Abstract
While magnetoencephalography (MEG) is of increasing utility in the assessment of pediatric patients with seizure disorders, this indication reflects only a part of the clinical potential of the technology. Beyond epilepsy, a broad range of developmental psychiatric disorders require the combined offerings of spatial and temporal resolution, along with direct sensitivity to neural electrical activity, that are offered by MEG. This article reviews the application of MEG in the study of auditory processing as an aspect of language impairment in children. Potential application is elaborated in the clinical case of autism spectrum disorders, a devastating disorder with prevalence of 1 in 150, poorly served by alternative imaging modalities. MEG offers both spatial and temporal insights, tentatively described as “electrophysiological signatures”. Results demonstrate the sensitivity of MEG for detection of abnormalities of auditory processing in ASD and their clinical correlates. These findings offer promise for the comprehensive assessment of developmental neuropsychiatric disorders such as autism, but also suggest avenues for the development of MEG technology to adequately meet the needs of such populations, as well as providing conduits to basic sciences including neurobiology and genetics.
INTRODUCTION
Autism spectrum disorders (ASDs) are a set of developmental disabilities diagnosed in childhood, that have significant impact throughout the child and adolescent’s development. Several characteristic behavioral symptoms may be manifest, including impaired social interaction, language and communication difficulties, and repetitive movements and stereotyped behaviors. Data from the Center for Disease Control estimates the prevalence of this devastating family of conditions to be as high as 1 in 150 children (Rice, 2007).
Implied by the name, ASD covers a spectrum of conditions, and thus one observes profound phenotypic heterogeneity in individuals with an ASD diagnosis. Although ASD is recognized as having a significant genetic component, genetic heterogeneity is suspected (Lamb et al., 2002). Additionally, children with an ASD diagnosis often exhibit symptoms of other neurological or psychiatric conditions (Leyfer et al., 2006), or at least manifestations sufficiently characteristic of other disorders as to engender difficulty establishing a clear diagnosis (Reiersen et al., 2007). Finally, although diagnosed in childhood, individuals with ASD show impairments into adulthood, especially with regard to communication and social integration.
At present, behavioral and pharmacological treatments are only moderately effective. Considered a neurodevelopmental disorder, it is likely that better understanding of the neurobiological abnormalities associated with ASD is needed to develop strategies for early identification and possible treatments. Thus, whereas clinical diagnosis and ongoing assessment is presently made on the basis of observed behavioral characteristics, underlying structural and functional brain abnormalities may better characterize this heterogeneous disorder, allowing more effective treatment and therapy monitoring (Edgar et al., 2007). To date, however, conventional radiologic imaging technologies, such as MRI and CT, have proven largely inadequate, due to the general absence of structural or physiologically identifiable lesions (Lewis 1996).
Given that brain activity is marked by rapid electrical events progressing rapidly through networks of functionally linked brain areas, comprehensive characterization of brain function should consist of spatial and temporal measures. Thus, a role exists for spatial imaging methodologies sensitive to neural electrical activity in real time. Functional imaging modalities such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) provide excellent spatial localization of functional activity, but image functional brain activity via the relatively slow hemodynamic response associated with neural activity (with significantly delayed temporal response characteristics).
Electroencephalography (EEG) offers a real-time method of measuring electrical neural activity, either through analysis of ongoing oscillatory activity or analysis of discrete phase-locking of these oscillations to the processing of specific events, resulting in event-related brain potentials (ERPs). Examined at the scalp, ERPs have been used to characterize specific events in the time domain of a functional response to a stimulus. ERP interpretation is often limited as most cognitive research is conducted using low channel counts (although this is rapidly changing). Analysis of ERPs, however, is also compromised by the smearing of electrical brain activity due to the varying electrical conductivities of intervening tissues (e.g., cerebrospinal fluid (CSF), scalp, skull). The temporal resolving power, or degree to which latencies of separate evoked responses can be precisely determined, is limited given the blurring and phase errors associated with the differing electrical conductivity paths. For auditory ERPs, scoring of left and right ERP auditory components is often difficult given the superposition of left and right hemisphere auditory activity at midline EEG sites.
Magnetoencephalography (MEG), the “magnetic cousin” of EEG, provides a methodology with high temporal resolution (~ms), relatively sharper component peak definition, and reasonable spatial resolution (~mm), especially for cortical activity. For example, Leahy et al. (1999) showed an average spatial localization error of 3 mm with 61-channel MEG and 7–8 mm with up to 64-channel EEG across 32 dipole sources. Whereas the ability of MEG to resolve and localize brain activity is under continuous discussion, evaluation, and improvement, numerous studies have demonstrated the utility of MEG source localization and this technique is now commonly used in the clinical definition of eloquent cortex prior to neurosurgical procedures (Nakasato & Yoshimoto, 2000; Alberstone et al., 2000; Schiffbauer et al., 2002; Ganslandt et al., 2004; Lee et al., 2006), as well as the localization of the source(s) of interictal epileptiform activity (Knowlton et al., 2006). However, separate from the ability to localize sources of brain activity, several other aspects of MEG offer attractive utility: (i) the temporal resolution (~ms), (ii) the fact that magnetic fields are not smeared as they travel through the scalp and skull, and (iii) the orientation of primary and secondary neurons in auditory areas in a way that allows examination of left and right auditory activity separately. Together, these facets of MEG hold promise for the study of brain disorders associated with impaired perception and processing of auditory signals.
Auditory neuromagnetic event related field (ERF) data are presented and reviewed below, with a focus on using MEG to accurately characterize auditory perception and processing, and thus provide insight into the underlying substrates and mechanisms of the language and communication impairments commonly observed in individuals with ASD. Specific attributes of the MEG signal that form dependent variables in studies of psychiatric disorders include the amplitude and, especially, the latency of auditory ERF components of neuromagnetic activity elicited by perception and processing of auditory stimuli.
Data presented and reviewed below focus on the role of MEG in approaching autism spectrum disorders by characterizing auditory processing as a potential mediator of language and communication impairment. Naturally, analogous experiments can be considered, and in some cases are underway, for characterizing other aspects of the autism phenotype (most notably assessing face-processing as an index of social interaction impairment; Bailey et al., 2005; Kylliainen et al., 2006). MEG research in these areas, although promising, is in its infancy, and thus results are necessarily preliminary. Present results, however, permit the development of specific hypotheses relating to the development of abnormal auditory processes and language impairment(s). Results also suggest strategies for a more thorough investigation of language impairments via incrementally more complex and abstract linguistic challenges.
Of note, most data presented are in children and adolescents 8 years old and above. The rationale for this is threefold. First, the emergence of clear auditory evoked components is a function of age, with a clear N1 observed around the ages of 8–10 years. N1 represents the electric analog of the M100 magnetic response described in many studies below. Second, sensitivity to subject motion precludes the study of children who are unable to maintain head position during the entire exam. This is very difficult for very young children. Third, the fixed sensor geometry in all whole-head MEG systems, which can accommodate both children’s and adult’s heads, is not optimal for very young children. Future developments in MEG technology hold promise for the extension of the approaches discussed in this manuscript to a younger population.
Frequency Encoding in the Brain of Children with Autism
Neural correlates of the recognition of basic features of auditory stimuli are observed in the amplitude and latency of auditory ERF components as early as 100ms (M100) (Roberts & Poeppel, 1996). In particular, the M100 latency shows a characteristic dependence on the acoustic properties of the stimuli, with an earlier M100 response to high frequency tones compared to low frequency tones (Roberts et al., 2000). These early properties define subsequent phonological identification and, as such, M100 latency modulation can be interpreted as an indicator of the integrity of early sound perception. It is assumed that accurate extraction and processing of basic auditory features is necessary for subsequent processing of more complex auditory and linguistic information.
M100 latency prolongation is most dramatic in the 100Hz to 1kHz range, with a ~30–40ms difference, or “dynamic range”, between earlier responses to high frequency tones and later responses to low frequency tones. The 100Hz to 1kHz spectral range encompasses the first formant (F1) position of most vowel sounds. As such, distinguishing between different frequencies within this range is of great importance for communication. In preliminary studies examining ASD, whereas the general form of the M100 latency response as a function of tone frequency was intact in children with ASD and typically developing controls (conforming to a 1/frequency model), the dynamic range in children with ASD (the latency difference between M100 responses to nominal high, 1kHz, and low, 100Hz, frequency stimuli) was markedly reduced in the right hemisphere of children with autism, showing only a 15–20 ms prolongation at low frequencies (100Hz) compared to high frequencies (1kHz) (Gage et al., 2003a). These observations led to the hypothesis that early detection systems and frequency analysis problems occurring at ~100ms may be impaired in children with autism, perhaps leading to imprecisely encoded auditory representations, and degraded “processed” data being input to subsequent neural systems. Findings also pointed to atypical hemispheric differences in the functional activity of auditory cortex processes in children with autism. M100 latency data from a representative subject with autism is shown as a function of stimulus frequency in Figure 1, and illustrates the general form of the latency dependence as well as the diminished dynamic range of latency variation in autism.
Figure 1.
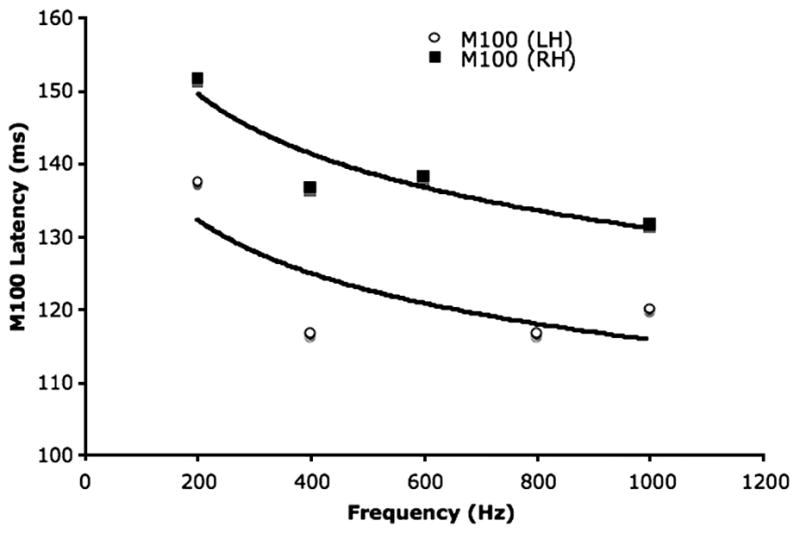
Latency of neuromagnetic evoked response, M100, as a function of tone frequency in a typical child with autism. Both left and right hemisphere responses show a characteristic latency inverse dependence on tone frequency, but note that the dynamic range (latency difference between M100 responses to low vs high frequency tones) is low compared with literature values in adults and typically developing children.
Although published studies have focused on the M100, both earlier and later ERF components can be examined. A recent report from our group confirmed the anecdotal observation that an earlier 50ms neuromagnetic component (M50), present but generally weaker than M100 in adults, is more robustly observed and is of greater amplitude than the M100 in a young typically developing population as well as in children with ASD (both groups 8–18 yrs) (Oram Cardy et al., 2004). Studies examining 50 and 100ms auditory responses provide an opportunity to characterize similar and/or distinct patterns of latency modulation in earlier (M50) vs. later (M100) stages of auditory sound perception. These analyses are being pursued as part of a comprehensive approach to study multiple components of the auditory ERF in order to identify the stage at which auditory/linguistic processes deviate from patterns observed in typical development.
Developmental trajectory of M100 latency
The use of ERPs and ERFs in developmental populations presents several obstacles that prevent testable hypotheses from appearing more often in the literature (for a detailed discussion of the problems associated with studying children and adolescents see DeBoer et al., (2005)). Whereas there is general consensus on how to score ERP/ERF auditory components, the latency and morphology of auditory components across development are only recently being defined. Paetau et al. (1995) discuss the changing form of electrophysiological responses to auditory stimulation (using both tones and speech elements) as a function of typical childhood and adolescence development. They noted that auditory components differ as a function of age, and observed a tendency for major ERPs (e.g., N1) to become stronger and to occur at an earlier latency with increasing age. Although longitudinal data in the same subjects is not yet available for a broad age range, horizontal snapshots across subjects of different ages confirm these broad observations (e.g. Oram Cardy et al., 2004). For research in this area to develop, understanding the normal changes in the auditory ERP/ERF as a function of age is needed so that findings across laboratories, studying different populations, can be legitimately compared.
As previously noted, in typical development, the M100 response shows a progressive and rapid decrease in latency during late childhood and adolescence (presumed secondary to ongoing white matter myelination). This latency shift is delayed in children with autism (and essentially not evident in the right hemisphere) (Gage et al. 2003b). Recent pilot work extended previous M100 latency findings, examining M100 latencies from each hemisphere in children with typical development and in children with an ASD using whole-head 275-channel MEG. MEG data was collected in four separate runs, each run consisting of over 100 trials (sampled at 1200Hz). Mean M100 amplitude (a root-mean square (RMS) measure) and standard error of the mean (SEM) measures were computed using a group of ~130 sensors covering each of the left and right hemispheres. In the left hemisphere (Figure 2a,b), both children with ASD and typically developing (TD) children showed earlier M100 latencies with increasing age (for TD, the correlation was r = −0.69; for children with ASD, the correlation was r = −0.43). As shown in Figure 2(a,b), M100 latencies, however, appear to be systematically prolonged in ASD compared to TD. This effect appears more pronounced for younger individuals. The group latency difference was significant in the right hemisphere (even in this small sample). Mean (± SEM) latency values for the left hemisphere were: TD = 120.7±1.5ms, children with ASD = 130.2±9.4ms, p=0.3; right hemisphere values were: TD = 117.4±3.4ms, children with ASD = 136.8±4.4ms, p<0.01 (Figure 2c). Although present findings show a developmental trajectory of M100 latency that is not significantly different than that of typical development, the finding reported in our previous study of longer M100 latencies in ASD (Gage et al., 2003b) was replicated. Furthermore, in these small sample studies, differences in the clinical and behavioral scores of the children with autism certainly exist, precluding direct comparison between studies but motivating further studies. Additionally, an influence of attention on M100 latency must be considered possible and it is not established in the above studies that the degree of attention is similar between children with autism and typical development. Studies focusing on directing attention are warranted. Nonetheless, differences in M100 latency between groups remain reproducibly resolved. Interestingly emerging from both studies are indications that the developmental latency abnormality occurs primarily in the right temporal lobe.
Figure 2.
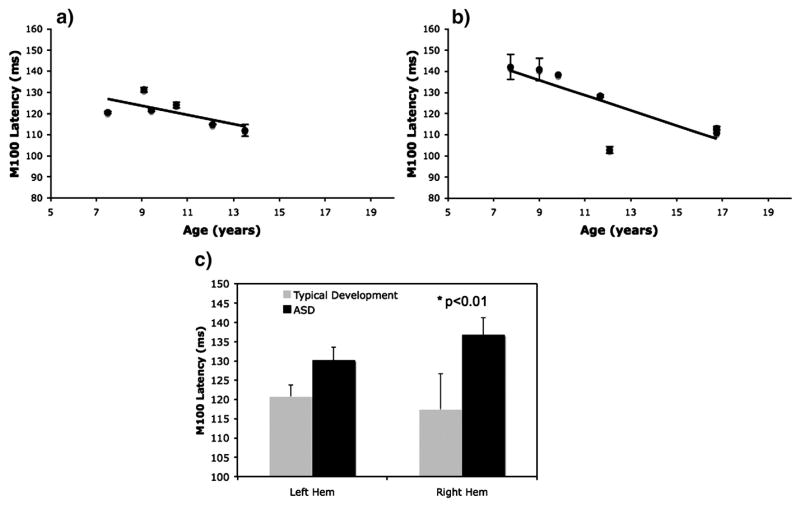
Age dependence of M100 latency. Left hemispheric M100 latency responses to 1kHz sinusoidal tones in (a) typical development, and (b) children with an ASD, both tend to show maturational shortening with increasing age, but note that (c) overall latencies (both left and right hemispheres) are prolonged for children with an ASD compared to age-matched controls.
Asymmetry in the Source Localization
We have also been examining structural asymmetries within the auditory system. Although the human brain is generally symmetric, the existence of specific structural and functional asymmetries is one of the foundations of modern neuroscience. From a structural perspective, a majority of the population shows greater width and length in right versus left frontal regions (Weinberger et al., 1982; Durara et al., 1991), but the left occipital region is wider and larger than the right occipital region (Wada et al., 1975; Lemay and Kido, 1978; Durara et al., 1991). Particular attention has been drawn to cerebral asymmetries in the region of the planum temporale (PT), especially because of this region’s importance in language function. The PT is a triangular region of cortex lying caudal to the transverse auditory gyrus of Heschl on the superior surface of the temporal lobe. It has been shown to be typically larger on the left than the right in both post mortem (Geschwind & Levitsky, 1968) and MRI (Larsen et al., 1989; Steinmetz et al., 1989) investigations; some reports suggest that PT asymmetry is explicitly related to left-hemisphere language dominance (Karbe et al., 1995).
Examining subjects without neurological or psychiatric dysfunction, Elberling and colleagues (1982) were the first to report that the pure-tone M100 source location for the right hemisphere is normally shifted anterior to that seen for the left hemisphere. This basic observation has been confirmed for right-handed male subjects by several other investigators (Reite et al., 1989; Makela et al., 1993; Eulitz, Diesch, Pantev, Hampson, & Elbert, 1995; Tiihonen et al., 1998; Ohtomo et al., 1998; Edgar et al., 1997; Rockstroh et al., 2001). Reite and colleagues further reported that right-handed male patients with schizophrenia fail to show the expected pattern of right-left asymmetry for the location of the M100 dipole source as evaluated using MEG (Reite et al., 1997; Rojas et al., 1997; Teale et al., 2000), and others have provided independent supporting data (Tiihonen et al. 1998; Edgar et al., 1997; Rockstroh et al., 2001).
At present, the neurogenetic and neurodevelopmental factors that guide formation of an asymmetric brain can only be speculated upon, an unfortunate situation since anomalies in cerebral asymmetries have been reported in several clinical populations. In particular, two recent studies have compared M100 structural asymmetries in individuals with schizophrenia and individuals with dyslexia. Heim et al., (2004) examined hemispheric asymmetry of the M100 in schizophrenia patients, dyslexic adults, and control subjects. Control subjects showed the typical finding of sources being more anterior in the right than in the left perisylvian region. In contrast, both schizophrenia patients and dyslexic subjects displayed a symmetrical M100 configuration. While in subjects with dyslexia the alteration appeared to originate in the right hemisphere, left-hemispheric deviations were thought to contribute to reduced asymmetry in patients with schizophrenia. Edgar et al. (2006) also replicated prior observations of a significant right-left M100 anterior-posterior positional asymmetry for normal control subjects and the reduction of this asymmetry for the male schizophrenic subjects. Subjects with dyslexia also showed reduced M100 positional asymmetries. Thus, this appears to be a reproducible observation in pathologic conditions, but may not be specific for any particular pathology or disorder.
Deviations from typical asymmetries have figured prominently in accounts of the etiologies of both schizophrenia and dyslexia, but given the similarities in M100 profiles for the two conditions, it is unlikely that M100 positional asymmetries account for the cardinal features of either condition. On the other hand, there may be (as yet unspecified) similarities in auditory processing dysfunction for the two conditions – similarities that are related to the observed M100 findings. For example, both schizophrenic and dyslexic subjects have been suggested to demonstrate abnormalities in auditory memory and the processing of rapidly presented auditory information (e.g., Todd et al., 2000 (schizophrenia); Tallal et al., 2000 (dyslexia)). Ongoing studies in our laboratory are examining both structural and functional measures in central auditory system measures to examine which structural and functional abnormalities are specific to ASD, and which relate to language impairment in general.
In a recent study (Schmidt et al., 2007a), we examined M100 source localization data from the left and right hemisphere of adults (n=10), typically developing children (n=8), and children with autism spectrum disorders (n=8). In control adults, replication of previously described anterior-posterior M100 asymmetry in adult auditory cortex sources was observed (Edgar et al., 2006), with an approximately 8mm anterior bias in the right hemisphere. Similar findings were observed in children with typical development (age 8–18 yrs), with a similar statistically significant 8mm difference. As typical M100 asymmetry was not observed in the two youngest subjects (<10yrs), a developmental trajectory to this phenomenon cannot be excluded (linear correlation of hemispheric source difference with age in this small sample, although positive, was not significant). In stark contrast, in the age-matched sample of children with autism spectrum disorders, no evidence for typically M100 source asymmetry was observed (in fact, the mean displacement was −1mm, slightly leftward biased). Although non-specific, these findings suggest that abnormal (or absent) hemispheric asymmetry in auditory cortex may be a sensitive marker for pathologic conditions, perhaps emerging in childhood or adolescence. Finally, pooling the children with typical development and autism, Schmidt et al. (2007) noted an interesting correlation between hemispheric M100 asymmetry and performance on an assessment of language performance (Clinical Evaluation of Language Fundamentals (CELF)-4 test), suggesting that decreased hemispheric asymmetry may be associated with language impairment, and thus is of functional and clinical significance.
Mismatch Field and Change Detection
Human speech consists of a stream of syllables, typically occurring at a rate of about 4Hz, with syllable durations of approximately 250ms. Recognizing and processing syllable changes is critical for the successful comprehension of human speech. It has been suggested that a deficiency in the processing of complex sounds may underlie language impairment in infants, children and adults (Gage et al., 2003a,b; Kuhl 1994; Tecchio et al., 2003; Ornitz 1989; Lincoln et al, 1995), including children with autism (Gage et al., 2003a,b). In particular, it is hypothesized that the successful processing of polysyllabic speech elements and recognizing differences between syllabic tokens is critical for speech comprehension. An electrophysiological correlate of the detection of a change in sensory stimuli exists in the form of the mismatch negativity (MMN), described by Näätänen (2001, 2003) and colleagues (Näätänen et al., 1987, 1997). The MMN provides a measure of the pre-attentive detection of a difference in the attributes of an infrequently presented “deviant” stimulus as compared to the stimulus properties represented in the memory trace of the frequently presented “standard” stimuli. Relevant stimulus differences might exist, for example, in the form of stimulus amplitude or spectral content. In fact any salient stimulus difference may serve to elicit a mismatch response. The MMN is obtained by subtracting the ERP elicited by the standard stimulus from the ERP elicited by the deviant. MMN responses can be obtained using visual, haptic, and auditory stimuli. For auditory stimuli, the MMN occurs approximately 200ms post-stimulus. The strength, or magnitude, of the MMN increases with larger differences between standard and deviant acoustic tokens.
In terms of language, phonological features may also provide differences, the detection of which is reflected in the MMN. It has been shown that the MMN response is greater in subjects for whom phonetic differences between stimuli cross a phoneme category boundary (Näätänen 2001) or is otherwise phonologically significant (Näätänen et al., 1997; Peltola et al., 2003; Winkler et al., 1990). As such, the MMN can be used to assess a subject’s ability to detect change in linguistic stimuli. Analogous to the MMN, the auditory MEG magnetic mismatch field (MMF) can be obtained. Brain areas generating the scalp recorded MMF have been localized near auditory cortex. MMN and MMF thus serve as passive, pre-attentive probes for abnormalities in auditory processing relevant to speech/language, providing neuronal indices of speech sound discrimination, processes crucial for language processing and language development.
Whereas studies of MMN/MMF in autism show conflicting results in children with ASD compared to TD controls (Ceponiene et al. 2003, Jansson-Verkasalo E 2003), studies from our group revealed delayed MMF responses in autism (with language impairment) compared to age-matched TD controls, with delays in subjects with autism up to 50ms (Oram Cardy et al. 2005a). Delays did not appear to be specific to speech, as delays were equivalent for vowel contrasts (/u/ vs. /a/) and acoustically matched tone contrasts (300Hz vs. 700Hz sinusoidal tones). This finding, nonetheless, is of interest as 50 ms represents a considerable fraction of syllable duration. Delays in neural detection of syllable change can potentially compound, leading to difficulties processing continuous streams of syllables. Interestingly, a subgroup of children with Asperger’s syndrome (with no language impairment) showed shortened (~30 ms) MMF latencies compared to TD controls. Given the findings of this study, and the conflicting results between Ceponiene (in children with autism) and Jansson-Verkasolo (children with Asperger Syndrome), auditory MMN/MMF signatures may be sensitive to, or predictive of, clinical manifestation of language impairment (which tend to be absent in Asperger Syndrome). However, an EEG study examining MMN in young children with ASD also observed shortened MMN latencies (Gomot et al., 2002). As such, It is not currently clear whether age or other group-selection differences account for reported MMN/MMF findings. In any case, mismatch negativity (and mismatch field) latency does appear to be a sensitive neural signature, warranting further study in children with autism with and without language impairment.
Related to the MMN/MMF is the component P3a, a later component attributed to automatic attentional switching to a sound change, and interpretable as reflecting the significance of such a change. While there is ERP data on the P3a in children with autism (Lepistö et al., 2005) and indeed magnetic recordings of analogous components in healthy subjects (Huotilainen et al. 2003, Alho et al. 1998), this remains a promising yet not fully explored line of investigation for MEG in autism.
Rapid Temporal Processing
A concept related to syllable change detection has been described in terms of rapid temporal processing (RTP), the ability to detect and process transient changes in the auditory stream. RTP impairment has been proposed to underlie language impairment and dyslexia (Tallal et al., 1993; Tallal, 1980). This hypothesis is controversial, with several authors arguing for deficits in the perception and processing of speech-specific sounds and not a deficit that generalizes to non-speech auditory processing in patients with reading and learning disabilities (Studdert-Kennedy & Mody, 1995; Watson & Miller, 1993; Mody et al., 1997). Behavioral assessment of RTP and deficits thereof are, however, confounded by performance issues in individuals with ASD, such as learning, memory and general task compliance. These difficulties motivate the search for a passively obtained neural signature of RTP ability. Indeed, in many ways the use of RTP paradigms to probe neural response to rapid changes in stimuli can be viewed as quite analogous to the above-described mismatch studies. An early MEG study from the PI’s laboratory (Nagarajan et al., 1999) demonstrated reduced neuronal responses to rapidly presented tones in adults who were poor readers, compared with age-matched adults who were good readers, again indicating RTP impairment in dyslexia. In a recent study from our group, Oram Cardy et al. (2005b) examined M50 and M100 responses, and observed absent responses to the second of two rapidly presented tones in subjects with autism (with language impairment). In particular, when the interstimulus interval (ISI) was long (e.g. 800ms), the first and second M50 and M100 responses were intact in both groups. However, at shorter ISIs (150ms), M50 and M100 responses to the second tone were intact in controls but absent in subjects with autism. RTP M50/M100 abnormalities appear to reflect a language impairment related phenotype of autism, as children with specific language impairment (SLI) also showed an absence of clear M50/M100 responses to the second tone at the short ISI, whereas a group with Asperger’s syndrome (on the autism spectrum but with no core language impairment) showed intact responses.
In a recent pilot study (Schmidt et al. 2007b), accuracy and reaction time data were obtained while subjects were presented paired-tone stimuli with ISIs of 150ms, 250ms, 500ms and 800ms. Stimuli were randomly interleaved, presented during a behavioral paradigm assessing the subject’s ability to discriminate transitions from high to low frequencies (2kHz and 1kHz) and vice versa. Behavioral accuracy and reaction time measures were recorded. Several children, particularly those with lower cognitive abilities, were unable to follow the instructions and perform the behavioral task, somewhat justifying a passive MEG-based correlate. In the passive MEG task, similar stimuli were presented, but no explicit task demands made. Dependent measures were the amplitude (RMS) and latency of left/right M100 responses evoked by the first and second tone. Recordings were made using a 275-channel whole head biomagnetometer, and responses from left and right hemispheres were separately determined from analysis of ~130 sensor channels covering each hemisphere. Amplitude and latency measures were associated with performance on neuropsychological assessment using the Clinical Evaluation of Language Fundamentals (CELF)-4 core index of language function. Three amplitude findings are beginning to emerge: 1) as expected, amplitude ratios (of responses to the second compared to the first tone) converge to 1 at long ISIs, 2) the amplitude ratio in the left hemisphere correlates positively with CELF-4, and 3) no significant correlation with CELF-4 scores was observed with right hemisphere activity. To further explore the association between M100 amplitude and CELF-4 scores, subjects were divided into those scoring above and below a cutoff of 100 on the CELF-4 core index. No effect of ISI on M100 amplitude was observed in the higher scoring group. In contrast, a significant amplitude increase to the second tone as a function of increasing ISI was observed in the group scoring below 100 on the CELF-4. At an ISI of 800ms, amplitude measures did not differ between the groups. Furthermore, this effect was only encountered in the left hemisphere. Finally, across all subjects, at short ISIs (150ms), the ratio of the left hemisphere M100 amplitude of the second compared to the first tone amplitude correlated positively with clinical language assessment, as indexed by the CELF-4 score (Figure 3). In other words, increased language ability was associated with stronger evoked responses to rapidly presented stimuli.
Figure 3.
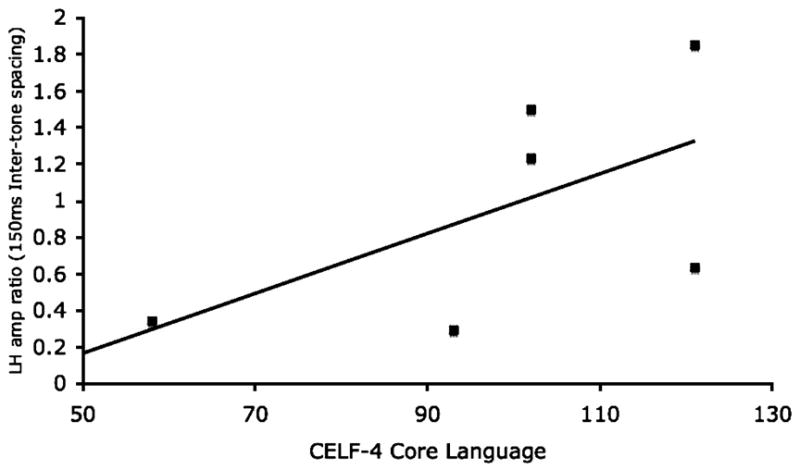
In a rapid temporal processing paradigm, it is noted that left hemispheric responses to closely spaced tones predict clinical language function assessment. Such correlation was absent in right hemispheric responses.
Taken together, present findings suggest that a sensitive neural correlate of language impairment is revealed at short ISIs and examining M100 amplitude ratios of evoked responses.
MEG COMPARED TO EEG
The studies above employed MEG to examine auditory brain activity. Given the potential utility of millisecond scale electrophysiological sensitivity, along with a typically-argued sensitivity of MEG and EEG to common neural generators, it is worth considering which of the above experimental approaches might be accomplished using EEG, given that EEG is a much less expensive technology. In addition, cognitive and clinical EEG labs increasingly obtain high-density EEG, recording from 64, 128, or even more electrodes, thus providing spatial coverage of the head similar to that obtained with current whole-head MEG systems. Despite the ability to obtain spatial coverage similar to MEG, however, fundamental physical considerations of electric and magnetic fields, as well as the location and orientation of primary and secondary auditory sources, underlie the different capabilities and limitations of MEG and EEG. The following paragraphs briefly illustrate differences between EEG and MEG which, in the auditory studies above, generally argue for the use of MEG over EEG.
A primary argument supporting the preferential use of MEG over EEG is the more straightforward identification of left and right hemisphere auditory activity. The measured signals (electrical potentials in EEG and magnetic fields in MEG) are distant from the neural generators and separated by tissues of the brain, CSF, skull, and scalp. These tissues have widely differing electrical conductivities, which mediate the scalp recorded electrical potentials and make them extremely sensitive to tissue composition and geometry. Thus, recorded electric potentials do not have a simple model to the source generator, distorted by the various intervening tissues. The magnetic permeabilities of these tissues are however similar, such that the pattern of magnetic fields recorded at the sensor positions does not depend significantly upon the intervening tissue composition. As such, simpler models may legitimately be used for source estimation. In casual parlance, some refer to the head and brain as “transparent” to magnetic fields. Thus the measures of M100 source asymmetry as discussed above might be expected to be less easily resolved using EEG compared to MEG.
Of importance to many of the applications and methodological strategies discussed above is the ability to identify, with appropriate precision, the latency of left and right hemisphere ERP/ERF components. Fundamental differences in the sensitivity of EEG and MEG often lead to a decision to choose one over the other modality, or to perform simultaneous recordings. For example, for recording auditory responses from the brainstem (e.g. BAEP, brainstem auditory evoked potentials), the superior sensitivity of EEG makes it a better choice than MEG. Specifically, although such far-field potentials are easily recorded with EEG, given the distance of the sources from the scalp they are difficult to record using MEG. On the other hand, MEG is well suited for studying individual superior temporal gyrus (STG) sources (e.g., see Edgar et al., 2003, Huang et al., 2003), which are typically active ~50–100ms post stimulus. With EEG, bilateral STG sources from the left and right hemisphere generate a maximum electric potential distribution on the top of the head (near Cz), and a minimum potential somewhere near the chin and neck area, a region where electrodes are generally not placed. Accordingly, only one pole of the electric field is measured, and when considering bilateral STG sources, recorded activity from mid- and near-midline vertex electrodes will reflect the combined activity from the two STG sources. Such conditions make localization and study of the individual STG generators using the traditional EEG montages difficult and indeed lead to temporally blurred “combined” responses if temporal morphology differences exist between individual hemispheric responses.
An example of the difficulty of separately scoring left and right primary auditory activity with EEG is shown in Figure 4. 275-channel MEG and 60-channel EEG were recorded from a single subject. Figure 4 shows MEG and EEG responses to a 200 Hz tone presented to the right ear for right MEG temporal sensors (top), left MEG temporal sensors (middle), and electrode Cz (bottom). The peak of the left hemisphere 100 ms contralateral response is observed at 116 ms (first solid vertical line). As expected, the right hemisphere ipsilateral response is delayed, occurring ~20 ms later (second solid vertical line). Maximal 100 ms activity at Cz is observed at 119 ms. Although the latency of Cz response is similar to the latency of the MEG left hemisphere response, Cz does not provide a clean measure of the left hemisphere activity, as left and right hemisphere activity linearly sum at the EEG midline sites. As the right hemisphere response is not observed as a distinct Cz peak, differentiating left from right hemisphere activity at Cz is not possible. Whereas EEG topography plots indicate greater right hemisphere activity at right hemisphere EEG sites (suggesting a better measure of right hemisphere activity could be obtained at more lateral EEG sites), the EEG topography plots do not indicate a similar lateral shift that would allow for easy identification of a distinct left hemisphere response. In contrast, as shown in the MEG topography plots, left and right MEG auditory activity is observed as distinct dipolar fields over each hemisphere.
Figure 4.
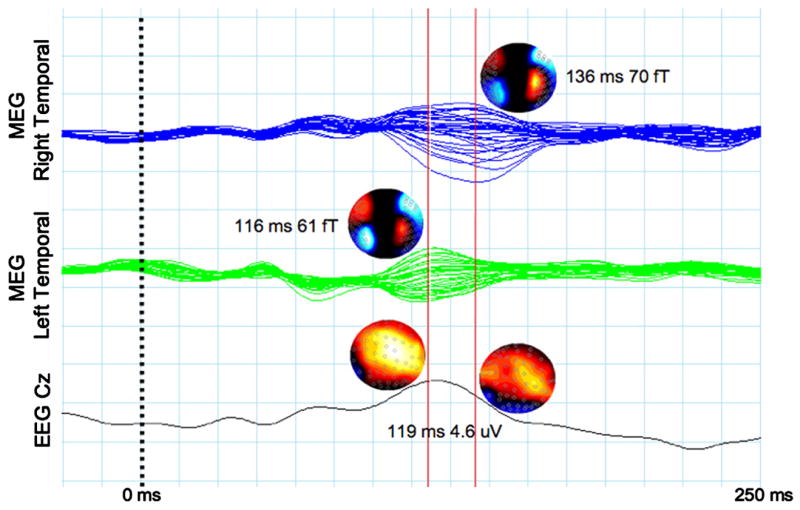
MEG and EEG responses to a 200Hz sinusoidal tone presented to the right ear of a healthy adult volunteer. 275-channel MEG and simultaneous 64-channel EEG was used. Responses are shown for right MEG temporal sensors (top), left MEG temporal sensors (middle), and electrode Cz (bottom). The peak of the left hemisphere 100 ms contralateral response is observed at 116 ms (first solid vertical line). As expected, the right hemisphere ipsilateral response is delayed, occurring ~20 ms later (second solid vertical line). Maximal 100 ms activity at Cz is observed at 119 ms. Although the latency of Cz response is similar to the latency of the MEG left hemisphere response, Cz does not provide a clean measure of the left hemisphere activity, as left and right hemisphere activity linearly sum at the EEG midline sites.
This 200Hz dataset provides an example of the difficulty inherent in using EEG voltage maps to assess auditory activity. Although interpreting voltage maps is problematic, assessing left and right EEG activity may be possible. For EEG, an alternative approach to locating generators of early auditory left and right hemisphere activity is to improve the spatial resolution of scalp EEG. To this end, high-resolution EEG methods such as skull current density estimates by means of a surface Laplacian algorithm are useful (Perrin et al., 1989). The surface Laplacian is the second spatial derivative of the voltage distribution in tissue and estimates the volume current flow out of the brain through the skull into the skin (also referred to as current source density or scalp current density maps). Figure 5 shows the surface Laplacian for the 100 ms EEG auditory response. In contrast to the EEG potential map (Figure 4), clear source and sink peaks are observed over each hemisphere, reflecting the existence of superficial and focal left and right hemisphere activity (because the STG sources are primarily tangential (in a fissure)), the surface Laplacian is weaker than a surface Laplacian obtained from a radial source (Srinivasan, 2005).
Figure 5.
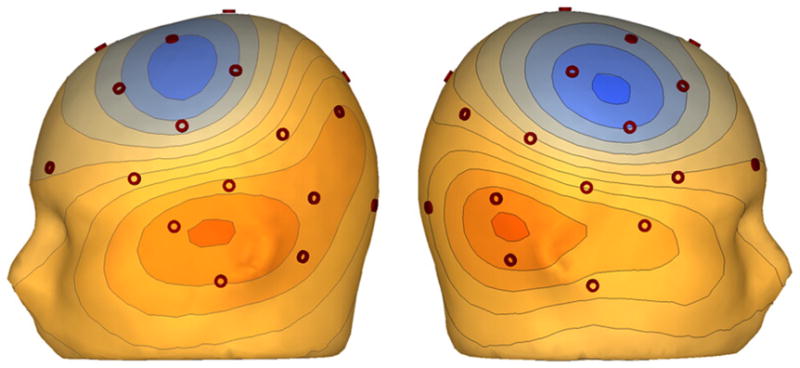
The surface Laplacian emphasizes superficial, localized sources. The surface Laplacian for the 100 ms EEG auditory response shows clear source and sink peaks over each hemisphere. As such, in this subject, separate scoring of left and right STG activity is possible.
Whereas most EEG studies compare control and patient potential activity only at midline sites (e.g., Fz, Cz, and Pz), high-resolution EEG methods require a more dense sampling of the head surface. Giard et al. (1994) used high-resolution EEG to distinguish left and right 100 ms STG activity, and radially oriented frontal activity also was observed. The frontal finding in the Giard et al. (1994) study highlights a limitation of MEG. As MEG is somewhat insensitive to radially oriented neural generators (Lewine & Orrison, 1995), radial frontal sources may invisible to MEG. In addition to early 100 ms activity, a radial STG source detected with EEG is present at approximately 140 ms (Wolpaw and Penry, 1975; Picton et al., 1999). Such findings indicate the need, in some instances, to obtain simultaneous EEG and MEG.
A complete review of the strengths and limitations of high-resolution EEG techniques is beyond the scope of this chapter. Srinivasan (2005) provides a detailed but accessible review of high-resolution EEG. One comment, however, is in order. For high-resolution EEG, electrode spacing of ~2 cm is thought to be ideal (Srinivasan et al., 1999). Given the availability of 64 or more channels EEG systems in many clinical and research labs, the use of high-resolution analysis to examine auditory processes in controls and patients is possible. Whole-head EEG (required for high-resolution EEG), however, requires placing many electrodes on the surface of the head. In some subjects with ASD, this may not be feasible. In particular, as correct placement of a whole-head EEG cap may take up to thirty minutes and may not be feasible in subjects hypersensitive to touch.
MEG has an advantage in this regard, as MEG is less physically invasive (requiring the placement of only 3–4 head coils). This advantage comes, however, at a cost. Because MEG sensors are located at a distance from the head, subjects need to stay still during the entire MEG exam so the position of their head with respect to MEG sensors remains constant. As a typical auditory task lasts 10 to 20 minutes, many patient subjects may be unable to hold still during the exam. Although the newest generation of MEG systems provide the ability to correct for head motion, current head motion correction procedures are robust only for a limited range of movement (perhaps 2.5 cm or less).
DISCUSSION
Reviewed findings suggest that abnormalities in early auditory processing may provide neural signatures of language impairment in ASD and that these may be revealed by magnetoencephalography. Open issues remain as to the specificity of these findings, as well as to the existence of analogous markers of abnormal functional activity during more complex linguistic tasks, such as analysis of words and sentences. It is our hypothesis that brain responses to a set of paradigms spanning sound perception, processing, and linguistic computation may provide an electrophysiological phenotype that characterizes the brain abnormalities associated with language impairment in ASD (Figure 6). Ongoing work is exploring the sensitivity and specificity of such a “phenotype vector” as well as its role in describing the broader autism phenotype in parents and siblings of ASD individuals. It is our hope that such a comprehensive neural phenotype will provide a valuable reference to aid interpretation of the extensive genotyping information now available. Finally, it is to be expected that such electrophysiological signatures or “traits” may provide concrete interfaces to genetic and other experimental models of disorders such as autism, disorders previously reliant on behavioral traits for diagnostic placement (see Edgar et al., 2007).
Figure 6.
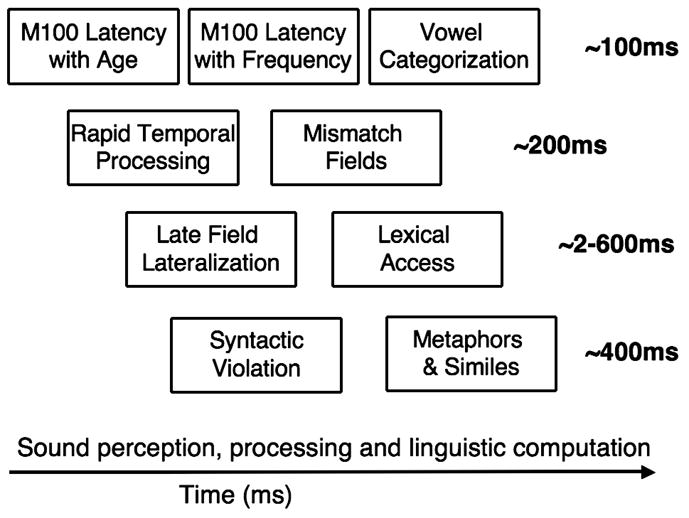
A strategy for use of MEG in studying language impairment in developmental disorders. Use of paradigms spanning sound perception, processing and linguistic computation directs focus to progressively later latencies in evoked response data.
Given the need for subjects to remain still while MEG data is obtained, it is necessary to design tasks subjects with autism or ASD can tolerate. For example, obtaining usable MEG data is more likely using short rather than long tasks. Less subject movement is sometimes obtained by placing the subject in a supine rather than upright position. If possible, for auditory tasks, allowing the subject to watch a movie (without audio) during the task may also reduce movement. Because most MEG machines are designed for adult heads, in for most child and adolescent subjects it is necessary to place something on each side of the head to hold the head stationary (e.g., foam wedges placed between the cheeks and the helmet). In subjects with verbal communication difficulties, a visual aid may help ensure their head is placed correctly in the helmet (Figure 7). Finally, although somewhat unrelated to reducing movement, a MEG lab should be welcoming and comfortable. MEG labs that focus on children populations typically have colorful posters and stuffed animals throughout the lab.
Figure 7.
In subjects with verbal communication difficulties, a visual aid may help ensure their head is placed correctly in the helmet.
CONCLUSION
Consideration of both spatial and temporal aspects of brain activity may be key in understanding neural dysfunction in developmental disorders such as autism. While evidence of structural anomalies exists in the form of auditory source localizations (decreased M100 asymmetry), other deviations from typical development appear in the fine structure of ERFs, especially component latencies. Temporal signatures of auditory and language impairment in ASD occur as early as 100ms in the form of delayed auditory responses. At 200–300ms, problems with change detection and rapid temporal processing are apparent. Hemispheric differences also exist, with some suggestion of right hemisphere abnormalities, perhaps consistent with emerging evidence from structural studies which implicate right hemisphere substrates in ASD (De Fosse et al., 2004; Herbert et al., 2002). That said, atypical left hemispheric responses in mismatch and RTP paradigms are also observed in ASD, reflective of language impairment. Extending the above measures to more sophisticated linguistic challenges will likely shed light on more specific aspects of impairment. While some of this will be achievable with EEG, for examining primary and secondary auditory activity, MEG may be the method of choice.
If the above described approach continues to show success in defining electrophysiological phenotypes of developmental disorders such as autism, it may be possible to develop clinical procedures to augment diagnosis in individuals with ASD. Rather than diagnose ASD, however, it is likely that development in this area will identify individuals with specific auditory and language deficits, thereby providing a way to parse the heterogeneity currently subsumed under the ASD diagnosis. Such procedures could be used to provide more informative diagnoses, identify patients for specific pharmaceutical and behavioral intervention, and monitor therapies that target change in neural function.
Acknowledgments
The authors thank Drs Nicole Gage, Elissa Flagg and Janis Oram Cardy for their collaboration and their contributions to this work. The authors also thank Prof John Welsh for valuable conversations and insights. The authors gratefully acknowledge the contributions of Tina Ahmadinejad, Sharon Orbach, Tali Lipper, John Dell, Ralph Magee, Tamara Lee, and all faculty, staff and students of the Penn/CHOP Center for Autism Research. Dr. Roberts would like to extend thanks to the Oberkircher Family for the Oberkircher Family Endowed Chair in Pediatric Radiology.
This work was supported in part by NIH R01DC008871, The Commonwealth of Pennsylvania, The Nancy Lurie Marks Family Foundation, The Christina and Jeffery Lurie Family Foundation, Autism Speaks, and the Canadian Institute for Health Research (CIHR) and NIH T32NS007413
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberstone CD, Skirboll SL, Benzel EC, Sanders JA, Hart BL, Baldwin NG, Tessman CL, Davis JT, Lee RR. Magnetic source imaging and brain surgery: presurgical and intraoperative planning in 26 patients. J Neurosurg. 2000;92(1):79–90. doi: 10.3171/jns.2000.92.1.0079. [DOI] [PubMed] [Google Scholar]
- Alho K, Winkler I, Escera C, Huotalainen M, Virtanen J, Jaaskalainen IP, Pekkonen E, Ilmoniemi RJ. Processing of novel sounds and frequency changes in human auditory cortex: magnetoencephalographic recordings. Psychophysiology. 1998;35(2):211–24. [PubMed] [Google Scholar]
- Bailey AJ, Braeutigam S, Jousmaki V, Swithenby SJ. Abnormal activation of face processing systems at early and intermediate latency in individuals with autism spectrum disorder: A magnetoencephalographic study. European Journal of Neuroscience. 2005;21:2575–85. doi: 10.1111/j.1460-9568.2005.04061.x. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Lepistö T, Shestakova A, Vanhala R, Alku P, Näätänen R, Yaguchi K. Proc Natl Acad Sci. 9. Vol. 100. 2003. Speech sound selective auditory impairment in children with autism: they can perceive but do not attend; pp. 5567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer T, Scott LS, Nelson CA. ERPs in developmental populations. In: Handy TC, editor. Event-related Potentials: A methods handbook. Cambridge, MA: The MIT Press; 2005. pp. 263–298. [Google Scholar]
- De Fosse L, Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr, McGrath L, Steele S, Ziegler DA, Herbert MR, Frazier JA, Tager-Flusberg H, Harris GJ. Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology. 2004;56:757–66. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- Duara R, Kushch A, Gross GK, Barker WW, Jallad B, Pascal S, Loewenstein DA, Sheldon J, Rabin M, Levin B, Lubs H. Neuroanatomic differences between dyslexic and normal readers on magnetic resonance imaging scans. Arch Neurol. 1991;48:410–416. doi: 10.1001/archneur.1991.00530160078018. [DOI] [PubMed] [Google Scholar]
- Edgar JC. Masters Thesis. 1997. Relationships between M100 antero-posterior differences and measures of developmental instability in normal, dyslexic and schizophrenic subjects. [Google Scholar]
- Edgar JC, Keller J, Heller W, Miller GA. Psychophysiology in Research on Psychopathology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. New York, NY: Cambridge University Press; 2007. pp. 665–687. [Google Scholar]
- Edgar JC, Yeo RA, Gangestad SW, Blake MB, Davis JT, Lewine JD, Ca–ive JM. Reduced auditory M100 asymmetry in schizophrenia and dyslexia: Applying a developmental instability approach to assess atypical brain asymmetry. Neuropsycholgia. 2006;44:289–299. doi: 10.1016/j.neuropsychologia.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Huang MX, Weisend MP, Sherwood A, Miller GA, Adler LE, Canive JM. Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biol Psych. 2003;65:1–20. doi: 10.1016/s0301-0511(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Elberling C, Bak C, Kofoed B, Lebech J, Saermark K. Auditory magnetic fields from the human cerebral cortex: location and strength of an equivalent current dipole. Acta Neurol Scand. 1982;65:553–569. doi: 10.1111/j.1600-0404.1982.tb03110.x. [DOI] [PubMed] [Google Scholar]
- Eulitz C, Diesch E, Pantev C, Hampson S, Elbert T. Magnetic and electric brain activity evoked by the processing of tone and vowel stimuli. Journal of Neuroscience. 1995;15:2748–2755. doi: 10.1523/JNEUROSCI.15-04-02748.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Roberts TPL. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Developmental Brain Research. 2003a;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Callen M, Roberts TPL. Cortical sound processing in children with Autism Disorder: an MEG investigation. Neuroreport. 2003b;14:2047–2051. doi: 10.1097/00001756-200311140-00008. [DOI] [PubMed] [Google Scholar]
- Ganslandt O, Buchfelder M, Hastreiter P, Grummich P, Fahlbusch R, Nimsky C. Magnetic source imaging supports clinical decision making in glioma patients. Clin Neurol Neurosurg. 2004;107(1):20–26. doi: 10.1016/j.clineuro.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Echallier JF, Thevenet M, Froment JC, Pernier J. Dissociation of temporal and frontal components in the human auditory N1 wave: a scalp current density and dipole model analysis. Electroencephalography and Clinical Neurophysiology. 1994;92:238–252. doi: 10.1016/0168-5597(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard MH, Adrien JL, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39:577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Heim S, Kissler J, Elbert T, Rockstroh B. Cerebral lateralization in schizophrenia and dyslexia: neuromagnetic responses to auditory stimuli. Neuropsychologia. 2004;42:692–697. doi: 10.1016/j.neuropsychologia.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J, Takeoka M, Tager-Flusberg H, Caviness VS., Jr Abnormal asymmetry in language association cortex in autism. Annals of Neurology. 2002;52:588–96. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Huang MX, Edgar JC, Thoma RJ, Hanlon FM, Moses SN, Lee RR, Paulson KM, Weisend MP, Irwin JG, Bustillo JR, Adler LE, Miller GA, Ca–ive JM. Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clinical Neurophysiology. 2003;114:835–850. doi: 10.1016/s1388-2457(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Huotilainen M, Kujala A, Hotakainen M, Shestakova A, Kushnerenko E, Parkkonen L, Fellman V, Näätänen R. Auditory magnetic responses of healthy newborns. Neuroreport. 2003;14:1871–1875. doi: 10.1097/00001756-200310060-00023. [DOI] [PubMed] [Google Scholar]
- Jansson-Verkasalo E, Ceponiene R, Kielinen M, Suominen K, Jantti V, Linna SL, Moilanen I, Näätänen R. Deficient auditory processing in children with Asperger Syndrome, as indexed by event-related potentials. Neurosci Lett. 2003;338(3):197–200. doi: 10.1016/s0304-3940(02)01405-2. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Elgavish R, Howell J, Blount J, Burneo JG, Faught E, Kankirawatana P, Riley K, Morawetz R, Worthington J, Kuzniecky RI. Magnetic source imaging versus intracranial electroencephalogram in epilepsy surgery: A prospective study. Ann Neurol. 2006;59(5):835–842. doi: 10.1002/ana.20857. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Learning and representation in speech and language. Curr Opin Neurobiol. 1994;4(6):812–22. doi: 10.1016/0959-4388(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Braeutigam S, Hietanen JK, Swithenby SJ, Bailey AJ. Face- and gaze-sensitive neural responses in children with autism: a magnetoencephalographic study. European Journal of Neuroscience. 2006;24:2679–90. doi: 10.1111/j.1460-9568.2006.05132.x. [DOI] [PubMed] [Google Scholar]
- Lamb JA, Parr JR, Bailey AJ, Monaco AP. Autism: in search of susceptibility genes. Neuromolecular Medicine. 2002;2:11–28. doi: 10.1385/NMM:2:1:11. [DOI] [PubMed] [Google Scholar]
- Larsen JP, Odegaard H, Grude TH, Hoien T. Magnetic resonance imaging-a method of studying the size and asymmetry of the planum temporale. Acta Neurol Scand. 1989;80:438–443. doi: 10.1111/j.1600-0404.1989.tb03906.x. [DOI] [PubMed] [Google Scholar]
- Leahy RM, Mosher JC, Spencer ME, Huang MX, Lewine JD. Los Alamos Tech. Rep. No. LA-UR-98-1442. Los Alamos, NM: Los Alamos National Laboratories; 1998. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. [DOI] [PubMed] [Google Scholar]
- Lee D, Sawrie SM, Simos PG, Killen J, Knowlton RC. Reliability of language mapping with magnetic source imaging in epilepsy surgery candidates. Epilepsy Behav. 2006;8(4):742–749. doi: 10.1016/j.yebeh.2006.02.012. [DOI] [PubMed] [Google Scholar]
- LeMay M, Kido DK. Asymmetries of the cerebral hemispheres on computed tomograms. J Comp Assist Tomography. 1978;2:471–476. doi: 10.1097/00004728-197809000-00018. [DOI] [PubMed] [Google Scholar]
- Lepistö T, Kujala T, Vanhala R, Alku P, Huotilainen M, Näätänen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Res. 2005;1066:147–57. doi: 10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Orrison WW. Magnetoencephalography and magnetic source imaging. In: Orrison WW, Lewine JD, Sanders JA, Hartshorne MF, editors. Functional Brain Imaging. St. Louis: Mosby; 1995. pp. 369–417. [Google Scholar]
- Lewis S. Structural brain imaging in biological psychiatry. British Medical Bulletin. 1996;52:465–73. doi: 10.1093/oxfordjournals.bmb.a011560. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36:849–61. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Courchesne E, Harms L, Allen M. Sensory modulation of auditory stimuli in children with Autism and receptive developmental language disorder: Event related brain potential evidence. Journal of Autism & Developmental Disorders. 1995;25(5):521–539. doi: 10.1007/BF02178298. [DOI] [PubMed] [Google Scholar]
- Mäkelä JP, Ahonen A, HŠmŠlŠinen M, Hari R, Ilmoniemi R, Kajola M, Knuutila J, Lounasmaa OV, McEvoy L, Salmeilin R, et al. Functional differences between auditory corticies of the two hemispheres revealed by whole-head Neuromagnetic recordings. Human Brain Mapping. 1993;1:48–56. [Google Scholar]
- Mody M, Studdert-Kennedy M, Brady S. Speech perception deficits in poor readers: auditory processing or phonological coding? J Exp Child Psych. 1997;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Mismatch negativity: clinical research and possible applications. Int J Psychophysiol. 2003;48:179–188. doi: 10.1016/s0167-8760(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The perception of speech sounds by the human brain as reflected by the mismatch negativity (MMN) and its magnetic equivalent (MMNm) Psychophysiol. 2001;38:1–21. doi: 10.1017/s0048577201000208. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Lehtokoski A, Lennes M, Cheour M, Huotilainen M, et al. Language specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich MM. Cortical auditory signal processing in poor readers. Proc Nat Acad Sci. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasato N, Yoshimoto T. Somatosensory, auditory, and visual evoked magnetic fields in patients with brain diseases. J Clin Neurophysiol. 2000;17(2):201–211. doi: 10.1097/00004691-200003000-00009. [DOI] [PubMed] [Google Scholar]
- Ohtomo S, Nakasato N, Kanno A, Hatanaka K, Shirane R, Mizoi K, Yoshimoto T. Hemispheric asymmetry of the auditory evoked N100m response in relation to the crossing point betwen the central sulcus and Sylvian fissure. Electroencephalography and Clinical Neurophysiology. 1998;108:219–225. doi: 10.1016/s0168-5597(97)00065-8. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Ferrari P, Flagg EJ, Roberts W, Roberts TPL. Prominence of M50 auditory evoked response over M100 in childhood and Autism. Neuroreport. 2004;15(12):1867–1870. doi: 10.1097/00001756-200408260-00006. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TPL. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 2005a;16(5):521–525. doi: 10.1097/00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Brian J, Roberts TPL. Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. Neuroreport. 2005b;16(4):329–332. doi: 10.1097/00001756-200503150-00005. [DOI] [PubMed] [Google Scholar]
- Ornitz EM. Autism at the interface between sensory and information processing. In: Geraldine Dawson E, et al., editors. Autism: Nature, diagnosis, and treatment. New York, NY, USA.: 1989. pp. 174–207. [Google Scholar]
- Paetau R, Ahonen A, Salonen O, Sams M. Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. J Clin Neurophysiol. 1995;12(2):177–85. doi: 10.1097/00004691-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Peltola M, Kujala T, Tuomainen J, Ek M, Aaltonen O, NŠŠtŠnen R. Native and foreign vowel discrimination as indexed by the mismatch negativity (MMN) response. Neurosci Lett. 2003;352:25–28. doi: 10.1016/j.neulet.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echalier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Alain C, Woods DL, John MS, Scherg M, Valdes-Sosa P, Bosch-Bayard J, Trujillo NJ. Intracerebral sounces of human auditory-evoked potentials. Audiology Neuro-Otology. 1999;4:64–79. doi: 10.1159/000013823. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology and Psychiatry. 2007;48:464–72. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Reite M, Sheeder J, Teale P, Adams M, Richardson D, Simon J, Jones RH, Rojas DC. Magnetic source imaging evidence of sex differences in cerebral lateralization in schizophrenia. Achieves of General Psychiatry. 1997;54:433–440. doi: 10.1001/archpsyc.1997.01830170059009. [DOI] [PubMed] [Google Scholar]
- Reite M, Teale P, Goldstein L, Whalen J, Linnville S. Late auditory magnetic sources may differ in the left-hemisphere of schizophrenic patients: A preliminary report. Achieves of Gen Psychiatry. 1989;46:565–572. doi: 10.1001/archpsyc.1989.01810060087013. [DOI] [PubMed] [Google Scholar]
- Rice C. Prevalence of autism spectrum disorders – autism and developmental disorders monitoring network, six sites, United States, 2000. Morbidity and Mortality Weekly Reports, Centers for Disease Control and Prevention. 2007;56(SS01):1–11. [PubMed] [Google Scholar]
- Roberts TPL, Poeppel D. Latency of auditory evoked M100 as a function of tone frequency. Neuroreport. 1996;7(6):1138–1140. doi: 10.1097/00001756-199604260-00007. [DOI] [PubMed] [Google Scholar]
- Roberts TPL, Ferrari P, Stufflebeam SM, Poeppel D. Latency of the auditory evoked neuromagnetic field components: Stimulus dependence and insights toward perception. Journal Of Clinical Neurophysiology. 2000;17(2):114–129. doi: 10.1097/00004691-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Rockstroh B, Kissler J, Mohr B, Eulitz C, Lommen U, Wienbruch C, Cohen R, Elbert T. Altered hemispheric asymmetry of auditory magnetic fields to tones and syllables in schizophrenia. Biological Psychiatry. 2001;49:694–703. doi: 10.1016/s0006-3223(00)01023-4. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Teale P, Sheeder J, Simon J, Reite M. Sex-specific expression of Heschl’s gyrus functional and structural abnormalities in paranoid schizophrenia. American Journal of Psychiatry. 1997;154:1655–1662. doi: 10.1176/ajp.154.12.1655. [DOI] [PubMed] [Google Scholar]
- Schiffbauer H, Berger MS, Ferrari P, Freudenstein D, Rowley HA, Roberts TP. Preoperative magnetic source imaging for brain tumor surgery: a quantitative comparison with intraoperative sensory and motor mapping. J Neurosurg. 2002;97(6):1333–1342. doi: 10.3171/jns.2002.97.6.1333. [DOI] [PubMed] [Google Scholar]
- Schmidt GL, Rey MM, Roberts TPL. Anatomical asymmetry of the M100 source in typically developing children and children with autism spectrum disorders. Paper presented at the annual meeting of the International Society for the Advancement of Clinical Magnetoencephalography; Sendai, Japan. 2007a. [Google Scholar]
- Schmidt GL, Blaskey LG, Rey MM, Levy SE, Roberts TPL. Hemispheric differences in the neural correlates of rapid temporal processing in autism spectrum disorders. Abstract, annual meeting of the Society for Neuroscience; San Diego, CA. 2007b. [Google Scholar]
- Srinivasan R. High-resolution EEG: theory and practice. In: Handy TC, editor. Event-related potentials: A handbook. Cambridge, MA: MIT Press; 2005. pp. 167–188. [Google Scholar]
- Srinivasan R. Methods to improve the spatial resolution of EEG. International Journal of Bioelectromagnetism. 2005;1:102–111. [Google Scholar]
- Steinmetz H, Rademacher J, Huang YX, Hefter H, Ziles K, Thron A, Freund HJ. Cerebral asymmetry: MR planimetry of the human planum temporale. J Comp Assist Tomography. 1989;13:996–1005. [PubMed] [Google Scholar]
- Studdert-Kennedy M, Mody M. Auditory temporal perception deficits in the reading-impaired: a critical review of the evidence. Psychonom Bull Rev. 1995;2:508–514. doi: 10.3758/BF03210986. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: a case for the preeminence of temporal processing. Ann NY Acad Sci. 1993;682:27–47. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- Tallal P. Language disabilities in children: a perceptual or linguistic deficit? J Pediatr Psychol. 1980;5(2):127–40. doi: 10.1093/jpepsy/5.2.127. [DOI] [PubMed] [Google Scholar]
- Tallal P. The science of literacy: from the laboratory to the classroom. Proceedings of the National Academy of Science USA. 2000;97:2402–2404. doi: 10.1073/pnas.97.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale P, Reite M, Rojas DC, Sheeder J, Arciniegas D. Fine structure of the auditory M100 in schizophrenia and schizoaffective disorder. Biological Psychiatry. 2000;48:1109–1112. doi: 10.1016/s0006-3223(00)00941-0. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Benassi F, Zappasodi F, Gialloreti LE, Palermo M, et al. Auditory sensoryprocessing in autism: a magnetoencephalographic study. Biol Psychiatry. 2003;54:647–654. doi: 10.1016/s0006-3223(03)00295-6. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kartila H, Pekkonen E, Jaaskelainen IP, Huotilainen M, Aronen HJ, Ilmoniemi RJ, Rasanen P, Virtanen J, Salli E, Karhu J. Reversal of cerebral aymmetry in schizophrenia measured with magnetoencephalography. Schizophrenia Research. 1998;10:209–219. doi: 10.1016/s0920-9964(97)00154-0. [DOI] [PubMed] [Google Scholar]
- Todd J, Michie PT, Budd TW, Rock D, Jablensky AV. Auditory sensory memory in schizophrenia: inadequate trace formation? Psychiatry Research. 2000;30:99–115. doi: 10.1016/s0165-1781(00)00205-5. [DOI] [PubMed] [Google Scholar]
- Vihla M, Lounasmaa OV, Salmelin R. Cortical processing of change detection: Dissociation between natural vowels and two-frequency complex tones. Proc Natl Acad Sci USA. 2000;97:10590–10594. doi: 10.1073/pnas.180317297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Archives of Neurology. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Watson BU, Miller TK. Auditory perception, phonological processing and reading ability/disability. J Speech Hear Res. 1993;36(4):850–63. doi: 10.1044/jshr.3604.850. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Luchins DJ, Morihisa J, Wyatt RJ. Asymmetric volumes of the right and left frontal and occipital regions of the human brain. Ann Neurol. 1982;11:97–100. doi: 10.1002/ana.410110118. [DOI] [PubMed] [Google Scholar]
- Winkler I, Kujala T, Tiitinen H, Sivonen P, Alku P, et al. Brain responses reveal the learning of foreign language phonemes. Psychophysiol. 1990;36:638–642. [PubMed] [Google Scholar]
- Wolpaw JR, Penry JK. A temporal component of the auditory evoked response. Electrophysiology and Clinical Neurophysiology. 1975;39:609–620. doi: 10.1016/0013-4694(75)90073-5. [DOI] [PubMed] [Google Scholar]


