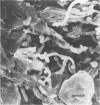
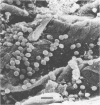
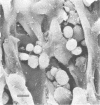
Abstract
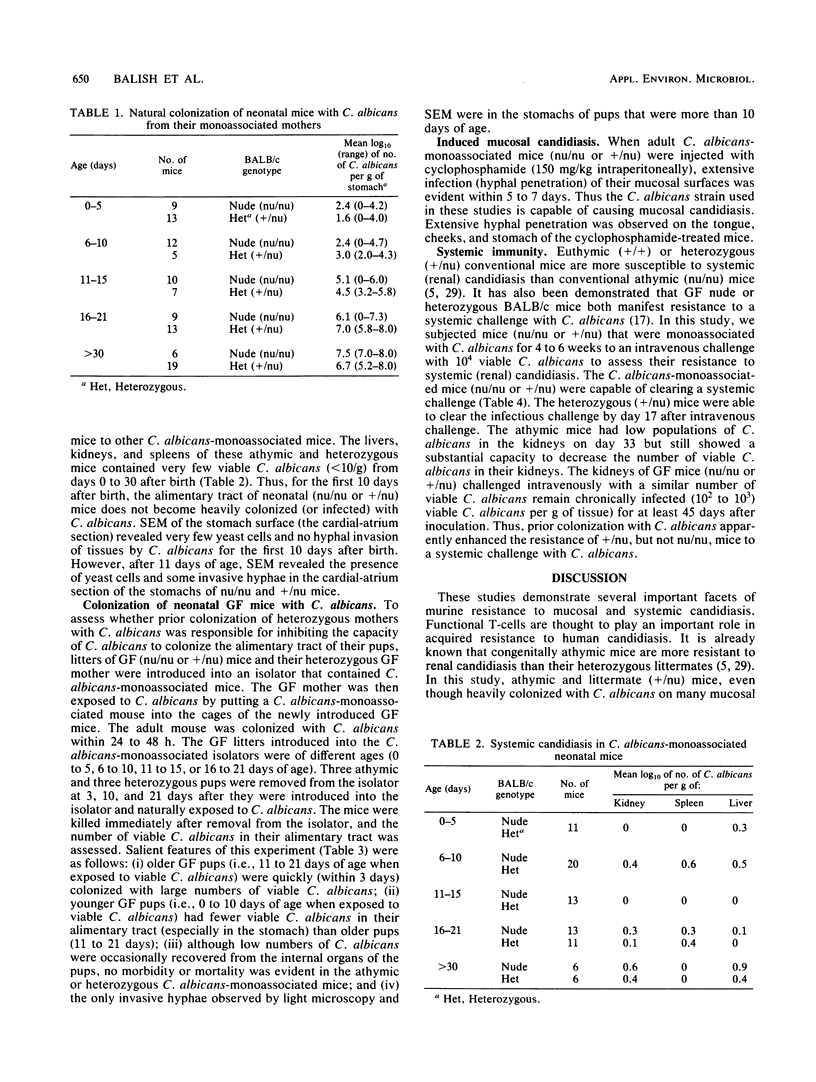
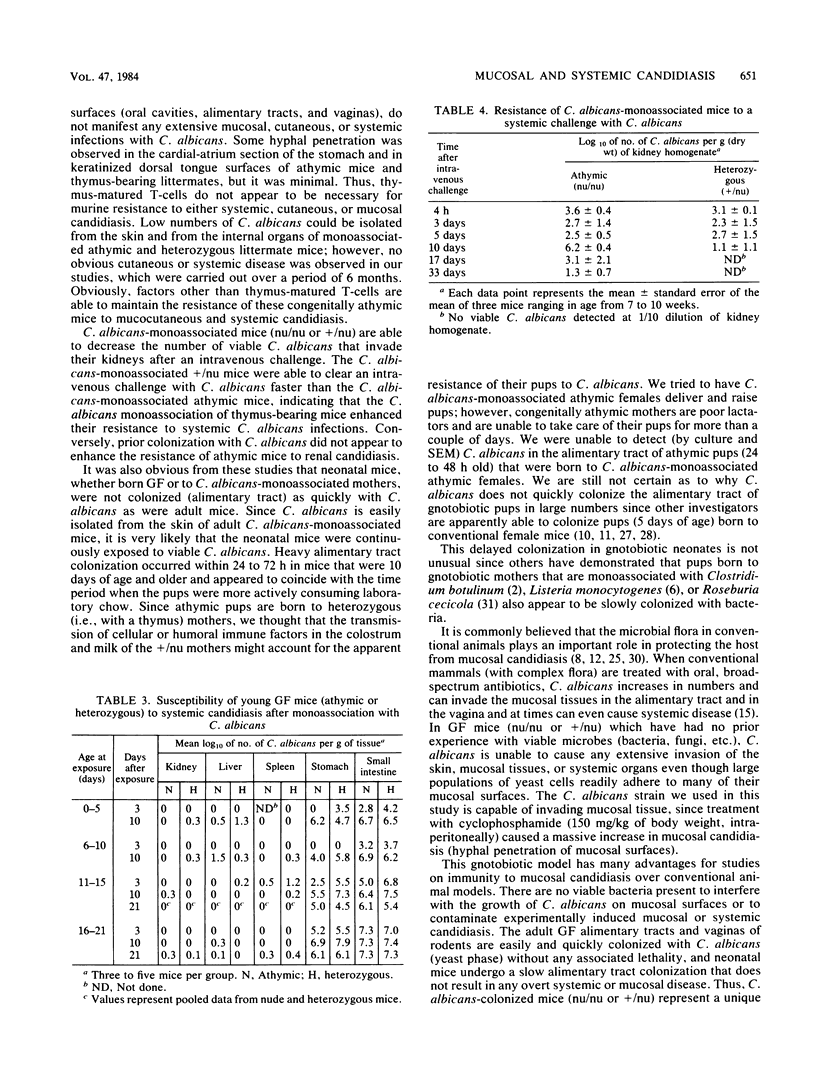
Colony counts, scanning electron microscopy, and light microscopy were used to assess the capacity of Candida albicans to colonize (naturally) and infect the alimentary tract of adult and neonatal (athymic [nu/nu] or heterozygous [+/nu] littermates) germfree BALB/c mice. When exposed to yeast-phase C. albicans, the alimentary tract of adult germfree mice (nu/nu or +/nu) is quickly (within 24 to 48 h) colonized with yeast cells. Neither morbidity nor mortality was evident in any mice that were colonized with a pure culture of C. albicans for 6 months. Yeast cells of C. albicans predominated on mucosal surfaces in the oral cavities and vaginas of adult athymic and heterozygous mice. In both genotypes, C. albicans hyphae were observed in keratinized tissue on the dorsal posterior tongue surface and in the cardial-atrium section of the stomach. Conversely, neonatal athymic or heterozygous mice, born to germfree or C. albicans-colonized mothers, do not become heavily colonized or infected with C. albicans until 11 to 15 days after birth. Although yeast cells adhered to some mucosal surfaces in vivo, neither widespread mucocutaneous candidiasis, i.e., invasion of mucosal surfaces with C. albicans hyphae, nor overwhelming systemic candidiasis was evident in neonatal (nu/nu or +/nu) mice. Thus, even in the absence of functional T-cells and a viable bacterial flora, athymic and heterozygous littermate mice (adult or neonatal BALB/c) that are colonized with a pure culture of C. albicans manifest resistance to extensive mucocutaneous and systemic candidiasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balish E., Phillips A. W. Growth and virulence of Candida albicans after oral inoculation in the chick with a monoflora of either Escherichia coli or Streptococcus faecalis. J Bacteriol. 1966 May;91(5):1744–1749. doi: 10.1128/jb.91.5.1744-1749.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Phillips A. W. Growth, morphogenesis, and virulence of Candida albicans after oral inoculation in the germ-free and conventional chick. J Bacteriol. 1966 May;91(5):1736–1743. doi: 10.1128/jb.91.5.1736-1743.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappler R. R., Maibach H. I., Conant M. A. Mucocutaneous candidiasis or mucocutaneous microbiosis? JAMA. 1978 Jan 30;239(5):428–429. [PubMed] [Google Scholar]
- Clark J. D. Influence of antibiotics or certain intestinal bacteria on orally administered Candida albicans in germ-free and conventional mice. Infect Immun. 1971 Dec;4(6):731–737. doi: 10.1128/iai.4.6.731-737.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Czuprynski C. J., Balish E. Pathogenesis of Listeria monocytogenes for gnotobiotic rats. Infect Immun. 1981 Apr;32(1):323–331. doi: 10.1128/iai.32.1.323-331.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria A., Buckley H., von Lichtenberg F. Gastrointestinal candidiasis in rats treated with antibiotics, cortisone, and azathioprine. Infect Immun. 1976 Jun;13(6):1761–1770. doi: 10.1128/iai.13.6.1761-1770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. H., Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Persistence and spread of Candida albicans after intragastric inoculation of infant mice. Infect Immun. 1981 Feb;31(2):783–791. doi: 10.1128/iai.31.2.783-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Herrera C. Effects of compromising agents on candidosis in mice with persistent infections initiated in infancy. Infect Immun. 1982 Jan;35(1):222–228. doi: 10.1128/iai.35.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector R. F., Domer J. E. Mammary gland contamination as a means of establishing long-term gastrointestinal colonization of infant mice with Candida albicans. Infect Immun. 1982 Nov;38(2):788–790. doi: 10.1128/iai.38.2.788-790.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helstrom P. B., Balish E. Effect of oral tetracycline, the microbial flora, and the athymic state on gastrointestinal colonization and infection of BALB/c mice with Candida albicans. Infect Immun. 1979 Mar;23(3):764–774. doi: 10.1128/iai.23.3.764-774.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C., Guentzel M. N. Mice with persistent gastrointestinal Candida albicans as a model for antifungal therapy. Antimicrob Agents Chemother. 1982 Jan;21(1):51–53. doi: 10.1128/aac.21.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel R. P., Oestreicher E. J., Maley M. P., Macmillan B. G. Inhibition of Candida albicans by Escherichia coli in vitro and in the germfree mouse. J Surg Res. 1973 Jul;15(1):53–58. doi: 10.1016/0022-4804(73)90163-7. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Lee K. W., Balish E. Effect of T-cells and intestinal bacteria on resistance of mice to candidosis. J Reticuloendothel Soc. 1982 Mar;31(3):233–240. [PubMed] [Google Scholar]
- Lee K. W., Balish E. Resistance of germfree mice to systemic candidosis. J Reticuloendothel Soc. 1981 Mar;29(3):241–248. [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Suppression of Candida albicans by human oral streptococci in gnotobiotic mice. Infect Immun. 1973 Nov;8(5):846–849. doi: 10.1128/iai.8.5.846-849.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier-Carpentier F., Kiehn T. E., Armstrong D. Fungemia in the immunocompromised host. Changing patterns, antigenemia, high mortality. Am J Med. 1981 Sep;71(3):363–370. doi: 10.1016/0002-9343(81)90162-5. [DOI] [PubMed] [Google Scholar]
- Moberg L. J., Sugiyama H. Microbial ecological basis of infant botulism as studied with germfree mice. Infect Immun. 1979 Aug;25(2):653–657. doi: 10.1128/iai.25.2.653-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T., Hatano H., Ohnishi N., Sasaki S., Nomura T. Establishment of Candida albicans in the alimentary tract of the germ-free mice and antagonism with Escherichia coli after oral inoculation. Jpn J Microbiol. 1969 Sep;13(3):263–276. doi: 10.1111/j.1348-0421.1969.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Phillips A. W., Balish E. Growth and invasiveness of Candida albicans in the germ-free and conventional mouse after oral challenge. Appl Microbiol. 1966 Sep;14(5):737–741. doi: 10.1128/am.14.5.737-741.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979 Aug;25(2):702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L. M., Cole G. T. SEM studies of adherence of candida albicans to the gastrointestinal tract of infant mice. Scan Electron Microsc. 1981;(Pt 3):73–80. [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Singer C., Kaplan M. H., Armstrong D. Bacteremia and fungemia complicating neoplastic disease. A study of 364 cases. Am J Med. 1977 May;62(5):731–742. doi: 10.1016/0002-9343(77)90876-2. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Savage D. C. Colonization of gnotobiotic mice by Roseburia cecicola, a motile, obligately anaerobic bacterium from murine ceca. Appl Environ Microbiol. 1983 May;45(5):1677–1684. doi: 10.1128/aem.45.5.1677-1684.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Butler T. F., Johnson M. E., Gordee R. S. Colonization of the intestinal tract of conventional mice with Candida albicans and treatment with antifungal agents. Antimicrob Agents Chemother. 1976 May;9(5):787–792. doi: 10.1128/aac.9.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Srivastava K. K. Decontamination of gnotobiotic mice experimentally monoassociated with Candida albicans. Infect Immun. 1975 Dec;12(6):1401–1404. doi: 10.1128/iai.12.6.1401-1404.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilborn W. H., Montes L. F. Scanning electron microscopy of oral lesions in chronic mucocutaneous candidiasis. JAMA. 1980 Nov 21;244(20):2294–2297. [PubMed] [Google Scholar]
- beta-Blockade and congestive cardiomyopathy. Lancet. 1981 Mar 14;1(8220 Pt 1):598–599. [PubMed] [Google Scholar]