Abstract
Electrospinning using natural proteins or synthetic polymers is a promising technique for the fabrication of fibrous scaffolds for various tissue engineering applications. However, one limitation of scaffolds electrospun from natural proteins is the need to cross-link with glutaraldehyde for stability, which has been postulated to lead to many complications in vivo including graft failure. In this study, we determined the characteristics of hybrid scaffolds composed of natural proteins including collagen and elastin, as well as gelatin, and the synthetic polymer poly(ε-caprolactone) (PCL), so to avoid chemical cross-linking. Fiber size increased proportionally with increasing protein and polymer concentrations, whereas pore size decreased. Electrospun gelatin/PCL scaffolds showed a higher tensile strength when compared to collagen/elastin/PCL constructs. To determine the effects of pore size on cell attachment and migration, both hybrid scaffolds were seeded with adipose-derived stem cells. Scanning electron microscopy and nuclei staining of cell-seeded scaffolds demonstrated complete cell attachment to the surfaces of both hybrid scaffolds, although cell migration into the scaffold was predominantly seen in the gelatin/PCL hybrid. The combination of natural proteins and synthetic polymers to create electrospun fibrous structures resulted in scaffolds with favorable mechanical and biological properties.
Keywords: Electrospinning, gelatin, collagen, elastin, PCL, tissue engineering
1. Introduction
Considerable effort has been made to develop hybrid scaffolds that recapitulate the extracellular matrix (ECM) for tissue engineering. Design and fabrication of scaffolds using appropriate biomaterials is a key but limiting step for the creation of functional engineered tissues and their clinical applications. The native ECM is comprised of a complex network of structural and regulatory proteins that are arrayed into a fibrous matrix. It also provides resident cells with specific ligands for cell adhesion and migration, and modulates cell proliferation and function [1–4]. Rather than recapitulating the complex structure of native ECM, investigators including ourselves have attempted to develop constructs that simply mimic native ECM by acting as a scaffold for initiating tissue regeneration upon cell seeding. Development of three-dimensional (3D) scaffolds that can replace the natural ECM will allow the diffusion of nutrient, metabolites and soluble factors until the seeded cells can produce a new functional matrix and regenerate the desired tissue structures [5–7]. There are a number of issues including; pore size and morphology, porosity, mechanical properties, surface properties and biodegradability, which must be considered in scaffold design. Of these, pore size and porosity are critical features that affect cell attachment, proliferation and migration [8–11].
Electrospinning has been used as an effective method to fabricate biomimetic non-woven scaffolds that are comprised of a large network of interconnected fibers and pores. This high porosity allows the efficient exchange of nutrients and metabolic waste between the scaffold and its environment, and provides a high surface area for local and sustained delivery of biochemical signals to the seeded cells [11–16]. On the other hand, the small pore size of reported electrospun scaffolds has thus far shown to be difficult for ingrowth and migration of seeded cells. To date, electrospinning has been used for the fabrication of scaffolds from numerous biodegradable polymers, such as poly(ε-caprolactone) (PCL), poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactide-co-glycolide) (PLGA) and polyurethane (PU); natural proteins have been used as well including collagen, elastin and gelatin [11, 13, 17–23]. Gelatin is a natural protein derived from collagen by controlled hydrolysis and is a heterogeneous mixture of single- or multistranded polypeptides containing between 300 – 4000 amino acids. There are generally two types of gelatin: Type A and Type B. Gelatin Type A is extracted and processed by acidic pretreatment of collagen, whereas gelatin Type B is obtained by alkaline pretreatment. The alkaline pretreatment converts glutamine and asparagine residues into glutamic and aspartic acids, respectively, which leads to higher carboxylic acid content for gelatin Type B than for gelatin Type A. Gelatin has several potential advantages over other natural proteins, such as its biological origin, biodegradability and commercial availability at low cost [24–33].
In this study, we created electrospun hybrid scaffolds that combined synthetic material with natural proteins to overcome limitations seen with scaffolds constructed with either one alone. We characterized the properties of these novel scaffolds and optimized for porosity, strength, cell seeding and migration. Gelatin/PCL hybrid fibrous scaffolds were determined to provide optimal fiber diameter, pore size and strength, leading to enhanced seeding of the electrospun scaffolds with cells. The biological and mechanical properties of these scaffolds should prove useful for their application in the field of tissue engineering and regenerative medicine.
2. Materials and Methods
2.1. Scaffold Fabrication
Unless otherwise noted all reagents were purchased from Sigma Aldrich (St. Louis, MO, USA). Electrospinning has been used to produce a scaffold with fibers that mimic the molecular and structural properties of the ECM. Collagen type I (calf skin), elastin (ligamentum nuchae, Elastin Products Company, Inc. Owensville, MO, USA) and gelatin type B (bovine skin) were dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFP), which has been used as a solvent to suspend proteins and simpler amino acid sequences for various conformational analysis studies [34]. To create the electrospun scaffold, different concentrations of collagen type I (5 and 10% w/v) with elastin (2.5 and 5% w/v) or gelatin alone (5–10% w/v) were dissolved in HFP. The solution containing collagen/elastin or gelatin was then loaded into a 10 ml-syringe, to which a 22-gauge blunt ended needle (spinning nuzzle) was attached. The syringe was raised above the benchtop with the needle pointed downward toward the table surface. The positive output lead of a high voltage supply (25 kV; Glassman High Voltage Inc., NJ, USA) was attached to the needle on the syringe. A thin jet was ejected from the syringe toward the target, i.e. grounded aluminum target (5 cm ×5 cm) coated with polyethylene glycol (PEG), which facilitated removal of the final electrospun 3D mat (100–200 μm thick) from the aluminum target (PEG is dissolved in the rinsing process described below). The target was placed ~15 cm under the needle tip. Various electrospinning parameters including needle gauge, voltage, and distance to target were tested to achieve smooth and uniform fibers free of beads. After scaffold fabrication, the electrospun constructs were exposed to glutaraldehyde vapor for 2 hours at room temperature in a sealed container and then rinsed through three changes of sterile PBS. The scaffold was then disinfected by soaking in 70% ethanol for 20 minutes followed by 3 ×5 min rinses in sterile water and PBS. At this point PEG had been dissolved in the aqueous solutions and the scaffold was detached from the aluminum as an intact sheet.
Due to the potential cytotoxicity of glutaraldehyde, we additionally electrospun scaffolds incorporating poly(ε-caprolactone) (PCL). This method does not use a chemical cross-linking reagent, and PCL scaffolds have been shown to tolerate aqueous solutions, as described below. PCL (average MW10–20000) in different concentrations (1–10% w/v) was added to mixtures of collagen/elastin or gelatin for scaffold fabrication.
2.2. Cell Culture and Scaffold Seeding
Human adipose derived stem cells (hASCs) were prepared as we have previously described [35] and were seeded at passage 2 onto the electrospun scaffolds at a density of 106 cells/cm2 to reach a confluent cell layer. The hASC-seeded scaffolds were then cultured for 2 weeks under dynamic conditions in DMEM-10% FBS at 37°C and 5% CO2.
2.3. Histological Analysis
To detect cell attachment on the electrospun scaffolds as well as cell migration throughout the scaffolds, scaffolds were analyzed after 2 weeks in culture. Cell-seeded scaffolds were fixed in 4% formaldehyde and paraffin-embedded. Sections were cut in 5 μm thicknesses, deparaffinized and stained with hematoxylin and eosin (H&E). Cell-seeded scaffolds were also fixed, permeabilized with 0.5% Triton X-100 and nuclei stained with 4′-6-Diamidino-2-phenylindole (DAPI). The number of DAPI-stained nuclei on the surface of the scaffold, was counted using ImageJ software (free download available at http://rsbweb.nih.gov/ij/).
2.4. Ultrastructural Scaffold Analysis
a) Scanning electron microscopy
For ultrastructural analysis, unseeded and seeded scaffold samples were processed for characterization by scanning electron microscopy (SEM) as described previously [36]. Fiber samples were cut from different locations on the electrospun mat to obtain representative fibers. The samples were mounted onto stubs and sputter coated by gold/palladium (Au/Pd, thickness of ~10nm) using Denton Desk II sputtering before scanning with a Cambridge 360 scanning electron microscope (Scientific. Instruments Ltd., Cambridge, England).
Fiber diameter in the electrospun scaffolds was measured on scanning electron micrographs. Average fiber diameter was determined from measurements taken perpendicular to the long axis of the fibers within representative microscopic fields (10 measurements per field). The size of the pores formed between the fibers was measured using ImageJ software. For each sample, at least 5 scanning electron micrographs were captured at a magnification of 2000x from random spots.
b) Transmission electron microscopy
In order to detect the separate collagen and elastin fibers, the electrospun scaffolds were prepared for transmission electron microscopy (TEM) by directly depositing the samples onto a copper grid, which was coated in advance with a supportive Formvar film followed by a carbon coating. Samples were rinsed in cacodylate buffer and post-fixed in 1% osmium tetroxide for 1 hour. Imbedded samples were thin-sectioned and examined with a JEOL-JEM-1200EX instrument (JEOL Inc., Peabody, MA, USA). The transmission electron microscope was operated at 100 kV.
c) Multiphoton imaging and SHG microscopy
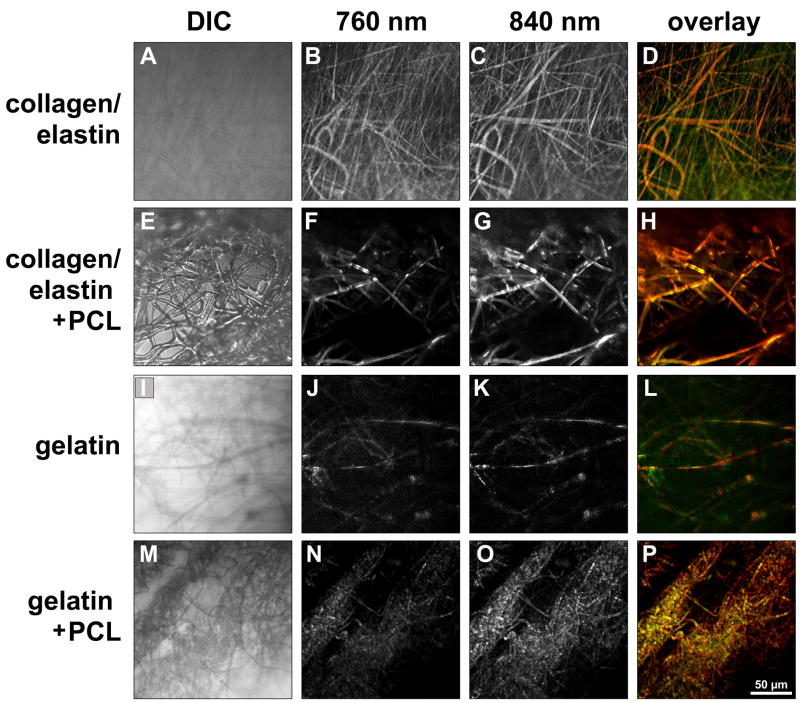
To determine the fiber composition in the electrospun scaffolds, multiphoton imaging and SHG microscopy were performed using a Zeiss LSM 510 META NLO femtosecond laser scanning system (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA), coupled to a software-tunable Coherent Chameleon titanium:sapphire laser (720 nm – 930 nm, 90 MHz; Coherent Laser Group, Santa Clara, CA, USA), and equipped with a high-resolution AxioCam HRc camera with 1300 × 1030 pixels (Carl Zeiss) as previously described [36–38]. Images were collected using an oil immersion Plan-Neofluar 40×/1.3 numerical aperture (NA) DIC, or an oil immersion Plan Apochromat 63×/1.4 NA objective lens (Carl Zeiss). All observations were made using non-treated samples of collagen10%/elastin5% (not cross-linked, cross-linked, and with PCL10%) and gelatin10% (not cross-linked, cross-linked, and with PCL10%) scaffolds. ECM structure-dependent autofluorescence and SHG were induced using wavelengths of 760 nm (elastin) and 840 nm (collagen) as described previously in more detail [36–38]. Non-invasive serial optical horizontal sections of six different areas of each of the specimens were taken in z-steps of 1 μm, 2.5 μm or 5 μm to depths of 50–100 μm.
2.5. Mechanical Testing
The tensile properties of the electrospun 3D fibrous scaffolds were determined in air at room temperature using an Instron 5564 mechanical testing instrument (Instron Corporation; Norwood, MA, USA). The ends of rectangular specimens were mounted vertically between two mechanical gripping units, leaving a 6 mm gauge length, and an extension rate of 1 mm/min was then applied. Data from the load-deformation and stress-strain curves were recorded and the tensile stress at maximal load was obtained from these data for each sample. For each fibrous scaffold, several rectangular specimens were taken and averaged to determine the tensile properties of the entire scaffold. Young’s modulus (E) values were calculated for all the measurements.
2.6. Statistical Analysis
Results are presented as mean ± standard error of mean. Statistical significance was tested using ANOVA. Values of P<0.05 were considered statistically significant.
3. Results
3.1. Scaffold Fabrication and Morphology
Collagen, elastin, gelatin and PCL could be dissolved in HFP and were electrospinnable, either separately or when mixed together. However, creation of electrospun scaffolds required a minimum concentration of protein and polymer. Various electrospinning parameters were tested and conditions that resulted in smooth and uniform fibers were then used throughout the study. Concentrations above a critical value of collagen (5% w/v), elastin (2.5% w/v) and gelatin (3% w/v) were required for fiber formation. SEM analysis of electrospun collagen/elastin and gelatin showed a 3D fibrous mat with fibers in random orientation (Fig. 1).
Figure 1.
Scanning electron micrographs of electrospun fibers consisting of collagen5%/elastin2.5% (A), collagen10%/elastin5% (B), collagen10%/elastin5% cross-linked in glutaraldehyde vapor for 2h (C), collagen10%/elastin5%/PCL10% (D), gelatin5% (E), gelatin10% (F), gelatin10% cross-linked in glutaraldehyde vapor for 2h (G) and gelatin10%/PCL10% (H). The scale bar shown applies to all images and equals 20 μm.
After scaffold fabrication, the electrospun collagen/elastin or gelatin mats were cross-linked in glutaraldehyde vapor for scaffolds not incorporating PCL. Glutaraldehyde was required to intermolecularly cross-link the fibers in the scaffolds to prevent dissolution when placed in the aqueous environment needed for cell culture. Although cross-linked electrospun scaffolds showed a significantly higher tensile strength compared to non-cross-linked scaffolds (cross-linked collagen10%/elastin5% 6.45 ± 0.49 MPa versus noncross-linked collagen10%/elastin5% 1.99 ± 0.28 MPa, P<0.0005 and cross-linked gelatin10% 15.87 ± 0.85 MPa versus non-cross-linked gelatin10% 0.74 ± 0.10, P<0.0001), the porosity decreased dramatically making subsequent cell seeding impossible. SEM images of cross-linked scaffolds showed markedly thickened fibers that in some cases melted into each other (Fig. 1C, G).
In order to avoid the need for cross-linking the electrospun scaffolds using a potential cytotoxic reagent such as glutaraldehyde, PCL was added to the mixture of collagen/elastin or gelatin for scaffold fabrication. A wide range of PCL concentrations (1%–15% w/v) could easily be electrospun into the hybrid fibers; however, higher concentrations of PCL (> 4% w/v) were necessary when used as the sole component to form distinct fibers (data not shown). The incorporation of PCL into the hybrid scaffolds maintained fiber width and porosity, which at the same time stabilized the scaffolds, obviating the need for further cross-linking (Fig. 1D, H).
To further characterize the fibrous scaffold mats we performed TEM analysis. Collagen/elastin scaffolds did not exhibit the 67 nm periodic banding typical of native collagen (data not shown) suggesting that each fiber might be a composite of the two proteins. To explore this hypothesis and further characterize the properties of the electrospun fibrous scaffold network, we performed multiphoton-induced autofluorescence and SHG microscopy on all scaffolds. Multiphoton imaging demonstrated that the fiber mesh included a minority of fibers, which were purely collagen- or elastin-containing fibers. The majority of the fibers in collagen/elastin, collagen/elastin/PCL, gelatin and gelatin/PCL scaffolds were a true hybrid of the base materials (Fig. 2).
Figure 2.
Simultaneous transmitted light-differential interference-contrast (DIC), multiphoton-induced autofluorescence and SHG imaging demonstrates that the majority of the electrospun fibers are autofluorescent at wavelengths of 760 nm (green: elastic fibers) and 840 nm (red: collagen), revealing that the fabricated fibers are composed of both collagen and elastin. Scale bar equals 50 μm.
3.2. Average Fiber Diameter, Pore Size and Tensile Strength
The average fiber diameter, pore size and tensile strength of the various scaffolds are shown in Tables 1 and 2. As shown, the average fiber size grew with increasing concentrations of collagen/elastin, gelatin and PCL. In detail, the average fiber size increased with increasing concentration of collagen and elastin (collagen5%/elastin2.5%: 0.11 ± 0.04 μm; collagen10%/elastin5%: 1.12 ± 0.53 μm) as well as gelatin (gelatin5%: 0.59 ± 0.28 μm; gelatin10%: 0.66 ± 0.50 μm). Similarly, average fiber size for collagen/elastin/PCL was also increased by increasing collagen, elastin and PCL concentrations (collagen5%/elastin2.5%/PCL1%: 0.47 ± 0.20 μm; collagen10%/elastin5%/PCL10%: 1.61 ± 1.10 μm). Average fiber size in gelatin/PCL scaffolds grew with increasing concentrations of gelatin and PCL (gelatin5%/PCL1%: 0.64 ± 0.45 μm; gelatin10%/PCL10%: 0.88 ± 0.28 μm). In contrast to fiber size, pore size decreased with increasing polymer concentration in most gelatin and gelatin/PCL scaffolds (gelatin5%: 50.45 ± 10.34 μm, gelatin10%: 35.01 ± 8.13 μm; and gelatin10%/PCL1%: 79.89 ± 15.55 μm, gelatin10%/PCL10%: 39.25 ± 7.24 μm). An opposite effect was observed in collagen/elastin and collagen/elastin/PCL scaffolds (collagen5%/elastin 2.5%: 1.5 ± 0.45 μm, collagen10%/elastin5%: 14.76 ± 3.19 μm; and collagen10%/elastin5%/PCL1%: 14.51 ± 5.12 μm, collagen10%/elastin5%/PCL10%: 39.06 ± 12.49 μm).
Table 1.
Fiber size, pore size, tensile strength and Young’s modulus of gelatin scaffolds alone and in different combinations with PCL. Data were expressed as mean% ± standard error of mean.
| Scaffold | Fiber size (μm) | Pore size (μm2) | Tensile strength (MPa) | Young's modulus (MPa) |
|---|---|---|---|---|
| Gelatin 5% | 0.59±0.09 | 50.45±10.34 | 2.31±0.23 | 24.54±3.41 |
| Gelatin 10% | 0.66±0.25 | 35.01±8.13 | 0.74±0.10 | 3.72±1.40 |
| Gelatin 5%-PCL 1% | 0.64±0.18 | 45.41±8.07 | NA | NA |
| Gelatin 7%- PCL 7% | 0.84±0.08 | 24.36±6.71 | 6.14±0.38 | 57.74±7.44 |
| Gelatin 10%- PCL 1% | 0.79±0.14 | 79.89±15.55 | NA | NA |
| Gelatin 10%- PCL 5% | 0.85±0.09 | 59.06±7.88 | NA | NA |
| Gelatin 10%- PCL 10% | 0.88±0.09 | 39.25±7.24 | 11.17±0.55 | 138.34±11,42 |
Table 2.
Fiber size, pore size, tensile strength and Young’s modulus of collagen/elastin scaffolds alone and in various combinations with PCL. Data were expressed as mean% ± standard error of mean.
| Scaffold | Fiber size (μm) | Pore size (μm2) | Tensile strength (MPa) | Young's modulus (MPa) |
|---|---|---|---|---|
| Col 5%-Eln 2.5% | 0.11±0.01 | 1.5±0.45 | 1.36±0.15 | 27.94±3.97 |
| Col 5%-Eln 2.5%-PCL 1% | 0.47±0.07 | 8.64±1.16 | NA | NA |
| Col 5%-Eln 2.5%-PCL 5% | 0.72±0.08 | 13.7±4.06 | NA | NA |
| Col 5%-Eln 2.5%-PCL 10% | 0.99±0.1 | 16.31±5.56 | NA | NA |
| Col 10%-Eln 5% | 1.12±0.2 | 14.76±3.19 | 1.99±0.28 | 35.28±7.62 |
| Col 10%-Eln 5%-PCL 1% | 1.07±0.2 | 14.51±5.12 | 1±0.07 | 23.70±0.89 |
| Col 10%-Eln 5%-PCL 5% | 1.19±0.1 | 38.87±11.09 | 0.89±0.09 | 22.43±1.15 |
| Col 10%-Eln 5%-PCL 10% | 1.61±0.2 | 39.06±12.49 | 0.97±0.03 | 20.35±0.80 |
Generally, the tensile strength of electrospun gelatin scaffolds was superior to those composed of collagen and elastin. Gelatin5% (2.31 ± 0.23 MPa) showed a higher tensile strength when compared to collagen/elastin (collagen5%/elastin2.5% 1.36 ± 0.15 MPa; collagen10%/elastin5% 1.99 ± 0.28 MPa). Moreover, electrospun collagen/elastin scaffolds were much more brittle than those made from gelatin. Overall, gelatin/PCL scaffolds were more stable and demonstrated improved tensile strength (gelatin10%/PCL10% 11.17 ± 0.55 MPa, collagen10%/elastin5%/PCL10% 0.97 ± 0.03 MPa).
3.3. Cell-Scaffold Interactions
Cell-scaffold interactions were studied in cell culture by seeding hASCs on the various electrospun fibrous scaffolds for 2 weeks. It was necessary to increase the PCL concentration to at least 5% in the scaffolds in order to maintain their three-dimensional and porous structure without the use of glutaraldehyde. No additional surface modification of the electrospun scaffolds was necessary to ensure robust attachment of hASCs to the scaffold. Histological staining of cell-seeded scaffolds demonstrated extensive cell attachment and confluent coverage of the entire scaffold surface of both collagen10%/elastin5%/PCL10% and gelatin10%/PCL10% scaffolds (Fig. 3–5). This result was confirmed by SEM (Fig. 3). The number of attached cells was higher on gelatin10%/PCL10% when compared to collagen10%/elastin5%/PCL10% (3.76×106 ± 0.20×106 versus 2.77×106 ± 0.22×106 cells/cm2; P<0.026); however, when the number of cells within the body of the scaffold was determined, improved cell migration was seen in gelatin10%/PCL10% when compared to collagen10%/elastin5%/PCL10% (Figs. 4 and 5).
Figure 3.

DAPI-stained hASCs cultured on collagen10%/elastin5%/PCL10% (A) and gelatin10%/PCL10% (C) for 2 weeks. Scanning electron micrographs confirm the attachment of hASCs on collagen10%/elastin5%/PCL10% (B) and gelatin10%/PCL10% (D).
Figure 5.
Images show DAPI-stained hASCs that migrated throughout collagen10%/elastin5%/PCL10% (A-E) and gelatin10%/PCL10% (F-J) scaffolds.
Figure 4.

H&E-stained hASCs cultured on collagen10%/elastin5%-PCL10% (A) and gelatin10%/PCL10% (B) for 2 weeks.
4. Discussion
Electrospinning is a promising fabrication technique that allows efficient and economical production of 3D fibrous scaffolds with high surface area to volume ratio. In the present study, we demonstrated the potential application of electrospun fibrous scaffolds for tissue engineering by studying the biological and physical properties of electrospun collagen/elastin, gelatin and hybrid fibers that include the synthetic polymer PCL. We observed that there is a range of different parameters in the electrospinning process that affect both the scaffold and fibers itself including solvent type, material concentration and viscosity, distance of the collecting target from the spinning nuzzle, the gauge of the needle and voltage, that allow us to obtain uniform fibrous scaffolds. Given that fiber diameter can affect cell attachment, proliferation, migration and cytoskeletal organization, it is likely that “optimal” conditions will need to be determined for each application [37–39]. Our results suggest that the gelatin10%/PCL10% scaffolds may be optimal for cardiovascular tissue engineering applications.
In this study, we used HFP as a solvent for the electrospinning process and fabrication of the fibrous scaffolds. HFP is an ideal organic solvent, in that it allows full extension of the polymer and it evaporates completely after the fiber formation process without leaving any residue on the formed fibers. In non-optimal conditions, we observed that the fibers were non-uniform or beaded with a different pattern of distribution. It has been previously reported that the bead formation occurs as a result of instability of the polymer solution jet, viscosity, surface tension and the net charge density induced by the electrospinning [40, 41]. Our results show that the polymer concentration in the HFP solvent plays a dominant role in determining the fiber morphology and distribution. The average size of the fibers was increased by increasing the concentration of gelatin, collagen and elastin, as well as PCL in the HFP solvent. Although the optimal pore size will likely vary depending on the seeded cell type, a pore enlargement could be expected in vivo as PCL is degraded, which should allow sufficient nutrient and gas exchange as well as further cell infiltration within the scaffold.
Although we could successfully electrospin pure collagen/elastin and gelatin to produce fibrous scaffolds that mimic the molecular and structural properties of the native ECM, these electrospun scaffolds dissolved and lost their 3D structure in aqueous conditions without the use of a cross-linking reagent or other stabilizing additive. It has been reported that the natural intermolecular cross-linking of the molecules is disrupted during processing, resulting in scaffold dissolution in aqueous solutions [3, 16]. Exposing the electrospun scaffold to glutaraldehyde intermolecularly cross-linked the scaffolds, making cell culturing possible; however, cross-linking reduced the porosity dramatically. Currently, only glutaraldehyde has been investigated as a cross-linking agent for electrospun collagen based structures [3, 16, 42, 43]. However, glutaraldehyde-treated materials can be cytotoxic [44]. As previously shown, pure PCL-based fibrous scaffolds were hydrolysis-resistant for culture periods over 40 days [45]. In order to avoid chemical cross-linking of electrospun scaffolds, PCL was added to the mixture of collagen and elastin as well as gelatin during scaffold fabrication. Adding PCL not only reduced the potential cytotoxicity that a chemical cross-linking reagent such as glutaraldehyde can cause, this approach also produced a novel biomaterial with improved mechanical and biological properties.
The mechanical properties of a scaffold are an important design parameter for maintaining stability of the scaffold before the cells can produce their own ECM. In this study the cross-linked electrospun fibrous scaffolds showed a higher tensile strength; however, these scaffolds shrank, and their pore size as well as porosity decreased dramatically during the cross-linking process in glutaraldehyde vapor. Furthermore, a dense layer of fibers hindered cell migration, most likely due to the smaller pore size [15, 46, 47]. The combination of PCL10% with gelatin10% resulted in significantly higher tensile strength compared to gelatin or collagen and elastin alone and resulted in a uniform and pliant fiber mat. Synthetic biodegradable polymers such as PCL, unlike natural ECM components, do not have specific cell-binding sides. Thus cell adhesion to pure synthetic polymers is poor and requires additional modifications such as adsorbing ECM proteins onto the polymer surface [48–51]. In our experiments, no additional surface modifications were necessary facilitating cell attachment onto the fibers of the hybrid scaffolds and both electrospun collagen/elastin/PCL and gelatin/PCL supported attachment and proliferation of hASCs. Similar to our results, Zhang et al. also reported improved migration when using gelatin/PCL fibrous scaffolds [15]. It is possible that the gradual degradation of PCL in the hybrid scaffold creates more space for cell migration and that gelatin, as a natural protein, provides binding sites for cellular attachment and proliferation. Gelatin/PCL hybrid fibrous scaffolds in this study exhibited suitable mechanical properties, which makes them capable as a scaffold for cell adhesion, proliferation and migration in different tissue engineering applications.
5. Conclusions
Native ECM is comprised of a complex network of structural and regulatory proteins that are arrayed into a fibrous matrix. The multifunctional nature of native ECM will need to be considered and hopefully reproduced in the design and fabrication of tissue-engineered scaffolds. The introduction of a protein/polymer hybrid such as gelatin/PCL provides both favorable mechanical properties and binding sites for cell attachment and proliferation. We believe that electrospinning with natural proteins and synthetic polymers can be used to produce tissue-engineered scaffolds that better recapitulate key features of the native ECM including its mechanical and biochemical properties.
Acknowledgments
We would like to thank Dr. Yuhuan Xu for technical assistance with electrospinning. This work was supported by gifts from the Laubisch Fund (WRM, REB) as well as grants AHA EIA 0340087N, P01 HL080111 and R01 HL62448 to WRM and the Deutsche Forschungsgemeinschaft (DFG; Sche701/2-1, 3-1) to KSL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nishimura I, Garrell RL, Hedrick M, Iida K, Osher S, Wu B. Precursor tissue analogs as a tissue-engineering strategy. Tissue Eng. 2003;9(Suppl 1):S77–89. doi: 10.1089/10763270360696996. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen LL, D’Amore PA. Cellular interactions in vascular growth and differentiation. Int Rev Cytol. 2001;204:1–48. doi: 10.1016/s0074-7696(01)04002-5. [DOI] [PubMed] [Google Scholar]
- 3.Shields KJ, Beckman MJ, Bowlin GL. Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Engineering. 2004;10:1510–1517. doi: 10.1089/ten.2004.10.1510. [DOI] [PubMed] [Google Scholar]
- 4.Schneck DJ. The Biomedical Engineering Handbook. Springer: CRC press, IEEE Press; 2000. [Google Scholar]
- 5.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 6.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(suppl 1):SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 7.Nerem RM. Tissue engineering: confronting the transplantation crisis. Proc Inst Mech Eng. 2000;214(1):95–9. doi: 10.1243/0954411001535273. [DOI] [PubMed] [Google Scholar]
- 8.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Holtorf HL, Datta N, Jansen JA, Mikos AG. Scaffold mesh size affects the osteoblastic differentiation of seeded marrow stromal cells cultured in a flow perfusion bioreactor. J Biomed Mater Res A. 2005;74(2):171–180. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 10.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues–state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12(1):107–124. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 11.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 12.Chew SY, Wen J, Yim EKF, Leong KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6:2017–2024. doi: 10.1021/bm0501149. [DOI] [PubMed] [Google Scholar]
- 13.Bhattarai SR, Bhattarai N, Yi HK, Hwang PH, Cha DI, Kim HY. Novel biodegradable electrospun membrane: scaffold for tissue engineering. Biomaterials. 2004;25:2595. doi: 10.1016/j.biomaterials.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Zong X, Ran S, Kim KS, Fang D, Hsiao BS, Chu B. Structure and morphology changes during in vitro degradation of electrospun poly(glycolide-co-lactide) nanofibers membrane. Biomacromolecules. 2003;4(2):416–423. doi: 10.1021/bm025717o. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater. 2005;72(1):156–165. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- 16.Boland ED, Matthews JA, Pawlowski KP, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Frontiers in Bioscience. 2004 May;(9):1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 17.Kim K, Yu M, Zong X, Chiu J, Fang D, Seo YS, et al. Control of degradation rate and hydrophilicity in electrospun non-woven poly(D,L-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24:4977–4985. doi: 10.1016/s0142-9612(03)00407-1. [DOI] [PubMed] [Google Scholar]
- 18.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res. 2004;70B(2):286–296. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 19.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res. 2003;67A(4):1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 21.Shin M, Yoshimoto H, Vacanti JP. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 2004;10(12):33–41. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- 22.Mo XM, Xu CY, Kotaki M, Ramakrishna S. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25(10):1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 23.Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res. 2003;67B(2):675–679. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
- 24.Rose P. Encyclopedia of polymer science andengineering. New York: Wiley; 1987. [Google Scholar]
- 25.Johns P, Courts A. The science and technology of gelatin. London: Academic; 1977. [Google Scholar]
- 26.Guidoin R, Marceau D, Rao TJ, King M, Merhi Y, Roy PE, et al. In vitro and in vivo characterization of an impervious polyester arterial prosthesis: The Gelseal Triaxial graft. Biomaterials. 1987;8:433–441. doi: 10.1016/0142-9612(87)90079-2. [DOI] [PubMed] [Google Scholar]
- 27.Jonas RA, Ziemer G, Schoen FJ, Britton L, Castaneda AR. A new sealant for knitted Dacron prostheses minimally crosslinked gelatin. J Vasc Surg. 1988;7:414–419. doi: 10.1067/mva.1988.avs0070414. [DOI] [PubMed] [Google Scholar]
- 28.Marois Y, Chakfe NL, Deng X, Marois M, How T, King MW, Guidoin R. Carbodiimide cross-linked gelatin: a new coating for porous polyester arterial prostheses. Biomaterials. 1995;16:1131–1139. doi: 10.1016/0142-9612(95)93576-y. [DOI] [PubMed] [Google Scholar]
- 29.Tabata Y, Hijikata S, Ikada Y. Enhanced vascularization and tissue granulation by basic fibroblast growth factor impregnated in gelatin hydrogels. J Control Release. 1994;31:189–199. [Google Scholar]
- 30.Cortesi R, Nastruzzi C, Davis SS. Sugar cross-linked gelatin for controlled release: microspheres and disks. Biomaterials. 1998;19:1641–1649. doi: 10.1016/s0142-9612(98)00034-9. [DOI] [PubMed] [Google Scholar]
- 31.Li JK, Wang N, Wu XS. Gelatin nanoencapsulation of protein/peptide drugs using an emulsifier-free emulsion method. J Microencapsul. 1998;15:163–172. doi: 10.3109/02652049809006846. [DOI] [PubMed] [Google Scholar]
- 32.Choi YS, Hong SR, Lee YM, Song KW, Park MH, Nam YS. Study on gelatin-containing artificial skin: I. Preparation and characteristics of novel gelatin-alginate sponge. Biomaterials. 1999;20:409–417. doi: 10.1016/s0142-9612(98)00180-x. [DOI] [PubMed] [Google Scholar]
- 33.Ulubayram K, Nur Cakar A, Korkusuz P, Ertan C, Hasirci N. EGF containing gelatin-based wound dressings. Biomaterials. 2001;22:1345–1356. doi: 10.1016/s0142-9612(00)00287-8. [DOI] [PubMed] [Google Scholar]
- 34.Hong DP, Hoshino M, Kuboi R, Goto Y. Clustering of fluorine-substituted alcohols as a factor responsible for their marked effects on proteins and peptides. J Am Chem Soc. 1999;121:8427–8433. [Google Scholar]
- 35.Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, Heydarkhan S, Xu Y, Zuk PA, et al. Cells Tissues Organs. Human Adipose Stem Cells: A Potential Cell Source for Cardiovascular Tissue Engineering. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 36.Opitz F, Schenke-Layland K, Richter W, Martin DP, Degenkolbe I, Wahlers T, et al. Tissue engineering of ovine aortic blood vessel substitutes using applied shear stress and enzymatically derived vascular smooth muscle cells. Ann Biomed Eng. 2004;32(2):212–222. doi: 10.1023/b:abme.0000012741.85600.f1. [DOI] [PubMed] [Google Scholar]
- 37.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 38.von Recum AF, van Kooten TG. The influence of micro-topography on cellular response and the implications for silicone implants. J Biomater Sci Polym Ed. 1995;7(2):181–198. doi: 10.1163/156856295x00698. [DOI] [PubMed] [Google Scholar]
- 39.Powell HM, Kniss DA, Lannutti JJ. Nanotopographic control of cytoskeletal organization. Langmuir. 2006;23:5087–5094. doi: 10.1021/la052993q. [DOI] [PubMed] [Google Scholar]
- 40.Deitzel J, Kleinmeyer J, Harris D, Tan N. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261. [Google Scholar]
- 41.Fong H, Chun I, Reneker D. Beaded nanofibers formed during electrospinning. Polymer. 1999;40:4585. [Google Scholar]
- 42.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of Collagen Nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 43.Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26(30):5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Vanwachem PB, Vanluyn MJA, Damink L, Dijkstra PJ, Feijen J, Nieuwenhuis P. Biocompatibility and issue regenerating capacity of cross-linked dermal sheep collagen. J Biomed Mater Res. 1994;28(3):353–356. doi: 10.1002/jbm.820280310. [DOI] [PubMed] [Google Scholar]
- 45.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2(4):377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 47.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12(5):1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 48.Smetana KJ. Cell biology of hydrogels. Biomaterials. 1993;14(14):1046–1450. doi: 10.1016/0142-9612(93)90203-e. [DOI] [PubMed] [Google Scholar]
- 49.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 50.Nikolovski J, Mooney DJ. Smooth muscle cell adhesion to tissue engineering scaffolds. Biomaterials. 2000;21(20):2025–2032. doi: 10.1016/s0142-9612(00)00079-x. [DOI] [PubMed] [Google Scholar]
- 51.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;1:531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]