Abstract
Visceral leishmaniasis (VL) is a fatal disease for humans, and no vaccine is currently available. Sand fly salivary proteins have been associated with protection against cutaneous leishmaniasis. To test whether vector salivary proteins can protect against VL, a hamster model was developed involving intradermal inoculation in the ears of 100,000 Leishmania infantum chagasi parasites together with Lutzomyia longipalpis saliva to mimic natural transmission by sand flies. Hamsters developed classical signs of VL rapidly, culminating in a fatal outcome 5–6 months postinfection. Saliva had no effect on the course of infection in this model. Immunization with 16 DNA plasmids coding for salivary proteins of Lu. longipalpis resulted in the identification of LJM19, a novel 11-kDa protein, that protected hamsters against the fatal outcome of VL. LJM19-immunized hamsters maintained a low parasite load that correlated with an overall high IFN-γ/TGF-β ratio and inducible NOS expression in the spleen and liver up to 5 months postinfection. Importantly, a delayed-type hypersensitivity response with high expression of IFN-γ was also noted in the skin of LJM19-immunized hamsters 48 h after exposure to uninfected sand fly bites. Induction of IFN-γ at the site of bite could partly explain the protection observed in the viscera of LJM19-immunized hamsters through direct parasite killing and/or priming of anti-Leishmania immunity. We have shown that immunity to a defined salivary protein (LJM19) confers powerful protection against the fatal outcome of a parasitic disease, which reinforces the concept of using components of arthropod saliva in vaccine strategies against vector-borne diseases.
Keywords: antisaliva immunity, Leishmania, sand fly saliva, vector-based vaccine
The bite of an infective sand fly transmits Leishmania parasites to a mammalian host. Together with the parasite, the sand fly injects saliva and promastigote secretory gel.
These components have been shown to enhance cutaneous leishmaniasis (CL) in mice (1–3). Saliva contains a variety of potent and pharmacologically active components that favorably change the environment at the feeding site (4–7). Exposure to sand fly bites or salivary proteins results in strong cellular and/or humoral immunity specific to these components (8–11). In animal models of CL, mice immunized with Phlebotomus papatasi salivary gland homogenate (SGH) or preexposed to uninfected sand fly bites were protected against Leishmania major infection delivered via needle inoculation (2) or by infected sand flies (12). Furthermore, immunization with PpSP15 and maxadilan, salivary proteins from P. papatasi and Lutzomyia longipalpis, respectively, also protected against L. major infection in mice (13, 14).
The protective effect of salivary proteins is not exclusive to sand flies and CL. It has been demonstrated that animals preexposed to ticks were protected from tularemia (15) and borreliosis (16, 17), and vaccination with a tick salivary cement protein protected mice against the lethal effect of tick-borne encephalitis virus (18). Preexposure to mosquito saliva through bites led to partial protection against Plasmodium berghei infection (19) and immunization with the saliva of an aquatic insect (Naucoris genus) protected mice against Mycobacterium ulcerans infection (20).
The established models of protection from CL by antisaliva immunity, together with the fact that all Leishmania infections, including visceral diseases, are initiated in the skin by the bite of an infective sand fly, led us to screen salivary proteins from a vector sand fly species to investigate whether some can protect against visceral disease.
L. infantum chagasi is the cause of visceral leishmaniasis (VL) in Latin America, and the only proven natural vector is Lu. longipalpis. Here, we test the hypothesis that immunity to Lu. longipalpis saliva can protect against VL caused by L. infantum chagasi in a hamster model. To date, progressive disease in hamsters, the model of choice for the study of VL, has been mostly achieved by the injection of a large number of parasites via the i.v., intracardial, or i.p. route (21–24). However, these routes of infection do not mimic natural transmission by sand fly bite where the parasites are delivered into the skin of a mammalian host in the presence of saliva. To our knowledge, apart from a single study reported over a decade ago (25) there is no animal model for VL that combines this natural route of transmission with fatal disease progression. In this work, we demonstrate the fatal outcome of VL in 3- to 4-month-old naïve hamsters after intradermal (i.d.) injection of parasites in the ear together with sand fly saliva and report that immunization with a defined salivary protein from the sand fly Lu. longipalpis protects hamsters from the fatal outcome of VL caused by L. infantum chagasi.
Results
A Model of VL in Hamsters to Test Salivary Vaccine Candidates.
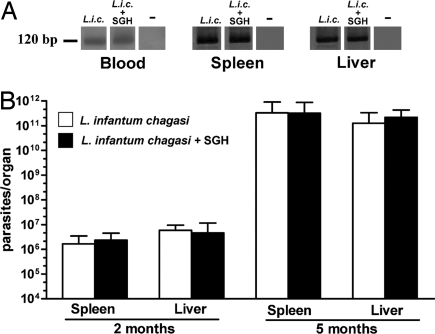
To date, no information exists regarding the effect of vaccination with sand fly salivary proteins on the outcome of VL to our knowledge. To test whether Lu. longipalpis salivary proteins can protect against VL, we developed a model that mimics the outcome of the disease and represents a more natural route of parasite inoculation in the skin in the presence of sand fly saliva. Male hamsters, aged 3–5 months, were infected i.d. in the ear with 105 stationary phase parasites and 0.5 pairs of SGH. Parasites were detectable in the blood, spleen, and liver 15 days postinfection (Fig. 1A). Thereafter, the parasite load increased exponentially to 106 and 1011 parasites per organ at 2 and 5 months postinfection, respectively, in both spleen and liver (Fig. 1B). Importantly, a similar progression of disease was noted in hamsters challenged with Leishmania in the absence of SGH (Fig. 1). Anti-Leishmania antibodies were detected at 2 and 5 months postinfection (data not shown). Infected hamsters presented clinical and pathological signs of parasite visceralization, including hepatosplenomegaly, hypergammaglobulinemia, and cachexia. All animals, challenged in the presence or absence of SGH, died of VL 5–6 months postinfection.
Fig. 1.
Parasite burden after challenge with 105 stationary phase L. infantum chagasi promastigotes in the presence or absence of 0.5 salivary gland pairs. (A) PCR amplification of Leishmania DNA from hamster blood, spleen, and liver 15 days postinfection with Leishmania alone (L.i.c.) and Leishmania and SGH (L.i.c.+SGH). Noninfected hamsters are indicated by −. (B) Parasite burden in the spleen and liver of hamsters (six animals per group) 2 and 5 months postinfection by using the limiting dilution assay. The bars represent the mean number of parasites per organ ± SEM.
Screening of Lu. longipalpis Sand Fly Salivary Proteins for Vaccine Candidates.
There is no information regarding the immune responses produced by Lu. longipalpis salivary proteins in hamsters and the consequences of these responses on the visceral form of leishmaniasis. Hamsters were immunized i.d. in the ear with DNA plasmids coding for the most abundant secreted proteins from Lu. longipalpis. Among the 16 plasmids tested, four (LJM17, LJM19, LJM11, and LJL11) induced an antibody response, a delayed-type hypersensitivity (DTH) response, or both responses in immunized hamsters (Table 1). Notably, the plasmids that were not immunogenic in hamsters were able to produce a cellular or antibody response in mice (data not shown). This finding suggests that the absence of an immune response to some of these plasmids in hamsters may be caused by host specificity (9). However, we cannot exclude a dose-related effect caused by differential expression of plasmids after hamster immunizations.
Table 1.
Immune response in hamsters by immunization with plasmids coding for the most abundant salivary proteins of Lu. longipalpis
| Sequence name | Predicted molecular mass, kDA | Protein annotation | National Center for Biotechnology Information accession no. | Antibody response | DTH response |
|---|---|---|---|---|---|
| LJL08 | 6.9 | Maxadilan | M77090 | + | − |
| LJS201 | 8.6 | Unknown | AY455919 | − | − |
| LJM19 | 10.7 | Unknown | AY438271 | − | +++ |
| LJM04 | 13.8 | Unknown | AF132517 | + | − |
| LJL18 | 16.3 | C-type lectin | DQ190947 | − | − |
| LJL91 | 16.3 | C-type lectin | AY445934 | − | − |
| LJL15 | 16.5 | C-type lectin | DQ190946 | − | − |
| LJM10 | 16.6 | C-type lectin | AAD33512 | − | − |
| LJS142 | 16.6 | C-type lectin | AY445934 | − | − |
| LJL13 | 26.4 | D7 protein | AF420274 | + | − |
| LJL34 | 28.8 | Ag5 protein | AF132511 | − | − |
| LJL23 | 35.0 | Apyrase | AF131933 | − | − |
| LJM111 | 43.0 | Yellow protein | DQ192488 | + | − |
| LJM11 | 43.2 | Yellow protein | AY445935 | +++ | +++ |
| LJM17 | 45.2 | Yellow protein | AF132518 | +++ | +++ |
| LJL11 | 60.6 | 5′-nucleotidase | AF132510 | +++ | − |
| SGH | +++ | +++ | |||
| Control DNA plasmid | − | − |
−, Negative response; +, weak response; +++, strong response.
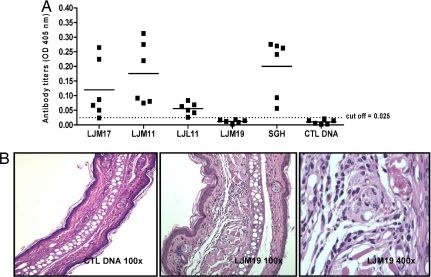
LJM17-, LJM11-, and LJL11-immunized hamsters showed high antibody titers comparable with those of animals immunized with SGH and considerably higher than control DNA-immunized hamsters (Fig. 2A). Moreover, 48 h after the challenge with SGH, LJM17-, LJM11-, and LJM19-immunized hamsters produced a DTH response with a significant increase in ear thickness as compared with negative controls (control DNA or naive groups) or ear thickness before challenge (data not shown). LJM19-immunized hamsters were the only group that produced a strong DTH response but did not produce a detectable antibody response (Fig. 2 A). The DTH response in LJM19-immunized hamsters was characterized by a mononuclear infiltration composed mainly of macrophages and lymphocytes and a minimal number of neutrophils (Fig. 2B). The DTH site in LJM19-immunized hamsters was representative of the DTH response observed in the other experimental groups (LJM17-, LJM11-, and SGH-immunized hamsters).
Fig. 2.
Immune response to Lu. longipalpis salivary proteins. (A) Antibody titers to Lu. longipalpis SGH and DNA plasmids coding for CTL DNA, LJM17, LJM11, LJL11, and LJM19 by using ELISA (six hamsters per group). The cutoff was determined by using sera from noninfected hamsters (mean + 2 SD). (B) Cellular infiltration in representative ears of hamsters immunized with LJM19 or CTL DNA 48 h after challenge with SGH in the contralateral ear (six animals per group, H&E staining). (Magnification: Left and Center, ×100; Right, ×400.)
Lu. longipalpis Salivary Molecule LJM19 Protects Against the Fatal Outcome of VL Caused by L. infantum chagasi.
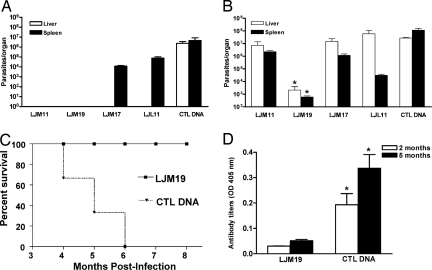
In three independent experiments, hamsters immunized with LJM17, LJM11, LJM19, or LJL11, the molecules producing immune responses in hamsters, were challenged i.d. in the ear by coinoculation of L. infantum chagasi and SGH. Two months postinfection, no parasites were detected in the liver and spleen of animals immunized with LJM11 and LJM19 (Fig. 3A). There was no significant difference in the parasite load in the spleen of LJM17- and LJL11-immunized animals compared with control DNA-immunized hamsters (Fig. 3A). Five months postinfection, however, only the group immunized with LJM19 had a significantly lower number of parasites in the spleen and the liver compared with the other immunized groups including LJM11 (Fig. 3B) and showed no clinical signs of VL. All remaining experimental groups, including control DNA-immunized animals, showed progressive cachexia and hepatosplenomegaly, classical signs of disease, and died 5–6 months postinfection. To establish the durability of protection, a fourth experiment followed the survival of 12 LJM19-immunized hamsters for 8 months when they had to be euthanized according to the Animal Care and Use Committee protocol (Fig. 3C). LJM19-immunized hamsters survived the fatal progression of disease and showed no clinical signs of VL. Hamsters immunized with LJM19 maintained a low anti-Leishmania IgG antibody level throughout the course of infection compared with control DNA-immunized hamsters (Fig. 3D).
Fig. 3.
DNA immunization with LJM19 protects hamsters from the fatal outcome of VL. (A and B) Parasite burden at two (A) and five (B) months postinfection in hamsters immunized with LJM11, LJM19, LJM17, LJL11 and CTL DNA after i.d. challenge in the ear with 105 stationary phase L. infantum chagasi promastigotes in the presence of 0.5 pairs of SGH. Parasite burden was determined by limiting dilution assay (six hamsters/group). (C) Percent survival of hamsters immunized with LJM19 and CTL DNA after i.d. challenge with L. infantum chagasi and SGH (12 hamsters/group). (D) Anti-Leishmania antibodies detected by ELISA in hamsters immunized with LJM19 and CTL DNA 2 and 5 months after i.d. challenge with L. infantum chagasi and SGH (six hamsters/group). *, P < 0.05. Data representative of three independent experiments with the exception of (C) where a fourth experiment was carried out to follow the survival of LJM19-immunized hamsters.
Expression of Cytokines in the Spleen and Liver of Protected LJM19-Immunized Hamsters After Challenge with L. infantum chagasi Plus SGH.
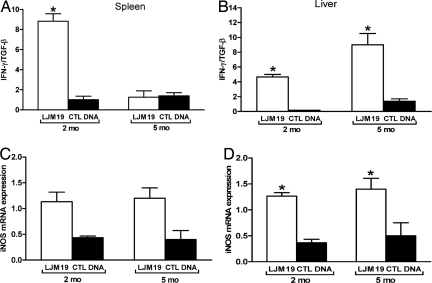
Expression of IFN-γ, IL-4, and TGF-β mRNA in the spleen and liver of LJM19-immunized hamsters was evaluated at 2 and 5 months postinfection. In the spleen, the ratio of IFN-γ/TGF-β expression levels was significantly higher in LJM19-immunized hamsters compared with control DNA-immunized hamsters 2 months postinfection (Fig. 4A). IL-4 expression was only detectable at 5 months postinfection but there was no difference observed in the IFN-γ/IL-4 ratio in LJM19 and control DNA-immunized hamsters (data not shown). In the liver, there was a consistently higher ratio of IFN-γ/TGF-β expression in LJM19-immunized hamsters compared with control DNA-immunized hamsters at 2 and 5 months (Fig. 4B). Moreover, there was a considerable increase in the ratio of IFN-γ/TGF-β expression from 2–5 months postinfection in the LJM19-immunized animals (Fig. 4B).
Fig. 4.
Protection against the fatal outcome of VL correlates with hepatic and splenic IFN-γ and iNOS expression. (A and B) Ratio of IFN-γ/TGF-β mRNA in the spleen (A) and liver (B) of hamsters immunized with LJM19 and CTL DNA plasmids 2 and 5 months after i.d. challenge with 105 stationary-phase L. infantum chagasi promastigotes in the presence of 0.5 pairs of SGH. (C and D) mRNA iNOS expression in the spleen (C) and liver (D) 2 and 5 months after i.d. challenge with L. infantum chagasi and SGH. All cytokine expression levels were normalized to HPRT mRNA expression levels. *, P < 0.05 (six animals per group).
It has been established that Leishmania killing by macrophages is mediated by NO (22, 23). As predicted, the expression of inducible NOS (iNOS) in the spleen and liver of LJM19-immunized hamsters was considerably higher at 2 and 5 months postinfection compared with control DNA-immunized hamsters (Fig. 4 C and D).
Immunity to LJM19 at the Site of Sand Fly Bites.
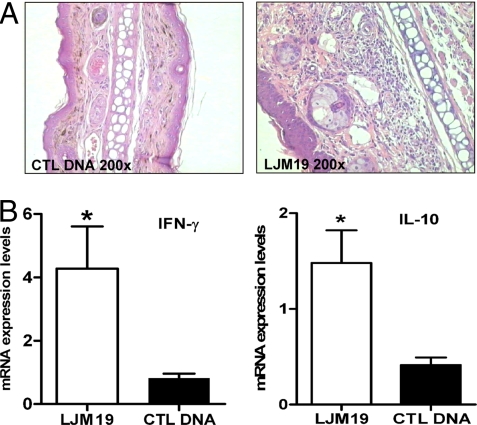
Having observed a strong T helper 1 (Th1) immunity associated with parasite killing and protection of LJM19-immunized hamsters, we investigated whether LJM19-immunized animals are able to mount a similar immune profile in the skin after sand fly bites. A DTH response characterized by mononuclear cell infiltration similar to that observed after the injection of SGH was detected in the skin of LJM19-immunized hamsters 48 h after sand fly bites (Fig. 5A). Moreover, at this time point a significantly higher level of IFN-γ and IL-10 expression was observed at the bite site in LJM19-immunized hamsters compared with control DNA-immunized hamsters (Fig. 5B). No difference was observed in IL-4 and TGF-β expression in these groups (data not shown).
Fig. 5.
Immune response in the ear tissue of hamsters immunized with LJM19 and CTL DNA after uninfected sand fly bites. (A) Cellular infiltration in representative ears of hamsters immunized with LJM19 or CTL DNA 48 h after challenge with uninfected sand fly bites in the contralateral ear (six animals per group, H&E staining). (B) IFN-γ and IL-10 mRNA expression in hamsters immunized with LJM19 and CTL DNA 48 h after uninfected sand fly bites. The data were normalized to HPRT expression. *, P < 0.05.
Discussion
Immune responses to sand fly salivary proteins have been repeatedly shown to protect mice against the cutaneous form of leishmaniasis caused by L. major (2, 12, 13) and L. amazonensis (26). To our knowledge, there are no studies pertaining to the potential role of sand fly salivary proteins in protection from the most aggressive and fatal form of this disease, VL. Furthermore, there are no animal models that incorporate sand fly saliva, and inherent component of natural transmission, to permit the evaluation of such vaccines.
In the present study, we report a model that mimics the natural course of VL infection in hamsters by the i.d. inoculation of 105 parasites in the presence of sand fly saliva. Parasites were detected in the spleen and liver of naïve hamsters at 2 and 5 months postinfection. These animals showed clinical signs similar to those observed in symptomatic individuals with VL, including hepatosplenomegaly, cachexia, and hyperglobulinemia. Hamsters infected in the absence of sand fly SGH showed comparable disease progression, indicating that saliva has no exacerbative effect on the course of infection (Fig. 1).
A significant improvement of the hamster model with this approach was that adult hamsters (3–4 months old) reproducibly died of VL 5–6 months postinfection. To our knowledge, clinical symptoms of VL in hamsters have been observed only 10 months after i.d. inoculation of L. donovani in the abdomen (25). Moreover, it has been established that adult hamsters (3–4 months old) are less susceptible to VL infection (27, 28). For the purpose of this study it was important to establish disease in older hamsters to account for the vaccination schedule. In the current model, the rapid onset of clinical symptoms in older hamsters could be the result of the inoculation of parasites in a highly vascularized tissue (the ear) facilitating visceralization and establishment of disease. An additional factor in the success of the current model is the use of male hamsters (29). It was previously demonstrated that host gender has a significant influence on the clinical evolution and immunological response to Leishmania (Viannia) infection (29). Taken together, we were able to reliably and reproducibly bring about a rapid onset of progressive disease in 3- to 4-month-old hamsters.
In nature, an infected sand fly deposits saliva and parasites into the skin of the animal while feeding. The presence of saliva at the feeding site is a permanent feature of natural transmission by sand fly bite. To mimic this mode of transmission and permit us to test the hypothesis that immunity to sand fly salivary molecules protects against VL, we focused on the i.d. delivery of parasites into the ear of hamsters in the presence of vector saliva where the presence of sand fly saliva is required to induce an immune response against salivary antigens.
In recent years, massive cDNA sequencing, proteomic and bioinformatic efforts targeting sand fly salivary glands permitted the identification and isolation of the most abundant salivary proteins from the sand fly Lu. longipalpis (30). This process provided the opportunity to investigate whether the protection observed by P. papatasi saliva in CL (2, 12, 13) could be achieved against VL by using salivary molecules from a natural vector. A powerful protection was observed against the fatal outcome of infection with L. infantum chagasi in hamsters immunized with the plasmid coding for the Lu. longipalpis salivary protein LJM19. Interestingly, this was the only molecule that produced a DTH and no detectable antibodies after DNA immunization. This finding reinforces the importance of cellular immunity to sand fly salivary antigens for protection from leishmaniasis, including the visceral form of disease. Induction of humoral response by these molecules does not seem to be a prerequisite for protection. LJM19-immunized animals maintained a controlled and low parasite load in the spleen and liver, surviving up to 8 months when they were euthanized according to the Animal Care and Use Committee protocol (Fig. 3C). Up to this point LJM19-immunized hamsters showed no outward signs of disease. It is worth noting that these hamsters also maintained a low level of anti-Leishmania IgG antibodies compared with CTL DNA-immunized group (Fig. 3D). High anti-Leishmania IgG titers have been associated with active VL (31).
The protection from VL observed in LJM19-immunized hamsters was associated with a considerably higher IFN-γ/TGF-β ratio and iNOS expression in their spleen and liver compared with control DNA-immunized hamsters (Fig. 4). In the spleen, the level of TGF-β increased significantly at 5 months postinfection, whereas that of IFN-γ was sustained, accounting for the decrease in the IFN-γ/TGF-β ratio (Fig. 4A). The level of TGF-β probably increased to serve a protective function to counteract possible excessive immonopathology caused by sustained IFN-γ production. This result was not noted in the liver (Fig. 4B), supporting the observation that the liver and spleen show organ specific immunity reaching a fine balance between parasite clearance and parasite pathology (32). IFN-γ plays an important role in limiting the growth of Leishmania in murine and human macrophages and limiting leishmaniasis progression (21, 33). Moreover, NO generation through IFN-γ is the critical macrophage effector mechanism in the control of parasite replication in mice (34). Recently, it was reported that iNOS was produced by macrophages with concomitant high levels of NO production in protected hamsters vaccinated with a kinetoplastid membrane protein-11 (KMP-11) and challenged intracardially with Leishmania donovani (21).
The protective immunity observed in the spleen and liver of LJM19-immunized hamsters may be the consequence of an antisaliva immune response initiated in the skin of challenged animals. This idea is supported by the DTH response and the high expression of IFN-γ produced at the bite site of LJM19-immunized hamsters (Fig. 5). The presence of IL-10 together with IFN-γ 48 h after sand fly bites in LJM19-immunized hamsters could be a regulatory mechanism to control possible immunopathology caused by IFN-γ (35–37).
To explain the protection observed in LJM19-immunized hamsters against a visceral infection, we propose that the initial anti-LJM19 immune response at the site of parasite inoculation in the ear dermis has a dual effect: (i) it creates an inhospitable environment for the establishment of Leishmania infection that may involve direct killing of the parasite, and (ii) it primes the initial host immune response to Leishmania that could also have resulted in acceleration of anti-Leishmania immunity. We have recent evidence that immunity to a salivary protein primes the host toward a protective anti-Leishmania immune response. In a cutaneous model of infection, mice immunized with a salivary molecule from P. papatasi (PpSP15) primed the immune response toward a Th1 type anti-L. major immunity (38). We hypothesize that immunization with LJM19 produces similar results. Analysis of early time points in the skin at the site of challenge will elucidate the contribution of direct parasite killing versus the indirect effect on the acceleration of anti-Leishmania immunity.
In humans, exposure to Lu. longipalpis sand flies in an endemic area for VL was correlated with the appearance of anti-Leishmania DTH, indicative of protection against VL (39, 40). These studies, together with the observed protection in LJM19-immunized hamsters, suggest that immunity to certain sand fly salivary proteins could have practical implications for the protection of humans from visceral disease. Studies need to be undertaken to determine the salivary molecules that are immunogenic in humans. Based on observed host specificity of immune responses to salivary antigens, these are likely to differ from protective molecules identified with animal models.
In summary, we have demonstrated the ability of a defined salivary protein from Lu. longipalpis (LJM19) to confer powerful protection against the fatal outcome of L. infantum chagasi infection in a hamster model. These data reinforce the concept of using components of arthropod saliva in vaccination strategies against vector-borne diseases, including VL, and underscore the importance of the salivary molecules for the induction of immunity irrespective of any exacerbative role.
Materials and Methods
Animals.
Two-month-old male Syrian golden hamsters (Mesocricetus auratus) were obtained from the Centro de Pesquisas Gonçalo Moniz/Fundaçao Oswaldo Cruz (FIOCRUZ) animal facility and Taconic. The experimental procedures used in this study were reviewed and approved by the Animal Care and Use Committees of the Centro de Pesquisas Gonçalo Moniz (CPqGM)/FIOCRUZ and the National Institute of Allergy and Infectious Diseases (NIAID).
Sand Flies and Preparation of SGH.
Lu. longipalpis, Cavunge strain (captured at Cavunge in Bahia, northeastern Brazil), was reared in the laboratory as described (41) at the Laboratório de Imunoparasitologia/CPqGM and the Laboratory of Malaria and Vector Research, NIAID. Salivary glands were dissected from 5- to 7-day-old females and stored in PBS at −70°C. Before use, salivary glands were sonicated and centrifuged at 12,000 × g for 2 min. The supernatant was collected and used immediately.
Intradermal Challenge with Parasites Plus SGH.
L. infantum chagasi (MHOM/BR00/MER/ STRAIN2) promastigotes were cultured in Schneider's medium (Sigma) supplemented with 20% of inactivated FBS, 2 mM l-glutamine, 100 units/ml penicillin, 100 μl/ml streptomycin, and 2% sterile human urine. Three- to 4-month-old hamsters were inoculated i.d. with105 stationary phase promastigotes in the absence or presence of 0.5 pairs SGH by using a 29-gauge needle (BD Ultra-Fine) in a volume of 20 μl.
Construction of DNA Plasmids Coding for Lu. longipalpis Salivary Proteins and Immunization of Hamsters.
Sixteen DNA plasmids coding for Lu. longipalpis salivary proteins were cloned into the VR2001-TOPO vector and purified as described (8). Two-month-old hamsters were immunized i.d. in the right ear three times at 2-week intervals with 20 μg of DNA plasmid or with the equivalent of 0.5 salivary gland pairs in 20 μl of saline.
Parasite Burden.
For early time points parasite burden was determined by PCR. DNA was extracted from 300 μl of blood and 100 mg of spleen and liver tissue from infected and control hamsters by using the Wizard Genomic DNA purification kit (Promega) following the manufacturer's instructions. PCR was performed with primers 5′-GGG(G/T)AGGGGCGTTCT(G/C)CGAA-3′ and 5′- (G/C) (G/C) (G/C) (A/T)CTAT(A/T)TTACACCAACCCC-3′, which amplify a 120-bp conserved region of the Leishmania kDNA minicircle. Conditions were as follows: 94°C for 3 min, 30 cycles at 94°C for 30 s, 55°C at 30 s, and 94°C for 45 sec with a final extension of 72°C for 10 min. For later time points, the parasite burden was evaluated from the spleen and liver by using the quantitative limiting dilution assay as described (42).
Antibody Detection.
Total IgG responses to L. infantum chagasi or Lu longipalpis DNA plasmids were measured by ELISA as described (40, 43).
Ear Thickness and Histology.
Ear thickness was measured 48 h after i.d. injection of Lu. longipalpis SGH or sand fly bites by using a vernier caliper (Mitutoyo). For histology, the ears were fixed in 10% phosphate-buffered formalin and embedded in paraffin. Five-micrometer sections were stained with hematoxylin–eosin.
Cytokine Determination by Semiquantitative PCR and Real-Time PCR.
Total RNA was extracted from the spleen and liver of infected hamsters by using TRIzol reagent (Invitrogen). First-strand cDNA synthesis was performed with ≈1–2 μg of RNA by using a SuperScript II reverse transcriptase (Invitrogen). The reaction mixture was incubated at 42°C for 50 min. DNA was amplified by using TaqDNA polymerase (Invitrogen) in a PTC-100 thermal cycler (MJ Research). Reaction conditions were 40 cycles of 1 min at 94°C, 1 min at 55°C, and 2 min at 72°C, with a final extension step of 7 min at 72°C. The band intensity of the amplified products was analyzed by using EagleSight, version 3.2 software (Stratagene). The results are expressed as the ratio of cytokine over hypoxanthine phosphoribosyltransferase (HPRT). For isolation of RNA from ears, tissue was homogenized on a Magna lyser (Roche) with three cycles at 7,000 rpm, 60 s each. Total RNA was isolated by using QIAshreder (Qiagen) and RNeasy Mini Kit (Qiagen) following the manufacturer's instructions. First-strand cDNA synthesis was performed with ≈1–2 μg of RNA in a total volume of 20 μl by using SuperScript III reverse transcriptase. DNA was amplified by using the LightCycler 480 Probes Master kit (Roche). Amplification conditions consisted of an initial preincubation at 95°C for 10 min, followed by amplification of the target DNA for 40 cycles of 95°C for 15 s and 60°C for 1 min with the LightCycler 480 (Roche Diagnostics). A standard curve was generated for each set of primers, and efficiency of each reaction was determined. The expression levels of genes of interest were normalized to HPRT levels. The results are expressed in fold change over control.
Oligonucleotide Primers and Probes.
Oligonucleotide primers used for semiquantitative PCR were: HPRT, reverse, TGT TTC ACC AAC AAG TTT GCA ATC, forward, ATG GTA GAG ATG GGA GGC CAT CAC); IFN-γ, reverse, TCA AAT ATT GCT GGC AAG AAT ATT CTT, forward, ATG CAC ACC ACA CGT TGC ATC TTG; IL-4, reverse, TCA CAT TGC AGC TCT TCT GAG GAA3, forward, ACG GAG AA A GAC CTC ATT TGC AG; IL-10, reverse, TCA CAG GGG AGA AAT CGA TGA CA, forward, TGG ACA ACA TAC TAC TCA GTC ATC; iNOS, reverse, CTCGAYCTGGTAGTAGTAGAA, forward, GCAGAATGTGACCATCATGG; and TGF-β, reverse, CTT GGG CTT GCG ACC CAC GTA GTA, forward, TTC AGC TCC ACG GAG AAG AAC TGC. These primers were obtained from the National Cancer Institute/SAIC Research Technology Program and Operon Biotechnologies. Oligonucleotide primers used for real time PCR were: HPRT, reverse, GGG AGT GGA TCT ATC ACA ATT TCT, forward, CCA TCA CAT TAT GGC CCT CT; IFN-γ, reverse, CAG GTC TGC CTT GAT GGT G, forward, GAA GCC TTG AAG GAC AAC CA; TGF-β, reverse, TGG TTG TAG AGG GCA AGG AC, forward GGC CCT GTC CCT ACA TTT G; IL-10, reverse, TCC AGC TGG TCC TTC TTT TG, forward, ACA TGC TCC GAG AGC TGA G; IL-4, reverse, CGG TAC ATG CTA GAA GGC AGA, forward, GAG ATC TAT TGA TGG GTC TCA GG. Primers were obtained from Operon, and probes for IL-4, IL10, TGF-β, IFN-γ, and HPRT were from Roche Diagnostics.
Exposure of Immunized Hamsters to Sand Fly Bites.
Immunized hamsters were exposed to 15 uninfected Lu. longipalpis bites in the left ear according to Kamhawi et al. (12) and Valenzuela et al. (13).
Statistical Analysis.
Statistical tests were performed with Graph Pad 4.0 Prism software. One-way nonparametric ANOVA was performed followed by the Dunn posttest. Dual comparisons were made with the Mann–Whitney test.
Acknowledgments.
We thank Dr. Aldina Barral, Dr. Manoel Barral Netto, Dr. José M. C. Ribeiro, and Ryan Jochim for critical review of this manuscript; Dr. Robert Gwadz for continuous support; Sheila Dreher for construction of DNA plasmids; Edivaldo Passos for technical assistance; and Nancy Shulman for editorial assistance. This research was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Fundação de Amparo à Pesquisa do Estado da Bahia, Conselho Nacional de Pesquisas, and Comissao de Aperfeiçoamento de Pessoal de Nivel Superior. C.I.B. and C.I.d.O. are Senior Investigators of Conselho Nacional de Pesquisas. R.G. received a fellowship from Conselho Nacional de Pesquisas during his Ph.D. studies.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid Y, et al. Development of a natural model of cutaneous leishmaniasis: Powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers ME, Ilg T, Nikolaev AV, Ferguson MA, Bates PA. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature. 2004;430:463–467. doi: 10.1038/nature02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlab R, Valenzuela JG, Rowton ED, Ribeiro JM. Toward an understanding of the biochemical and pharmacological complexity of the saliva of a hematophagous sand fly, Lutzomyia longipalpis. Proc Natl Acad Sci USA. 1999;96:15155–15160. doi: 10.1073/pnas.96.26.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro JM, Rossignol PA, Spielman A. Blood-finding strategy of a capillary-feeding sand fly, Lutzomyia longipalpis. Comp Biochem Physiol A. 1986;83:683–686. doi: 10.1016/0300-9629(86)90709-7. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro JM, Vachereau A, Modi GB, Tesh RB. A novel vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. Science. 1989;243:212–214. doi: 10.1126/science.2783496. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro MC, et al. Effect of Lutzomyia longipalpis salivary gland extracts on leukocyte migration induced by Leishmania major. Am J Trop Med Hyg. 2007;76:88–94. [PubMed] [Google Scholar]
- 8.Oliveira F, et al. From transcriptome to immunome: Identification of DTH-inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 9.Rohousova I, Volf P. Sand fly saliva: Effects on host immune response and Leishmania transmission. Folia Parasitol (Praha) 2006;53:161–171. [PubMed] [Google Scholar]
- 10.Rohousova I, Ozensoy S, Ozbel Y, Volf P. Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology. 2005;130:493–499. doi: 10.1017/s003118200400681x. [DOI] [PubMed] [Google Scholar]
- 11.Silva F, et al. Inflammatory cell infiltration and high antibody production in BALB/c mice caused by natural exposure to Lutzomyia longipalpis bites. Am J Trop Med Hyg. 2005;72:94–98. [PubMed] [Google Scholar]
- 12.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 13.Valenzuela JG, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: Characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 15.Bell JF, Stewart SJ, Wikel SK. Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: Allergic klendusity. Am J Trop Med Hyg. 1979;28:876–880. [PubMed] [Google Scholar]
- 16.Nazario S, et al. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am J Trop Med Hyg. 1998;58:780–785. doi: 10.4269/ajtmh.1998.58.780. [DOI] [PubMed] [Google Scholar]
- 17.Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labuda M, et al. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2006;2:e27. doi: 10.1371/journal.ppat.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donovan MJ, et al. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect Immun. 2007;75:2523–2530. doi: 10.1128/IAI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsollier L, et al. Protection against Mycobacterium ulcerans lesion development by exposure to aquatic insect saliva. PLoS Med. 2007;4:e64. doi: 10.1371/journal.pmed.0040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu R, et al. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J Immunol. 2005;174:7160–7171. doi: 10.4049/jimmunol.174.11.7160. [DOI] [PubMed] [Google Scholar]
- 22.Bories C, et al. Lack of a nitric oxide response during the course of Leishmania infantum infection in the golden hamster (Mesocricetus auratus), with or without treatment with liposomal amphotericin B. Ann Trop Med Parasitol. 1998;92:685–692. doi: 10.1080/00034989859140. [DOI] [PubMed] [Google Scholar]
- 23.Melby PC, Yang YZ, Cheng J, Zhao W. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect Immun. 1998;66:18–27. doi: 10.1128/iai.66.1.18-27.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palatnik-de-Sousa CB, Paraguai-de-Souza E, Gomes EM, Borojevic R. Experimental murine Leishmania donovani infection: Immunoprotection by the fucose-mannose ligand (FML) Braz J Med Biol Res. 1994;27:547–551. [PubMed] [Google Scholar]
- 25.Wilson ME, Innes DJ, Sousa AD, Pearson RD. Early histopathology of experimental infection with Leishmania donovani in hamsters. J Parasitol. 1987;73:55–63. [PubMed] [Google Scholar]
- 26.Thiakaki M, et al. Sand fly specificity of saliva-mediated protective immunity in Leishmania amazonensis-BALB/c mouse model. Microbes Infect. 2005;7:760–766. doi: 10.1016/j.micinf.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Giannini MS. Effects of promastigote growth phase, frequency of subculture, and host age on promastigote-initiated infections with Leishmania donovani in the golden hamster. J Protozool. 1974;21:521–527. doi: 10.1111/j.1550-7408.1974.tb03692.x. [DOI] [PubMed] [Google Scholar]
- 28.Singh N, Samant M, Gupta SK, Kumar A, Dube A. Age-influenced population kinetics and immunological responses of Leishmania donovani in hamsters. Parasitol Res. 2007;101:919–924. doi: 10.1007/s00436-007-0562-3. [DOI] [PubMed] [Google Scholar]
- 29.Travi BL, et al. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect Immun. 2002;70:2288–2296. doi: 10.1128/IAI.70.5.2288-2296.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valenzuela JG, Garfield M, Rowton ED, Pham VM. Identification of the most abundant secreted proteins from the salivary glands of the sand fly Lutzomyia longipalpis, vector of Leishmania chagasi. J Exp Biol. 2004;207:3717–3729. doi: 10.1242/jeb.01185. [DOI] [PubMed] [Google Scholar]
- 31.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85:138–147. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho EM, et al. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J Infect Dis. 1992;165:535–540. doi: 10.1093/infdis/165.3.535. [DOI] [PubMed] [Google Scholar]
- 34.Murray HW, et al. Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J Immunol. 1992;148:1858–1863. [PubMed] [Google Scholar]
- 35.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4+CD25−Foxp3− Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jankovic D, et al. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nylen S, et al. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204:805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira F, Lawyer PG, Kamhawi S, Valenzuela JG. Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response toward protection or exacerbation of disease. PLoS Negl Trop Dis. 2008;2:e226. doi: 10.1371/journal.pntd.0000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barral A, et al. Human immune response to sand fly salivary gland antigens: A useful epidemiological marker? Am J Trop Med Hyg. 2000;62:740–745. doi: 10.4269/ajtmh.2000.62.740. [DOI] [PubMed] [Google Scholar]
- 40.Gomes RB, et al. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis. 2002;186:1530–1534. doi: 10.1086/344733. [DOI] [PubMed] [Google Scholar]
- 41.Modi GB, Tesh RB. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. J Med Entomol. 1983;20:568–569. doi: 10.1093/jmedent/20.5.568. [DOI] [PubMed] [Google Scholar]
- 42.Lima HC, Bleyenberg JA, Titus RG. A simple method for quantifying Leishmania in tissues of infected animals. Parasitol Today. 1997;13:80–82. doi: 10.1016/s0169-4758(96)40010-2. [DOI] [PubMed] [Google Scholar]
- 43.Melby PC, Chandrasekar B, Zhao W, Coe JE. The hamster as a model of human visceral leishmaniasis: Progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol. 2001;166:1912–1920. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]