Abstract
Human T-cell leukemia virus type-I expresses Tax, a 40-kDa oncoprotein that activates IκB kinase (IKK), resulting in constitutive activation of NFκB. Herein, we have developed an in vitro signaling assay to analyze IKK complex activation by recombinant Tax. Using this assay in combination with reporter assays, we demonstrate that Tax-mediated activation of IKK is independent of phosphatases. We show that sustained activation of the Tax-mediated activation of the NFκB pathway is dependent on an intact Hsp90-IKK complex. By acetylating and thereby preventing activation of the IKK complex by the Yersinia effector YopJ, we demonstrate that Tax-mediated activation of the IKK complex requires a phosphorylation step. Our characterization of an in vitro signaling assay system for the mechanism of Tax-mediated activation of the IKK complex with a variety of mutants and inhibitors results in a working model for the biochemical mechanism of Tax-induced activation.
The first identified pathogenic retrovirus, human T-cell leukemia virus type-1, the causal agent for adult T-cell leukemia, expresses Tax, a 40-kDa phospho-oncoprotein, that plays a pivotal role in the growth and transformation of T-cells (1). Tax chronically stimulates the IκB kinase (IKK)3 complex via NEMO/IKKγ, resulting in sustained phosphorylation and degradation of IκB and activation of NFκB (2–4). Tax, unlike all the other upstream stimuli such as NFκB-inducing kinase, MEK kinase 1, and transforming growth factor β-activated kinase 1, is not a kinase that phosphorylates and activates the IKK complex. It activates the complex by direct interaction via a mechanism not clearly understood. It has been proposed that Tax activates the NFκB pathway by inducing a conformational change onto IKK (5).
Previous studies have identified several binding partners for Tax, such as MEK kinase 1 (6) and IKKγ in the IKK complex (3). The catalytic subunit of the serine/threonine protein phosphatase 2A (PP2A) was recently identified to bind Tax directly, and it was demonstrated that Tax forms a ternary complex together with IKKγ and PP2A (7). The role of PP2A in inhibiting IKK activation has been established in the past (8). Based on these studies, two models have been proposed to explain the regulation of the IKK complex by Tax in conjunction with PP2A (7, 9). According to one model, Tax binding to PP2A relieves negative inhibition on the IKK complex, resulting in an active IKK complex (7). In contrast, another group has demonstrated that PP2A positively regulates IκB kinase signaling, i.e. IKK-PP2A complexes are essential for IKK activation (9). This gives rise to a second model wherein Tax-mediated activation of IKK requires the association of active PP2A with the IKK complex (9).
In contrast to the viral pathogen human T-cell leukemia virus type-1, the bacterial pathogen Yersinia pestis, the causal agent for bubonic plague, inhibits the NFκB pathway (10, 11). Yersinia species use the bacterial virulence factor YopJ to block all MAPK and NFκB pathways at a common point (11, 12). Recent studies revealed that YopJ functions as an acetyltransferase (13, 14). It blocks the activation of all MAPK kinases, including IKKβ, by the addition of an acetyl group to the highly conserved serine and threonine residues in the activation loop of the kinase. Acetylation of these residues by YopJ prevents the activation of these kinases by inhibiting phosphorylation. Previously, overexpression studies have shown that YopJ inhibits Tax-mediated activation of the IKK complex (15).
To decipher the requirements of Tax-induced NFκB signaling, we have developed an in vitro signaling assay to analyze the activation of the IKK complex by Tax. The assay utilizes wild type and mutant recombinant Tax proteins, an S100 lysate containing unstimulated IKK complex, and a readout for the activation of the NFκB pathway, phospho-IκB. The mutant Tax proteins that we have used in our assays include M22, H41Q, H43Q, and K85N. Fu et al. (7) demonstrated that the ability to induce NFκB activation is abrogated in the M22 mutant whereas the other three mutants are defective in binding PP2A and also fail to activate the NFκB pathway. Based on their study, they proposed that binding of Tax to PP2A is essential for NFκB activation. Herein, we find that activation of the NFκB pathway by recombinant Tax does not require binding to or the activity of PP2A but is dependent on an intact Hsp90-IKK complex. Recombinant Tax is unable to activate the IKK complex in an S100 lysate that contains the acetyltransferase YopJ. Based on results from transcription reporter assays and our in vitro signaling system, we propose that the activation of the IKK complex by human T-cell leukemia virus type-1 Tax requires both a signaling complex containing functional chaperone Hsp90 and an activation loop on IKKβ that is required for phosphorylation. This in vitro signaling assay will thus allow us to dissect the mechanism of Tax-dependent activation of IKK.
MATERIALS AND METHODS
Plasmids and Reagents—Tax was cloned into pET28a and pcDNA3 vectors using 5′-BamH1 and 3′-Xho1. The four Tax mutant constructs, M22, H41Q, H43Q, and K85N, in pET28a and pcDNA3 vectors were created using the Stratagene mutagenesis kit with the following pair of oligonucleotides: M22, 5′-ACC CTT GGG CAG CAC CTC CCA AGC GCG TCT TTT CCA GAC CCC GGA CTC, M22, 3′-GAG TCC GGG GTC TGG AAA AGA CGC GCT TGG GAG GTG CTG CCC AAG GGT; H41Q, 5′-GGA CTA TGT TCG GCC CGC CTA CAG CGT CAC GCC CTA CTG GCC ACC, H41Q, 3′-GGT GGC CAG TAG GGC GTG ACG CTG TAG GCG GGC CGA ACA TAG TCC; H43Q, 5′-GGA CTA TGT TCG GCC CGC CTA CAT CGT CAG GCC CTA CTG GCC ACC, H43Q, 3′-GGT GGC CAG TAG GGC CTG ACG ATG TAG GCG GGC CGA ACA TAG TCC; K85N, 5′-TTC CCC ACC CAG AGA ACC TCT AAT ACC CTC AAG GTC CTT ACC CCG, K85N, 3′-CGG GGT AAG GAC CTT GAG GGT ATT AGA GGT TCT CTG GGT GGG GAA. pcDNA3-FLAG-IκB was kindly provided by Dr. Zhijian Chen. pSFFV YopJ-FLAG, pSFFV YopJC172A-FLAG, and 5xNFκB luciferase reporter were described previously (8).
Anti-phospho-IκB antibody was obtained from Cell Signaling, and anti-FLAG antibody was obtained from Sigma. Antibodies to Hsp90 and IKKβ were purchased from Santa Cruz Biotechnology. Protein A-agarose beads were purchased from Invitrogen. Geldanamycin and okadaic acid were purchased from Alexis. pNPP was obtained from Sigma.
Expression and Purification of Recombinant Proteins—Wild type and the various mutants of Tax (M22, H41Q, H43Q, and K85N) were expressed as His-tagged proteins in Rosetta (Invitrogen) cells, grown to an O.D. of 0.6–0.8, and induced at room temperature with 0.2 mm isopropyl-1-thio-β-d-galactopyranoside for 8–12 h. The bacterial pellet was then lysed by an Emulsiflex C5 cell disruptor (Avestin) and purified using standard protocols for Ni2+-nitrilotriacetic acid purification (Qiagen). Recombinant TRAF6 was used as a positive control and was purified from Sf9 cells as described in Li et al. (16). Anti-Tax was used as previously described (17).
Tissue Culture and Lysate Preparation—HEK293 or HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% cosmic calf serum and 100 units/ml penicillin/streptomycin/glutamine (Invitrogen) in the presence of 5% CO2. For the in vitro assays, untransfected HeLa cells or HEK293 cells transfected with 10 μg of pSFFV, pSFFV-YopJ-FLAG, or pSFFV-YopJC172A-FLAG using FuGENE (Roche Applied Science) were grown up to 100% confluency, harvested using phosphate-buffered saline-EDTA, and lysed with equal volume of HTX lysis buffer (10 mm Hepes, pH 7.4, 10 mm MgCl2, 1 mm MnCl2, 0.5% Triton X-100, 0.1 mm EGTA) containing protease inhibitor mixture tablet (Roche Applied Science) by incubating on ice for 20–60 min. Lysate was spun sequentially at 800, 10,000, and then 100,000 × g to obtain a cleared S100 lysate that was then stored at -80 °C at a protein concentration of 10 mg/ml.
Luciferase and Transfection Assays—HEK293 cells were transfected with 200 ng of WT Tax or the different Tax mutants (M22, H41Q, H43Q, and K85N) and 5xNFκB luciferase reporter in the absence or presence of pSFFV YopJ-FLAG or pSFFV YopJC172A-FLAG for 24 h using FuGENE transfection reagent. Each plate was also transfected with pRSV-Renilla to serve as the internal standard control. Lysates were prepared using passive lysis buffer (Promega), and luciferase assays were performed using Fluostar Optima (BMG Labtech).
For in vivo activation of the NFκB pathway, HEK293 cells were transfected with pcDNA3-WT-Tax or mutant Tax and pcDNA3-FLAG-IκB in the absence or presence of 100 ng of pSFFV-YopJ-FLAG or pSFFV-YopJC172A-FLAG. 36 h post-transfection, cells were lysed with HNT lysis buffer (10 mm HEPES, pH 7.4, 50 mm NaCl, and 1% Triton X-100) containing protease inhibitor mixture tablet (Roche Applied Science), 1 mm dithiothreitol, 20 mm NaF, 20 mm β-glycerophosphate, 0.5 mm sodium vanadate, and 0.5 mm EGTA. Lysates were immunoblotted with anti-phospho-IκB, anti-FLAG, and anti-Tax antibodies.
pNPP Assay—A 1:50 dilution of Hela-S3 cell-free lysate was incubated in the absence or presence of 10 nm okadaic acid. The 96-well microtiter plate assay (Upstate Biotechnology) was carried out in triplicate according to the manufacturer's description. The absorbance at wavelength 410 nm was read using a FluoStar Optima (BMG Labtech).
In Vitro Assay Using Recombinant Tax—Cleared lysates (10 mg/ml) were incubated with or without 2 μm recombinant WT Tax and/or all the mutants or 1 μm TRAF6 in the presence of an ATP-regenerating system (10× stock: 10 mm ATP, 350 mm creatine phosphate, 20 mm Hepes, pH 7.2, 10 mm MgCl2, and 500 μg/ml creatine kinase) for 10 min at 37 °C. 0.5 μm okadaic acid and 2 μm geldanamycin were added to cleared lysates before the addition of the recombinant protein. Reactions were terminated by addition of 5× SDS sample buffer. Proteins from the reaction samples were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. IKK activation was detected by immunoblotting with anti-phospho-IκB antibody. For in vitro assays using Hsp90-immunodepleted lysate, lysates were incubated with anti-Hsp90 antibody for 1 h at 4 °C, followed by incubation with 30 μl of Protein A-agarose beads for 30 min at 4 °C. This step was performed twice to ensure complete immunodepletion of Hsp90. The lysates were finally incubated with Tax or TRAF6 recombinant proteins. In vitro kinase assays were performed on immunoprecipitated complexes as previously described using [γ-32P]ATP and GST-IkBα-(1–52) as the recombinant substrate (11).
RESULTS
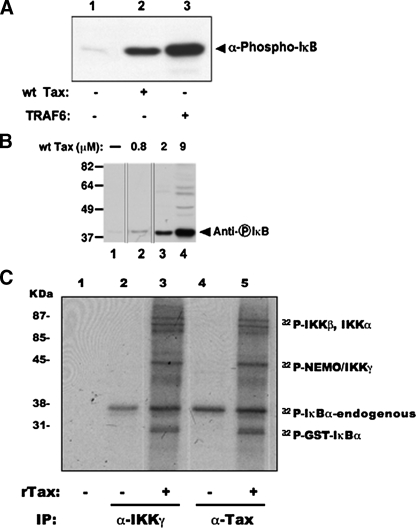
In Vitro Activation of the NFκB Pathway by Recombinant Human T-cell Leukemia Virus Type-1 Tax—We previously established an assay to study the activation of MAPK and NFκB signaling pathways in vitro (13). The assay is initiated by the addition of a partially purified activator of a signaling pathway to an S100 lysate in the presence of an ATP-regenerating system, followed by termination of the reaction by addition of SDS sample buffer. The activation of the signaling pathway is assessed by analysis of a downstream indicator by immunoblotting for a phosphorylated protein. Herein, we analyzed the activation of the NFκB pathway by the addition of partially purified recombinant WT His6-Tax protein to the unstimulated S100 lysate (Fig. 1A, lane 1). Addition of Tax protein (2 μm) to cleared lysate results in the activation of the NFκB pathway as indicated by robust phosphorylation of IκB (Fig. 2A, lane 2). Similarly, addition of 1 μm partially purified recombinant TRAF6 (Fig. 1A, lane 6), which served as the positive control in this assay, also activates the NFκB pathway (Fig. 2A, lane 3). Using serial dilutions of WT recombinant Tax added to the in vitro signaling system, we demonstrate that the activation of the NFκB pathway is detected with as little as 800 nm Tax (Fig. 2B).
FIGURE 1.
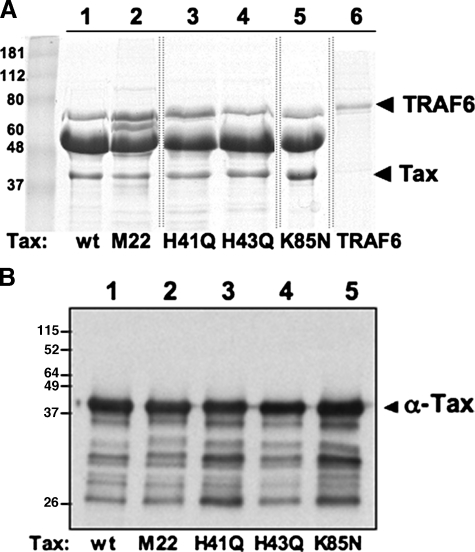
Recombinant Tax and TRAF6. Wild type and mutant forms of Tax (lanes 1–5) were expressed as His6-tagged proteins in BL21-DE3 cells and purified from BL21-DE3 cells using standard protocols. Recombinant TRAF6 (lane 6) was purified from Sf9 cells as previously described (16). Sample of each recombinant protein was resolved on SDS-PAGE and stained with Coomassie Blue (A) or subjected to Western blotting using anti-Tax antibody (B). Thin lines denote where lanes have been deleted from the scanned image.
FIGURE 2.
In vitro activation of NFκB pathway by recombinant Tax and TRAF6. A, IKK activation by wild type (wt) Tax and TRAF6 using in vitro signaling assay. Lane 1, lysate control; lanes 2 and 3, addition of 2 μm Tax and 1 μm TRAF6, respectively, to lysate. B, concentration dependence of Tax-mediated IκB phosphorylation. Lane 1, lysate control; lanes 2–4, increasing amounts of Tax (0.8–9.0 μm) added to lysate. Thin lines denote where lanes have been deleted from the scanned image. For all experiments, activation of IKK complex was detected by immunoblotting with anti-phospho-IκB antibody. C, in vitro kinase assay of complexes isolated with anti-IKKγ and anti-Tax from unstimulated (lanes 1, 2, and 4) and Tax-stimulated (lanes 3 and 5) lysates. Immuno-isolated IKK complexes were incubated with [γ-32P]ATP and GST-IκBα-(1–52), separated by SDS-PAGE, and analyzed by autoradiography. The data shown are representative of three independent experiments.
To confirm that WT His6-Tax protein is activating the IKK complex specifically, we activated lysates with WT His6-Tax protein followed by immunoprecipitation of the IKK complex using antibodies for IKKγ or Tax (Fig. 2C). The isolated signaling complexes were tested for radioactive kinase activity in an in vitro kinase assay using GST-IκBα-(1–52) as the substrate. Identical phosphorylated protein profiles were observed from signaling complexes isolated using either anti-IKKγ or anti-Tax antibodies, demonstrating that Tax is binding to and activating the IKK complex (Fig. 2C). As expected, only complexes isolated from the Tax-activated lysates were able to phosphorylate GST-IκBα (Fig. 2, B and C).
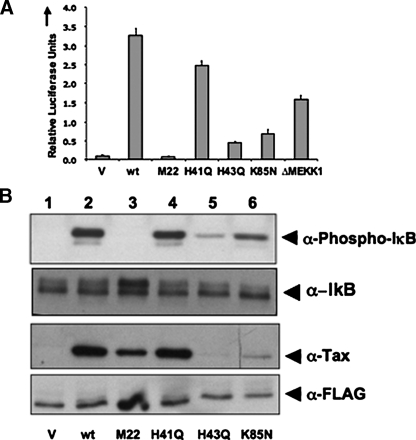
Tax-mediated Activation of NFκB Pathway Is Independent of Binding to Phosphatases—To determine the role of phosphatases in the activation of the NFκB pathway by Tax, several previously described mutants of Tax (7) were used in our in vivo and in vitro signaling assays. The effect on the activation of the NFκB pathway by wild type and mutant Tax proteins was first analyzed using transfection-based luciferase assays with an NFκB luciferase reporter (Fig. 3A). The activation of the NFκB pathway by the various Tax mutants was also analyzed by immunoblotting cell lysates with anti-phospho-IκB antibody (Fig. 3B). We observed that the M22 mutant, which has previously been described as the inactive form of Tax, fails to induce activation of the NFκB pathway, and these findings are consistent with previous studies in which this mutant was shown to be unable to activate the NFκB pathway (Fig. 3, A and B) (7). Tax mutants H41Q, H43Q, and K85N activated the NFκB pathway to varying levels, based on both the luciferase assays and the immunoblotting for phospho-IκB (Fig. 3, A and B). For the latter experiment, cells were constitutively expressed with FLAG-IκB to detect phosphorylation of exogenous IκB, because endogenous IκB is degraded upon activation of the NFκB pathway (13). The mutants H43Q and K85N were not detected using the anti-Tax antibody because of their decreased expression levels in the mammalian system (Fig. 3B) (17). Because H41Q, H43Q, and K85N, which are defective in binding PP2A, were able to activate the NFκB pathway, the binding of PP2A to Tax does not appear to be essential for Tax-mediated activation of the IKK complex (7).
FIGURE 3.
Activation of the NFκB pathway by wild type and mutant Tax. A, activation of a NFκB luciferase reporter gene by Tax. HEK293 cells were transfected with an empty vector (V), wild type Tax (wt), or the different Tax mutants (M22, H41Q, H43Q, and K85N), 5xNFκB luciferase reporter, and pRSV-Renilla (to serve as the internal standard control) for 24 h. The luciferase assay was performed using Fluostar Optima. Error bars denote mean ± S.D. of triplicates; results shown are representative of four independent experiments. B, HEK293 cells were transfected with empty vector (lane 1), wild type Tax (wt; lane 2), M22 (lane 3), H41Q (lane 4), H43Q (lane 5), or K85N (lane 6) and FLAG-IκB. Activation of IKK complex, Tax expression, and FLAG-IκB were detected by immunoblotting with anti-phospho-IκB antibody, anti-IκB antibody, anti-Tax antibody, and anti-FLAG antibody, respectively. The data shown are representative of three independent experiments.
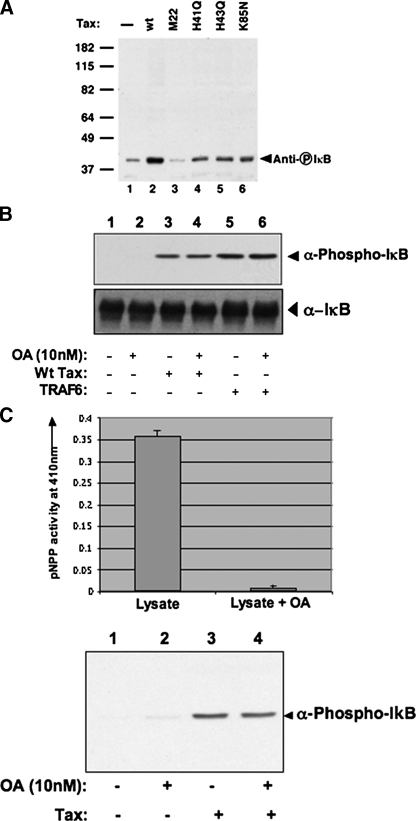
The Tax mutants were also expressed as recombinant His-tagged proteins in bacteria and purified using nickel affinity chromatography (Fig. 1A). The anti-Tax antibody was able to immunoreact with all the recombinant proteins (Fig. 1B). Consistent with previous in vivo observations, addition of the recombinant inactive mutant form of Tax (M22) to the in vitro signaling system was unable to induce phosphorylation of IκB (Fig. 4A, lane 5). However, addition of the three recombinant mutant forms of Tax including H41Q, H43Q, and K85N, which are unable to bind PP2A, resulted in activation of the NFκB pathway as indicated by phosphorylation of IκB in vitro, albeit with varying potency (Fig. 4A, lanes 3, 4, and 6). The profiles of partially purified wild type Tax and the inactive mutant M22 proteins (Fig. 1, lanes 1 and 2) appear the same, supporting the proposal that differences in the activities can be attributed to the mutated amino acids in the various Tax proteins. Overall, these observations do not support the first model, in that binding of PP2A to Tax is not essential for Tax activation of the IKK complex (7).
FIGURE 4.
In vitro activation of NFκB pathway by Tax is independent of phosphatases. A, activation of the IKK complex by buffer (lane 1) wild type (wt) Tax (lane 2), M22 (lane 3), H41Q (lane 4), H43Q (lane 5), or K85N (lane 6) using the in vitro signaling assay. Activation of IKK complex was detected by immunoblotting with anti-phospho-IκB antibody. B, in vitro activation of the IKK complex by buffer (lanes 1 and 2), Tax (lanes 3 and 4), or TRAF6 (lanes 5 and 6) in the absence (lanes 1, 3, and 5) or presence (lanes 2, 4, and 6) of 10 nm okadaic acid (OA). Activation of the IKK complex was detected by immunoblotting with anti-phospho-IκB antibody. To confirm equal amounts of IκBin all lanes, the same lysates were blotted with α-IκB antibody. C, activity of PP2A in S100 lysate in the presence or absence of 10 nm OA. PP2A activity was measured using pNPP as a substrate. The data shown are representative of three independent experiments. Error bars denote mean ± S.D. of triplicates; results shown are representative of four independent experiments.
Tax-mediated Activation of the NFκB Pathway Is Independent of Active Phosphatases—To further assess whether PP2A plays a positive role in the in vitro activation of the IKK complex by Tax, the in vitro activation of the NFκB pathway was analyzed in the presence of okadaic acid (OA). Addition of 10 nm OA (or 500 nm, data not shown) did not alter the ability of wild type Tax to activate the IKK complex as observed by phosphorylation of IκB (Fig. 4B, lane 4). At this very low concentration of OA (10 nm), PP2A activity is effectively repressed in the lysate (Fig. 4C). Therefore, when PP2A activity is inhibited, Tax is still able to activate the IKK complex. As expected, there was no change in IκB phosphorylation levels upon addition of TRAF6 to the lysate in the presence of OA (Fig. 4B, lane 6). Based on these observations, Tax can activate the IKK complex independent of PP2A activity, thereby discounting the second model that predicted that the interaction between IKK and PP2A is essential for Tax to be able to activate the IKK complex (9).
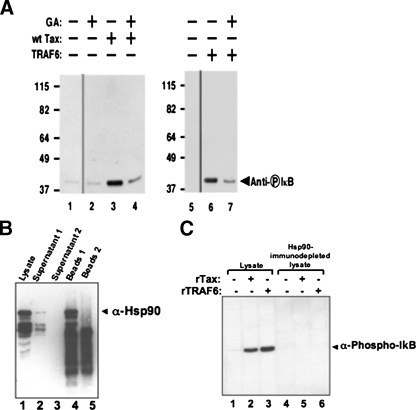
Hsp90 Is Essential for Tax-mediated Activation of the IKK Complex—Studies were initiated to further understand the requirements for activation of the IKK complex by Tax. The in vitro signaling system recapitulated the activation of the NFκB pathway by Tax and also demonstrated that Tax-mediated IKK activation is independent of phosphatases. It is possible that by binding to IKKγ, Tax is inducing a conformational change that results in the activation of the IKK complex (5). Hsp90, which is known to maintain the structural integrity of protein complexes (18), is an integral component of the IKK complex (19). To test whether Hsp90 plays a role in Tax-mediated activation of the NFκB pathway in vitro, geldanamycin (GA), an Hsp90-specific inhibitor, was used in the in vitro signaling system. Upon addition of 2 μm GA to the in vitro signaling assay, it was observed that Tax could no longer activate IKK as indicated by the lack of IκB phosphorylation (Fig. 5A, lane 4). GA also inhibited the TRAF6-dependent activation of the IKK complex (Fig. 5A, lane 7). This is consistent with previous observations where GA-dependent Hsp90 inhibition has been shown to interfere with IKK activation (19). To further assess the effect of Hsp90 on Tax-mediated NFκB activation, we used lysate, immunodepleted for Hsp90 (Fig. 5B), in our in vitro cell-free signaling assay. Addition of recombinant Tax and TRAF6 proteins to Hsp90-immunodepleted lysate failed to activate the NFκB pathway as shown by immunoblotting against phospho-IκB (Fig. 5C, lanes 5 and 6). Because Hsp90 is known to maintain the structural integrity of protein complexes, these observations support a new model that a preformed native complex requiring active Hsp90 is essential for the activation of the IKK complex by Tax. This supports the proposed model that binding of Tax to IKKγ in the IKK complex causes a conformational change that induces autoactivation of the kinases in the complex (5).
FIGURE 5.
Inhibition of Tax-mediated activation of the NFκB pathway by geldanamycin. A, in vitro activation of the IKK complex by buffer (lanes 1 and 2), Tax (lanes 3 and 4), or TRAF6 (lanes 5 and 6) in the absence (lanes 1, 3, and 5) or presence (lanes 2, 4, and 6) of geldanamycin (GA). Thin lines denote where lanes have been deleted from the scanned image. Activation of the IKK complex was detected by immunoblotting with anti-phospho-IκB antibody. B, cell-free lysates (1 mg/ml) were immunodepleted with Hsp90 by performing two successive rounds of immunoprecipitation with anti-Hsp90 antibody (1:100). The levels of Hsp90 and IKKβ in these lysates were assessed by immunoblotting against anti-Hsp90 (upper panel) and anti-IKKβ antibodies. C, lysates immunodepleted with Hsp90 (lanes 4–6) or not (lanes 1–3) were incubated with 2 μm Tax and 1 μm TRAF6 for 10 min at 37 °C. Samples were subjected to SDS-PAGE followed by immunoblotting with anti-phospho-IκB antibody. The data shown are representative of three independent experiments.
Tax Cannot Bypass Inhibition of the IKK Complex by YopJ Acetylation—Tax is one of the upstream activators of the IKK complex; however, the mechanism by which it activates IKK has not been completely deciphered. The effector protein YopJ from Yersinia was recently shown to inhibit MAPK kinase and IKK activation by acetylating the conserved serine and threonine residues in the activation loop of the kinase (13, 14, 20). Consistent with previous findings, YopJ inhibited IKK activation by Tax, as shown by using a NFκB luciferase reporter (Fig. 6A) (15). The catalytically inactive mutant YopJC172A, however, had no effect on Tax-mediated activation of NFκB (Fig. 6A). Transfection experiments with YopJ and Tax followed by immunoblotting with antibody against phospho-IκB further confirmed these results because YopJ, but not YopJC172A, inhibited Tax-mediated IκB phosphorylation (Fig. 6B). As before, cells constitutively expressed FLAG-IκB to detect phosphorylation of exogenous IκB.
FIGURE 6.
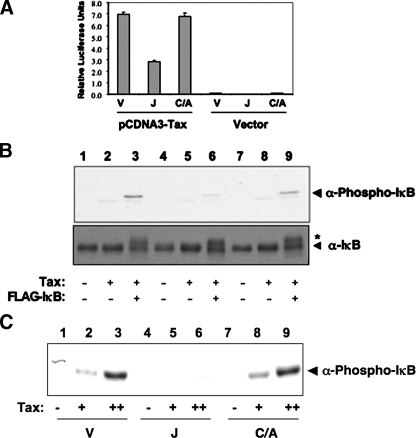
Activation of the NFκB pathway by Tax is inhibited by the acetyltransferase YopJ. A, activation of an NFκB luciferase reporter gene by Tax. HEK293 cells were transfected with an empty vector (Vector) or wild type Tax (pcDNA3-Tax) in the presence of an empty vector (V), YopJ (J), or catalytically inactive YopJC172A (C/A), the 5xNFκB luciferase reporter, and pRSV-Renilla (to serve as the internal standard control) for 24 h. The luciferase assay was performed using Fluostar Optima. B, HEK293 cells were cotransfected with Tax and either empty vector (V), YopJ (J), or catalytically inactive YopJC172A (C/A) in the presence or absence of FLAG-IκB followed by immunoblotting cell lysates with anti-phospho-IκB antibody and anti-IκB antibody. The asterisk indicates transfected FLAG-IκB. C, in vitro activation of the IKK complex by Tax (+, 0.8 μm; ++, 1.6 μm) in lysates isolated from cells containing empty vector (V), YopJ (J), or catalytically inactive YopJC172A (C/A). Activation of IKK complex was detected by immunoblotting with anti-phospho-IκB antibody. The data shown are representative of three independent experiments.
YopJ Inhibits in Vitro Activation of IKK by Tax—To gain insight into the mechanism used by Tax, in vitro assay of Tax-mediated IKK activation was utilized. 10 mg/ml membrane-cleared lysate was prepared from cells transfected with vector (V), YopJ (J), or YopJC172A (C/A) plasmids (13). Recombinant WT Tax was then used to activate these lysates. Tax was able to phosphorylate endogenous IκBin both V- and C/A-transfected cell lysates (Fig. 6C). By contrast and as expected, Tax had lost its ability to phosphorylate IκB from cells expressing YopJ. The results from this in vitro assay confirm the above in vivo results and are in accordance with the work by Carter et al. (15). Just like all other upstream stimuli, YopJ is able to block the Tax-mediated activation of the IKK complex.
DISCUSSION
In this study, an in vitro signaling assay was used to analyze the activation of IKK by Tax. Recombinant Tax, purified from bacteria, was able to efficiently induce IκB phosphorylation in cleared lysates. The phosphorylation profiles of the Tax-activated complexes isolated by immunoprecipitation with IKKγ or Tax appear the same, supporting the accepted hypothesis that Tax, IKKγ, and the IKKs are parts of the same signaling complex. The PP2A binding-deficient mutants of Tax were also able to activate NFκB signaling, although not as strongly as WT Tax. These observations do not support the model that the binding of PP2A to Tax is essential for Tax-mediated activation of the IKK complex (7). In addition, experiments with okadaic acid added to the in vitro assay did not alter the ability of WT Tax to activate the IKK complex and therefore do not support the alternative model that Tax-mediated IKK activation is positively regulated by PP2A (9). By contrast, Tax was unable to activate the NFκB pathway when Hsp90, an integral component of the IKK complex, was inhibited by the addition of geldanamycin to the assay. Binding of Tax to IKKγ in the IKK complex may cause a conformational change that induces autoactivation of the kinases in the complex. As more mutants associated with the other activities of viral Tax are discovered (21), this system can be used to diagnose their role in the activation of IKK.
Both in vivo and in vitro studies revealed that YopJ blocks the activation of IKK by Tax. YopJ leads to the acetylation of the conserved serine and threonine in the activation loop of kinases, including IKK. When the activation loop residues are acetylated, they can no longer be phosphorylated and the kinase cannot be activated (13, 14, 20). The acetyltransferase activity of YopJ on IKK thus competes against phosphorylation of IKK by upstream kinases. As YopJ inhibits Tax-mediated IKK activation, these studies strongly indicate that phosphorylation of IKK is a key intermediate step in the activation of IKK by Tax.
Our results support previously postulated mechanisms for Tax-mediated activation of the IKK complex, including induction of a conformational change or recruitment of an upstream kinase (5). The in vitro signaling system used in this study in combination with a number of inhibitors and activators has been useful for the elucidation of the biochemical mechanism of Tax-induced activation of the IKK complex. Based on these studies, a model is proposed whereby Tax-dependent activation of the IKK complex requires active Hsp90 and phosphorylation of IKK. Future biochemical studies that further dissect this mechanism may reveal other factors that are essential for the activation of the IKK complex by Tax.
Acknowledgments
We thank Dr. Charles Bangham, Dr. Julie Pfeiffer, and the members of the Orth laboratory for support and helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-AI056404 and R21-DK072134 from NIAID. This work was also supported by the Welch Research Foundation (I-1561). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IKK, IκB kinase; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; PP2A, protein phosphatase 2A; MAPK, mitogen-activated protein kinase; HEK, human embryonic kidney; WT, wild type; OA, okadaic acid; GA, geldanamycin; pNPP, p-nitrophenyl phosphate.
References
- 1.Yoshida, M. (2001) Annu. Rev. Immunol. 19 475-496 [DOI] [PubMed] [Google Scholar]
- 2.Chu, Z. L., Shin, Y. A., Yang, J. M., DiDonato, J. A., and Ballard, D. W. (1999) J. Biol. Chem. 274 15297-15300 [DOI] [PubMed] [Google Scholar]
- 3.Jin, D. Y., Giordano, V., Kibler, K. V., Nakano, H., and Jeang, K. T. (1999) J. Biol. Chem. 274 17402-17405 [DOI] [PubMed] [Google Scholar]
- 4.Xiao, G., Harhaj, E. W., and Sun, S. C. (2000) J. Biol. Chem. 275 34060-34067 [DOI] [PubMed] [Google Scholar]
- 5.Harhaj, E. W., and Harhaj, N. S. (2005) IUBMB Life 57 83-91 [DOI] [PubMed] [Google Scholar]
- 6.Yin, M. J., Christerson, L. B., Yamamoto, Y., Kwak, Y. T., Xu, S., Mercurio, F., Barbosa, M., Cobb, M. H., and Gaynor, R. B. (1998) Cell 93 875-884 [DOI] [PubMed] [Google Scholar]
- 7.Fu, D. X., Kuo, Y. L., Liu, B. Y., Jeang, K. T., and Giam, C. Z. (2003) J. Biol. Chem. 278 1487-1493 [DOI] [PubMed] [Google Scholar]
- 8.DiDonato, J. A., Hayakawa, M., Rothwarf, D. M., Zandi, E., and Karin, M. (1997) Nature 388 548-554 [DOI] [PubMed] [Google Scholar]
- 9.Kray, A. E., Carter, R. S., Pennington, K. N., Gomez, R. J., Sanders, L. E., Llanes, J. M., Khan, W. N., Ballard, D. W., and Wadzinski, B. E. (2005) J. Biol. Chem. 280 35974-35982 [DOI] [PubMed] [Google Scholar]
- 10.Viboud, G. I., and Bliska, J. B. (2005) Annu. Rev. Microbiol. 59 69-89 [DOI] [PubMed] [Google Scholar]
- 11.Orth, K., Palmer, L. E., Bao, Z. Q., Stewart, S., Rudolph, A. E., Bliska, J. B., and Dixon, J. E. (1999) Science 285 1920-1923 [DOI] [PubMed] [Google Scholar]
- 12.Orth, K., Xu, Z., Mudgett, M. B., Bao, Z. Q., Palmer, L. E., Bliska, J. B., Mangel, W. F., Staskawicz, B., and Dixon, J. E. (2000) Science 290 1594-1597 [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee, S., Keitany, G., Li, Y., Wang, Y., Ball, H. L., Goldsmith, E. J., and Orth, K. (2006) Science 312 1211-1214 [DOI] [PubMed] [Google Scholar]
- 14.Mittal, R., Peak-Chew, S. Y., and McMahon, H. T. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 18574-18579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter, R. S., Pennington, K. N., Ungurait, B. J., Arrate, P., and Ballard, D. W. (2003) J. Biol. Chem. 278 48903-48906 [DOI] [PubMed] [Google Scholar]
- 16.Deng, L., Wang, C., Spencer, E., Yang, L., Braun, A., You, J., Slaughter, C., Pickart, C., and Chen, Z. J. (2000) Cell 103 351-361 [DOI] [PubMed] [Google Scholar]
- 17.Lee, B., Tanaka, Y., and Tozawa, H. (1989) Tohoku J. Exp. Med. 157 1-11 [DOI] [PubMed] [Google Scholar]
- 18.Pratt, W. B., and Toft, D. O. (2003) Exp. Biol. Med. (Maywood) 228 111-133 [DOI] [PubMed] [Google Scholar]
- 19.Broemer, M., Krappmann, D., and Scheidereit, C. (2004) Oncogene 23 5378-5386 [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee, S., Hao, Y. H., and Orth, K. (2007) Trends Biochem. Sci. 32 210-216 [DOI] [PubMed] [Google Scholar]
- 21.Nejmeddine, M., Barnard, A. L., Tanaka, Y., Taylor, G. P., and Bangham, C. R. (2005) J. Biol. Chem. 280 29653-29660 [DOI] [PubMed] [Google Scholar]