Abstract
Modular proteins such as titin, fibronectin, and cadherin are ubiquitous components of living cells. Often involved in signaling and mechanical processes, their architecture is characterized by domains containing a variable number of heterogeneous “repeats” arranged in series, with either flexible or rigid linker regions that determine their elasticity. Cadherin repeats arranged in series are unique in that linker regions also feature calcium-binding motifs. While it is well known that the extracellular repeats of cadherin proteins mediate cell-cell adhesion in a calcium-dependent manner, the molecular mechanisms behind the influence of calcium in adhesion dynamics and cadherin's mechanical response are not well understood. Here we show, using molecular dynamics simulations, how calcium ions control the structural integrity of cadherin's linker regions, thereby affecting cadherin's equilibrium dynamics, the availability of key residues involved in cell-cell adhesion, and cadherin's mechanical response. The all-atom, multi-nanosecond molecular dynamics simulations involved the entire C-cadherin extracellular domain solvated in water (a 345,000 atom system). Equilibrium simulations show that the extracellular domain maintains its crystal conformation (elongated and slightly curved) when calcium ions are present. In the absence of calcium ions, however, it assumes a disordered conformation. The conserved residue Trp2, which is thought to insert itself into a hydrophobic pocket of another cadherin molecule (thereby providing the basis for cell-cell adhesion), switches conformation from exposed to intermittently buried upon removal of calcium ions. Furthermore, the overall mechanical response of C-cadherin's extracellular domain is characterized at low force by changes in shape (tertiary structure elasticity), and at high force by unraveling of secondary structure elements (secondary structure elasticity). This mechanical response is modulated by calcium ions at both low and high force, switching from a stiff, rod-like to a soft, spring-like behavior upon removal of ions. The simulations provide an unprecedented molecular view of calcium-mediated allostery in cadherins, also illustrating the general principles of linker-mediated elasticity of modular proteins relevant not only for cell-cell adhesion and sound transduction, but also muscle elasticity.
INTRODUCTION
Development of complex multicellular organs and tissues relies on selective and robust adhesion between cells (1–4). Cadherin proteins are responsible for calcium-mediated cell-cell adhesion and have been implicated in various biologically relevant processes related to tissue morphogenesis and maintenance of tissue integrity, such as neuronal connectivity or prevention of tumor cell propagation (3–8). Members of the cadherin family of proteins have also been suggested to form part of the mechanotransduction apparatus of the inner ear (9–12). Classical cadherins feature a cytoplasmic domain, a single transmembrane segment, and a long extracellular domain made of five, tandemly arranged, heterogeneous cadherin repeats (1,13–15). The repeats are labeled EC1–EC5, with EC1 being the most distant from the membrane (see Fig. 1 A).
FIGURE 1.
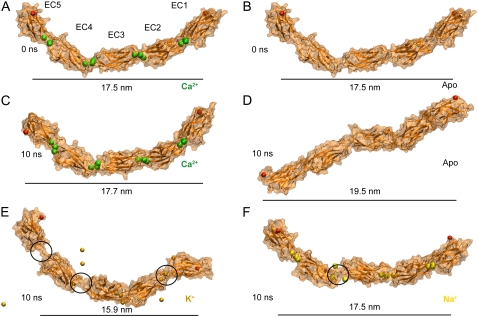
Influence of ions on C-cadherin equilibrium dynamics. (A and B) Models used as starting conformations in simulations of the complete C-cadherin extracellular domain with and without Ca2+ ions, respectively. The protein is shown in cartoon representation and its surface is drawn in transparent orange. Crystallographic Ca2+ and terminal Cα atoms are shown as green and red spheres. Water and bulk ions are not shown. (C–F) Snapshots of the complete extracellular domain of C-cadherin after 10 ns of equilibration in the presence of Ca2+ ions (SimCa1, C), in the absence of Ca2+ ions (SimApo1, D), with K+ (SimK1, E), and with Na+ ions (SimNa1, F), respectively. While C-cadherin remains curved when simulated in the presence of Ca2+, its shape is lost after 10 ns of dynamics in the absence of Ca2+. Both K+ and Na+ ions begin to unbind from C-cadherin during 10 ns of dynamics (black circles).
Selective adhesion is achieved through trans interactions between cadherin extracellular domains coming from two adjacent cells (8,15,16). In addition, cis interactions arising from dimerization of cadherin molecules that belong to the same cell have been suggested to be necessary for the formation of trans bonds (16–22). The interaction between cadherin extracellular domains likely involves one or more repeats from each cadherin molecule (8,23–29). Multiple studies using different experimental techniques such as mutagenesis, electron microscopy, force measurements, NMR, and x-ray crystallography have been used to postulate and probe different models of trans- and cis- interactions (see (8) for a recent review). In all cases it has been shown that Ca2+ and the EC1 repeat are both essential for at least the initial stages of trans-bond formation.
Calcium ions seem to stabilize and rigidify extracellular domains. Indeed, electron microscopy (EM) and other experiments have shown that the E-cadherin extracellular domain forms an elongated, semicurved rod in the presence of Ca2+, while it collapses upon Ca2+ removal (17,30–34). The crystal structure of a complete C-cadherin extracellular domain (35) depicts in atomic detail the elongated rod observed for E-cadherin with EM and also reveals all Ca2+ binding spots found in linker regions between repeats (Fig. 1 A). Biochemical assays, mutagenesis, and quantitative force and bead aggregation measurements have shown that disruption of some Ca2+ binding spots cooperatively influence large regions of the protein structure, often abolish adhesion, and affect the stability of individual repeats (36–38). Furthermore, molecular dynamics simulations of a single C-cadherin repeat's mechanical unfolding and of the EC1-EC2 E-cadherin equilibrium dynamics have confirmed the relevant role of Ca2+ in the stability of cadherin repeats (39–41).
While calcium ions provide rigidity, the EC1 repeat provides an anchor residue (Trp2) that may intramolecularly dock into a hydrophobic pocket of EC1, or insert itself into the same hydrophobic pocket but of a neighboring EC1 coming from an adjacent cell, thereby facilitating cell-cell adhesion (17,33,35,42–44). Antibody binding and mutagenesis combined with force measurements suggest that Ca2+ binding allosterically modulates the availability of Trp2 (38,45). However, a molecular dynamic view of how calcium ions control Trp2 availability and the flexibility of cadherin repeats is missing. Here we present molecular dynamics simulations that reveal how Ca2+ controls the elasticity and adhesive property of C-cadherin, switching the cadherin ectodomain's mechanical response from a stiff, rod-like regime to one that is soft and spring-like. Moreover, the simulations depict, for the first time at the atomic level, the Ca2+ modulation of Trp2 availability.
METHODS
Systems
The psfgen VMD (46) plug-in was utilized to build four systems containing the entire crystal structure of C-cadherin (Protein Data Bank code 1L3W) solvated in water with the VMD solvate plug-in. The first system with a total of 345,467 atoms included 12 crystallographically resolved and protein-bound Ca2+ ions and eight bulk Na+ ions randomly placed for cell neutralization with the autoionize VMD plug-in. The second system encompassing 345,407 atoms did not include Ca2+ ions, but contained 32 bulk Na+ ions. The last two systems included 12 K+ or Na+ ions replacing crystallographically resolved Ca2+ ions, respectively. In addition, these two systems included 20 bulk Na+ ions and encompassed 345,443 atoms. In all systems, 39 crystallographic water molecules were kept as part of the model while n-acetylglucosamine residues were excluded. Disulfide bonds for cysteines 448–532 and 530–539 were explicitly modeled for all systems. Residues Asp, Glu, Lys, and Arg were assumed to be charged throughout the protein, while the protonation states of His residues were chosen favoring the formation of evident hydrogen bonds. Before solvation, the C-cadherin molecule was spatially aligned such that the vector joining the Cα atoms of the terminal residues was oriented along the x axis. The size of the resulting systems was ∼36.4 × 10.5 × 9.4 nm3.
Molecular dynamics simulations
All molecular dynamics simulations were performed using NAMD 2.6 (47), the CHARMM22 force field for proteins with the CMAP correction (48–50) (see validation using lysozyme (51,52) in Supplementary Materials, Data S1, Fig. S23), and the TIP3P model for water (53). The standard set of CHARMM parameters for ions was utilized in all simulations. Parameters for Ca2+ correspond to those obtained to reproduce their experimental free energy of hydration (54). Parameters for K+ and Na+ did not include the NBFIX correction, used in simulation of potassium ion channels to describe ion-backbone interactions more accurately, as C-cadherin Ca2+ binding sites involve ion-backbone as well as ion-to-side-chain interactions. A summary of all simulations carried out in our study totaling >150 ns is presented in Table 1.
TABLE 1.
Summary of simulations
| Label | tsim (ns) | Type | Ensemble | γ (ps−1) | Velocity (nm/ns) | Start |
|---|---|---|---|---|---|---|
| SimCa1 | 10.00 | EQ | NpT/NVE* | — | — | — |
| SimCa2 | 0.67 | PCV | NV | — | 5 × 2 | SimCa1 (5.0 ns) |
| SimCa2E | 1.07 | PCV | NV | — | 5 × 2 | SimCa2 |
| SimCa3 | 10.00 | REL | NVE | — | — | SimCa2 |
| SimCa4 | 4.04 | EQ | NpT | 5.0 | — | SimCa1 (1.1 ns) |
| SimCa5 | 1.70 | PCV | NpT | 5.0 | 5 × 2 | SimCa4 |
| SimCa6 | 2.15 | PCV | NpT | 0.1 | 5 × 2 | SimCa4 |
| SimCa7 | 0.50 | PCV | NV | — | 50 × 2 | SimCa1 (5.0 ns) |
| SimCa8 | 0.50 | PCV | NV | — | 25 × 2 | SimCa1 (5.0 ns) |
| SimCa9 | 14.96 | PCV | NV | — | 0.5 × 2 | SimCa1 (5.0 ns) |
| SimCa10 | 1.30 | PCV† | NV | — | 5 × 2 | SimCa1 (5.0 ns) |
| SimCa11 | 1.30 | PCV† | NV | — | 5 × 2 | SimCa1 (5.0 ns) |
| SimCa12 | 5.00 | PCL | NV | — | 10/13 nm/C | SimCa1 (5.0 ns) |
| SimCa13 | 5.00 | PCL | NV | — | 10/8 nm/C | SimCa1 (5.0 ns) |
| SimCa14 | 3.60 | PCL | NV | — | 10/15 nm/C | SimCa1 (5.0 ns) |
| SimCa15 | 2.90 | PCL | NV | — | 10/13 nm/N | SimCa1 (5.0 ns) |
| SimCa16 | 2.30 | PCL | NV | — | 10/14 nm/N | SimCa1 (5.0 ns) |
| SimCa17 | 3.10 | PCL | NV | — | 10/8 nm/N | SimCa1 (5.0 ns) |
| SimApo1 | 10.00 | EQ | NpT/NVE* | — | — | — |
| SimApo2 | 0.65 | PCV | NV | — | 5 × 2 | SimApo1 (5.0 ns) |
| SimApo2E | 1.05 | PCV | NV | — | 5 × 2 | SimApo2 |
| SimApo3 | 5.00 | REL | NVE | — | — | SimApo2 |
| SimApo4 | 9.34 | EQ | NpT | 5.0 | — | SimApo1 (1.1 ns) |
| SimApo5 | 14.61 | EQ | NpT | 0.1 | — | SimApo1 (1.1 ns) |
| SimApo6 | 2.00 | PCV | NpT | 5.0 | 5 × 2 | SimApo1 (5.0 ns) |
| SimApo7 | 2.10 | PCV | NpT | 0.1 | 5 × 2 | SimApo1 (5.0 ns) |
| SimApo8 | 12.10 | PCV | NV | — | 0.5 × 2 | SimApo1 (5.0 ns) |
| SimApo9 | 3.40 | PCL | NV | — | 10/8 nm/C | SimApo1 (5.0 ns) |
| SimK1 | 10.00 | EQ | NpT/NVE* | — | — | — |
| SimK2 | 5.00 | EQ | NpT/NVE* | — | — | — |
| SimNa1 | 10.00 | EQ | NpT/NVE* | — | — | — |
| SimNa2 | 1.77 | PCV | NV | — | 5 × 2 | SimNa1 (5.0 ns) |
Labels indicate the presence (Ca) or absence (Apo) of crystallographic Ca2+ ions in the system. Replacement of Ca2+ by Na+ or K+ is indicated by labels Na and K, respectively. EQ denotes equilibrium simulations, PCV denotes constant velocity SMD simulations, and REL denotes free dynamics simulations in the corresponding ensemble. PCL denotes constant velocity SMD simulations in which one end of the protein is held fixed while the other end of the protein (N- or C-terminus) is pulled until a predefined elongation has being achieved. Then, the steering atom is held in space and the protein is allowed to relax in a so-called length-clamp steering protocol (see Methods). Initial coordinates and velocities were obtained from the last frame of the simulations mentioned in the Start column. All SMD simulations were performed by attaching steering springs to Cα atoms of residues 1 and 540, unless otherwise stated.
These simulations consisted of 1000 steps of minimization, 100 ps of dynamics with the backbone of the protein restrained (k = 1 Kcal/mol/Å2), and the remaining time as free dynamics in the NpT (1 ns with γ = 5 ps−1) and NVE ensembles.
These SMD simulations were performed by pulling repeats one and three (SimCa10) or three and five (SimCa11) in opposite directions (see Methods).
A uniform integration time step of 1 fs was assumed for all types of interactions throughout all simulations. In all cases a cutoff of 12 Å (switching function starting at 10 Å) for van der Waals interactions was assumed, and the particle-mesh Ewald method was used to compute long-range electrostatic forces without cutoff (55). The density of grid points for particle-mesh Ewald was at least 1/Å3. Periodic boundary conditions were assumed in all cases.
Langevin dynamics was utilized to maintain a constant temperature of T = 300 K when indicated, with the damping coefficient set to 5 or 0.1 ps−1 for all heavy atoms (see Table 1). Constant-pressure simulations at 1 atm were conducted using a hybrid Nosé-Hoover-Langevin piston method with a decay period of 200 fs and a damping timescale of 50 fs.
Constant velocity stretching simulations were performed using the steered molecular dynamics method (SMD) and the NAMD Tcl Forces interface. The stretching direction was set along the x axis, which matched the vector connecting the Cα atoms of the N- and C-terminal residues when the systems were built. The SMD simulations were performed by attaching the 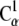 and
and 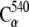 atoms of the N- and C-terminal residues to virtual (independent) springs of stiffness ks = 1 (kcal/mol)/Å2 each. The free ends of the mentioned virtual springs were then moved away from the protein at a constant velocity of v each in opposite directions. Different velocities were used throughout simulations (see Table 1). The force applied at each end was computed using the extension of the virtual springs.
atoms of the N- and C-terminal residues to virtual (independent) springs of stiffness ks = 1 (kcal/mol)/Å2 each. The free ends of the mentioned virtual springs were then moved away from the protein at a constant velocity of v each in opposite directions. Different velocities were used throughout simulations (see Table 1). The force applied at each end was computed using the extension of the virtual springs.
Two additional SMD protocols were used. In the first protocol, the center of mass of all Cα atoms of repeats EC1 and EC3 (SimCa10) or repeats EC3 and EC5 (SimCa11) were each attached to virtual springs of stiffness ks = 1 (kcal/mol)/Å2 each. The free ends of the springs were moved as in the standard SMD protocol described above, effectively stretching repeats EC2 and EC4 (see Data S1, Fig. S19).
The second SMD protocol (length clamp) consisted of two phases. First, a standard constant velocity SMD simulation is performed in which one end of the protein (Cα atom of C- or N-terminus) is held fixed while the other end of the protein (Cα atom of N- or C-terminus) is pulled by a virtual spring as described above. The second phase begins when a predetermined elongation has been achieved. Then, the free end of the virtual spring is held fixed in space and the protein is allowed to relax while the force applied on the spring by the protein is obtained by monitoring the extension of the virtual spring.
Analysis tools
Coordinates of all atoms of the system were saved every picosecond of simulation for later analysis. Overall structural deformation of the protein was monitored by computing root mean-square deviations (RMSD) over entire trajectories using VMD. The crystallographic structure served as the reference point, and positions of protein Cα atoms were compared. End-to-end distances were computed as the distance between the Cα atoms of the N- and C-terminal residues. Root mean-square fluctuations (RMSF) of Cα atom positions were computed using VMD and trajectories in which each cadherin repeat (EC1–EC5) was individually aligned to its crystal conformation. Alignment of structures for visualization purposes and for computation of RMSD or RMSF was performed by comparing positions of Cα atoms to their original positions in the crystal using the VMD Tcl interface. The solvent-accessible surface area for Trp2 was computed using VMD and assuming a probe radius of 0.14 nm.
The total energy ET of the system (reported as TOTAL3 in the NAMD output) was computed using the total potential energy U, the total kinetic energy Kc obtained from step-centered velocities (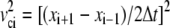 ), and the total kinetic energy Kh obtained from half-step velocities averaged (
), and the total kinetic energy Kh obtained from half-step velocities averaged (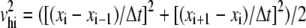 ). The final form of ET used was
). The final form of ET used was
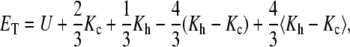 |
(1) |
where the first three terms correspond to a total energy that is numerically well behaved in long timescales, while the last two terms correct short-timescale fluctuations with a weighted exponential average (〈X〉new = 0.9375 〈X〉old + 0.0625 X).
Local deformations or strain (see Supplementary Material, Movie S1, movies mIII and mIV) were computed following a method similar to that described in Ortiz et al. (56). First, for each 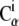 atom of the protein, a list of neighboring
atom of the protein, a list of neighboring 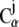 atoms (within 1 nm) was created using positions of the crystal conformation. Then, the local strain ui(t) was computed every time frame (t) for every
atoms (within 1 nm) was created using positions of the crystal conformation. Then, the local strain ui(t) was computed every time frame (t) for every 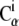 atom using
atom using
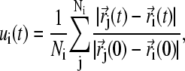 |
(2) |
where 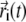 is the position of the Cα atom i at time t and Ni is the total number of neighboring
is the position of the Cα atom i at time t and Ni is the total number of neighboring 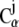 atoms for Cα atom i (determined as described above). The strain value ui(t) was then used to color each Cα atom.
atoms for Cα atom i (determined as described above). The strain value ui(t) was then used to color each Cα atom.
RESULTS
Molecular dynamics simulations were carried out using the complete extracellular domain of C-cadherin, a Xenopus cadherin involved in the processes that drive morphogenesis during amphibian gastrulation (57). The corresponding crystal structure (35), solved at 3.1 Å resolution (PDB code 1L3W), features five cadherin repeats, each made of ∼110 amino acids and folded in a Greek-key motif characterized by seven β-strands forming two β-sheets (13). Four sets of simulations of this structure were performed. The first set (labeled “SimCaX”) utilized a system containing the protein, water molecules, and 12 crystallographically resolved Ca2+ ions (three ions located at each linker region). The second set (labeled “SimApoX”) was performed without Ca2+ ions. The last two sets, labeled “SimKX” and “SimNaX”, involved systems in which crystallographically resolved Ca2+ ions were replaced by potassium (K+) and sodium (Na+) ions, respectively (Fig. 2). Further details of the systems and simulations can be found in Methods and Data S1.
FIGURE 2.
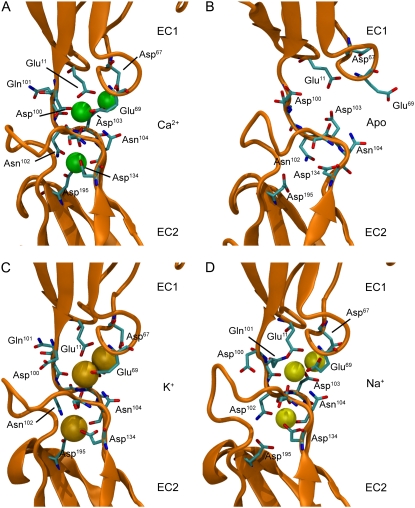
Linker region of C-cadherin repeats EC1-EC2. (A–D) Snapshots of the linker region between repeats EC1-EC2 of C-cadherin after 1.1 ns of simulation for systems with crystallographically resolved Ca2+, without Ca2+, with K+, and with Na+ ions, respectively. The protein is shown in orange cartoon representation and ions are shown as green (Ca2+), dark yellow (K+), and light yellow (Na+) spheres. Residues originally involved in Ca2+ binding are labeled and shown for all snapshots in licorice representation.
Influence of ions on C-cadherin equilibrium dynamics
Equilibrium simulations showed that the overall curved shape of the protein was maintained throughout 10 ns of dynamics in which Ca2+ ions were present in the model (SimCa1, Fig. 1 C), while the cadherin extracellular domain adopted a disordered shape when calcium ions were removed (SimApo1, Fig. 1 D). Indeed, the absence of Ca2+ resulted in strong repulsion between negatively charged residues (previously coordinating ions) and partial disruption of linker regions (Fig. 2 B). Complete disruption and unfolding of linker regions was prevented by performing an equilibration step in which 1000 steps of minimization and 100 ps of dynamics were carried out with backbone atoms harmonically restrained to their crystal conformation (as in all our initial equilibrations, see Methods and Table 1). The linker disruption, most clearly seen at the linker between EC3 and EC4, favored independent motion of individual repeats leading to the observed disordered shape (Fig. 3). The same behavior was reproduced in simulations that used different temperature control protocols (SimCa4, SimApo4, and SimApo5; Data S1, Figs. S8 and S9). Whether protonation states of charged amino acids change upon Ca2+ removal remains to be elucidated. However, our results are in agreement with experiments showing that linkers without calcium can destabilize cadherin repeats through electrostatic interactions (37). Additional simulations were performed in which Ca2+ ions were replaced by K+ or Na+ ions (SimK1 and SimNa1, respectively). The replacement of divalent ions by monovalent ions likely reduced artifacts introduced by simple elimination of Ca2+. These control simulations showed that although the extracellular domain of C-cadherin partially retains its curved conformation on a 10-ns timescale (Fig. 1, E and F), interrepeat motion was clearly enhanced and in some cases similar to that observed in the absence of Ca2+ (Fig. 3). Neither K+ nor Na+ ions seemed to maintain the rigidity of the C-cadherin extracellular domain. Furthermore, unbinding of both types of ions was observed throughout the simulations. Current limitations in the timescale of MD simulations and the accuracy of force fields would likely prevent a quantitative characterization of the cadherin binding sites' selectivity, especially when comparing more subtle effects arising from the use of two different types of divalent ions. Thus, we refrained from performing further simulations in which Ca2+ is replaced by another divalent ion (such as Mg2+).
FIGURE 3.
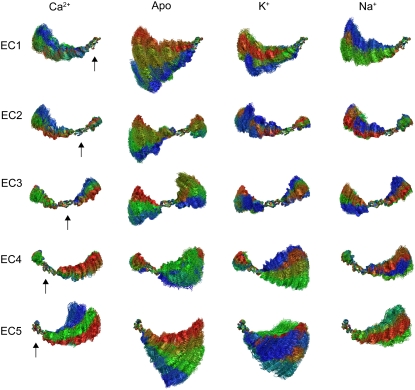
C-Cadherin equilibrium dynamics and influence of ions on interrepeat motion. Conformations of the extracellular domain of C-cadherin during simulations SimCa1, SimApo1, SimK1, and SimNa1 (lasting 10 ns each) were aligned so as to illustrate the relative motion of individual repeats with respect to their neighbors. Individual repeats (EC1–EC5 as indicated by arrows) were aligned throughout the trajectories using the crystal conformation as a reference. The rest of the protein was moved using the same matrix transformation required to align the corresponding repeat. The resulting aligned molecules are shown superimposed every 40 ps. Color indicates time, with red being early stages of the simulations and blue indicating the latest stages of the simulations. Interrepeat motion is readily observed in the absence of Ca2+ and when K+ replaces Ca2+. Even in the presence of Ca2+ the extracellular domain of C-cadherin is not completely rigid, as suggested by limited but significant interrepeat motion (particularly for repeat EC5).
Root mean-square deviations (RMSD), computed for individual repeats during SimCa1, SimApo1, SimK1, and SimNa1 (see Data S1, Fig. S10), reached stable values below 0.3 nm in all cases except one, indicating that individual repeats maintained their secondary structure on a nanosecond timescale even in the absence of Ca2+. Repeat EC5 exhibited large RMSD values (>0.3 nm) during a simulation in which Ca2+ was replaced by K+ (SimK1), likely reflecting deformations induced by unbinding of this large monovalent ion. RMSD values for repeats EC1 and EC2 were found to be consistently smaller than those for other repeats in simulations with Ca2+, K+, and Na+. The slight increase in RMSD observed when calcium was removed (SimApo1) suggests that EC1 and EC2 are the most sensitive to Ca2+ binding.
The outcome of our equilibrium simulations are in line with those presented by Cailliez and Lavery (40,41) (using a different force-field and simulation engine) for only two repeats of E-cadherin and agree well with previous experimental results indicating that Ca2+ rigidifies the extracellular cadherin domain (30–34). Although our simulations span a short timescale which precludes observation of a full collapse of cadherin repeats (as seen in electron microscopy and AFM images (30,58)) or possible unfolding, the simulations permitted us to clearly see interrepeat motion that could lead to a collapsed conformation in the absence of Ca2+ (Fig. 3, and see Data S1, Figs. S8 and S9).
Tertiary structure elasticity of C-cadherin
We further probed cadherin stability by using conformations from equilibrium simulations as the starting points for constant-velocity steered molecular dynamics simulations (59–63). The SMD simulations were performed on systems with and without Ca2+ ions and on a system in which Na+ replaced the crystallographically resolved Ca2+. Our SMD setup was similar to that used in Gräter et al. (64) as we attached both ends of the protein (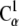 and
and 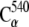 ) to virtual springs (ks =1 kcal/mol/Å2). The free ends of the springs moved in opposite directions at a constant velocity along the axis defined by the vector joining the protein termini. Different temperature control protocols and stretching velocities were utilized.
) to virtual springs (ks =1 kcal/mol/Å2). The free ends of the springs moved in opposite directions at a constant velocity along the axis defined by the vector joining the protein termini. Different temperature control protocols and stretching velocities were utilized.
The SMD simulations revealed that upon stretching, the complete C-cadherin extracellular domain becomes straight by rearranging the relative orientation of individual repeats with respect to each other (see Fig. 4, B and E). This so-called tertiary structure elasticity (TSE) demonstrated earlier in other protein systems (39,63,65) was observed here in SMD simulations of C-cadherin with and without Ca2+, and with Na+ replacing Ca2+. While the apo SMD simulation shows that the linkers between repeats extend fairly easily in this case, the simulation with Ca2+ reveals that these ions act as molecular bearings, such that linker regions behave as stiff hinges and the extracellular domain responds as one unit.
FIGURE 4.
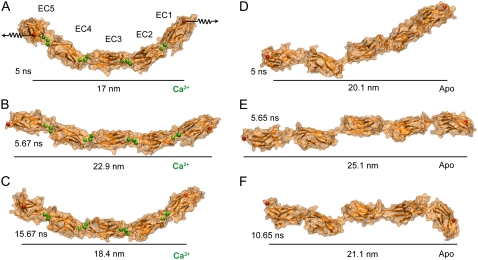
C-Cadherin tertiary structure elasticity. (A–C) Snapshots of the complete extracellular domain of C-cadherin simulated in the presence of Ca2+ ions after 5 ns of equilibration (SimCa1), after 0.67 ns of a constant velocity stretching (SimCa2), and after 10 ns of a subsequent relaxation (SimCa3), respectively. The protein is shown in cartoon representation and its surface is drawn in transparent orange. Crystallographic Ca2+ and termini Cα atoms are shown as green and red spheres, respectively. (D–F) Snapshots of the C-cadherin extracellular domain simulated in the absence of Ca2+ after 5 ns of equilibration (SimApo1), after 0.65 ns of constant velocity stretching (SimApo2), and after 5 ns of a subsequent relaxation (SimApo3), respectively. The extracellular domain of C-cadherin exhibits limited flexibility in the presence of Ca2+ and independent interrepeat mobility in the absence of Ca2+.
We ask then whether cadherin's TSE is reversible. We turned off the applied forces at the end of simulations SimCa2 and SimApo2, and continued with equilibrium dynamics in two simulations labeled SimCa3 and SimApo3 lasting 10 ns and 5 ns, respectively. In both cases the protein relaxed and the end-to-end distance partially recovered the value observed before stretching (see Fig. 4, C and F, and see Data S1, Fig. S11 A). However, while C-cadherin partially recovered curvature in the presence of Ca2+ (SimCa3, see Fig. 4 C), in the absence of Ca2+ (SimApo3) it relaxed into a conformation even more disordered than the one observed before stretching (disorder meaning that relative orientation of individual repeats was not uniform as seen in Fig. 4 F and in Movie S1, movies mI and mII; and Data S1, Fig. S12). Reversibility for the Apo simulation arises only in regard to end-to-end distance, as confirmed by the RMSD of the complete structure computed during stretching and relaxation (see Data S1, Fig. S11 B). The latter result suggests that TSE has different molecular origins in both cases. Interestingly, RMSD of individual repeats remained below 0.3 nm throughout the whole stretching and relaxation trajectories in the presence and the absence of calcium, confirming that linkers are responsible for the observed shape changes. Furthermore, partial recovery of C-cadherin's shape in the presence of divalent ions suggests that its curvature is not an artifact caused by crystallographic packing.
Local deformations of the C-cadherin structure were also monitored by computing the average strain per residue (see Methods and (56)) during equilibration, short stretching, and relaxation simulations in the presence and absence of Ca2+ (see Movie S1, movies mIII and mIV). In the presence of Ca2+, the largest strains were observed in loops of repeats EC3, EC4, and EC5 and the corresponding linker regions. In the absence of Ca2+, the largest strains were observed in all linker regions and loops, confirming once again the role of Ca2+ ions in interrepeat motion and TSE.
Tertiary structure elasticity was first predicted through simulations of the protein Ankyrin-R (9,39), and subsequently confirmed through AFM experiments involving Ankyrin-B (66). Both ankyrin proteins feature 24 repeat units but, unlike cadherin, ankyrin repeats interact with each other through extensive hydrophobic surfaces, the parallel stack of repeats forming a curved superhelical arrangement (67). The reversible, nonentropic TSE observed for ankyrin resembles better the one observed here for cadherin with calcium ions, while the TSE observed for cadherin without ions seems to be rather entropic in origin, although some residual interactions at the linkers may remain. Whether such difference in the origin of the observed TSE can be distinguished through experimental force spectroscopy or if it is relevant at all for adhesion are questions that remain to be answered. However, a simple calculation can set some limits for the elasticity in both cases. A freely jointed chain with n segments of length b each would exhibit an entropic spring constant of 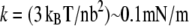 (lower limit of k for cadherin without Ca2+ at low force using n = 5 and b = 5 nm), while a straight rigid rod 23 nm (L) long and 2 nm (2r) wide would exhibit a longitudinal stiffness of
(lower limit of k for cadherin without Ca2+ at low force using n = 5 and b = 5 nm), while a straight rigid rod 23 nm (L) long and 2 nm (2r) wide would exhibit a longitudinal stiffness of 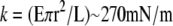 (upper limit of k for cadherin with Ca2+ using a Young's modulus for collagen of E = 2 GPa).
(upper limit of k for cadherin with Ca2+ using a Young's modulus for collagen of E = 2 GPa).
A rough estimate of the elastic constant of C-cadherin with and without Ca2+ was obtained using a novel SMD protocol termed “length-clamp” (SimCa13/SimCa17 and SimApo9; see Methods and Data S1, Fig. S13). The estimated spring constants from elastic forces (68), 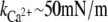 (400/8 pN/nm) and kApo ∼ 25 mN/m (200/8 pN/nm), are within the boundaries computed above. These simulations also reveal that the complete C-cadherin extracellular domain exhibits a viscoelastic behavior.
(400/8 pN/nm) and kApo ∼ 25 mN/m (200/8 pN/nm), are within the boundaries computed above. These simulations also reveal that the complete C-cadherin extracellular domain exhibits a viscoelastic behavior.
Secondary structure elasticity of C-cadherin
The simulations described above depict the extracellular domain of cadherin switching from a rodlike to a flexible-chain-like behavior upon removal of Ca2+ at low force regimes. While unfolding of individual domains may not play a physiological role in adhesion (44), we explored the effect of calcium and sodium on the stability of individual domains when subject to large forces inducing mechanical unfolding. Two SMD simulations (SimCa2 and SimApo2) were further continued until stretching forces unfolded one repeat (SimCa2E and SimApo2E) and eight new SMD simulations (SimCa5, SimCa6, SimCa7, SimCa8, SimCa9, SimApo6, SimApo7, and SimApo8) were performed using different steering velocities and thermodynamic ensemble protocols.
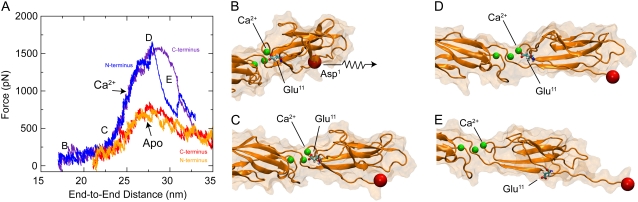
The SMD simulations showed what is perhaps the most dramatic difference between the elastic response of the protein with or without Ca2+. While the EC1 repeat unfolded fairly easily in the absence of Ca2+ (see Fig. 5 A), unfolding of EC1 in the presence of Ca2+ required a considerably larger force (1664 pN compared to 858 pN at v = 10 nm/ns) that breaks a bond between residue Glu11 and a Ca2+ ion (Fig. 5, B–E, and see Data S1, Figs. S14 and S15, and Movie S1and Movie S2, movies mV–mVII). Peak forces arising in a SMD simulation unfolding cadherin in which Ca2+ ions were replaced by Na+ (SimNa) were only slightly larger than those observed for the simulations performed in the absence of Ca2+, reinforcing the relevance of Ca2+ for the stability of C-cadherin (see Data S1, Fig. S16).
FIGURE 5.
C-Cadherin secondary structure elasticity. (A) Force versus end-to-end distance profile for constant-velocity stretching simulations with crystallographic Ca2+ ions (blue and indigo, SimCa2-SimCa2E) and without Ca2+ (red and orange, SimApo2-SimApo2E). (B–E) Snapshots of the unfolding pathway during simulation SimCa2E. Views show the EC1-linker-EC2 domain in cartoon representation with the corresponding surface drawn in transparent orange. The Cα atom of the N-terminus is shown as a red sphere and Ca2+ ions as green spheres. Residue Glu11 is shown in licorice representation. Disruption of secondary structure elements (secondary structure elasticity) proceeds only upon rupture of a bridge formed by Glu11 and a Ca2+ ion.
Additional simulations performed at different stretching speeds (SimCa7, SimCa8, SimCa9, and SimApo8 in Table 1) confirmed a similar unfolding scenario and a reduction in unfolding force peaks upon decrease of v, as expected (69,70) (see Data S1, Fig. S17). It remains to be elucidated whether the difference observed in peak forces for C-cadherin between simulations with and without Ca2+ is as dramatic at slow pulling speeds as it is at the speeds used in this study. Even the slowest stretching velocity used in our SMD simulations is large compared to those used in AFM or other experimental techniques (1 nm/ns vs. 10−5 nm/ns), since all-atom molecular dynamics simulations can usually achieve only a submicrosecond timescale. Despite this limitation, multiple SMD studies, all similarly exceeding experimental pulling speeds, have provided qualitative and quantitative predictions confirmed by experiments (39,63,71–77).
The SMD simulations mentioned above were performed in the NVE ensemble. Further simulations performed in the NpT ensemble (SimCa5, SimCa6, SimApo6, SimApo7) confirmed the Ca2+-dependent mechanical stability of the C-cadherin domain. However, even when using a small damping coefficient, Langevin dynamics (NpT) resulted in an artificial increase of unfolding peak forces and, in the worst case, complete decoupling of forces measured at opposite ends of the protein (see Data S1, Fig. S18).
Unfolding forces for EC1 in all cases are considerably smaller than the forces required to unfold a single EC2 repeat simulated under similar conditions but isolated from the rest of the structure (reported in our earlier SMD simulations (39)). The results are consistent with temperature- and denaturant-induced unfolding experiments of E-cadherin showing that Ca2+ stabilizes cadherin repeats and that EC1 is weaker than EC2 (36). However, the mechanical unfolding of the EC2 repeat within the entire C-cadherin extracellular domain may differ from that of the isolated repeat. We therefore probed the mechanical response of repeats EC2 and EC4 by steering the center-of-mass of repeats EC3/EC1 and repeats EC5/EC3 in opposite directions (effectively stretching repeats EC2 and EC4, see Data S1, Fig. S19 A). The simulations (SimCa10 and SimCa11) revealed that the EC4-EC5 linker is weaker than the EC1-EC2 linker, indicating a possible mechanical hierarchy for cadherin repeats/linkers. Interestingly, the unfolding force for repeat EC2 within the complete domain is comparable to that of the isolated repeat (see Data S1, Fig. S19 B), and both unfolding pathways exhibit an intermediate state in which the repeat is partially unfolded due to rupture of interactions between conserved residues and Ca2+ ions. Such a state may be particularly relevant under physiological conditions, as it provides a safety mechanism in which cadherin can extend in response to sustained mechanical stimuli and easily refold when the external force is turned off.
Previous SMD simulations identified two types of mechanical unfolding processes for immunoglobulin-like domains (78) in which water mediates rupture of hydrogen bonds concertedly (sheering mode) or one-by-one (zipper mode). In both types of unfolding, rupture of hydrogen bonds between β-strands (concertedly or one-by-one) gives rise to the unfolding force-peak. Based on the results presented here and in Sotomayor et al. (39), we add a third mechanical unfolding category in which a bridge between charged amino acids and divalent ions produces the unfolding force-peak. Thus, Ca2+ is not only acting as a molecular bearing that facilitates cooperative motion of cadherin repeats, but also as a staple that maintains the stability of the structure providing further resistance to mechanical unfolding.
Unfolding as observed with the SMD methodology employed here permitted us to also estimate a characteristic time for stress propagation through the protein. This example shows how an unfolding event that occurred at one end of a protein is perceived at the other end (by the second steering spring considered now as a sensor) after some time. The force peaks measured through spring extension at both ends of the protein are not synchronous (see Data S1, Fig. S14). In fact, in SimCa2E, a delay between the appearances of force peaks at both ends of the protein can be used to compute a velocity v ∼ 285 Å/38 ps ∼ 747 m/s at the corresponding extension of the protein. This velocity may depend on multiple factors including the thermodynamic ensemble used in the simulation, stretching velocity, spring constants used for stretching springs, and criteria used to determine the characteristic time. Indeed, our simulations show that damping artificially introduced by temperature control algorithms may decouple different zones of the protein from each other (see Data S1, Fig. S18). Although protein motions are largely overdamped (79), it has been hypothesized that stress signals could propagate over large distances in the cell (80). The two-end stretching approach outlined here may serve to test this hypothesis experimentally and theoretically.
Ca2+ allosteric control of residues involved in cell adhesion
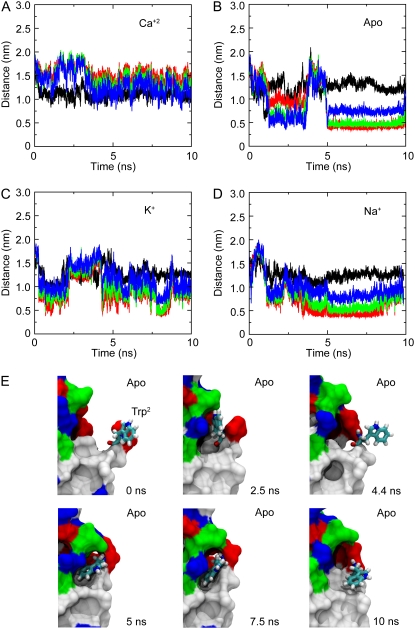
The dynamics of the extracellular domain, controlled by Ca2+, determines the availability of repeat EC1 to form adhesive contacts with proteins coming from neighboring cells. There are also subtle changes in C-cadherin dynamics controlled by Ca2+ that will affect its adhesive properties. For instance, we monitored the dynamics of the conserved residue Trp2 throughout equilibrium simulations in presence and absence of Ca2+, as well as with K+ and Na+ replacing Ca2+. As mentioned above, Trp2 is thought to mediate trans-interactions of cadherin by inserting itself in a hydrophobic pocket of another EC1 repeat coming from either the same cell or an adjacent cell (cis versus trans). The conformation of Trp2 in the C-cadherin structure is that of an exposed residue (see first snapshot in Fig. 6 E), since the crystallographic arrangement of the cadherin molecule leads to a proposed trans interaction (strand-exchanged) between molecules of different crystallographic unit cells. During the simulations in which calcium ions were present (SimCa1, SimCa3, and SimCa4), the side chain of Trp2 remained partially exposed at all times (Fig. 6 A and see Data S1, Fig. S20 A). However, when calcium ions were removed or replaced by monovalent ions, the EC1 domain was more mobile (see root mean-square fluctuations in Data S1, Fig. S21 A) and the side chain of Trp2 fluctuated between two states: exposed, and partially buried (see Fig. 7, snapshots in Fig. 6 E, and see Data S1, Fig. S20, B and C, and Movie S2, movies mVIII–mXI). In the latter state, the side chain is hidden in a hydrophobic pocket (intramolecular docking), close to residues 24 and 25. We termed this state partially buried since interactions with E90 (D90 in E-cadherin) seen in other structures and NMR experiments with E-cadherin are not observed here, perhaps due to the short timescale of the simulation. The solvent-accessible surface area for Trp2 computed throughout different equilibrium simulations also confirms the greater availability of this side chain when Ca2+ is bound to C-cadherin (see Data S1, Fig. S22). Our results strongly support experimental work using antibodies and independent mutagenesis experiments combined with force measurements suggesting that Trp2 availability is allosterically modulated by Ca2+ (38,45).
FIGURE 6.
Dynamics of Trp2. The state of Trp2 is characterized by the distance to its neighboring residues during simulations with (A) and without (B) Ca2+, as well as with K+ (C) and Na+ (D) ions replacing Ca2+. Distances between the Cα atom of Trp2 and Cα atoms of residues Ile24, Lys25, and Ser26 are shown in red, green, and blue, respectively. The distance between atom Nɛ of Trp2 and the carbonyl oxygen atom of Glu90 is shown in black. Intermittent bound states can be clearly observed during simulations performed without Ca2+. (E) Snapshots show Trp2 in licorice representation at times t = 0, 2.5, 4.4, 5, 7.5, and 10 ns during SimApo1. The rest of the EC1 domain is shown in surface representation and colored according to hydrophobicity (white, hydrophobic residues; green, polar residues; red or blue, charged residues).
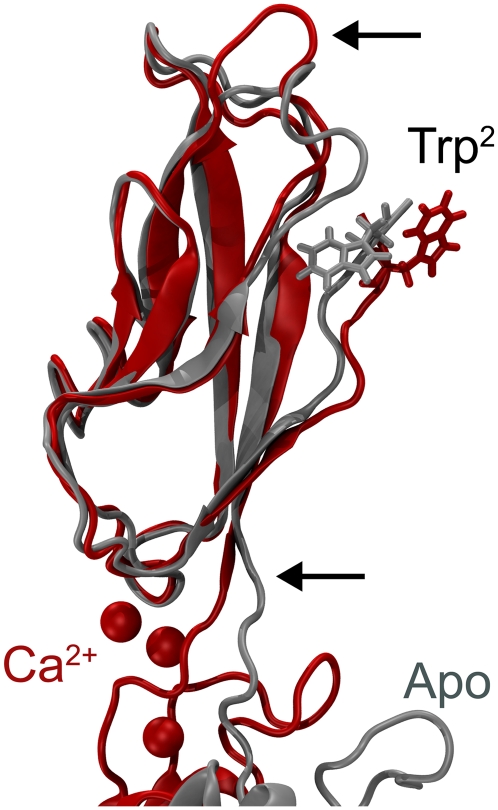
FIGURE 7.
Allosteric switch of Trp2. Snapshots of C-cadherin repeat EC1 and EC1-EC2 linker region at the end of SimCa1 (red) and after 5 ns of dynamics in SimApo1 (gray) are superimposed and shown in cartoon representation. Calcium ions are shown as spheres. The two conformations adopted by Trp2 are evident. Arrows indicate regions of the protein backbone where significant differences between the two conformations can be observed.
The dynamic switching of Trp2 availability is also compatible with the strand-exchanged adhesive interface (81), as backbone atoms of residues 25 and 27 (involved in hydrogen bonds with backbone atoms of residues 1 and 3 of a neighboring molecule) are more exposed when Ca2+ is present and the Trp2 side chain is in its extended conformation. The Trp2 side chain may directly and indirectly mediate the formation of the strand-exchanged interface. Interestingly, the behavior observed here for Trp2 is similar to that observed for a Trp185/187 residue in rat/human Annexin V, where Ca2+ induces exposure of the Trp side chain (involved in membrane-protein interactions) (82,83), thus hinting at a more general mechanism behind calcium-mediated adhesion.
DISCUSSION
The results presented here qualitatively validate molecular dynamics simulations as a tool to study the mechanical function of cadherin. The simulations, using standard and widely used parameters, account for experimentally known facts such as the role of Ca2+ in shaping the extracellular cadherin domain, the putative allosteric effect of Ca2+ on the key binding residue Trp2, and the Ca2+-dependent stability of individual repeats probed here through mechanical unfolding. At the same time, the simulations provide a unique atomistic view of cadherin dynamics. Such a detailed view is a necessary complement to experiments investigating cadherin function.
The reversible TSE observed here for C-cadherin provides support for the multiple conformations observed in desmosomal cadherins through electron tomography (84) and also suggest that the extracellular domains of cadherin molecules should not be considered as completely rigid units, even in the presence of Ca2+. Moreover, the allosteric control of Trp2 conformations by Ca2+ observed, to the best of our knowledge, for the first time at the atomic level in our simulations, provides a conceptual framework to interpret mutations that modify sites located far from the strand-exchanged adhesive interface (81) but may allosterically affect residues involved in adhesion as well as binding selectivity.
This study serves as a step toward future molecular dynamics studies of cadherin that should address:
The relevance of cadherin elasticity and mechanical intermediates for adhesion;
The robustness of the intrinsic curvature of C-cadherin in response to multiple stretching and compressing cycles;
The mechanical stability and complete unfolding of all repeats;
The selectivity of adhesion molecules (85,86) in the complete adhesion complex; and, eventually,
The mechanical or allosteric role of mutations affecting calcium binding sites in cadherin-23 and protocadherin-15 resulting in hereditary deafness (12,87,88).
Our simulations strongly suggest that deafness may arise through mutations that affect the cadherin-23/protocadherin-15 hair-cell tip link in two ways: abolishing tip-link formation by directly or allosterically interfering with residues involved in adhesion or dimerization; and/or by modifying the mechanical strength of the tip link by favoring unfolding or precluding mechanical intermediates by breaking bridges between protein atoms and Ca2+ ions at linker regions.
The results presented here have implications for other proteins featuring tandemly-arranged repeats, the archetypical examples being spectrin and titin. Linkers have been shown to be important in spectrin elasticity (56,89,90), while interdomain motion and divalent ions may likely play a role in titin elasticity (91). Cadherin may serve as an exceptional example of the design principles behind linker-mediated elasticity, as it features unique linker regions that can behave as stiff or flexible hinges, depending on the availability of calcium ions.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Supplementary Material
Acknowledgments
We thank Deborah Leckband, Alek Aksimentiev, David P. Corey, Bechara Kachar, and members of the Theoretical and Computational Biophysics Group for helpful discussions. The molecular images in this article were created with the molecular graphics program VMD.
This work was supported by funds from the National Institutes of Health (grants No. P41 RR05969 and No. 1 R01 GM073655). The authors also acknowledge computer time provided by the National Science Foundation through LRAC grant No. MCA93S028.
Editor: Angel E. Garcia.
References
- 1.Takeichi, M. 1990. Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 59:237–252. [DOI] [PubMed] [Google Scholar]
- 2.Hynes, R. O. 1999. Cell adhesion: old and new questions. Trends Cell Biol. 9:M33–M37. [PubMed] [Google Scholar]
- 3.Takeichi, M. 2005. Looking for cell-cell adhesion molecules: a cadherin story. Proc. Jpn. Acad. 81:321–328. [Google Scholar]
- 4.Gumbiner, B. M. 2005. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6:622–634. [DOI] [PubMed] [Google Scholar]
- 5.Takeichi, M. 1991. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 251:1451–1455. [DOI] [PubMed] [Google Scholar]
- 6.Takeichi, M. 1995. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 7:619–627. [DOI] [PubMed] [Google Scholar]
- 7.Takeichi, M. 2007. The cadherin superfamily in neuronal connections and interactions. Nat. Rev. Neurosci. 8:11–20. [DOI] [PubMed] [Google Scholar]
- 8.Leckband, D., and A. Prakasam. 2006. Mechanism and dynamics of cadherin adhesion. Annu. Rev. Biomed. Eng. 8:259–287. [DOI] [PubMed] [Google Scholar]
- 9.Corey, D. P., and M. Sotomayor. 2004. Tightrope act. Nature. 428:901–902. [DOI] [PubMed] [Google Scholar]
- 10.Söllner, C., G.-J. Rauch, J. Siemens, R. Geisler, S. C. Schuster, U. Müller, and T. Nicolson. 2004. The Tübingen 2000 Screen Consortium. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 428:955–958. [DOI] [PubMed] [Google Scholar]
- 11.Siemens, J., C. Lillo, R. A. Dumont, A. Reynolds, D. S. Williams, P. G. Gillespie, and U. Müller. 2004. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 428:950–955. [DOI] [PubMed] [Google Scholar]
- 12.Kazmierczak, P., H. Sakaguchi, J. Tokita, E. M. Wilson-Kubalek, R. A. Milligan, U. Müller, and B. Kachar. 2007. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 449:87–91. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro, L., P. D. Kwong, A. M. Fannon, D. R. Colman, and W. A. Hendrickson. 1995. Consideration on the folding topology and evolutionary origin of cadherin domains. Proc. Natl. Acad. Sci. USA. 92:6793–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nollet, F., P. Kools, and F. van Roy. 2000. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 299:551–572. [DOI] [PubMed] [Google Scholar]
- 15.Patel, S. D., C. P. Chen, F. Bahna, B. Honig, and L. Shapiro. 2003. Cadherin-mediated cell-cell adhesion: sticking together as a family. Curr. Opin. Struct. Biol. 13:690–698. [DOI] [PubMed] [Google Scholar]
- 16.Koch, A. W., D. Bozi, O. Pertz, and J. Engel. 1999. Homophilic adhesion by cadherins. Curr. Opin. Struct. Biol. 9:275–281. [DOI] [PubMed] [Google Scholar]
- 17.Nagar, B., M. Overdulin, M. Ikura, and J. Rini. 1996. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 380:360–364. [DOI] [PubMed] [Google Scholar]
- 18.Tomschy, A., C. Fauser, R. Landwehr, and J. Engel. 1996. Homophilic adhesion of E-cadherin occurs by a co-operative two-step interaction of N-terminal domains. EMBO J. 15:3507–3514. [PMC free article] [PubMed] [Google Scholar]
- 19.Yap, A. S., W. M. Brieher, M. Pruschy, and B. M. Gumbiner. 1997. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr. Biol. 7:308–315. [DOI] [PubMed] [Google Scholar]
- 20.Takeda, H., Y. Shimoyama, A. Nagafuchi, and S. Hirohashi. 1999. E-cadherin functions as a cis-dimer at the cell-cell adhesive interface in vivo. Nat. Struct. Biol. 6:310–312. [DOI] [PubMed] [Google Scholar]
- 21.Klingelhöfer, J., O. Y. Laur, R. B. Troyanovsky, and S. M. Troyanovsky. 2002. Dynamic interplay between adhesive and lateral E-cadherin dimers. Mol. Cell. Biol. 22:7449–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahrens, T., M. Lambert, O. Pertz, T. Sasaki, T. Schulthess, R.-M. Mège, R. Timpl, and J. Engel. 2003. Homoassociation of VE-cadherin follows a mechanism common to “classical” cadherins. J. Mol. Biol. 325:733–742. [DOI] [PubMed] [Google Scholar]
- 23.Sivasankar, S., W. Brieher, N. Lavrik, B. Gumbiner, and D. Leckband. 1999. Direct molecular force measurements of multiple adhesive interactions between cadherin ectodomains. Proc. Natl. Acad. Sci. USA. 96:11820–11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivasankar, S., B. Gumbiner, and D. Leckband. 2001. Direct measurements of multiple adhesive alignments and unbinding trajectories between cadherin extracellular domains. Biophys. J. 80:1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chappuis-Flament, S., E. Wong, L. D. Hicks, C. M. Kay, and B. M. Gumbiner. 2001. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J. Cell Biol. 154:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu, B., S. Chappuis-Flament, E. Wong, I. E. Jensen, B. M. Gumbiner, and D. Leckband. 2003. Functional analysis of the structural basis of homophilic cadherin adhesion. Biophys. J. 84:4033–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perret, E., A. Leung, and E. Evans. 2004. Trans-bonded pairs of E-cadherin exhibit a remarkable hierarchy of mechanical strengths. Proc. Natl. Acad. Sci. USA. 101:16472–16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayas, M. V., A. Leung, E. Evans, and D. Leckband. 2006. Lifetime measurements reveal kinetic differences between homophilic cadherin bonds. Biophys. J. 90:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsukasaki, Y., K. Kitamura, K. Shimizu, A. H. Iwane, Y. Takai, and T. Yanagida. 2007. Role of multiple bonds between the single cell adhesion molecules, nectin and cadherin, revealed by high sensitive force measurements. J. Mol. Biol. 367:996–1006. [DOI] [PubMed] [Google Scholar]
- 30.Pokutta, K., S. Herrenknecht, R. Kemler, and J. Engel. 1994. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur. J. Biochem. 223:1019–1026. [DOI] [PubMed] [Google Scholar]
- 31.Alattia, J.-R., J. B. Ames, T. Porumb, K. I. Tong, Y. M. Heng, P. Ottensmeyer, C. M. Kay, and M. Ikura. 1997. Lateral self-assembly of E-cadherin directed by cooperative calcium binding. FEBS Lett. 417:405–408. [DOI] [PubMed] [Google Scholar]
- 32.Koch, A. W., S. Pokutta, A. Lustig, and J. Engel. 1997. Calcium binding and homoassociation of E-cadherin domains. Biochemistry. 36:7696–7705. [DOI] [PubMed] [Google Scholar]
- 33.Pertz, O., D. Bozic, A. W. Koch, C. Fauser, A. Brancaccio, and J. Engel. 1999. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 18:1738–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Häussinger, D., T. Ahrens, H.-J. Sass, O. Pertz, J. Engel, and S. Grzesiek. 2002. Calcium-dependent homoassociation of E-cadherin by NMR spectroscopy: changes in mobility, conformation and mapping of contact regions. J. Mol. Biol. 324:823–839. [DOI] [PubMed] [Google Scholar]
- 35.Boggon, T. J., J. Murray, S. Chappuis-Flament, E. Wong, B. M. Gumbiner, and L. Shapiro. 2002. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 296:1308–1313. [DOI] [PubMed] [Google Scholar]
- 36.Prasad, A., and S. Pedigo. 2005. Calcium-dependent stability studies of domains 1 and 2 of epithelial cadherin. Biochemistry. 44:13692–13701. [DOI] [PubMed] [Google Scholar]
- 37.Prasad, A., H. Zhao, J. M. Rutherford, H. N. C. Nichols, and S. Pedigo. 2006. Effect of linker segments on the stability of epithelial cadherin domain 2. Proteins Struct. Funct. Bioinform. 62:111–121. [DOI] [PubMed] [Google Scholar]
- 38.Prakasam, A., Y.-H. Chien, V. Maruthamuthu, and D. E. Leckband. 2006. Calcium site mutations in cadherin: impact on adhesion and evidence of cooperativity. Biochemistry. 45:6930–6939. [DOI] [PubMed] [Google Scholar]
- 39.Sotomayor, M., D. P. Corey, and K. Schulten. 2005. In search of the hair-cell gating spring: elastic properties of ankyrin and cadherin repeats. Structure. 13:669–682. [DOI] [PubMed] [Google Scholar]
- 40.Cailliez, F., and R. Lavery. 2005. Cadherin mechanics and complexation: the importance of calcium binding. Biophys. J. 89:3895–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cailliez, F., and R. Lavery. 2006. Dynamics and stability of E-cadherin dimers. Biophys. J. 91:3964–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shapiro, L., A. M. Fannon, P. D. Kwong, A. Thompson, M. S. Lehmann, G. Grübel, J. F. Legrand, J. Als-Nielsen, D. R. Colman, and W. A. Hendrickson. 1995. Structural basis of cell-cell adhesion by cadherins. Nature. 374:327–336. [DOI] [PubMed] [Google Scholar]
- 43.Häussinger, D., T. Ahrens, T. Aberle, J. Engel, J. Stetefeld, and S. Grzesiek. 2004. Proteolytic E-cadherin activation followed by solution NMR and x-ray crystallography. EMBO J. 23:1699–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayas, M. V., K. Schulten, and D. Leckband. 2004. Forced dissociation of the strand dimer interface between C-cadherin ectodomains. Mech. Chem. Biosystems. 1:101–111. [PubMed] [Google Scholar]
- 45.Harrison, O. J., E. M. Corps, T. Berge, and P. J. Kilshaw. 2005. The mechanism of cell adhesion by classical cadherins: the role of domain 1. J. Cell Sci. 118:711–721. [DOI] [PubMed] [Google Scholar]
- 46.Humphrey, W., A. Dalke, and K. Schulten. 1996. VMD—visual molecular dynamics. J. Mol. Graph. 14:33–38. [DOI] [PubMed] [Google Scholar]
- 47.Phillips, J. C., R. Braun, W. Wang, J. Gumbart, E. Tajkhorshid, E. Villa, C. Chipot, R. D. Skeel, L. Kale, and K. Schulten. 2005. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26:1781–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacKerell, A. D., Jr., D. Bashford, M. Bellott, J. R. L. Dunbrack, J. Evanseck, M. J. Field, S. Fischer, J. Gao, H. Guo, S. Ha, D. Joseph, L. Kuchnir, K. Kuczera, F. T. K. Lau, C. Mattos, S. Michnick, T. Ngo, D. T. Nguyen, B. Prodhom, B. Roux, M. Schlenkrich, J. Smith, R. Stote, J. Straub, M. Watanabe, J. Wiorkiewicz-Kuczera, D. Yin, and M. Karplus. 1992. Self-consistent parameterization of biomolecules for molecular modeling and condensed phase simulations. FASEB J. 6:A143. [Google Scholar]
- 49.MacKerell, A., Jr., D. Bashford, M. Bellott, R. L. Dunbrack, Jr., J. Evanseck, M. J. Field, S. Fischer, J. Gao, H. Guo, S. Ha, D. Joseph, L. Kuchnir, K. Kuczera, F. T. K. Lau, C. Mattos, S. Michnick, T. Ngo, D. T. Nguyen, B. Prodhom, I. W. E. Reiher, B. Roux, M. Schlenkrich, J. Smith, R. Stote, J. Straub, M. Watanabe, J. Wiorkiewicz-Kuczera, D. Yin, and M. Karplus. 1998. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 102:3586–3616. [DOI] [PubMed] [Google Scholar]
- 50.MacKerell, A. D., M. Feig, and C. L. Brooks III. 2004. Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 25:1400–1415. [DOI] [PubMed] [Google Scholar]
- 51.Young, A., J. Dewan, C. Nave, and R. Tilton. 1993. Comparison of radiation-induced decay and structure refinement from x-ray data collected from lysozyme crystals at low and ambient temperatures. J. Appl. Cryst. 26:309–319. [Google Scholar]
- 52.Buck, M., S. Bouguet-Bonnet, R. W. Pastor, and A. D. MacKerell. 2006. Importance of the CMAP correction to the CHARMM22 protein force field: dynamics of hen lysozyme. Biophys. J. 90:L36–L38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jorgensen, W. L., J. Chandrasekhar, J. D. Madura, R. W. Impey, and M. L. Klein. 1983. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79:926–935. [Google Scholar]
- 54.Marchand, S., and B. Roux. 1998. Molecular dynamics study of calbindin D9k in the apo and singly and doubly calcium-loaded states. Proteins Struct. Funct. Genet. 33:265–284. [PubMed] [Google Scholar]
- 55.Essmann, U., L. Perera, M. L. Berkowitz, T. Darden, H. Lee, and L. G. Pedersen. 1995. A smooth particle mesh Ewald method. J. Chem. Phys. 103:8577–8593. [Google Scholar]
- 56.Ortiz, V., S. O. Nielsen, M. L. Klein, and D. E. Discher. 2005. Unfolding a linker between helical repeats. J. Mol. Biol. 349:638–647. [DOI] [PubMed] [Google Scholar]
- 57.Zhong, Y., W. M. Brieher, and B. M. Gumbiner. 1999. Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. J. Cell Biol. 144:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baumgartner, W., P. Hinterdorfer, W. Ness, A. Raab, D. Vestweber, H. Schindler, and D. Drenckhahn. 2000. Cadherin interaction probed by atomic force microscopy. Proc. Natl. Acad. Sci. USA. 97:4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Izrailev, S., S. Stepaniants, B. Isralewitz, D. Kosztin, H. Lu, F. Molnar, W. Wriggers, and K. Schulten. 1998. Steered molecular dynamics. In Computational Molecular Dynamics: Challenges, Methods, Ideas, Vol. 4 of Lecture Notes in Computational Science and Engineering. P. Deuflhard, J. Hermans, B. Leimkuhler, A. E. Mark, S. Reich, and R. D. Skeel, editors. Springer-Verlag, Berlin.
- 60.Isralewitz, B., J. Baudry, J. Gullingsrud, D. Kosztin, and K. Schulten. 2001. Steered molecular dynamics investigations of protein function. J. Mol. Graphics Modeling. 19:13–25. [DOI] [PubMed] [Google Scholar]
- 61.Isralewitz, B., M. Gao, and K. Schulten. 2001. Steered molecular dynamics and mechanical functions of proteins. Curr. Opin. Struct. Biol. 11:224–230. [DOI] [PubMed] [Google Scholar]
- 62.Gao, M., M. Sotomayor, E. Villa, E. Lee, and K. Schulten. 2006. Molecular mechanisms of cellular mechanics. Phys. Chem. Chem. Phys. 8:3692–3706. [DOI] [PubMed] [Google Scholar]
- 63.Sotomayor, M., and K. Schulten. 2007. Single-molecule experiments in vitro and in silico. Science. 316:1144–1148. [DOI] [PubMed] [Google Scholar]
- 64.Gräter, F., J. Shen, H. Jiang, M. Gautel, and H. Grubmüller. 2005. Mechanically induced titin kinase activation studied by force-probe molecular dynamics simulations. Biophys. J. 88:790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee, E. H., J. Hsin, O. Mayans, and K. Schulten. 2007. Secondary and tertiary structure elasticity of titin Z1Z2 and a titin chain model. Biophys. J. 93:1719–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee, G., K. Abdi, Y. Jiang, P. Michaely, V. Bennett, and P. E. Marszalek. 2006. Nanospring behavior of ankyrin repeats. Nature. 440:246–249. [DOI] [PubMed] [Google Scholar]
- 67.Michaely, P., D. R. Tomchick, M. Machius, and R. G. W. Anderson. 2002. Crystal structure of a 12 ANK repeat stack from human ankyrin-R. EMBO J. 21:6387–6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silver, F. H., D. Christiansen, P. B. Snowhill, Y. Chen, and W. J. Landis. 2000. The role of mineral in the storage of elastic energy in turkey tendons. Biomacromolecules. 1:180–185. [DOI] [PubMed] [Google Scholar]
- 69.Izrailev, S., S. Stepaniants, M. Balsera, Y. Oono, and K. Schulten. 1997. Molecular dynamics study of unbinding of the avidin-biotin complex. Biophys. J. 72:1568–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evans, E., and K. Ritchie. 1997. Dynamic strength of molecular adhesion bonds. Biophys. J. 72:1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu, H., B. Isralewitz, A. Krammer, V. Vogel, and K. Schulten. 1998. Unfolding of titin immunoglobulin domains by steered molecular dynamics simulation. Biophys. J. 75:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marszalek, P. E., H. Lu, H. Li, M. Carrion-Vazquez, A. F. Oberhauser, K. Schulten, and J. M. Fernandez. 1999. Mechanical unfolding intermediates in titin modules. Nature. 402:100–103. [DOI] [PubMed] [Google Scholar]
- 73.Krammer, A., H. Lu, B. Isralewitz, K. Schulten, and V. Vogel. 1999. Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proc. Natl. Acad. Sci. USA. 96:1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu, H., and K. Schulten. 1999. Steered molecular dynamics simulation of conformational changes of immunoglobulin domain I27 interpret atomic force microscopy observations. Chem. Phys. 247:141–153. [Google Scholar]
- 75.Gao, M., D. Craig, V. Vogel, and K. Schulten. 2002. Identifying unfolding intermediates of FN-III10 by steered molecular dynamics. J. Mol. Biol. 323:939–950. [DOI] [PubMed] [Google Scholar]
- 76.Gao, M., D. Craig, O. Lequin, I. D. Campbell, V. Vogel, and K. Schulten. 2003. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc. Natl. Acad. Sci. USA. 100:14784–14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craig, D., M. Gao, K. Schulten, and V. Vogel. 2004. Tuning the mechanical stability of fibronectin type III modules through sequence variation. Structure. 12:21–30. [DOI] [PubMed] [Google Scholar]
- 78.Lu, H., and K. Schulten. 1999. Steered molecular dynamics simulations of force-induced protein domain unfolding. Proteins Struct. Funct. Genet. 35:453–463. [PubMed] [Google Scholar]
- 79.Howard, J. 2001. Mechanics of Motor Proteins and the Cytoskeleton. Sinauer Associates, Sunderland, MA.
- 80.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687. [DOI] [PubMed] [Google Scholar]
- 81.Patel, S. D., C. Ciatto, C. P. Chen, F. Bahna, N. A. Rajebhosale, I. Schieren, T. M. Jessell, B. Honig, S. R. Price, and L. Shapiro. 2006. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 124:1255–1268. [DOI] [PubMed] [Google Scholar]
- 82.Concha, N. O., J. F. Head, M. A. Kaetzel, J. R. Dedman, and B. A. Seaton. 1993. Rat annexin V crystal structure: Ca2+-induced conformational changes. Science. 261:1321–1324. [DOI] [PubMed] [Google Scholar]
- 83.Sopkova, J., M. Renouard, and A. Lewit-Bentley. 1993. The crystal structure of a new high-calcium form of annexin V. J. Mol. Biol. 234:816–825. [DOI] [PubMed] [Google Scholar]
- 84.He, W., P. Cowin, and D. L. Stokes. 2003. Untangling desmosomal knots with electron tomography. Science. 302:109–113. [DOI] [PubMed] [Google Scholar]
- 85.Chen, C. P., S. Posy, A. Ben-Shaul, L. Shapiro, and B. H. Honig. 2005. Specificity of cell-cell adhesion by classical cadherins: critical role for low-affinity dimerization through β-strand swapping. Proc. Natl. Acad. Sci. USA. 102:8531–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prakasam, A. K., V. Maruthamuthu, and D. E. Leckband. 2006. Similarities between heterophilic and homophilic cadherin adhesion. Proc. Natl. Acad. Sci. USA. 103:15434–15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bork, J. M., L. M. Peters, S. Riazuddin, S. L. Bernstein, Z. M. Ahmed, S. L. Ness, R. Polomeno, A. Ramesh, M. Schloss, C. R. S. Srisailpathy, S. Wayne, S. Bellman, D. Desmukh, Z. Ahmed, S. N. Khan, V. M. der Kaloustian, X. C. Li, A. Lalwani, S. Riazuddin, M. Bitner-Glindzicz, W. E. Nance, X.-Z. Liu, G. Wistow, R. J. H. Smith, A. J. Griffith, E. R. Wilcox, T. B. Friedman, and R. J. Morell. 2001. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am. J. Hum. Genet. 68:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Brouwer, A. P. M., R. J. Pennings, M. Roeters, P. van Hauwe, L. M. Astuto, L. H. Hoefsloot, P. L. M. Huygen, B. van den Helm, A. F. Deutman, J. M. Bork, W. J. Kimberling, F. P. M. Cremers, C. W. R. J. Cremers, and H. Kremer. 2003. Mutations in the calcium-binding motifs of CDH23 and the 35DELG mutation in gjb2 cause hearing loss in one family. Hum. Genet. 112:156–163. [DOI] [PubMed] [Google Scholar]
- 89.Mirijanian, D. T., J. W. Chu, G. S. Ayton, and G. A. Voth. 2007. Atomistic and coarse-grained analysis of double spectrin repeat units: the molecular origins of flexibility. J. Mol. Biol. 365:523–534. [DOI] [PubMed] [Google Scholar]
- 90.Paramore, S., and G. A. Voth. 2006. Examining the influence of linkers and tertiary structure in the forced unfolding of multiple-repeat spectrin molecules. Biophys. J. 91:3436–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marino, M., P. Zou, D. Svergun, P. Garcia, C. Edlich, B. Simon, M. Wilmanns, C. Muhle-Goll, and O. Mayans. 2006. The Ig doublet Z1Z2: a model system for the hybrid analysis of conformational dynamics in Ig tandems from titin. Structure. 14:1437–1447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.