Abstract
The purine nucleoside inosine has been shown to induce axon outgrowth from primary neurons in culture through a direct intracellular mechanism. For this study, we investigated the effects of inosine in vivo by examining whether it would stimulate axon growth after a unilateral transection of the corticospinal tract. Inosine applied with a minipump to the rat sensorimotor cortex stimulated intact pyramidal cells to undergo extensive sprouting of their axons into the denervated spinal cord white matter and adjacent neuropil. Axon growth was visualized by anterograde tracing with biotinylated dextran amine and by immunohistochemistry with antibodies to GAP-43. Thus, inosine, a naturally occurring metabolite without known side effects, might help to restore essential circuitry after injury to the central nervous system.
Under normal circumstances, neurons of the mature central nervous system (CNS) are unable to regenerate injured axons. This failure generally has been ascribed to inhibitory proteins in myelin (1–4), an insufficiency of essential trophic factors (5–7), or the presence or absence of other substrate molecules (8). With alterations in the extracellular environment, however, many neurons that otherwise show no potential for growth can extend injured axons over long distances (9).
In cell culture studies, the purine nucleoside inosine was found to act through a direct intracellular mechanism to promote neurite outgrowth in retinal ganglion cells (10) and sympathetic neurons (11). Its likely target is a serine-threonine kinase previously shown to be critical for neurite outgrowth in PC12 cells (12–14). To investigate whether inosine can stimulate axon growth in vivo, we examined its effects on the injured rat corticospinal tract (CST). The rat CST arises from layer 5 pyramidal cells in the sensorimotor cortex and mediates fine movements of the extremities (15), though its trajectory in the spinal cord and pattern of terminations are somewhat different from those of humans (16). Limited sprouting or regenerative growth has been achieved in this pathway by altering the glial environment, applying growth factors, and/or by neutralizing myelin inhibitory proteins (5–7, 17–22). We show here that after unilateral CST transections, inosine induces a massive compensatory sprouting of axon collaterals from uninjured pyramidal cells into the denervated spinal cord white matter and adjacent neuropil.
Methods
Surgery and Minipump Implantation.
Male Sprague–Dawley rats (n = 22; Charles River Breeding Laboratories; 250–300 g) were weighed and anesthetized by metofane inhalation followed by i.p. sodium pentobarbital (Ayerst Laboratories; 50 mg/kg). Some of the animals (n = 8) either died as a result of surgery or, as determined by macroscopic inspection, did not have adequate transections and were not processed further. Through a ventral midline incision in the neck, the trachea and paratracheal musculature were retracted to expose the skull base. The left CST was exposed on the ventral surface of the medulla via a 1.5- to 2.0-mm craniotomy. By using the basilar artery as the medial boundary, the CST was transected with a microknife, creating a lesion of the medulla 1.0–1.5 mm wide and 0.5 mm deep (23). Exposed brain tissue was covered with Gelfoam (Upjohn), and the skin was sutured closed (3-0 Ethilon). Animals were reanesthetized 24 h later and implanted with minipumps (Model 2002, Alzet, Palo Alto, CA) placed subcutaneously between the scapulae to deliver either inosine (Sigma; 10 mM in sterile PBS; n = 8) or PBS (n = 6) at a rate of 0.5 μl/h for 14 days into the right (nonaxotomized) sensorimotor cortex (ref. 24; stereotaxic coordinates: 0.8 mm posterior to bregma, 2.2 mm lateral, 1.5-mm depth). The concentration of inosine used was approximately 1,000 times the ED50 found to stimulate axon outgrowth from neurons in culture (10). Infusion needles were held in place with cyanoacrylate.
Axon Tracing with Biotin Dextran Amine (BDA).
On day 15 after lesions were made, animals were again anesthetized and placed in the stereotaxic frame; the infusion needle and pump were removed, and a large craniotomy was performed to expose the right (i.e., nonaxotomized, inosine-treated) sensorimotor cortex. By using a Nanoject injector (Drummond Scientific, Broomall, PA), BDA [Molecular Probes: 10% (wt/vol) solution in sterile saline; ref. 25] was injected into 10 points in the sensorimotor cortex (6). At each coordinate, 70 nl of BDA was injected at depths of 0.5, 1.0, and 2.0 mm from the surface of the cortex. The craniotomy was covered with Gelfoam, and the skin was sutured in place. An additional group of animals (n = 4) underwent transections of the left CST and were then injected with BDA on the axotomized (ipsilateral) side 2 weeks later to verify the extent of the transections. Body temperature was maintained at 37°C with a warming blanket during surgery. All procedures were approved by the Children’s Hospital Institutional Animal Care and Use Committee.
Animals were anesthetized and perfused transcardially with heparinized saline (100 ml; 100,000 units/liter; 4°C) and 4% (vol/vol) paraformaldehyde (120 ml; 4°C) 2 weeks after BDA injections. The brain and spinal cord were removed, postfixed for 24 h, impregnated with 30% (wt/vol) sucrose, frozen, and cut at 40 μm. Free-floating sections (240-μm spacing) were incubated sequentially in 0.1% H2O2 in methanol (10 min), PBS plus 0.5% Triton X-100 (PBS-T; 30 min), avidin-biotin-peroxidase complex (Vector Laboratories; 1 h), PBS-T (twice for 10 min), and diaminobenzidine (DAB Kit; Vector Laboratories; 5 min). Sections were mounted (Superfrost Plus slides, Fisher Scientific), air-dried, dehydrated, and coverslipped (Permount, Fisher Scientific). Sections were examined first under low power magnification, and the midline was identified by using the central canal and dorsal median fissure as anatomical landmarks. Some sections split along this fissure, affording an additional criterion for the midline. For each case, we counted individual axons under ×400 magnification in several coronal sections that showed a high density of reinnervation, and obtained an average from the three sections with the greatest number of crossed axons. These numbers were then used to generate population histograms for the inosine- and PBS-treated groups.
GAP-43 Immunohistochemistry.
In alternate coronal sections from the above series, newly growing CST axons were visualized with a polyclonal antibody to the growth-associated protein GAP-43 (1:2,500), followed by a fluorescein-conjugated secondary antibody (Vector Laboratories). Sections were mounted and covered by using Vectashield (Vector Laboratories) medium. Immunohistochemistry with this antibody has been described (26).
Results
The rat CST originates in the sensorimotor cortex, descends through the brainstem, decussates in the caudal medulla, and continues its course in the contralateral dorsal funiculus (16). We used a ventral approach to transect the left CST before the decussation on the ventromedial surface of the rostral medulla (ref. 23; Fig. 1a). The success of our lesions was visualized in several animals in which we injected the tracer BDA into the left (i.e., axotomized) sensorimotor cortex (n = 4). Histochemistry performed after a 2-week labeling period indicated that our lesions typically transected over 90% of the CST fibers (Fig. 1 b and c).
Figure 1.
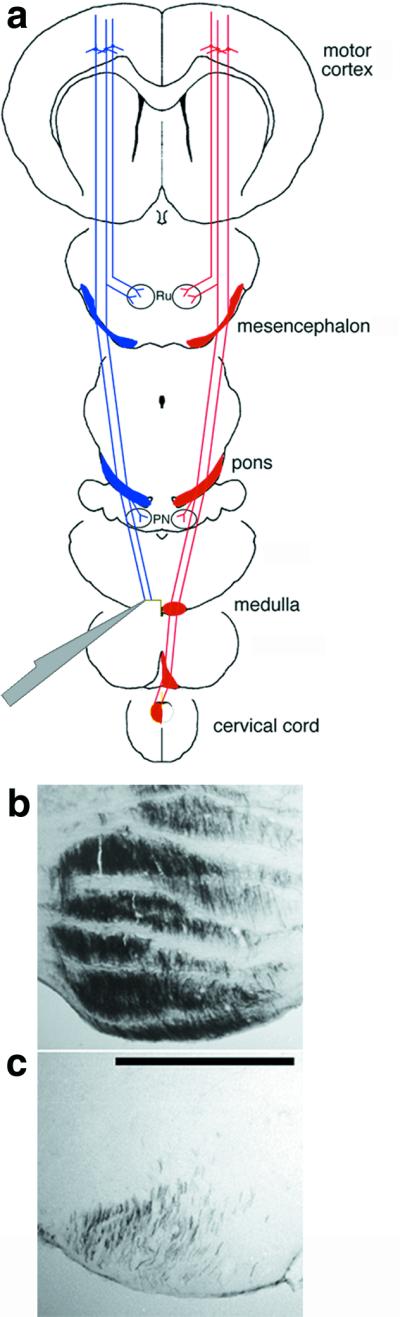
Unilateral transection of the CST. (a) CST axons arise from layer 5 pyramidal cells in the sensorimotor cortex, extend projections to the mesencephalic nucleus ruber (Ru) and pontine nuclei (PN), then assume a ventromedial position in the lower brainstem. In the caudal medulla, CST axons decussate and course in the dorsal funiculus of the contralateral spinal cord. Axons were transected via a ventral approach in the left medulla before the decussation. (b and c) Controls to verify the extent of surgery. Numerous fibers are seen in the left rostral medulla after left-sided BDA labeling (b); caudal to the transection, fewer than 10% of the original fibers remain. (c: bar = 500 μm.)
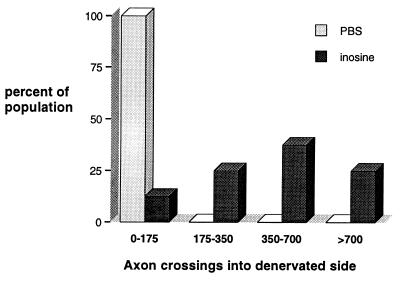
To investigate compensatory sprouting from intact CST axons, animals underwent left-sided CST transections and were treated with inosine in the right (nonaxotomized) sensorimotor cortex for 2 weeks (n = 8). Axon growth was evaluated by injecting BDA into the treated cortex and allowing another 2 weeks for the label to be transported along the length of the axons. As expected, right-sided BDA injections labeled uninjured CST axons that coursed through the right ventral brainstem (Fig. 2a), crossed over through the decussation to the left caudal medulla (Fig. 2b), and continued through the left dorsal funiculus in the rostral spinal cord (Fig. 2c). At the level of the cervical enlargement, however, hundreds of BDA-stained axon collaterals crossed the midline to the denervated side (Figs. 2d and 3a). This staining pattern is indicative of experimentally induced sprouting. Control animals treated with PBS instead of inosine after unilateral CST lesions (n = 6) showed only limited axon crossing (Fig. 2e). Crossed fibers in inosine-treated animals were initially clustered close to the midline but spread out laterally in the dorsal funiculus in more caudal sections. In some instances, labeled fibers were also seen coursing laterally into the denervated gray matter (Fig. 3b). Axon counts revealed that in the inosine-treated group, the maximum number of crossed axons per section had a median value of 402, and two cases had several times this number. With the exception of one outlier with few crossed axons, axon counts ranged from 340 to 2567 (Fig. 4). At cervical levels above the region of massive sprouting, the median number of crossed axons in the inosine-treated cases was only 27. Controls implanted with minipumps delivering PBS into the right cortex had, on the average, 10% of the number of crossed fibers of inosine-treated animals (median = 41; range of 28–170; the difference between the two groups was significant at P = 0.015; χ2 test; Pearson correction; P < 0.02; nonparametric Mann–Whitney U test; two-sided).
Figure 2.
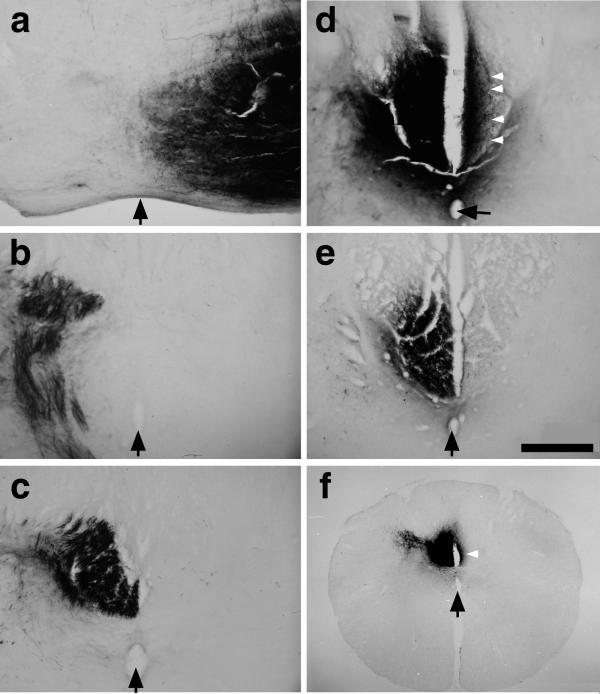
Inosine induces collateral sprouting in the adult rat CST. (a–d) Axon trajectories in a case in which we transected the CST in the left medulla (before the decussation), treated the right (nonaxotomized) sensorimotor cortex with inosine for 2 weeks, then traced fiber trajectories by injecting BDA into the right sensorimotor cortex. At more rostral levels, BDA-labeled axons remain strictly lateralized and are seen in the right ventral brainstem (a), left caudal medulla (b), and left dorsal funiculus (c). At the level of the cervical enlargement, however, numerous labeled axons also appear in the denervated (right-sided) dorsal funiculus (d; white arrowheads point to individual crossed fibers). (e) Controls treated with PBS instead of inosine after unilateral CST surgery show few crossed fibers. (Bar = 200 μm.) (f) A low-magnification image of the cervical cord from a case with left CST transection, inosine treatment in the right sensorimotor cortex, and BDA labeling in the right cortex. The white arrow points to dense axon staining on the denervated side. The midline is indicated in all sections with black arrows (pointing to the central canal in c–f).
Figure 3.
Axon crossing and growth into the spinal cord gray matter. At the level of the cervical enlargement, labeled fibers (black arrowheads in a) are seen crossing the midline (black arrow). (b) In addition to numerous crossed fibers that remain close to the midline (left side of image), some crossed fibers course laterally into the denervated neuropil (arrowheads). (Bar = 100 μm.)
Figure 4.
Axon crossings: frequency distributions for inosine- and PBS-treated groups. For each animal, we determined the mean number of crossed axons in the three coronal sections that showed the highest density of reinnervation. The x axis of the histogram shows the mean number of crossed axons, whereas the y axis represents the percentage of animals with axon numbers in each range. The distributions for numbers of crossed fibers show little overlap between the inosine-treated (n = 8; darkly shaded bars) and PBS-treated (n = 6; lightly shaded) groups.
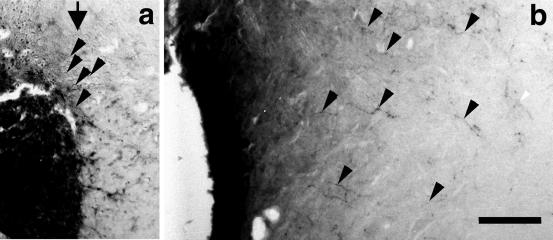
In the rat CST, as in most CNS pathways, expression of the growth-associated phosphoprotein GAP-43 is high during development but diminishes with maturation (27, 28). Because GAP-43 is generally up-regulated during axon regeneration (29, 30), we used an antibody to this protein to investigate whether crossed axons in inosine-treated cases were in a growth state. In the normal CST, GAP-43 immunoreactivity was modest bilaterally (Fig. 5a) and decreased on the denervated side in unilaterally transected control animals treated with PBS (right side, Fig. 5b). However, in unilaterally transected rats treated with inosine, GAP-43 immunoreactivity was elevated bilaterally (Fig. 5c). Intensely labeled axons on the intact (left) side presumably reflect undamaged fibers that are in a growth state, whereas those on the denervated (right) side correspond in their distribution with the crossed fibers visualized by BDA labeling (compare Fig. 2d). Because inosine treatment had ended 2 weeks before the animals were killed, the intensity of GAP-43 observed here may have declined from earlier, higher levels, as reflected in the moderate background staining seen on the denervated side of Fig. 5b.
Figure 5.
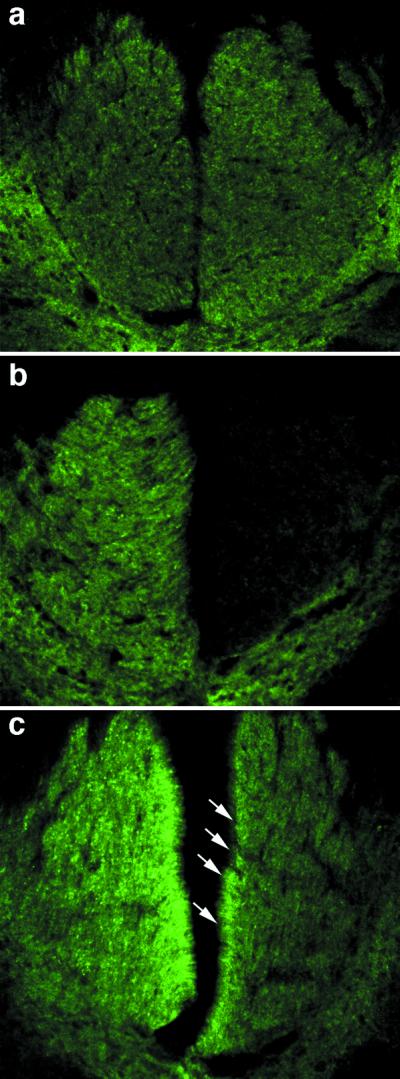
Axon growth visualized by GAP-43 immunohistofluorescence. (a) At the midcervical level, modest levels of GAP-43 immunoreactivity appear bilaterally in the dorsal funiculi. (b) In control animals with unilateral CST transections and a 2-week infusion of PBS, GAP-43 immunoreactivity disappears from the denervated (right) dorsal funiculus, though a small number of fibers with increased staining appear in the intact side. (c) In animals with unilateral CST injury and inosine treatment, GAP-43 immunoreactivity intensifies on the intact (left) side and is also present in many fibers in the denervated (right) dorsal funiculus (white arrows show crossed fibers near the midline).
Discussion
These findings show that inosine has potent axon-promoting effects in vivo. After CST injury, inosine stimulated uninjured pyramidal cells of the sensorimotor cortex to extend collaterals into denervated areas. This compensatory growth occurred over considerable distances in the white matter of the spinal cord, a terrain generally viewed as being inhibitory to growth.
Inosine, a naturally occurring product of adenosine hydrolysis, becomes abundant in the nervous system after injury (31, 32). The small but significant amount of axon crossing found in controls might be due in part to inosine released from cells injured by performing transections, implanting catheters, or injecting BDA, though other trophic factors are also stimulated by injury and are likely to play a significant role (33–35). Inosine must be transported across the cell membrane to induce axon growth (10, 11). One hint about its mechanism of action comes from cell culture studies with the purine analog, 6-thioguanine (6-TG). In PC12 pheochromocytoma cells and primary sympathetic neurons, 6-TG blocks the effects of nerve growth factor on neurite formation but does not affect cell viability (12, 13). The target of 6-TG’s action seems to be a 47- to 50-kDa serine-threonine kinase (N-kinase) that is normally activated within minutes of treating PC12 cells with nerve growth factor (14); these effects are specific, because 6-TG does not affect other signal transduction molecules (e.g., ras, mitogen-activated protein kinase, protein kinase A, protein kinase C, or Ca2+-calmodulin) or the induction of several immediate-early genes (e.g., c-fos or zif-268). Inosine competitively reverses the inhibitory effects of 6-TG on axon outgrowth in retinal ganglion cells (10) and in PC12 cells (L.I.B. and N.I., unpublished observations). Thus, inosine may act as an agonist at the same site at which 6-TG exerts its inhibitory effects, i.e., N-kinase. Another possibility is that inosine could be converted to cyclic nucleotides that enable advancing nerve endings to overcome the inhibitory effects of myelin (36, 37). However, in retinal ganglion cells at least, the effects of inosine on neurite outgrowth are unrelated to cyclic nucleotide generation (10). Inosine has also been reported to stimulate differentiation in rat sympathetic neurons (11), augment nerve growth factor-induced neuritogenesis in PC12 cells (38), and promote the survival of astrocytes (39).
Prior studies have shown that severed CST axons can be induced to regenerate into spinal cord gray matter when animals are treated with antibodies to myelin inhibitory proteins (22, 40). With growth factor stimulation, CST axons can regenerate into implants of fetal tissue (20), peripheral nerve grafts (5), or Schwann cells (19), though they seldom continue to grow into white matter beyond the implant. The only other instance in which CST axons have been shown to regenerate into white matter is after implants of olfactory ensheathing cells (17, 18). Thus, the extensive growth of CST axons in the dorsal funiculus after inosine treatment is notable. Significantly, some of the crossed fibers extend laterally into the denervated spinal cord gray matter and may therefore form synapses with appropriate target neurons, a question that needs to be addressed with electron microscopy. We have not yet determined whether axon collaterals continue to cross in the thoracic and lumbar cord nor how far they extend in the dorsal funiculus, although we observe BDA-labeled axons in sections spanning a rostrocaudal length of over 1 cm. Other systems in which neurons have been shown to extend injured axons through mature, myelinated CNS tracts include the growth of retinal ganglion cell axons through the optic nerve after intravitreal sciatic nerve implants (41) and the growth of dorsal root ganglion axons over the corpus callosum (8) or through the dorsal column after a priming lesion of the peripheral nerve (42). In addition, CNS neurons can regenerate their axons into a peripheral nerve graft (9), and this regeneration can be stimulated by growth factors in the rubrospinal system (43) or the retina (44).
Besides enabling uninjured CST axons to sprout collaterals into the denervated side of the cord, inosine may also stimulate collateral sprouting from uninjured axons ipsilateral to a partial transection and even from damaged axons. However, because it is technically difficult to distinguish newly growing sprouts from spared fibers on the denervated side, we have concentrated on the less ambiguous case of fibers crossing over to the denervated side from the intact dorsal funiculus (22). This approach takes advantage of the fact that in the rat, unlike the situation in humans, there are very few uncrossed CST fibers, and only a handful of these course in the ipsilateral dorsal funiculus (22, 45). Thus, the background staining caused by the normal ipsilateral component of the CST is negligible. Inosine may induce some of these normal ipsilateral CST axons to sprout on the denervated side or induce some of the normal contralateral fibers to form sprouts that remain on the innervated side of the cord, but again, such growth would be more difficult to recognize experimentally. The possibility that labeled fibers in the right dorsal funiculus might have resulted from BDA leaking over to the left sensorimotor cortex is excluded by the near-absence of bilateral labeling at more rostral levels (Fig. 2 a–c). Finally, it is conceivable that inosine may induce sprouting even without injury; however, in the absence of unilateral CST injury, such growth might occur elsewhere in the neuraxis and be more difficult to identify.
After unilateral transection of the CST in newborn hamsters, intact CST axons cross into the denervated half of the spinal cord at the segmental levels at which they innervate their targets (46, 47). This fact suggests that inductive signals for crossing might arise from the denervated target area. We likewise observed CST collaterals crossing only at midcervical levels, suggesting that our findings may reflect mechanisms that had operated during an earlier stage of development. Another possibility is that signals arising from the denervated tract serve as a costimulus for sprouting, because crossing first occurs at the level at which the two CSTs come into close apposition. In any event, our results support the idea that inosine, a normally occurring metabolite, may be useful clinically in the treatment of spinal cord injury and other CNS insults.
Acknowledgments
We thank Drs. Marc Lanser (Boston Life Sciences) for insightful observations, David Zurakowski (Children’s Hospital) for statistical help, Raymond Grill (University of California San Diego) for advice on BDA labeling, and Evan Snyder, Bruce Yankner, and Steven Finkbeiner (Children’s Hospital) for helpful comments on the manuscript. We are grateful for the support of National Institutes of Health Grant EY 05690, with supplemental funding for the Neurodegenerative Research Initiative from the Office of the Director; of Boston Life Sciences; and of the Boston Neurosurgical Foundation.
Abbreviations
- CNS
central nervous system
- CST
corticospinal tract
- BDA
biotin dextran amine
- 6-TG
6-thioguanine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Caroni P, Schwab M E. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnell L, Schwab M E. Nature (London) 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 3.McKerracher L, David S, Jackson D L, Kottis V, Dunn R J, Braun P E. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay G, Doherty P, Walsh F S, Crocker P R, Filbin M T. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H, Cao Y, Olson L. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 6.Grill R, Murai K, Blesch A, Gage F H, Tuszynski M H. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnell L, Schneider R, Kolbeck R, Barde Y A, Schwab M E. Nature (London) 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 8.Davies S J, Fitch M T, Memberg S P, Hall A K, Raisman G, Silver J. Nature (London) 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 9.Aguayo A J, Rasminsky M, Bray G M, Carbonetto S, McKerracher L, Villegas-Perez M P, Vidal-Sanz M, Carter D A. Philos Trans R Soc London B. 1991;331:337–343. doi: 10.1098/rstb.1991.0025. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz L I, Jing Y, Tabibazar R, Rosenberg P A, Jo S, Petrausch B, Stuermer C, Irwin N. J Biol Chem. 1998;273:29626–29634. doi: 10.1074/jbc.273.45.29626. [DOI] [PubMed] [Google Scholar]
- 11.Zurn A, Do K. Proc Natl Acad Sci USA. 1988;85:8301–8305. doi: 10.1073/pnas.85.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene L A, Volonte C, Chalazonitis A. J Neurosci. 1990;10:1479–1485. doi: 10.1523/JNEUROSCI.10-05-01479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batistatou A, Volonte C, Greene L A. Mol Biol Cell. 1992;3:363–371. doi: 10.1091/mbc.3.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volonte C, Rukenstein A, Loeb D M, Greene L A. J Cell Biol. 1989;109:2395–2403. doi: 10.1083/jcb.109.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whishaw I Q, Gorny B, Sarna J. Behav Brain Res. 1998;93:167–183. doi: 10.1016/s0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 16.Tracey D. In: The Rat Nervous System. Paxinos G, editor. Vol. 2. New York: Academic; 1985. pp. 311–324. [Google Scholar]
- 17.Li Y, Field P M, Raisman G. J Neurosci. 1998;18:10514–10524. doi: 10.1523/JNEUROSCI.18-24-10514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Field P M, Raisman G. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 19.Guest J D, Hesse D, Schnell L, Schwab M E, Bunge M B, Bunge R P. J Neurosci Res. 1997;50:888–905. doi: 10.1002/(SICI)1097-4547(19971201)50:5<888::AID-JNR24>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.Diener P S, Bregman B S. J Neurosci. 1998;18:779–793. doi: 10.1523/JNEUROSCI.18-02-00779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bregman B S, Kunkel-Bagden E, Schnell L, Dai H N, Gao D, Schwab M E. Nature (London) 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 22.Z’Graggen W J, Metz G A, Kartje G L, Thallmair M, Schwab M E. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro A. Brain Res. 1972;44:313–323. doi: 10.1016/0006-8993(72)90305-8. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Boston: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 25.Wouterlood F G, Jorritsma-Byham B. J Neurosci Methods. 1993;48:75–87. doi: 10.1016/s0165-0270(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz L I, Apostolides P J, Perrone-Bizzozero N, Finklestein S P, Zwiers H. J Neurosci. 1988;8:339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis R, Averill S, Priestley J V, Wilkin G P. J Neurocytol. 1993;22:39–50. doi: 10.1007/BF01183974. [DOI] [PubMed] [Google Scholar]
- 28.Gorgels T G, Oestreicher A B, de Kort E J, Gispen W H. Neurosci Lett. 1987;83:59–64. doi: 10.1016/0304-3940(87)90216-3. [DOI] [PubMed] [Google Scholar]
- 29.Skene J H. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- 30.Benowitz L I, Routtenberg A. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 31.Bell M, Kochanek P, Carcillo J, Mi Z, Schiding J, Wisniewski S, Clark R, Dixon C, Marion D, Jackson E. J Neurotrauma. 1998;15:163–170. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson P, Hillered L, Ponten U, Ungerstedt U. J Cereb Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz J P, Nishiyama N. Brain Res Bull. 1994;35:403–407. doi: 10.1016/0361-9230(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 34.Nieto-Sampedro M, Lim R, Hicklin D J, Cotman C W. Neurosci Lett. 1988;86:361–365. doi: 10.1016/0304-3940(88)90511-3. [DOI] [PubMed] [Google Scholar]
- 35.Rudge J S, Manthorpe M, Varon S. Brain Res. 1985;351:161–172. doi: 10.1016/0165-3806(85)90188-9. [DOI] [PubMed] [Google Scholar]
- 36.Cai D, Shen Y, Bellard M D, Tang S, Filbin M T. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 37.Song H, Ming G, He Z, Lehmann M, Tessier-Lavigne M, Poo M. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 38.Braumann T, Jastorff B, Richter-Landsberg C. J Neurochem. 1986;47:912–919. doi: 10.1111/j.1471-4159.1986.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 39.Haun S E, Segeleon J E, Trapp V L, Clotz M A, Horrocks L A. J Neurochem. 1996;67:2051–2059. doi: 10.1046/j.1471-4159.1996.67052051.x. [DOI] [PubMed] [Google Scholar]
- 40.Bregman B S, Kunkel-Bagden E, Schnell L, Dai H N, Gao D, Schwab M E. Nature (London) 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 41.Berry M, Carlile J, Hunter A. J Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- 42.Neumann S, Woolf C J. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi N R, Fan D P, Giehl K M, Bedard A M, Wiegand S J, Tetzlaff W. J Neurosci. 1997;17:9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui Q, Lu Q, So K F, Yip H K. Invest Ophthalmol Visual Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- 45.Brosamle C, Schwab M E. J Comp Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 46.Kuang R Z, Kalil K. J Comp Neurol. 1990;302:461–472. doi: 10.1002/cne.903020304. [DOI] [PubMed] [Google Scholar]
- 47.Keifer J, Kalil K. Exp Neurol. 1991;111:98–105. doi: 10.1016/0014-4886(91)90055-h. [DOI] [PubMed] [Google Scholar]