Abstract
Aquaporin-4 (AQP4) is a water transport protein expressed in glial cell plasma membranes, including glial cell foot processes lining the blood-brain barrier. AQP4 deletion in mice reduces cytotoxic brain edema produced by different pathologies. To determine whether AQP4 is rate-limiting for brain water accumulation and whether altered AQP4 expression, as occurs in various pathologies, could have functional importance, we generated mice that overexpressed AQP4 in brain glial cells by a transgenic approach using the glial fibrillary acid protein promoter. Overexpression of AQP4 protein in brain by ∼2.3-fold did not affect mouse survival, appearance, or behavior, nor did it affect brain anatomy or intracranial pressure (ICP). However, following acute water intoxication produced by intraperitoneal water injection, AQP4-overexpressing mice had an accelerated progression of cytotoxic brain swelling, with ICP elevation of 20 ± 2 mmHg at 10 min, often producing brain herniation and death. In contrast, ICP elevation was 14 ± 2 mmHg at 10 min in control mice and 9.8 ± 2 mmHg in AQP4 knock-out mice. The deduced increase in brain water content correlated linearly with brain AQP4 protein expression. We conclude that AQP4 expression is rate-limiting for brain water accumulation, and thus, that altered AQP4 expression can be functionally significant.
Aquaporin-4 (AQP4)2 is a water-selective membrane transport protein expressed in glial cells in brain, particularly at the borders between brain parenchyma and the major fluid compartments in brain (1, 2). Strong AQP4 expression is found in astroglial cell foot processes at the blood-brain barrier, in glia lining the subarachnoid cerebrospinal fluid space, and in ependyma and subependymal glia lining the ventricular cerebrospinal fluid space. AQP4 expression in glial cells is up-regulated in various brain pathologies, including trauma (3, 4), tumor (5, 6), subarachnoid hemorrhage (7), ischemia (8), and inflammation (9, 10). Whether increased AQP4 expression is functionally significant for water movement between brain and cerebrospinal fluid or blood is not known, as is whether AQP4 is rate-limiting for water movement across the blood-brain barrier. Because water movement from blood to brain parenchyma involves water transport across a tight endothelial cell layer, which does not express AQPs, followed by transport across AQP4-expressing glial cell foot processes surrounding brain microvessels, water movement into the brain may be limited by the endothelial barrier.
Evidence from AQP4 knock-out mice indicates slowed water movement both into and out of the brain in AQP4 deficiency, resulting in opposite consequences for cytotoxic versus vasogenic brain edema (reviewed in Ref. 11). AQP4 knock-out mice have reduced brain swelling and improved survival when compared with control, wild-type mice following water intoxication and reduced hemispheric swelling after focal cerebral ischemia (12). AQP4-null mice also have greatly improved survival in a mouse model of bacterial meningitis (9). According to the Klatzo classification of brain edema, these are primarily models of cytotoxic (cell swelling) edema in which excess water moves from the vasculature into the brain parenchyma through an intact blood-brain barrier. AQP4 also facilitates the elimination of excess brain water. When the blood-brain barrier becomes disrupted as in brain tumor or abscess, water moves from the vasculature into the brain extracellular space in an AQP4-independent manner to form vasogenic edema. Excess water is eliminated primarily through the glia limiting membranes into the cerebrospinal fluid. Greater brain water accumulation and intracranial pressure were found in AQP4-null versus wild-type mice with brain tumor, brain abscess, focal cortical-freeze injury, and after infusion of normal saline directly into brain extracellular space (10, 13), indicating that vasogenic edema fluid is eliminated by an AQP4-dependent route. Also, in a kaolin injection model of obstructive hydrocephalus, AQP4-null mice develop more marked hydrocephalus than wild-type mice (14), probably due to reduced water clearance in AQP4-null mice through the ependymal and blood-brain barriers. These studies support the view that AQP4 is a bidirectional water channel that facilitates water transport into and out of the brain.
The studies using AQP4 knock-out mice, however, do not address whether increased AQP4 expression, as occurs in various types of brain injuries and pathologies, could have functional consequences. If endothelial cells in brain microvessels are rate-limiting for water transport under normal conditions, then further increases in AQP4 expression in glial cell foot processes would be without effect. To address this question, we generated glial cell-targeted, AQP4-overexpressing mice using a glial fibrillary acid protein (GFAP) promotor strategy and investigated whether AQP4 overexpression would accelerate cytotoxic brain edema by increasing water transport across the blood-brain barrier. GFAP is expressed in most glial cells, including those lining the blood-brain barrier. The data show accelerated brain water accumulation following acute water intoxication, indicating that under normal conditions, glial cell AQP4 is rate-limiting for water movement into the brain, and thus, that altered AQP4 expression can be functionally significant.
EXPERIMENTAL PROCEDURES
Transgenic Mice—A 1.54-kb fragment of mouse AQP4 cDNA (121–1661, GenBank™ accession number NM_009700), containing the full coding region (including the ATG translational start site and the TAG stop codon) and the 3′-untranslated and poly-A sequences, was PCR-amplified from mouse brain cDNA and subcloned into pGEM3Z (Promega). A cytomegalovirus enhancer sequence (∼0.3 kb, subcloned from pcDNA3, Invitrogen) and a PCR-amplified ∼2.2-kb GFAP promoter sequence (4–2177, GenBank accession number M67446) were inserted upstream of the AQP4 cDNA sequence (see Fig. 1A). A ∼4-kb EcoRI/HindIII fragment containing the cytomegalovirus enhancer, GFAP promoter, AQP4 cDNA coding region, and 3′-untranslated and poly-A sequences was isolated, purified, and injected into pronuclei of C57 BL/6JxCBA F1 fertilized oocytes. Fertilized oocytes were transferred into pseudopregnant C57 BL/6JxCBA F1 mice. F0 generation mice carrying the GFAP-AQP4 transgene construct were identified by genomic PCR analysis of tail DNA using sense primer (5′-TGGTCTGGCTCCAGGTACCAC-3′) from the GFAP promoter and antisense primer (5′-AGCAATGCTGAGTCCAAAGC-3′) from the AQP4 cDNA coding region. PCR-positive mice were bred with wild-type CD1 to establish heterozygotic GFAP-AQP4 transgenic strains. Mouse lines with the strongest AQP4 expression were identified by immunoblot analysis of brain homogenates in F1 generation offspring. AQP4 knock-out mice in a CD1 genetic background, generated by targeted gene disruption (15), were used as well. Protocols were approved by the University of California, San Francisco, Committee on Animal Research.
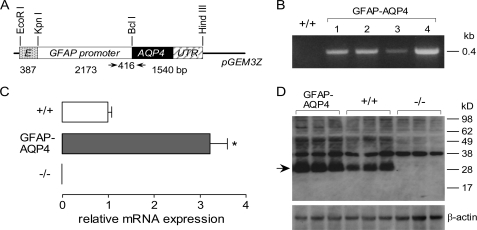
FIGURE 1.
Generation and initial characterization of glial cell-targeted, AQP4-overexpressing transgenic mice. A, schematic of GFAP-AQP4 transgene expression vector. Gray box, cytomegalovirus enhancer fragment. Open box, GFAP promoter fragment. Filled box, mouse AQP4 cDNA coding region. Striped box, AQP4 cDNA 3′-untranslated and poly-A sequences. The thin line represents pGEM3Z vector sequences. The arrows indicate the primer sites for genotyping. The numbers indicate the length of fragments. B, genomic PCR of four GFAP-AQP4 founder mice. C, relative AQP4 mRNA expression in brain quantified by real-time PCR, using as template cDNA reversed-transcribed from total brain RNA (S.E., n = 5, *, p < 0.01). D, AQP4 immunoblot of whole brain homogenates, 5 μg of protein/lane (top). The arrow indicates AQP4 protein monomer. β-actin immunoblot was used for normalization.
Histology and Immunofluorescence—Tissues for histological examination was immersion-fixed in neutral buffered formalin, paraffin-embedded, sectioned, and stained with hematoxylin and eosin according to standard protocols. For immunofluorescence, brain tissue was fixed in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4) for 24 h and then equilibrated with 30% sucrose in phosphate-buffered saline overnight at 4 °C. Frozen (6-μm) sections were blocked with 3% nonfat milk and then incubated overnight at 4 °C with rabbit anti-AQP4 polyclonal antibody (1:500 dilution, Santa Cruz Biotechnology) and mouse anti-GFAP antibody (1:1000 dilution, Chemicon), washed with phosphate-buffered saline, and incubated with Cy3-labeled anti-rabbit IgG secondary antibody (Sigma) and fluorescein isothiocyanate-labeled anti-mouse secondary antibody (Invitrogen). Sections were also 4′,6-diamidino-2-phenylindole-stained and mounted with Vectashield mounting medium (Vector Laboratories).
Immunoblot Analysis—Brains were homogenized by 20 strokes of a glass Dounce homogenizer in 250 mm sucrose, 10 mm Tris-HCl, pH 7.4, 0.2 mm EDTA, 20 μg/ml phenylmethylsulfonyl fluoride. Nuclei were removed by centrifugation at 500 × g for 10 min at 4 °C. Protein concentration was determined in the supernatant using the DC protein assay kit (Bio-Rad). Proteins (5 μg of protein/lane) were resolved by SDS-PAGE, transferred to Hybond-P membranes (GE Healthcare), blocked with 5% nonfat milk, and incubated with rabbit anti-AQP4 (1:1000 dilution) or rabbit anti-β-actin (1:2000 dilution, Santa Cruz Biotechnology). Detection was done using the ECL Plus Western blotting detection system (GE Healthcare).
Reverse Transcription-Polymerase Chain Reaction—Brain tissues were stored in RNA Later™ solution (Ambion). Total RNA was isolated using the RNeasy Mini Kit (Qiagen). cDNA was reverse-transcribed from mRNA using random primers (SuperScript III first-strand synthesis system, Invitrogen). Fluorescence-based real-time PCR was carried out using the LightCycler with LightCycler FastStart DNA MasterPLUS SYBR Green I kit (Roche Diagnostics) using primers: 5′-TGTATGCCTCTGGTCGTACC-3′ (sense) and 5′-CAGGTCCAGACGCAGGATG-3′ (antisense) for β-actin and 5′-GAGTCACCACGGTTCATGGA-3′ (sense) and 5′-CGTTTGGAATCACAGCTGGC-3′ (antisense) for AQP4. Primer sequences were derived from GenBank accession numbers NM_007393 (β-actin) and NM_009700 (AQP4). Real-time PCR was carried out according to the manufacturer's instructions, using β-actin as reference gene and pooled wild-type cDNA as the calibrator. Results are reported as normalized, calibrated ratios, normalized to the reference gene.
Intracranial Pressure (ICP) Measurements—Mice were anesthetized by intraperitoneal administration of Avertin (2,2,2-tribromoethanol, 125 mg/kg) and immobilized in a stereotaxic frame (MyNeuroLab). Core temperature was monitored and maintained at 37 °C. In some experiments, the jugular vein was cannulated using PE-10 polyethylene tubing (BD Biosciences) to obtain blood samples (∼40 μl). A ∼1-mm-long sagittal skin incision was made 1.5 mm lateral to the superficial projection of the superior sagittal suture. After exposing the skull, a burr hole of 1-mm diameter was made 1 mm posterior to bregma and 1 mm lateral to midline. A low compliance pressure probe (Millar) was inserted through the craniotomy, and the gap between the edge of the burr hole and the probe was sealed with tissue glue. ICP was recorded at 100 Hz using a Biopac recording system.
Water Intoxication Studies—Female GFAP-AQP4 transgenic mice with matched wild-type mice and AQP4 knock-out mice (age ∼10 weeks) were used for functional studies, with genotype information blinded until completion of measurements. Brain water accumulation across the blood-brain barrier was measured using the acute water intoxication model (12, 16), in which intraperitoneally administered water is absorbed through mesenterial vessels producing hypo-osmotic hyponatremia. In anesthetized mice prepared for ICP monitoring as described above, a bolus of water (10% body weight) was administered intraperitoneally along with desmopressin acetate (DDAVP, 0.4 μg/kg) to prevent renal free water excretion. The osmotic gradient drives water from the vascular bed into the cerebral parenchyma through the blood-brain barrier, which is monitored continuously by ICP. To measure the development of hyponatremia following water intoxication, serum sodium concentration was determined at 5-min intervals following intraperitoneal water administration. Blood samples (∼40 μl) were drawn through a jugular cannula, serum protein was removed by trichloroacetic acid, and sodium concentration was determined by flame photometry after 5-fold dilution of serum. ICP curves were analyzed to compute: baseline ICP, ΔICP (from baseline) at 10 and 20 min after water intoxication, the rate of ICP increase (d(ICP)/dt) at 10 min, maximum (d(ICP)/dt) (from first-derivative plot), and time to reach maximum (d(ICP)/dt).
RESULTS
Generation and Characterization of AQP4-overexpressing Transgenic Mice—To generate GFAP-AQP4-overexpressing transgenic mice, 100 C57 BL/6JxCBA embryos injected with the transgene construct in Fig. 1A were implanted into six C57 BL/6JxCBA foster mothers, producing 32 F0 generation mice. Four of the 32 F0 mice carried the GFAP-AQP4 transgene as determined by genomic PCR analysis using primers specific for the transgene sequence (four examples are shown in Fig. 1B). Genotype analysis of offspring from breeding of F0 generation GFAP-AQP4 mice with wild-type mice gave F1 generation mice with >50% carrying the GFAP-AQP4 transgene (22 GFAP-AQP4 mice, 8 wild-type mice), indicating unimpaired neonatal survival. The mouse line producing offspring with the highest brain AQP4 protein expression (by immunoblot analysis) was used as the founder to establish an AQP4-overexpressing transgenic mouse line. The GFAP-AQP4 mice had normal survival, appearance, activity, and behavior and developed and bred normally. There was no significant difference in mouse weights of GFAP-AQP4 versus wild-type mice over the first 10 weeks of life (data not shown). It was found, however, that in subsequent generations of breeding (with wild-type mice), although brain AQP4 expression remained high, the fraction of GFAP-AQP4-positive offspring was progressively reduced. By the fourth generation, only ∼20% of mice carried the GFAP-AQP4 transgene, indicating exclusion of the GFAP-AQP4 transgene from the genome in some germ cells. However, despite the genetic instability of the mouse line chosen for analysis, a sufficient number of AQP4-overexpressing mice could be generated for their characterization and brain swelling studies.
Real-time PCR analysis of RNA from brain homogenates showed that the GFAP-AQP4 mice had ∼3.2-fold greater expression of mRNA encoding AQP4 than wild-type mice (Fig. 1C). Full-length AQP4 mRNA was not detected in AQP4 knock-out mice, as expected. Fig. 1D shows immunoblot analysis of brain homogenates from mice at age 12 weeks. The ∼30-kDa AQP4-specific protein band, which was absent in AQP4-null mice, was increased 2.3 ± 0.3-fold (by quantitative densitometry using dilution standards) in the GFAP-AQP4 mice. AQP4 protein expression was not different in kidneys of GFAP-AQP4 mice and wild-type mice (data not shown), as expected, since kidney collecting duct cells do not express GFAP. These data indicate that AQP4 transgene expression driven by the GFAP promoter increases AQP4 protein expression in glial cells.
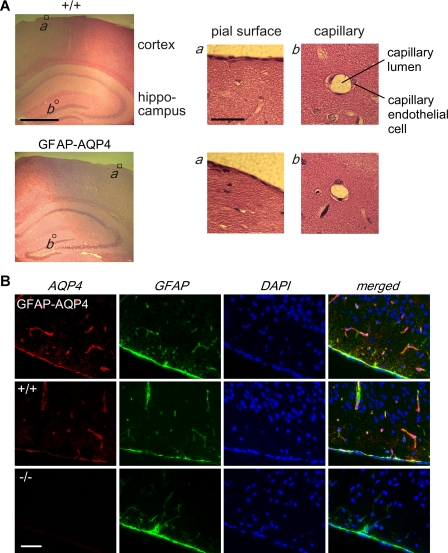
To ensure that GFAP-AQP4 transgene expression had no effect on glial cell development, morphology, or distribution, brain histology and GFAP immunoreactivity were compared in GFAP-AQP4 and wild-type mice. The appearance of major cerebral regions as well as the finer structure of pial membranes and cerebral capillaries was indistinguishable in wild-type versus GFAP-AQP4 mice (Fig. 2A). AQP4 immunofluorescence showed a similar AQP4 protein distribution pattern in the GFAP-AQP4 and wild-type mice, although with higher expression in the GFAP-AQP4 mice. AQP4 and GFAP protein were co-localized in the same cells (Fig. 2B). Co-immunofluorescence with AQP4 and neuN antibody confirmed the expected absence of AQP4 protein in neurons in the GFAP-AQP4 mice (data not shown). These results indicate glial cell-specific AQP4 overexpression in brain in GFAP-AQP4 mice.
FIGURE 2.
Brain anatomy and AQP4 expression in mice. A, brain histology in hematoxylin and eosin-stained sections from GFAP-AQP4 and wild-type (+/+) mice. Left, low magnification micrograph of cerebral cortex and hippocampus. Scale bar, 1 mm. Right, higher magnification micrographs of indicated regions. Scale bar, 100 μm. B, AQP4 (red, Cy3-labeled) and GFAP (green, fluorescein isothiocyanate-labeled) immunofluorescence, with cell nuclei-stained blue (4′,6-diamidino-2-phenylindole (DAPI)). Scale bar, 100 μm.
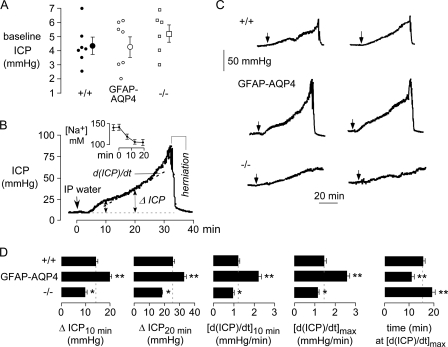
Functional Brain Water Accumulation Studies—Fig. 3A shows baseline ICP in a series of mice measured using an implanted pressure microtransducer. ICP did not differ significantly in GFAP-AQP4 versus wild-type mice or in AQP4 knock-out mice. Fig. 3B shows an original ICP curve (from a GFAP-AQP4 mouse) in which a bolus of water (10% body weight) was infused intraperitoneally as indicated by the arrow. Following a lag period where ICP remained nearly constant, ICP increased progressively, with large increases seen at ∼30 min, just prior to brain herniation and death. Fig. 3B (inset) shows the rapid development of serum hyponatremia following intraperitoneal water administration measured in a separate group of mice.
FIGURE 3.
Accelerated brain water accumulation in AQP4-overexpressing mice in a water intoxication model of cytotoxic brain edema. A, baseline ICP in mice of indicated genotype. Data from individual mice are shown, with averaged data shown with error bars (S.E., differences not significant). B, original ICP curve for GFAP-AQP4 mouse. The arrow indicates the time of intraperitoneal administration of water (IP water, 10% body weight). The inset shows the time course of serum sodium after intraperitoneal administration of water (S.E., n = 4). C, representative ICP curves for two mice of each genotype. D, summary of ICP curve analysis: ΔICP at 10 and 20 min, d(ICP)/dt at 10 min after water intoxication, maximal d(ICP)/dt, and time to reach maximal d(ICP)/dt (S.E., 5–8 mice/group, *, p < 0.05, **, p < 0.01 when compared with +/+ mice).
Fig. 3B shows representative ICP curves for two mice of each genotype. ICP increases were consistently greater in GFAP-AQP4 mice than in wild-type mice or AQP4-null mice. Brain herniation and death were seen by 40 min in 80% of GFAP-AQP4 mice, 50% of wild-type mice, and 30% of AQP4 knock-out mice.
Several parameters were deduced from ICP kinetic data obtained from a series of mice. Fig. 3D summarizes the increase in ICP from baseline (ΔICP) at 10 and 20 min after water administration, the rate of ICP increase (d(ICP)/dt) at 10 min, the maximal rate of ICP increase, and the time at which the maximum rate of ICP increase was seen. AQP4 overexpression significantly increased ΔICP at 10 and 20 min, as well as d(ICP)/dt at 10 min and maximum d(ICP)/dt, and shortened the time to reach maximum d(ICP)/dt. Significant differences, in the opposite direction, were seen comparing AQP4-null and wild-type mice.
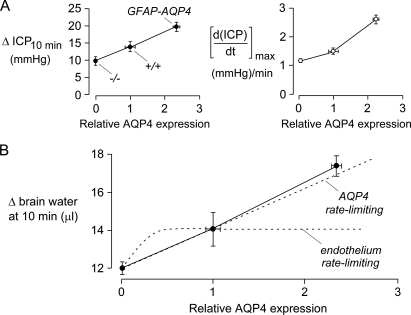
Fig. 4A shows correlations between two ICP parameters with brain AQP4 protein expression: ΔICP at 10 min following intraperitoneal administration of water (left) and maximum d(ICP)/dt (right). Fig. 4B shows a near linear relationship between brain AQP4 expression and the increase in brain water at 10 min, deduced from ΔICP and brain pressure-volume index. Pressure-volume index (∼18.3 in mice) is defined by the following relation: pressure-volume index = dV/log(ICP2/ICP1), where dV is the increase in brain water at 10 min, ICP1 is baseline ICP, and ICP2 is ICP at 10 min (17).
FIGURE 4.
Correlations between ICP parameters and brain AQP4 protein expression. A, ΔICP at 10 min (left) and maximal d(ICP)/dt (right) determined from ICP curve analysis plotted against AQP4 protein expression determined by immunoblot analysis (S.E.). B, deduced increase in brain water at 10 min following intraperitoneal administration of water (Δ brain water, in μl), as a function of brain AQP4 expression. Δ brain water at 10 min is a measure of osmotic water permeability of the blood-brain barrier. Hypothetical curves (dashed) show predictions if AQP4 is rate-limiting for blood-brain barrier water permeability versus if the endothelium is rate-limiting for blood-brain water permeability.
An approximately linear relationship between Δ brain water at 10 min (a measure of osmotic water permeability of the blood-brain barrier) and AQP4 protein expression, as found in Fig. 4B, is predicted if glial foot-process AQP4 is rate-limiting for brain water accumulation across the blood-brain barrier (the dashed line labeled AQP4 rate-limiting). In contrast, a saturating relation is predicted if the endothelial cell is rate-limiting (the dashed curve labeled endothelium rate-limiting). Where saturation occurs depends on the relative endothelial cell versus glial foot-process water permeabilities.
DISCUSSION
The main experimental finding here is that glial cell-targeted, AQP4-overexpressing mice manifest accelerated brain water accumulation in a water intoxication model of cytotoxic brain edema. Intraperitoneal water administration produces acute serum hyponatremia, creating an osmotic driving force for water entry into the brain across an intact blood-brain barrier. The accelerated increase in ICP and hence water entry into the brain in AQP4-overexpressing mice indicates that the enhanced AQP4 expression over baseline levels in control mice increases the osmotic water permeability of the blood-brain barrier, implying that AQP4 and glial water permeability are rate-limiting.
One consequence of our finding is that altered AQP4 expression in various brain pathologies can be functionally significant by increasing water movement into the brain. Cell culture and in vivo studies show altered AQP4 expression in response to various stresses. AQP4 transcript and protein expression were reduced by 60–80% in astrocyte cultures exposed to hypoxia (18). AQP4 expression was increased ∼5-fold in human astrocyte cultures with inflammation produced by interferon-γ exposure (19). In mice, greatly increased AQP4 expression was found following stab wound injury (13), brain abscess (10), and bacterial meningitis (9). AQP4 expression correlated with brain water content in a mouse model of transient cerebral ischemia (8). Brain water content assessed from T2 weighted magnetic resonance images correlated with AQP4 expression in the tumor tissue (5, 6). Up-regulated AQP4 expression was also found in penetrating brain injury in rats (4) and in cortical contusion injury (3). Other studies show reduced AQP4 expression in response to various insults, including transient middle cerebral artery occlusion in rats (20, 21) and diffuse (22) and focal (23) head trauma. Notwithstanding some conflicting results in the literature regarding the direction of change in AQP4 expression in various pathologies, it is concluded that brain AQP4 expression is sensitive to many types of insults, which, from the functional data here, translates to altered brain water balance.
A second consequence of our finding is that the glial cell membrane is a rate-limiting barrier for water movement across the blood-brain barrier, as supported by the correlation between brain water accumulation and AQP4 expression in Fig. 4B. However, it is not possible from the measurements here to determine absolute glial and endothelial cell osmotic water permeabilities. The measured increase in ICP in the acute water intoxication model is a composite function of intracranial compliance, the extent of AQP4 polarization to glial cell foot processes, the kinetics of serum and brain parenchymal osmolalities, and unstirred layer effects. Also, glial and endothelial cell surface areas at the blood-brain barrier are not known.
We used a GFAP promoter strategy to generate glial cell AQP4-overexpressing transgenic mice. GFAP is an intermediate filament protein found almost exclusively in glial cells (24, 25). Because of its specificity, the GFAP promoter has been used as a tool to direct the expression of other genes to glial cells for a variety of studies of glial cell function, brain injury, and other brain pathologies (26–28). The GFAP promoter drives glial cell-specific expression of a variety of foreign proteins not normally expressed in glial cells, such as lacZ (25), green fluorescent protein (29), apoE3 and apoE4 (30), interleukin-12 (31), hemagglutinin (32), erbB (33), interferon-βR1 (34), and tau (35). Both the mouse and the human GFAP promoters have been used in transgenic mouse models (36), with the highest levels of expression found in human GFAP promoter-transgene mouse lines. Smith et al. (37) reported that the human GFAP promoter expressed a transgene to greater than 0.1% of total brain protein. AQP4 protein is normally expressed in glial cells at high levels to increase their water permeability, as the intrinsic (single-channel) water permeability of AQP4 is low (38). To obtain high levels of AQP4 transgene expression, we used the cytomegalovirus enhancer and human GFAP promoter, as done by Wang and Wang (39). Out of four GFAP-AQP4-positive mouse lines, the line with highest AQP4 expression was expanded for functional measurements. However, as mentioned under “Results,” the decreasing percentage of AQP4-positive mice over several generations suggests an unstable integration site in genome. The expression pattern of a transgene is highly dependent on its integration site (36). Generation of genetically stable AQP4-overexpressing mice will require propagation of additional founder mouse lines or use of site-specific integration, as has been done in some models (40).
We used an established model of cytotoxic brain edema, acute water intoxication produced by intraperitoneal injection of water, to investigate AQP4-dependent water movement into the brain across the blood-brain barrier. The acute water intoxication model has been used extensively, including in studies of AQP4 gene deletion in mice (12, 40) and of AQP4 cellular mistargeting produced by α-syntrophin gene deletion (41). Various outcome measures have been used to assess brain water accumulation, including clinical score in awake mice, ICP, brain water content, glial cell foot-process swelling, and near infrared light scattering. Brain water accumulation occurs by an osmotic mechanism in which reduced serum osmolality drives water influx into the brain through an intact blood-brain barrier. Following intraperitoneal water injection, water moves rapidly into the blood compartment, which equilibrates with total body water, generating an osmotic gradient for brain water uptake. As shown in Fig. 3B (inset), serum hyponatremia rapidly develops following intraperitoneal water injection. We chose intraperitoneal water injection and ICP monitoring for studies of cytotoxic brain water accumulation because it is an established, highly reproducible model that provides continuous, quantitative information about brain water uptake. Although direct intravenous or intraarterial administration of hypotonic fluid might provide more rapid control of serum osmolality, large quantities of fluid would need to be delivered rapidly, which would equilibrate with total body water and produce transient circulatory overload. Another practical consideration in the selection of the model was, as mentioned above, the limited numbers of AQP4-overexpressing mice available for these studies, mandating the use of a model with low failure rate and minimal variability.
In conclusion, analysis of glial cell AQP4-overexpressing mice supports the conclusion that AQP4 expression in glial cell foot processes at the blood-brain barrier is rate-limiting, and thus, that altered expression of AQP4 under pathological conditions is predicted to influence brain water balance. It will be interesting to examine the effect of AQP4 overexpression on other causes of cytotoxic edema, such as ischemic stroke and spinal cord injury, as well as on various forms of vasogenic edema and hydrocephalus.
Acknowledgments
We acknowledge the Stanford Transgenic Research Facility for DNA microinjections and Liman Qian for mouse breeding and genotyping.
This work was supported, in whole or in part, by National Institutes of Health Grants DK35124, EY13574, HL59198, EB00415, DK72517, and HL73856. This work was also supported by Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: AQP4, aquaporin-4; GFAP, glial fibrillary acid protein; ICP, intracranial pressure.
References
- 1.Rash, J. E., Yasumura, T., Hudson, C. S., Agre, P., and Nielsen, S. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 11981-11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman, C. S., Gorelick-Feldman, D. A., Davidson, K. G., Yasumura, T., Neely, J. D., Agre, P., and Rash, J. E. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 13609-13614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghabriel, M. N., Thomas, A., and Vink, R. (2006) Acta Neurochir. Suppl. 96 402-406 [DOI] [PubMed] [Google Scholar]
- 4.Neal, C. J., Lee, E. Y., Gyorgy, A., Ecklund, J. M., Agoston, D. V., and Ling, G. S. (2007) J. Neurotrauma 24 1609-1617 [DOI] [PubMed] [Google Scholar]
- 5.Saadoun, S., Papadopoulos, M. C., Davies, D. C., Krishna, S., and Bell, B. A. (2002) J. Neurol. Neurosurg. Psychiatry 72 262-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warth, A., Simon, P., Capper, D., Goeppert, B., Tabatabai, G., Herzog, H., Dietz, K., Stubenvoll, F., Ajaaj, R., Becker, R., Weller, M., Meyermann, R., Wolburg, H., and Mittelbronn, M. (2007) J. Neurosci. Res. 85 1336-1346 [DOI] [PubMed] [Google Scholar]
- 7.Badaut, J., Brunet, J. F., Grollimund, L., Hamou, M. F., Magistretti, P. J., Villemure, J. G., and Regli, L. (2003) Acta Neurochir. Suppl. 86 495-498 [DOI] [PubMed] [Google Scholar]
- 8.Badaut, J. (2006) FASEB J. 20 2423-2424 [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos, M. C., and Verkman, A. S. (2005) J. Biol. Chem. 280 13906-13912 [DOI] [PubMed] [Google Scholar]
- 10.Bloch, O., Papadopoulos, M. C., Manley, G. T., and Verkman, A. S. (2005) J. Neurochem. 95 254-262 [DOI] [PubMed] [Google Scholar]
- 11.Verkman, A. S., Binder, D. K., Bloch, O., Auguste, K., and Papadopoulos, M. C. (2006) Biochim. Biophys. Acta 1758 1085-1093 [DOI] [PubMed] [Google Scholar]
- 12.Manley, G. T., Fujimura, M., Ma, T., Noshita, N., Filiz, F., Bollen, A. W., Chan, P., and Verkman, A. S. (2000) Nat. Med. 6 159-163 [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos, M. C., Manley, G. T., Krishna, S., and Verkman, A. S. (2004) FASEB J. 18 1291-1293 [DOI] [PubMed] [Google Scholar]
- 14.Bloch, O., Auguste, K. I., Manley, G. T., and Verkman, A. S. (2006) J. Cereb. Blood Flow Metab. 26 1527-1537 [DOI] [PubMed] [Google Scholar]
- 15.Ma, T., Yang, B., Gillespie, A., Carlson, E. J., Epstein, C. J., and Verkman, A. S. (1997) J. Clin. Investig. 100 957-962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amiry-Moghaddam, M., Xue, R., Haug, F. M., Neely, J. D., Bhardwaj, A., Agre, P., Adams, M. E., Froehner, S. C., Mori, S., and Ottersen, O. P. (2004) FASEB J. 18 542-544 [DOI] [PubMed] [Google Scholar]
- 17.Marmarou, A., Shulman, K., and Rosende, R. (1978) J. Neurosurg. 48 332-344 [DOI] [PubMed] [Google Scholar]
- 18.Fu, X., Li, Q., Feng, Z., and Mu, D. (2007) Glia 55 935-941 [DOI] [PubMed] [Google Scholar]
- 19.Satoh, J., Tabunoki, H., Yamamura, T., Arima, K., and Konno, H. (2007) Neuropathology 27 245-256 [DOI] [PubMed] [Google Scholar]
- 20.Meng, S., Qiao, M., Lin, L., Del Bigio, M. R., Tomanek, B., and Tuor, U. I. (2004) Eur. J. Neurosci. 19 2261-2269 [DOI] [PubMed] [Google Scholar]
- 21.Frydenlund, D. S., Bhardwaj, A., Otsuka, T., Mylonakou, M. N., Yasumura, T., Davidson, K. G., Zeynalov, E., Skare, O., Laake, P., Haug, F. M., Rash, J. E., Agre, P., Ottersen, O. P., and Amiry-Moghad-dam, M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 13532-13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke, C., Poon, W. S., Ng, H. K., Pang, J. C., and Chan, Y. (2001) Neurosci. Lett. 301 21-24 [DOI] [PubMed] [Google Scholar]
- 23.Kiening, K. L., van Landeghem, F. K., Schreiber, S., Thomale, U. W., von Deimling, A., Unterberg, A. W., and Stover, J. F. (2002) Neurosci. Lett. 324 105-108 [DOI] [PubMed] [Google Scholar]
- 24.Besnard, F., Brenner, M., Nakatani, Y., Chao, R., Purohit, H. J., and Freese, E. (1991) J. Biol. Chem. 266 18877-18883 [PubMed] [Google Scholar]
- 25.Brenner, M., Kisseberth, W. C., Su, Y., Besnard, F., and Messing, A. (1994) J. Neurosci. 14 1030-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner, M., and Messing, A. (1996) Methods (Amst.) 10 351-364 [DOI] [PubMed] [Google Scholar]
- 27.de Leeuw, B., Su, M., ter Horst, M., Iwata, S., Rodijk, M., Hoeben, R. C., Messing, A., Smitt, P. S., and Brenner, M. (2006) J. Neurosci. Res. 83 744-753 [DOI] [PubMed] [Google Scholar]
- 28.Lee, Y., Messing, A., Su, M., and Brenner, M. (2008) Glia 56 481-493 [DOI] [PubMed] [Google Scholar]
- 29.Zhuo, L., Sun, B., Zhang, C. L., Fine, A., Chiu, S. Y., and Messing, A. (1997) Dev. Biol. 187 36-42 [DOI] [PubMed] [Google Scholar]
- 30.Sun, Y., Wu, S., Bu, G., Onifade, M. K., Patel, S. N., LaDu, M. J., Fagan, A. M., and Holtzman, D. M. (1998) J. Neurosci. 18 3261-3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagenstecher, A., Lassmann, S., Carson, M. J., Kincaid, C. L., Stalder, A. K., and Campbell, I. L. (2001) J. Immunol. 164 4481-4492 [DOI] [PubMed] [Google Scholar]
- 32.Cornet, A., Savidge, T. C., Cabarrocas, J., Deng, W. L., Colombel, J. F., Lassmann, H., Desreumaux, P., and Liblau, R. S. (2001) Proc Proc. Natl. Acad. Sci. U. S. A. 98 13306-13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prevot, V., Rio, C., Cho, G. J., Lomniczi, A., Heger, S., Neville, C. M., Rosenthal, N. A., Ojeda, S. R., and Corfas, G. (2003) J. Neurosci. 23 230-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hindinger, C., Gonzalez, J. M., Bergmann, C. C., Fuss, B., Hinton, D. R., Atkinson, R. D., Macklin, W. B., and Stohlman, S. A. (2005) J. Neurosci. Res. 82 20-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forman, M. S., Lal, D., Zhang, B., Dabir, D. V., Swanson, E., Lee, V. M., and Trojanowski, J. Q. (2005) J. Neurosci. 25 3539-3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su, M., Hu, H., Lee, Y., d'Azzo, A., Messing, A., and Brenner, M. (2004) Neurochem. Res. 29 2075-2093 [DOI] [PubMed] [Google Scholar]
- 37.Smith, J. D., Sikes, J., and Levin, J. A. (1998) Neurobiol. Aging 19 407-413 [DOI] [PubMed] [Google Scholar]
- 38.Yang, B., and Verkman, A. S. (1997) J. Biol. Chem. 272 16140-16146 [DOI] [PubMed] [Google Scholar]
- 39.Wang, C. Y., and Wang, S. (2006) Gene Ther. 13 1447-1456 [DOI] [PubMed] [Google Scholar]
- 40.Thiagarajah, J. R., Papadopoulos, M. C., and Verkman, A. S. (2005) J. Neurosci. Res. 80 293-299 [DOI] [PubMed] [Google Scholar]
- 41.Amiry-Moghaddam, M., Williamson, A., Palomba, M., Eid, T., de Lanerolle, N. C., Nagelhus, E. A., Adams, M. E., Froehner, S. C., Agre, P., and Ottersen, O. P. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 13615-13620 [DOI] [PMC free article] [PubMed] [Google Scholar]