Abstract
EET between the two circular bacteriochlorophyll compartments B800 and B850 in native (containing the carotenoid rhodopin) and carotenoidless LH2 isolated from the photosynthetic purple sulfur bacterium Allochromatium minutissimum was investigated by femtosecond time-resolved transient absorption spectroscopy. Both samples were excited with 120-fs laser pulses at 800 nm, and spectral evolution was followed in the 720–955 nm range at different delay times. No dependence of transient absorption in the B800 band on the presence of the carotenoid rhodopin was found. Together with the likewise virtually unchanged absorption spectra in the bacteriochlorophyll Qy region, these observations suggest that absence of rhodopin does not significantly alter the structure of the pigment-protein complex including interactions between bacteriochlorophylls. Apparently, rhodopin does also not accelerate B800 to B850 EET in LH2, contrary to what has been suggested previously. Moreover, “carotenoid-catalyzed internal conversion” can also be excluded for the bacteriochlorophylls in LH2 of A. minutissimum. Together with previous results obtained with two-photon fluorescence excitation spectroscopy, it can also be concluded that there is neither EET from rhodopin to B800 nor (back-)EET from B800 to rhodopin.
INTRODUCTION
Carotenoids play important roles in photosynthetic organisms: 1), as usually indispensable integral structural components of pigment-protein complexes, 2), as accessory light-harvesting pigments in spectral regions where BChl absorption is low, and 3), in protection against potentially harmful BChl singlet and derived triplet excited states as well as reactive oxygen species. Moreover, recently, Scholes and Fleming (1) have suggested that the bound carotenoids may enhance EET between the two circular BChl compartments, BChl B800 and B850 in LH2. Additionally, it has been proposed by Razi Naqvi that carotenoids may induce enhanced relaxation of the first excited singlet state (S1) of BChls in photosynthetic light-harvesting antenna complexes by “carotenoid-catalyzed internal conversion” (2).
Absorption of carotenoids in the visible region is the result of an electronic transition from the ground state, 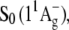 to a higher excited singlet state usually denoted
to a higher excited singlet state usually denoted 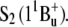 The lowest-lying excited singlet state,
The lowest-lying excited singlet state, 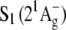 is optically “dark”; i.e., its radiative combination with S0 is forbidden by parity (e.g., g ←//→ g) and pseudoparity (e.g., −←//→−) selection rules analogous to C2h symmetric all-trans polyenes (3–7).
is optically “dark”; i.e., its radiative combination with S0 is forbidden by parity (e.g., g ←//→ g) and pseudoparity (e.g., −←//→−) selection rules analogous to C2h symmetric all-trans polyenes (3–7).
After excitation to the carotenoid S2 state, rapid internal conversion to S1 occurs (within 100–200 fs) in solution as well as in pigment-protein complexes (8,9). Thus, to account for the observed efficient EET from carotenoids to (B)Chls (4), a significant proportion of the excitation energy has to be assumed to flow through the carotenoid S1 state (10). Moreover, the carotenoid S1 state has also been invoked to be involved in a possible reverse (B)Chl-to-carotenoid EET. The latter has been proposed as a possible mechanism underlying photoprotective quenching of excess excitation energy input (11).
The issues related to EET between carotenoids and (B)Chls, in particular when dark states of the former are involved, have become accessible only recently as a result of advances in (laser) spectroscopy, as reviewed in (3–6).
To understand the role(s) of the carotenoid S1-to-(B)Chl EET channel (in both directions), knowledge of the energetic location of the optically “dark” S1 state of the respective carotenoids is required. It is generally accepted that carotenoid S1 energies correlate with the extension of the conjugated C=C bond system, but the precise locations remain uncertain (6,7). Considerable effort has been devoted to determine the S1 energies of relevant carotenoids (for a recent discussion, see Polivka and Sundström (6)). However, consistent results obtained with carotenoids, in particular with those bound to photosynthetic pigment-protein complexes, are still very scarce or controversial (6).
Because of the important roles of carotenoids for stable assembly and maintainance of the respective pigment-protein complexes, the isolation of structurally intact complexes retaining native spectral features from photosynthetic organisms that are devoid of carotenoids is usually precluded (12). On removal of the native carotenoid by mutation, carotenoid biosynthesis inhibitor treatment, or extraction, LH2 complexes from purple bacteria generally lose the characteristic B800 absorption (12), indicating a concomitant loss of the monomeric B800 BChls. The photosynthetic purple sulfur bacterium Allochromatium minutissimum (previously denoted Chromatium minutissimum), however, allows isolation of stable carotenoidless peripheral LH2 with apparently native structure and spectral features from cells in which the native carotenoids (essentially rhodopin, a carotenoid with 11 conjugated double bonds) are absent because of inhibition of carotenoid biosynthesis (13–15).
In the following, a femtosecond time-resolved TA study with native and carotenoidless LH2 from A. minutissimum is described. The results clearly demonstrate that the structure of LH2 from A. minutissimum is not significantly disturbed in the absence of carotenoids. Moreover, similarity of TA spectra and kinetics for both native and carotenoidless complexes indicate that EET from B800 to B850 is not affected by the lack of carotenoids and that there is neither EET from rhodopin to B800 nor back-EET from B800 to rhodopin.
MATERIALS AND METHODS
Sample preparation
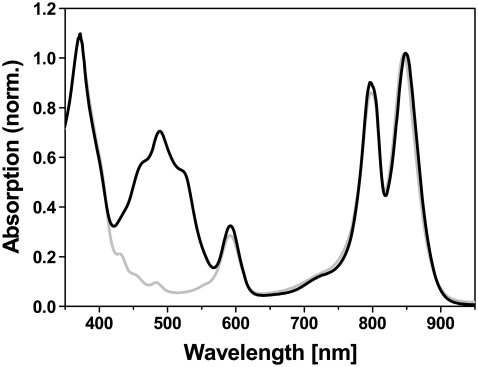
Cells of A. minutissimum (strain MSU) were grown for 3–4 days at 30°C in a luminostate. To obtain carotenoidless cells, 0.7 μM diphenylamine was added to the growth medium (13). LH2 was purified by preparative polyacrylamide gel electrophoresis using a home-made apparatus as previously described (14). The carotenoid complement in native LH2 is composed of mainly rhodopin (80.0%), with additional 9.5% spirilloxanthin, 5.2% anhydrorhodovibrin, 2.9% lycopene, and 2.4% dimethylspirilloxanthin (13). LH2 from diphenylamine-treated cells contained only trace amounts of mature (colored) carotenoids (Fig. 1). Absorption spectra were recorded using a Lambda-19 spectrometer (Perkin-Elmer, Norwalk, CT).
FIGURE 1.
Absorption spectra of native (solid) and carotenoidless LH2 (shaded) from A. minutissimum. The spectra are normalized to unity at the long-wavelength maximum (850 nm).
TA measurements
TA measurements were carried out using a home-made femtosecond spectrometer with a time resolution of ∼300 fs. The principal features of the setup are described elsewhere (16). A diode-pumped frequency-doubled Nd:YVO-laser (Millenia, Spectra Physics, Mountain View, CA) is used for pumping a Ti:Sa-Oscillator (Tsunami, Spectra Physics) which produces femtosecond pulses at 800 nm. The pulse energy is amplified from some nanoJoules to ∼1 mJ with a pulse duration of 120 fs FWHM) using a regenerative amplifier system (Spitfire, Spectra Physics). The output of the amplifier system is split: ∼1% of the intensity with a spectral width of ∼12 nm (FWHM) and a repetition rate of 1 kHz is used as pump pulses. After passing a variable translation stage (minimum step 0.5 μm), the pulses are directed to the sample in the pump-probe apparatus. Pulse energies were varied between 0.4 and 3.5 μJ. During the experiments, the pulse duration of 120 fs is continuously controlled with a home-built online frequency-resolved optical gating (FROG) system (17).
The main output of the amplifier is used for pumping a white-light-seeded, two-stage collinear optical parametric amplifier (OPA 800F, Spectra Physics) to generate laser pulses at a center wavelength of 1200 nm with pulse energies of 80 μJ. The beam is focused on a rotating 5-mm optical pathway cuvette containing D2O to generate a nearly flat (between 700 nm and 950 nm) white light continuum used for probing the absorption changes in the sample.
The white light beam overlaps with the pump beam at an angle of 5° in the center of a rotating fused silica cuvette (2 mm optical path length) so that at a repetition rate of 1 kHz, the irradiated sample volume is exchanged after each pulse. The polarization difference between the beams is set to the magic angle of 54.7° to minimize polarization effects. A small fraction of the white light is split off in front of the sample and used as reference beam. The transmitted probe beam and the reference beam are collected with a charge-coupled device camera (CCD576-TE, Princton Instruments, Trenton, NJ) with 16-bit dynamic range mounted on a polychromator (McPherson, Chelmsford, MA). This allows the simultaneous detection of absorption changes in a 190-nm section with a spectral resolution of ∼0.5 nm. Data from 36,000 laser shots per time delay step between pump and probe pulse in a time window from 0 to 200 ps were accumulated, and transient absorption changes were calculated with a resolution of ΔA > 8 × 10−4. No sample degradation was observed during the experiments.
RESULTS AND DISCUSSION
The steady-state absorption spectra of native and carotenoid-less LH2 from A. minutissimum are shown in Fig. 1. Obviously, except for the region where carotenoids absorb in the native complex (∼400–550 nm), the spectra are essentially identical. In particular, the characteristic B800 band is conserved. This observation clearly indicates that there are no significant perturbations of the structure in the carotenoid-less complex. In particular, the intricate BChl-BChl interactions giving rise to the characteristic B800 and B850 bands in the BChl-Qy-region are retained. When the native carotenoid is removed, LH2 complexes from other purple bacteria usually lose the B800 compartment (12), indicating a disturbed arrangement of the pigments.
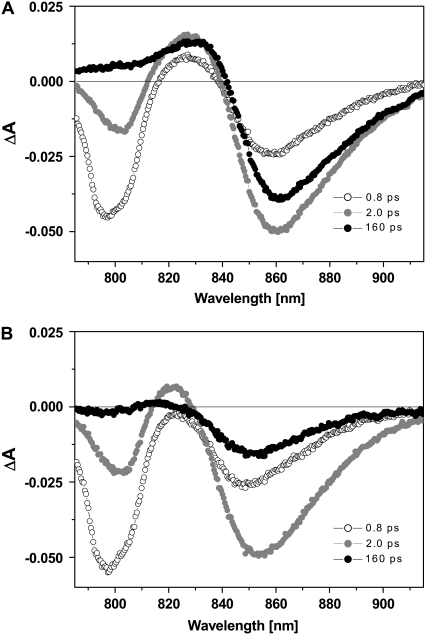
TA spectra of native and carotenoidless LH2 were obtained at room temperature on excitation at 800 nm. Three selected TA spectra of native (A) and carotenoidless LH2 (B) recorded at different delay times are shown in Fig. 2. Under these experimental conditions B800 is preferentially excited. The spectrum at 0.8 ps delay between the pump and probe pulses (Fig. 2, open symbols) indicates that the state formed on excitation exhibits a bleaching with a maximum at 797 nm. Additional bleaching at longer wavelengths (with a maximum at 852 nm) in the same time domain may originate either from direct excitation of the B850 subcomplex, because of the relatively broad laser pulses (12 nm FWHM) or, alternatively, may be caused by a very fast B800-to-B850 EET process. Additionally, there is also a weak absorption of the B850 BChls in the 800-nm region as a result of an upper excitonic band. Bleaching in the region of the B850 band increases up to a delay of ∼2.5 ps, whereas the B800 bleaching concomitantly decreases (Fig. 2, shaded symbols). This behavior is indicative of fast EET from B800 to B850. The TA spectra exhibit a positive feature between 815 nm and 830 nm (with a maximum at 821 nm) at ∼2 ps after excitation. The positive signal is characterized by similar rise kinetics as the bleaching of the B850 band. Hence, this feature can be assigned to an excited state absorption of BChls a in the B850 subcomplex (18).
FIGURE 2.
Transient absorption spectra of native (A) and carotenoidless LH2 (B) from A. minutissimum at three selected delay times between pump and probe pulse (0.8, 2.0, and 160 ps) on excitation at 800 nm.
At longer delay times (up to the limits of the time window of ∼200 ps), no further spectral features occur, and the bleaching of the B800 band, excited state absorption as well as the B850 bleaching, decreases (see Fig. 2, solid symbols). The lack of residual bleaching of the B800 band at long delay times corroborates the above-mentioned notion of a very efficient EET from B800 onto the B850 ring, also in carotenoidless LH2.
TA spectra of both samples, carotenoid-less as well as the carotenoid-containing native LH2, were measured under the same experimental conditions and the results obtained at different delay times between pump and probe pulses were compared: No principal differences in the spectral characteristics of both - carotenoid-less as well as native LH2 - were found in the entire measuring range, except for a minor red shift of the bleaching of the B850 band (peaking at ∼860 nm) in the native sample.
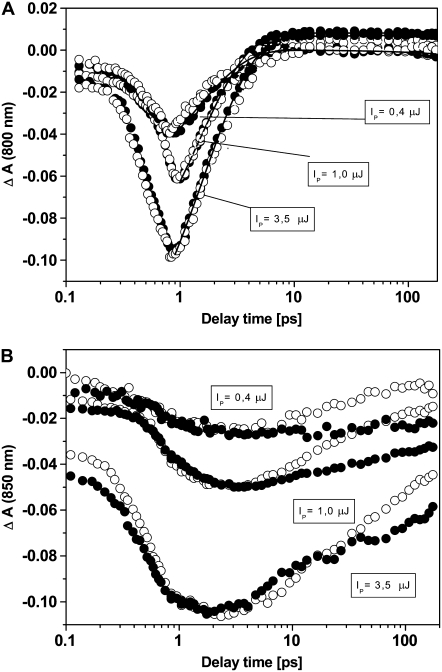
Time courses of B800 bleaching upon excitation with different excitation energies are shown for both samples in Fig. 3 A. Within the error limits, no significant differences can be discerned. The decay of the B800 bleaching as a function of the delay between pump and probe pulse can be described by a single-exponential decay with a time constant of 1.6 ps in all measurements performed.
FIGURE 3.
Transient absorption spectra of native (solid symbols) and carotenoidless (open symbols) LH2 from A. minutissimum obtained on 800-nm excitation with different excitation energies. (A) Time course of B800 bleaching. (B) Time course of B850 bleaching.
A similar time course was observed for the rise kinetics of the B850-bleaching in both carotenoidless and native LH2; it was in full agreement with the decay kinetics of the B800 subcomplex (Fig. 3 B). These observations are indicative of an (almost) identical B800-to-B850 EET in both samples. Previous modeling studies have suggested that carotenoids may accelerate B800-to-B850 EET in LH2 (1). Moreover, there were also experimental indications that the carotenoid (depending on the extension of the conjugated C=C double bond system) may influence the B800 to B850 EET rate in LH2 (19).
Marked differences, however, were observed in the decays of the B850 bleaching when native and carotenoidless LH2 were compared. Biexponential decay kinetics were observed for the decay of the B850 bleaching in all samples examined and were strongly dependent on excitation energy. In addition to a nonresolvable nanosecond-scale decay, the B850 bleaching shows fast biexponential decay kinetics with the time constants τ1 = 6 ± 2 ps and τ2 = 30 ± 4 ps. The relative proportion of the faster component of the picosecond-scale decay kinetics increases strongly at higher pump intensites. Therefore, we ascribe the fast decay mainly to singlet-singlet annihilation, i.e., the case that several singlet excitations are created simultaneously in an antenna domain.
A striking observation is that the decay of the B850 bleaching is apparently slower for native LH2 than for the carotenoidless complex under the same excitation conditions. This observation indicates that carotenoid-catalyzed internal conversion as proposed previously by Razi Naqvi (2) can be excluded for BChls in native LH2 of A. minutissimum.
The observed faster decay of the B850 bleaching in carotenoidless LH2, however, may be explained as being caused by the accumulation of BChl triplet excited states: BChl triplets that have been generated by intersystem crossing may well quench BChl singlet states via singlet-triplet annihilation and, thus, may considerably shorten BChl singlet excited state lifetimes in carotenoidless LH2. These BChl triplets would have been rapidly quenched by the nearby carotenoids in native LH2.
Apparently, the influence of rhodopin on TA in the BChl absorption region is negligibly small in almost the entire measuring range.
Moreover, if previous results obtained with two-photon fluorescence excitation spectroscopy (15) are also taken into account, it can be concluded that no EET from rhodopin onto the energetically nearby BChl-B800 level occurs. Back-EET from B800 onto rhodopin (which might have a photoprotective function in other antenna complexes) can also be excluded.
Acknowledgments
This work has been supported by the Deutsche Forschungsgemeinschaft (SFB 429, TPs A1 and A2) as well as by the joint Bundesministerium für Bildung und Forschung project RUS 99/174 and by grants from the Russian Foundation for Basic Research (06-04-49072 and 06-04-48516) to A.P.R. and A.A.M.
Abbreviations used: EET, excitation energy transfer; B800, B850, bacteriochlorophyll absorption bands at 800 and 850 nm; BChl, Chl, (bacterio)chlorophyll; LH2, purple bacterial peripheral light-harvesting complex; FWHM, full width at half-maximum; TA, transient absorption.
Editor: Brian R. Dyer.
References
- 1.Scholes, G. D., and G. R. Fleming. 2000. On the mechanism of light harvesting in photosynthetic purple bacteria: B800 to B850 energy transfer. J. Phys. Chem. B. 104:1854–1868. [Google Scholar]
- 2.Razi Naqvi, K. 1998. Carotenoid-induced electronic relaxation of the first excited state of antenna chlorophylls. In Photosynthesis: Mechanisms and Effects, Vol. 1. G. Garab, editor. Kluwer Academic Publisher, Dordrecht. 265–270.
- 3.Frank, H. A. 2001. Spectroscopic studies of the low-lying singlet excited states and photochemical properties of carotenoids. Arch. Biochem. Biophys. 385:53–60. [DOI] [PubMed] [Google Scholar]
- 4.Ritz, T., A. Damjanovic, K. Schulten, J.-P. Zhang, and Y. Koyama. 2000. Efficient light harvesting through carotenoids. Photosynth. Res. 66:125–144. [DOI] [PubMed] [Google Scholar]
- 5.Fraser, N. J., H. Hashimoto, and R. J. Cogdell. 2001. Carotenoids and bacterial photosynthesis: The story so far.. Photosynth. Res. 70:249–256. [DOI] [PubMed] [Google Scholar]
- 6.Polivka, T., and V. Sundström. 2004. Ultrafast dynamics of carotenoid excited states from solution to natural and artificial systems. Chem. Rev. 104:2021–2071. [DOI] [PubMed] [Google Scholar]
- 7.Tavan, P., and K. Schulten. 1987. Electronic excitations in finite and infinite polyenes. Phys. Rev. B. 36:4337–4358. [DOI] [PubMed] [Google Scholar]
- 8.Macpherson, A. N., J. B. Arellano, N. J. Fraser, R. J. Cogdell, and T. Gillbro. 2001. Efficient energy transfer from the carotenoid S2 state in a photosynthetic light-harvesting complex. Biophys. J. 80:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gradinaru, C. C., J. T. M. Kennis, E. Papagiannakis, I. H. M. van Stokkum, R. J. Cogdell, G. R. Fleming, R. A. Niederman, and R. van Grondelle. 2001. An unusual pathway of excitation energy deactivation in carotenoids: Singlet-to-triplet conversion on an ultrafast timescale in a photosynthetic antenna. Proc. Natl. Acad. Sci. USA. 98:2364–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krueger, B. P., J. Yom, P. J. Walla, and G. R. Fleming. 1999. Observation of the S1 state in LH2 by two-photon fluorescence excitation. Chem. Phys. Lett. 310:57–64. [Google Scholar]
- 11.Frank, H. A., A. Cua, V. Chynwat, A. Young, D. Gosztola, and M. R. Wasielewski. 1994. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth. Res. 41:389–395. [DOI] [PubMed] [Google Scholar]
- 12.Limantara, L., R. Fujii, J. P. Zhang, T. Kakuno, H. Hara, A. Kawamori, T. Yagura, R. J. Cogdell, and Y. Koyama. 1998. Generation of triplet and cation-radical bacteriochlorophyll a in carotenoidless LH1 and LH2 antenna complexes from Rhodobacter sphaeroides. Biochemistry. 37:17469–17486. [DOI] [PubMed] [Google Scholar]
- 13.Moskalenko, A. A., G. Britton, A. Connor, A. Young, and O. A. Toropygina. 1991. Composition of carotenoids in chromatophores and pigment-protein complexes isolated from Chromatium minutissimum. Biol. Membrany (Russ.). 8:249–260. [Google Scholar]
- 14.Moskalenko, A. A., and Y. E. Erokhin. 1974. Isolation of pigment-lipoprotein complexes from purple photosynthesizing bacteria by preparative electrophoresis in polyacrylamide-gel. Microbiologia (Russ.). 43:162–171. [PubMed] [Google Scholar]
- 15.Krikunova, M., A. Kummrow, B. Voigt, M. Rini, H. Lokstein, A. Moskalenko, H. Scheer, A. Razjivin, and D. Leupold. 2002. Fluorescence of native and carotenoid-depleted LH2 from Chromatium minutissimum, originating from simultaneous two-photon absorption in the spectral range of the presumed (optically ‘dark’) S1 state of carotenoids. FEBS Lett. 528:227–229. [DOI] [PubMed] [Google Scholar]
- 16.Theiss, C., I. Trostmann, S. Andree, F. J. Schmitt, T. Renger, H. J. Eichler, H. Paulsen, and G. Renger. 2007. Pigment-pigment and pigment-protein interactions in recombinant water-soluble chlorophyll proteins (WSCP) from cauliflower. J. Phys. Chem. B. 111:13325–13335. [DOI] [PubMed] [Google Scholar]
- 17.O'Shea, P., M. Kimmel, X. Gu, and R. Trebino. 2001. Highly simplified device for ultrashort pulse measurement. Opt. Lett. 26:932–934. [DOI] [PubMed] [Google Scholar]
- 18.Leupold, D., H. Stiel, K. Teuchner, F. Nowak, W. Sandner, B. Ücker, and H. Scheer. 1996. Size enhancement of transition dipoles to one- and two-exciton bands in a photosynthetic antenna. Phys. Rev. Lett. 77:4675–4678. [DOI] [PubMed] [Google Scholar]
- 19.Liu, W., Y. Liu, L. Guo, C. Xu, and S. Qian. 2006. The mutation of carotenoids affects the energy transfer in LH2 light harvesting complexes from Rhodobacter sphaeroides 601 at room temperature. J. Lumin. 119–120:350–355. [Google Scholar]