD/E-protein heterodimers.
Figure 4.
Transcriptional activation by wild-type E12 and CaM-resistant E12 mutants in synergy with MyoD and effects of Ca2+ channel blockers. (A) NIH-3T3 fibroblasts were transiently transfected with 1.5 μg of expression vector for the indicated proteins together with 0.5 μg MyoD responsive luciferase reporter 4x(MEF1)-luciferase and 0.5 μg hCMV-β-gal. Empty expression vector was added where necessary to give 4 μg of total DNA. Twenty-four hours after transfection, the cells were transferred to differentiation medium for 4 d before analysis. Mean normalized luciferase values are shown, ±SD from three independent experiments. The activity of wild-type E12 together with MyoD was set at 100%. (B) Twenty-four hours after the start of transfections of MyoD with E12, m8N47 mutant, or empty expression vector as in A, the medium was replaced with differentiation medium with or without the indicated Ca2+ channel blocker (nif., nifedipine, μM; ver., verapamil, μM), and the cells were harvested for analysis 4 d later. Mean normalized luciferase values are shown, ±SD from three independent experiments. Activity from the cotransfection of E12 with MyoD in the absence of Ca2+ channel blocker was set at 100%.
Ca2+ Modulators Differentially Affect Transcriptional Activation of a MyoD-responsive Reporter by Coexpressed MyoD and Wild-Type or CaM-resistant E12
To challenge our model further, we compared the effects of Ca2+ modulators on wild-type and CaM-resistant E12. The L-type Ca2+ channel blockers nifedipine and verapamil have been shown to inhibit myogenic differentiation of a myoblast cell line (Porter et al., 2002). We analyzed the effects of these Ca2+ channel blockers on the activity of the MyoD reporter in cells transfected with MyoD alone or MyoD together with either wild-type E12 or mutant m8N47. Both Ca2+ channel blockers inhibited expression from the MyoD responsive reporter in a dose-dependent manner, in cotransfection of MyoD together with wild-type E12, whereas transcriptional activation in cotransfection of MyoD with CaM-resistant mutant m8N47 was not affected (Figure 4B).
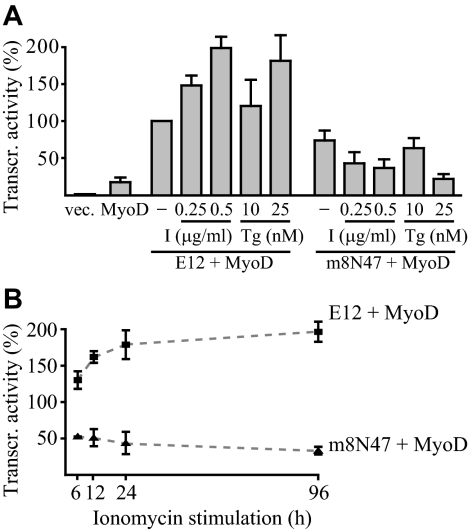
Because a decrease in intracellular Ca2+ resulted in an inhibition of expression from the MyoD responsive promoter by coexpressed MyoD and wild-type—but not mutant—E12 (Figure 4B), we were prompted to analyze the effects of an increase in intracellular Ca2+. Stimulation of cells with either a Ca2+ ionophore, ionomycin, or a Ca2+ pump inhibitor, thapsigargin, increased reporter activity in transfections of MyoD together with wild-type E12 but not together with the CaM-resistant mutant m8N47 (Figure 5A). To determine the duration of increased Ca2+ levels required for transcriptional activation by MyoD/E12 heterodimers, transfected cells were treated with ionomycin for the first 6, 12, or 24 h of differentiation only, and, as a control, for all 4 d. Ionomycin stimulation for 12 h was sufficient to obtain most of the induction, and 24 h was sufficient to give an induction level that was not significantly lower than the stimulation during the whole 4-d differentiation period (Figure 5B). These results suggest that activation of MyoD/E12 by Ca2+ stimulation is a relatively fast response and that this activation could subsequently be maintained throughout differentiation by one or more other mechanisms.
Figure 5.
Effects of Ca2+ inducers on transcription from a MyoD-responsive reporter. (A) NIH-3T3 fibroblasts were transiently transfected with 0.75 μg expression vector for MyoD together with 0.75 μg empty expression vector or expression vector for E12 or m8N47 mutant, together with 0.25 μg of 4x(MEF1)-luciferase reporter and 0.25 μg of hCMV-β-gal. Twenty-four hours after the start of transfection, the medium was replaced with differentiation medium containing, where indicated, Ca2+ stimulators (I, ionomycin; Tg, thapsigargin), and the cells were harvested for analysis 4 d later. Mean normalized luciferase values are shown, ±SD from three independent experiments. Activity from transfection of E12 together with MyoD in the absence of Ca2+ stimulator was set at 100%. (B) NIH-3T3 fibroblasts were transfected as in A. The medium was replaced with differentiation medium with or without 0.5 μg/ml ionomycin 24 h after transfection, and the cells were cultured in the presence of the Ca2+ inducer for the indicated period of time. In stimulations lasting 6, 12, and 24 h, the medium was replaced with differentiation medium without ionomycin after the period of stimulation, and the cells were further cultured for a total time of 96 h (4 d). The effects of ionomycin stimulations on mean normalized luciferase activities are shown, ±SD from three independent experiments. Activities obtained from transfections of E12 together with MyoD in the absence of ionomycin were set at 100%.
Tethered MyoD-E12 Dimers Are Insensitive to Ca2+/CaM Regulation and Myogenesis Is Efficient with Tethered CaM-resistant Mutant
We had not formally excluded the possibility that the inhibition of myogenesis by CaM-resistant E12 and the differential effects on transcriptional activation by E12 homodimers and heterodimers of E12 with MyoD described above could have another, unknown cause instead of the differential Ca2+/CaM sensitivity of homo- and heterodimers of E-proteins. To challenge whether the effects were indeed dependent on alternative dimerizations, MyoD was forced into dimerization with E12 by tethering MyoD and E12 in fusion proteins. Previous analysis of fusion proteins of MyoD and E47 (MyoD–E47), created by inserting a flexible polypeptide linker between the two proteins, showed that they were myogenic, resistant to inhibition by Id proteins and had a substantial ability to bypass negative regulation of differentiation by serum growth factors (Neuhold and Wold, 1993). We created expression plasmids for fusion proteins of MyoD tethered by a flexible linker to either wild-type E12 or the m847 mutant. Both of these fusion protein constructs resulted in a higher transcriptional activation of the MyoD reporter than coexpressed MyoD plus E12. The MyoD–E12 “tether” was somewhat more efficient in activating transcription than the MyoD–m847 tether (Figure 6A), which is in line with the somewhat higher activation seen with the nontethered E12 wild-type protein than with the m847 mutant (Figures 3A and 4A). In contrast to ionomycin stimulation after cotransfection of MyoD plus E12 (Figures 5 and 6A), ionomycin did not affect transcriptional activation by MyoD–E12 or MyoD–m847 (Figure 6A). This suggests that Ca2+ stimulation no longer regulates MyoD activity positively when Ca2+ stimulation cannot regulate competition between E12 homodimers and MyoD-E12 heterodimers on the promoter, which strongly argues in favor of the effect of the mutation being on Ca2+ regulation of alternative dimerizations.
Figure 6.
No transcriptional regulation by Ca2+/CaM or inhibition of myogenesis with tethered MyoD–E12 fusion proteins. (A) NIH-3T3 fibroblasts were transiently transfected with 0.75 μg expression vectors for MyoD and E12 or with 1.5 μg expression vector for MyoD–E12 or MyoD–m847 tether together with 0.25 μg of 4x(MEF-1)-luciferase reporter and 0.25 μg of hCMV-β-gal. Twenty-four hours after transfection, the medium was replaced with differentiation medium with or without 0.5 μg/ml ionomycin, and the cells were harvested for analysis 4 d later. The mean normalized reporter activities are shown, ±SD from at least three independent experiments. (B) NIH-3T3 fibroblasts were transiently transfected with 2 μg expression vector for MyoD together with 2 μg empty expression vector (vec.) or expression vector for E12 or the E12 basic sequence mutant m847, or 4 μg of expression vector for the indicated tether. The transfected cells were allowed to differentiate for 4 d and were immunostained for MyoD and myosin. Bars, mean values of counts of myosin-positive cells, ±SD from at least three independent experiments. The number of myosin-positive cells from transfection of MyoD together with empty expression vector (vec.) was arbitrarily set at 100%. (C) Myogenesis indices indicating the percentage of cells stained for myosin in the population of cells stained for MyoD from transfections of B. Bars, mean values of myogenesis index, ±SD.
The MyoD–E12 and MyoD–m847 “tethers” were also compared with the corresponding cotransfections in myogenesis. The tethers produced much higher quantities of myosin-positive cells than MyoD alone, or cotransfection with MyoD and E12 (Figure 6B). Importantly, MyoD–m847 tether was not significantly less myogenic than MyoD-E12 tether, and it was two- to threefold more myogenic than cotransfection with MyoD and E12, and more than 10-fold more myogenic than cotransfection with MyoD and m847 (Figure 6B). Thus, remarkably, the m847 mutant of E12, which was strongly inhibitory for myogenesis in cotransfections with MyoD, was instead positive for myogenesis when forced to heterodimerize by fusion to MyoD (Figure 6B). In addition, the difference between wild-type and m847 disappeared upon tethering to MyoD when the myogenesis indices were analyzed (Figure 6C). Thus, in contrast to cotransfection with MyoD and E12, where Ca2+/CaM-resistant m847 mutation severely affected myogenesis (Figure 6, B and C), little effect of the same mutation was obtained in tethers with MyoD, which strongly indicates that the inhibitory effect of the mutation on myogenesis is on Ca2+ regulation of alternative dimerizations.
Rescue of Myogenesis by Wild-Type, But Not CaM-resistant, E12 after E12/E47 Depletion with siRNA
The inhibition of myogenesis by CaM-resistant, but not wild-type, E12 described above (Figures 2, B–E, 3D, and 6, B and C) was observed after overexpression experiments. To analyze whether Ca2+/CaM inhibition of E12 homodimers was important for myogenesis by MyoD–E12 heterodimers at low levels of E12 also, we used RNA interference in the NIH-3T3 fibroblasts to inhibit endogenous expression of the E2A gene products E12 and E47. As previously reported (Nie et al., 2003), the cell line expressed E12/E47 (Figure 7A). Including siRNA against E12 and E47 in MyoD transfections reduced the expression of E12/E47 by an average of 86.3 ± 3.1% and resulted in a 90.1 ± 1.7% reduced expression of MyoD (Figure 7A), which is in line with the existence of a positive-feedback loop of MyoD to transcriptionally activate its own expression (Zingg et al., 1994) and the previously reported need for heterodimerization with E12/E47 for MyoD function (Lassar et al., 1991; Massari and Murre, 2000). Restoring the amounts of wild-type E12 in the presence of E2A siRNA, by transfection of an E12 expression plasmid, restored the expression of MyoD (Figure 7A). In contrast, transfection of expression plasmid for m847 mutant of E12 did not restore expression of MyoD (20.3 ± 3.2% of wild-type level), and transfection of expression plasmid for the m8N47 mutant only partially restored the MyoD expression (Figure 7A). Thus, the positive-feedback loop of MyoD to activate its own expression does not function when the E12 that the cells express is CaM-resistant.
Figure 7.
Deficiency of CaM-resistant E12 mutants in rescue of myogenesis inhibition by siRNA against E2A and resistance of tethered MyoD–E12 fusion protein to myogenesis inhibition by CaM-resistant E12 mutants. (A) Levels of E12 and MyoD protein in transfected NIH-3T3 cells determined by Western blot analysis. NIH-3T3 cells were cotransfected with 0.8 μg expression vector for MyoD and 3.2 μg of the empty pcDNAI/amp vector or expression vector for E12 or E12 mutant, and 10 pmol of siRNA against murine E2A where indicated, using Lipofectamine 2000 in antibiotic-free growth medium according to the manufacturer's recommendations. Twenty-four hours after transfection, the medium was replaced with differentiation medium supplemented with 10 pmol E2A siRNA complexed with Lipofectamine 2000. The cells were allowed to differentiate for 4 d before harvest. Whole cell extracts were prepared and used in Western blot analysis to detect E12 and MyoD using the levels of α-tubulin as loading controls. (B) NIH-3T3 cells were grown on glass coverslips in 24-well plates and cotransfected with 0.2 μg expression vector for MyoD and 0.8 μg of the indicated E12 or SEF2-1 expression vectors and 10 pmol of siRNA against murine E2A, using Lipofectamine 2000. The amounts of E12, m847, and m8N47 expression plasmids were 0.05, 0.1, 0.3, and 0.8 μg, the amounts of SEF2-1 expression plasmid were 0.05, 0.1, and 0.8 μg, and the empty expression vector was included where necessary to a total of 1 μg of plasmid DNA. The cotransfections of MyoD expression vector and siRNA against murine E2A with SEF2-1 plus m847 expression plasmids were with 0.025, 0.05, and 0.4 μg each of the SEF2-1 and m847 expression plasmids. Vec. indicates that no E-protein expression plasmid was added. Twenty-four hours after transfection, the medium was replaced with differentiation medium supplemented with 10 pmol E2A siRNA complexed with Lipofectamine 2000. The cells were allowed to differentiate for 4 d and immunostained for MyoD and myosin. Bars, mean values of counts of myosin-positive cells, ±SD from at least three independent experiments. The number of myosin-positive cells from transfection of MyoD together with empty expression vector (vec.) was arbitrarily set at 100%. (C) Myogenesis indices indicating the percentage of cells stained for myosin in the population of cells stained for MyoD in transfections of (B). Bars, mean values of myogenesis index ± SD. (D) Resistance of tethered MyoD–E12 fusion protein to myogenesis inhibition by CaM-resistant E12 mutants. NIH-3T3 fibroblasts grown in 24-well plates were transiently cotransfected with 0.2 μg expression vector for tethered MyoD–E12 fusion protein and the indicated E12 expression vector. The amounts of E12, m847, and m8N47 expression plasmids were 0.1, 0.4, and 0.8 μg, and the empty expression vector was included where necessary to a total of 1 μg of plasmid DNA. Vec. indicates that no E-protein expression plasmid was added with the expression vector for the tether. Cotransfections of 0.5 μg expression vector for MyoD together with 0.5 μg empty expression vector (vec.) or expression vector for the E12 basic sequence mutant m847 were used as controls. The transfected cells were allowed to differentiate for 4 d and were immunostained for MyoD and myosin as in Figure 6B. Bars, mean values of counts of myosin-positive cells, ±SD from three independent experiments. The number of myosin-positive cells from transfection of MyoD together with empty expression vector (vec.) was arbitrarily set at 100%.
The RNA interference of E12 and E47 expression inhibited MyoD-initiated myogenesis (Figure 7, B and C), which confirms the previously reported (Lassar et al., 1991; Massari and Murre, 2000) need for heterodimerization with an E protein in myogenesis and shows that the predominant heterodimer partner for MyoD in this cell line is E12/E47. The siRNA against E2A reduced the number of myosin-positive cells to ∼10% and the myogenesis index to ∼40% (Figure 7, B and C). Importantly, in contrast to restoring the amounts of wild-type E12 in the presence of E2A siRNA, which resulted in a nearly complete rescue of myogenesis in a dose-dependent manner, no amount of expression vector for CaM-resistant E12 mutant m847 or m8N47 rescued myogenesis (Figure 7, B and C). Comparison of myogenesis with corresponding doses of expression plasmids for the wild type and mutant showed that CaM-resistant E12 was also inhibitory to myogenesis at levels that (owing to the siRNA) were the same as or lower than normal E12/E47 levels (Figure 7, B and C). Furthermore, the CaM-resistant mutants even reduced the averages of the myogenic indices below the level seen without adding mutant (Figure 7C), probably reflecting the fact that the mutants can reduce myogenic indices approximately as much as the siRNA against E12/E47 (cf. Figures 2B and 7C). To analyze if only E12 could rescue myogenesis, we compared the effect of transfection of expression plasmid for another E-protein, SEF2-1 (E2-2). The SEF2-1 expression plasmid was approximately as efficient as the E12 expression plasmid both in restoring the number of myosin-positive cells and in restoring the myogenesis index (Figure 7, B and C), showing that E12 is not the only E-protein with the ability to participate in myogenesis. Furthermore, coexpression of SEF2-1 with the CaM-resistant E12 mutant m847 reduced the ability of SEF2-1 to rescue myogenesis (Figure 7, B and C), supporting that they compete for the same target, MyoD. In summary, CaM-resistant E12, the homodimers of which cannot be inhibited by Ca2+, does not function as a heterodimer partner in the myogenesis, which strongly suggests that inhibition of homodimerization of E12/E47 by Ca2+-loaded CaM is also essential for myogenesis at low or normal levels of E12/E47.
Myogenesis by Tethered MyoD-E12 Dimers Is Insensitive to CaM-resistant E12 Mutants
The results above strongly suggest that the block of myogenesis by the CaM resistant mutants is due to Ca2+/calmodulin regulation of E-protein homodimers versus MyoD/E-protein heterodimers. However, persistent activity of homodimers of the CaM-resistant mutants could alternatively be hypothesized to block myogenesis through activation of nonmyogenic genes, provided the existence of genes activated by E12 homodimers and blocking myogenic differentiation in these cells. To challenge whether the effects were dependent on regulation by homodimers versus heterodimers or due to the hypothesized alternative, we analyzed whether myogenesis induced by the tethered MyoD-E12 protein was inhibited by the E12 mutants. Myogenesis by tethered MyoD-E12 protein should be resistant to the E12 mutants if alternative dimerizations are critical for the inhibitory effect of the CaM-resistant mutants, but it should be fully sensitive with the other hypothesis. To enable challenge with a large excess of expression vector for CaM-resistant mutants, the amount of transfected expression vector for tethered MyoD–E12 was reduced compared with the experiment in Figure 6, B and C, to a level where the number of myosin-positive cells was below that with transfection of MyoD expression plasmid (Figure 7D). Myogenesis by the tethered MyoD-E12 heterodimer was indeed resistant to the challenge of overexpression of E12 mutants. In contrast to cotransfection of MyoD with m847 mutant of E12, which reduced the number of myosin-positive cells by about fourfold at a 1:1 ratio (Figures 6B and 7D) and more than 10-fold at a 1:4 ratio (Figure 2D), the corresponding cotransfection of MyoD-E12 tether with m847 or m8N47 mutant did not significantly reduce the myosin-positive cells even when a fourfold excess of the mutant was added (Figure 7D). Furthermore, the myogenesis index did not decrease even with the fourfold excess of CaM-resistant mutant. The index was only varying between 71 and 81%, and there was no difference between overexpression of wild-type or CaM-resistant mutant of E12. These results show that myogenesis by the tethered heterodimer is indeed resistant to the challenge of overexpression of E12 mutants in agreement with Ca2+/calmodulin regulation of E-protein homodimers versus MyoD/E-protein heterodimers and in disagreement with interpretations not involving alternative dimers. Thus, alternative dimerizations are critical for the inhibitory effect of the CaM resistant mutants on the myogenesis.
DISCUSSION
Our studies of Ca2+/CaM inhibition of DNA binding of E-protein bHLH transcription factors (Corneliussen et al., 1994; Saarikettu et al., 2004) raise the possibility that Ca2+/CaM may selectively inhibit DNA binding of E-protein homodimers on E-boxes of muscle-specific genes, thereby promoting DNA binding of MyoD/E-protein heterodimers and myogenesis (Figure 1B). In this report, we have tested this hypothesis using E12 mutants that are resistant to inhibition by Ca2+/CaM to different extents. Our data support the idea that Ca2+/CaM enables myogenesis by inhibiting DNA binding of CaM-sensitive E-protein homodimers, thereby selectively promoting occupancy of myogenic bHLH protein/E-protein heterodimers on promoters of myogenic target genes during myogenesis. All of the CaM-resistant E12 basic sequence mutants analyzed were also inhibitory to MyoD-induced myogenic conversion of transfected fibroblasts (Figure 2, B–E) independently of the resistance or sensitivity of the mutant to Id inhibitors (Figure 3, A and C). Thus, the inhibitory effect of these basic sequence mutants on myogenesis was clearly not mediated through Id protein resistance.
The reduction in the ability of the E12 basic sequence mutants to activate the MyoD-responsive promoter in cooperation with MyoD was similar, but not identical, to their ability to interfere with MyoD in induction of myogenic conversion (cf. Figures 2B and 4A). Both MyoD-induced myogenic conversion of transfected cells and 4x(MEF1)-luciferase reporter activity rely on active MyoD in a heterodimer with E12. It should be noted, however, that the luciferase reporter activity is relatively high even 1 d after transfection in cells in growth medium, whereas myogenesis requires a period of several days of subsequent differentiation to be detectable. The data from the myogenesis and reporter assays should therefore be compared with caution, but they show that both myogenesis and synergistic transcriptional activation by MyoD are negatively affected by CaM-resistant E12 mutants (Figures 2B and 4A).
Reduction of intracellular Ca2+ concentration in C2C12 myoblast cells upon treatment with the L-type Ca2+ channel blockers nifedipine and verapamil has been shown to inhibit myogenic differentiation (Porter et al., 2002). The Ca2+ blockers also inhibited the expression of a luciferase reporter driven by the skeletal α-actin promoter without affecting the expression level of the myogenic bHLH protein Myf5. In this article we have shown that the Ca2+ channel blockers inhibit transcriptional activation by MyoD and by coexpressed MyoD together with wild-type E12 from a MyoD-responsive reporter containing MyoD-binding E-boxes (Figure 4B). In contrast, transcriptional activation by MyoD coexpressed with a CaM-resistant mutant of E12 was not affected by the Ca2+ blockers (Figure 4B). At least some of the inhibitory effect of Ca2+ channel blockers on myogenesis observed by Porter et al. (2002) could therefore be due to an increase in E-protein homodimers competing with the MyoD/E-protein heterodimers due to a reduction of Ca2+/CaM levels. Conversely, increases in intracellular Ca2+ concentration by treatment with either ionomycin or thapsigargin resulted in an increase in transcriptional activation of the MyoD reporter by MyoD coexpressed with wild-type E12, whereas transcriptional activation by MyoD together with a CaM-resistant mutant of E12 was slightly inhibited by the Ca2+ stimulators (Figure 5). This argues for the possibility that an increase in Ca2+ might have a negative effect on transcriptional activation by MyoD in the absence of the CaM regulation through interaction with the E-protein. Importantly, ionomycin stimulation did not induce transcriptional activation by MyoD–E12 or MyoD–m847 tethers (Figure 6A), showing that the effect of E12 mutation was dependent on alternative dimerization of E12.
We did not detect any increase in the reporter activity by stimuli that increase the Ca2+ concentration when stimulations were carried out in growth-promoting medium (data not shown). A possible explanation for this could be high levels of expression of the inhibitory Id proteins in cells cultured in growth medium (Iyer et al., 1999). Id proteins may inhibit activation of transcription by bHLH proteins under these conditions and override the effects gained from Ca2+ stimulation.
CaM was detected both in the cytoplasm and the nucleus of NIH-3T3 fibroblasts cultured in a medium containing 10% fetal bovine serum that promotes cell proliferation. The change to differentiation medium resulted in an increase in the proportion of CaM in the nucleus. The nuclear localization of CaM in nonproliferating fibroblasts supports its role as a regulator of transcription under conditions permissive for myogenic differentiation. Nuclear localization of CaM has been shown in other cellular systems to be induced by an increase in the intracellular Ca2+ concentration (Deisseroth et al., 1998; Teruel et al., 2000), and spontaneous Ca2+ spikes and oscillations in intracellular Ca2+ concentration have been shown to occur in differentiating C2C12 myoblast cells (Lorenzon et al., 1997). These observations suggest a possible relationship between conditions permissive for differentiation of myoblasts or MyoD-transfected fibroblasts and nuclear Ca2+/CaM, which can in turn modify the activity of nuclear transcription factors involved in regulation of muscle development. Several articles have demonstrated a positive role for Ca2+ and CaM in regulation of the activity of muscle-specific transcription factors, and myogenesis. MEF2 has been shown to be in a repressed state in myoblasts through interaction with the transcriptional corepressors HDAC4 and HDAC5 (Miska et al., 1999; Lu et al., 2000), and this repression of MEF2 was shown to be relieved by the Ca2+/CaM-dependent kinase I, which disrupts the MEF2-HDAC interaction through phosphorylation of HDAC (Lu et al., 2000; McKinsey et al., 2000). HDAC5 has also been shown to be a direct target of Ca2+/CaM (Berger et al., 2003). A positive role in regulation of myogenesis has also been suggested for the Ca2+/CaM-dependent phosphatase calcineurin through activation of NFAT (Delling et al., 2000), MEF2 and MyoD (Friday et al., 2003). The Ca2+/CaM inhibition of DNA binding of CaM-sensitive E-protein homodimers and thus selective occupancy of myogenic bHLH protein/E-protein heterodimers on promoters of myogenic target genes, as reported here, is therefore not the only role of Ca2+/CaM in myogenesis.
Myogenic differentiation of NIH-3T3 cells by MyoD was dependent on E2A, because it was inhibited by E2A depletion (Figure 7). This inhibition of myogenesis was rescued by wild-type E12, but not by calmodulin-resistant E12 mutants, demonstrating that calmodulin-sensitive E2A protein is critical for myogenesis in this system. However, also SEF2-1 (E2-2) is calmodulin sensitive (Corneliussen et al., 1994) and could rescue the myogenesis (Figure 7). Furthermore, the calmodulin-binding basic sequence is identical in SEF2-1 and in HEB. This argues that all three E-proteins have the potential to participate in calmodulin sensitivity–dependent promotion of myogenesis, and that their relative role as heterodimer partners in myogenic differentiation of a precursor cell will depend on other factors such as their abundance and functional activity in the cell.
The Ca2+ signaling through nuclear CaM in regulation of myogenesis by differential inhibition of bHLH transcription factors reported here can function as a trigger of differentiation. This activation of transcription by tissue-specific class II bHLH factors, such as MyoD, through inhibition of their competitor E-protein homodimers may function at an early stage of myogenic differentiation, thus resulting in increased transcriptional activity of MyoD. In support of this, Ca2+ stimulation for 1 d did not produce significantly less transcriptional activation by E12/MyoD than Ca2+ stimulation for all 4 d of the differentiation (Figure 5B). In addition, E47 has been suggested to be inactivated through phosphorylation in differentiating C2C12 myoblasts. The p38 kinase that is activated in differentiating myoblasts has been shown to phosphorylate E47, leading to improved heterodimerization with MyoD (Lluis et al., 2005). Furthermore, casein kinase II can phosphorylate E47 with similar effects (Johnson et al., 1996; Sloan et al., 1996). Signaling from the CDO cell surface receptor in developing muscle has been proposed to results in a modification of E-proteins, promoting heterodimerization with MyoD (Cole et al., 2004). Because expression of CDO was shown to be induced by MyoD, it was proposed that this signaling is involved in maintenance of the myogenic program (Cole et al., 2004). However, before MyoD/E-protein heterodimers can maintain MyoD activity through CDO, establishment of the initial ability of the heterodimers to activate transcription, including expression of CDO, is required. The importance of Ca2+/CaM inhibition of CaM-sensitive E-protein homodimers in myogenesis at a step before the positive-feedback loop in MyoD expression is supported by the loss of the feedback loop in cells expressing CaM-resistant E12 mutants (Figures 2F and 7A). In summary, the results reported here suggest that differential Ca2+/CaM inhibition of bHLH proteins is an initiation step in the decision to differentiate to myocytes, preceding the maintenance of the myogenic program through the previously mentioned phosphorylation of E-proteins.
We report here an activating effect of Ca2+/calmodulin regulation of E-proteins in myogenesis, in contrast to the inhibitory effect of this regulatory system that we recently reported in B lymphocytes (Hauser et al., 2008). Ca2+/calmodulin regulation of E-proteins was found to inhibit transcription of activation-induced cytidine deaminase (AID), the key mutagenic antibody diversification enzyme that enables highly specific and potent antibody responses, after successful antibody gene mutagenesis in B lymphocytes (Hauser et al., 2008). We have found transcriptional inhibition through Ca2+/calmodulin inhibition of E proteins also for other genes in this cell lineage (Hauser, Sveshnikova, Saarikettu, and Grundström, unpublished observations). The activation of transcription reported here in myogenesis, in contrast to this inhibitory effect, is consistent with Ca2+/CaM-resistant heterodimers between E-proteins and other types of bHLH proteins playing a key role in myogenesis, and not E-protein homodimers as in B-lymphocytes (Massari and Murre, 2000).
The number of mammalian genes encoding bHLH transcription factors is very high. The mouse genome has been estimated to have 116 genes for potential bHLH transcription factors (Gray et al., 2004). Because the ubiquitously expressed CaM-sensitive E-proteins are believed to be obligate heterodimer partners for many tissue-specific bHLH proteins in many differentiation processes apart from myogenesis (Massari and Murre, 2000), Ca2+/CaM may play a more universal role in regulation of differentiation through differential inhibition of bHLH proteins. For example, this regulation may also be found in neurogenesis, where bHLH proteins have important roles (Lee, 1997; Farah et al., 2000; Ik Tsen Heng and Tan, 2003) and spontaneous Ca2+ transients have been demonstrated to be required for differentiation (Gu and Spitzer, 1997).
ACKNOWLEDGMENTS
We thank Dr. Michael D. Walker for the generous gift of Id expression vectors. This work was supported by a grant from the Swedish Research Council.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-09-0886) on March 19, 2008.
REFERENCES
- Bain G., et al. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Berger I., Bieniossek C., Schaffitzel C., Hassler M., Santelli E., Richmond T. J. Direct interaction of Ca2+/calmodulin inhibits histone deacetylase 5 repressor core binding to myocyte enhancer factor 2. J. Biol. Chem. 2003;278:17625–17635. doi: 10.1074/jbc.M301646200. [DOI] [PubMed] [Google Scholar]
- Berkes C. A., Tapscott S. J. MyoD and the transcriptional control of myogenesis. Semin. Cell. Dev. Biol. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Cole F., Zhang W., Geyra A., Kang J. S., Krauss R. S. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev. Cell. 2004;7:843–854. doi: 10.1016/j.devcel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Conway K., Pin C., Kiernan J. A., Merrifield P. The E protein HEB is preferentially expressed in developing muscle. Differentiation. 2004;72:327–340. doi: 10.1111/j.1432-0436.2004.07207004.x. [DOI] [PubMed] [Google Scholar]
- Corneliussen B., Holm M., Waltersson Y., Onions J., Hallberg B., Thornell A., Grundström T. Calcium/calmodulin inhibition of basic-helix-loop-helix transcription factor domains. Nature. 1994;368:760–764. doi: 10.1038/368760a0. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H. Acquisition of myogenic specificity by replacement of three amino acid residues from MyoD into E12. Science. 1992;256:1027–1030. doi: 10.1126/science.1317057. [DOI] [PubMed] [Google Scholar]
- Deisseroth K., Heist E. K., Tsien R. W. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Delling U., Tureckova J., Lim H. W., De Windt L. J., Rotwein P., Molkentin J. D. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol. Cell. Biol. 2000;20:6600–6611. doi: 10.1128/mcb.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M. H., Olson J. M., Sucic H. B., Hume R. I., Tapscott S. J., Turner D. L. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- Friday B. B., Mitchell P. O., Kegley K. M., Pavlath G. K. Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation. 2003;71:217–227. doi: 10.1046/j.1432-0436.2003.710303.x. [DOI] [PubMed] [Google Scholar]
- Gray P. A., et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Gu X., Spitzer N. C. Breaking the code: regulation of neuronal differentiation by spontaneous calcium transients. Dev. Neurosci. 1997;19:33–41. doi: 10.1159/000111183. [DOI] [PubMed] [Google Scholar]
- Hauser J., Sveshnikova N., Wallenius A., Baradaran S., Saarikettu J., Grundström T. B-cell receptor activation inhibits AID expression through calmodulin inhibition of E-proteins. Proc. Natl. Acad. Sci. USA. 2008;105:1267–1272. doi: 10.1073/pnas.0708220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K., Edin S., Antonsson A., Grundström T. Calmodulin-dependent kinase II mediates T cell receptor/CD3- and phorbol ester-induced activation of IkappaB kinase. J. Biol. Chem. 2001;276:36008–36013. doi: 10.1074/jbc.M106125200. [DOI] [PubMed] [Google Scholar]
- Hughes K., Saarikettu J., Grundström T. Gene expression in transfected cells. Methods Mol. Biol. 2002;173:355–363. doi: 10.1385/1-59259-184-1:355. [DOI] [PubMed] [Google Scholar]
- Ik Tsen Heng J., Tan S. S. The role of class I HLH genes in neural development—have they been overlooked? Bioessays. 2003;25:709–716. doi: 10.1002/bies.10299. [DOI] [PubMed] [Google Scholar]
- Iyer V. R., et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- Johnson S. E., Wang X., Hardy S., Taparowsky E. J., Konieczny S. F. Casein kinase II increases the transcriptional activities of MRF4 and MyoD independently of their direct phosphorylation. Mol. Cell. Biol. 1996;16:1604–1613. doi: 10.1128/mcb.16.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991;66:305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Lee J. E. Basic helix-loop-helix genes in neural development. Curr. Opin. Neurobiol. 1997;7:13–20. doi: 10.1016/s0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- Lluis F., Ballestar E., Suelves M., Esteller M., Munoz-Canoves P. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005;24:974–984. doi: 10.1038/sj.emboj.7600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon P., Giovannelli A., Ragozzino D., Eusebi F., Ruzzier F. Spontaneous and repetitive calcium transients in C2C12 mouse myotubes during in vitro myogenesis. Eur. J. Neurosci. 1997;9:800–808. doi: 10.1111/j.1460-9568.1997.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Lu J., McKinsey T. A., Nicol R. L., Olson E. N. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc. Natl. Acad. Sci. USA. 2000;97:4070–4075. doi: 10.1073/pnas.080064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari M. E., Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T. A., Zhang C. L., Lu J., Olson E. N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T. A., Zhang C. L., Olson E. N. MEF 2, a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 2002;27:40–47. doi: 10.1016/s0968-0004(01)02031-x. [DOI] [PubMed] [Google Scholar]
- Miska E. A., Karlsson C., Langley E., Nielsen S. J., Pines J., Kouzarides T. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 1999;18:5099–5107. doi: 10.1093/emboj/18.18.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhold L. A., Wold B. HLH forced dimers: tethering MyoD to E47 generates a dominant positive myogenic factor insulated from negative regulation by Id. Cell. 1993;74:1033–1042. doi: 10.1016/0092-8674(93)90725-6. [DOI] [PubMed] [Google Scholar]
- Nie L., Xu M., Vladimirova A., Sun X. H. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 2003;22:5780–5792. doi: 10.1093/emboj/cdg567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onions J., Hermann S., Grundström T. Basic helix-loop-helix protein sequences determining differential inhibition by calmodulin and S-100 proteins. J. Biol. Chem. 1997;272:23930–23937. doi: 10.1074/jbc.272.38.23930. [DOI] [PubMed] [Google Scholar]
- Parker M. H., Perry R. L., Fauteux M. C., Berkes C. A., Rudnicki M. A. MyoD synergizes with the E-protein HEB beta to induce myogenic differentiation. Mol. Cell. Biol. 2006;26:5771–5783. doi: 10.1128/MCB.02404-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter G. A., Jr, Makuck R. F., Rivkees S. A. Reduction in intracellular calcium levels inhibits myoblast differentiation. J. Biol. Chem. 2002;277:28942–28947. doi: 10.1074/jbc.M203961200. [DOI] [PubMed] [Google Scholar]
- Puri P. L., Sartorelli V. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 2000;185:155–173. doi: 10.1002/1097-4652(200011)185:2<155::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Rutherford M. N., LeBrun D. P. Restricted expression of E2A protein in primary human tissues correlates with proliferation and differentiation. Am. J. Pathol. 1998;153:165–173. doi: 10.1016/S0002-9440(10)65557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzinova M. B., Benezra R. Id proteins in development, cell cycle and cancer. Trends. Cell Biol. 2003;13:410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Saarikettu J., Sveshnikova N., Grundström T. Calcium/calmodulin inhibition of transcriptional activity of E-proteins by prevention of their binding to DNA. J. Biol. Chem. 2004;279:41004–41011. doi: 10.1074/jbc.M408120200. [DOI] [PubMed] [Google Scholar]
- Sabourin L. A., Rudnicki M. A. The molecular regulation of myogenesis. Clin. Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- Sloan S. R., Shen C. P., McCarrick-Walmsley R., Kadesch T. Phosphorylation of E47 as a potential determinant of B-cell-specific activity. Mol. Cell. Biol. 1996;16:6900–6908. doi: 10.1128/mcb.16.12.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Trausch-Azar J. S., Ciechanover A., Schwartz A. L. E2A protein degradation by the ubiquitin-proteasome system is stage-dependent during muscle differentiation. Oncogene. 2007;26:441–448. doi: 10.1038/sj.onc.1209793. [DOI] [PubMed] [Google Scholar]
- Teruel M. N., Chen W., Persechini A., Meyer T. Differential codes for free Ca(2+)-calmodulin signals in nucleus and cytosol. Curr. Biol. 2000;10:86–94. doi: 10.1016/s0960-9822(00)00295-5. [DOI] [PubMed] [Google Scholar]
- Yun K., Wold B. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr. Opin. Cell Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Cheng P., Weintraub H. B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2, and HEB. Mol. Cell. Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Soriano P., Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Zingg J. M., Pedraza-Alva G., Jost J. P. MyoD1 promoter autoregulation is mediated by two proximal E-boxes. Nucleic Acids Res. 1994;22:2234–2241. doi: 10.1093/nar/22.12.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]