Abstract
The bioactive surface of scorpion β-toxins that interact with receptor site-4 at voltage-gated sodium channels is constituted of residues of the conserved βαββ core and the C-tail. In an attempt to evaluate the extent by which residues of the toxin core contribute to bioactivity, the anti-insect and anti-mammalian β-toxins Bj-xtrIT and Css4 were truncated at their N and C termini, resulting in miniature peptides composed essentially of the core secondary structure motives. The truncated β-toxins (ΔΔBj-xtrIT and ΔΔCss4) were non-toxic and did not compete with the parental toxins on binding at receptor site-4. Surprisingly, ΔΔBj-xtrIT and ΔΔCss4 were capable of modulating in an allosteric manner the binding and effects of site-3 scorpion α-toxins in a way reminiscent of that of brevetoxins, which bind at receptor site-5. While reducing the binding and effect of the scorpion α-toxin Lqh2 at mammalian sodium channels, they enhanced the binding and effect of LqhαIT at insect sodium channels. Co-application of ΔΔBj-xtrIT or ΔΔCss4 with brevetoxin abolished the brevetoxin effect, although they did not compete in binding. These results denote a novel surface at ΔΔBj-xtrIT and ΔΔCss4 capable of interaction with sodium channels at a site other than sites 3, 4, or 5, which prior to the truncation was masked by the bioactive surface that interacts with receptor site-4. The disclosure of this hidden surface at both β-toxins may be viewed as an exercise in “reverse evolution,” providing a clue as to their evolution from a smaller ancestor of similar scaffold.
Voltage-gated sodium channels (Navs)3 are critical for generation and propagation of action potentials in excitable cells and are targeted by a large variety of ligands that bind at distinct receptor sites on the pore-forming α-subunit (1). Most lipid-soluble Nav activators, including pyrethroid insecticides, toxic alkaloids (e.g. veratridine, from the plant family of Liliaceae, and batrachotoxin, from the skin of the Colombian frog Phyllobates aurotaenia), and marine cyclic polyether toxins (e.g. brevetoxins produced by “red tide” dinoflagellates), are effective in invertebrates as well as vertebrates. In addition, a wide array of proteinaceous Nav modifiers exists in the venom of scorpions, spiders, cone snails, and sea anemones, which are utilized for prey and defense (1, 2).
Nav gating modifiers from scorpion venom are divided into two classes, α and β, according to their mode of action and binding properties (3–5). α-Toxins prolong the action potential by inhibiting the fast inactivation of the Nav upon binding to receptor site-3 (4, 6), which involves extracellular loops in domains 1 and 4 (6). These toxins are divided into three groups (7): (i) anti-mammalian α-toxins (e.g. Lqh2 from Leiurus quinquestriatus hebraeus), which are highly toxic in mammalian brain and bind with high affinity to rat brain Navs; (ii) anti-insect α-toxins (e.g. LqhαIT), which are highly toxic to insects and show weak activity in mammalian brain; and (iii) α-like toxins (e.g. Lqh3), which are toxic to insects and mice (by intracerebroventricular and peripheral injections) but bind weakly to rat brain synaptosomes (3). Alterations in the binding of α-toxins to receptor site-3 on rat brain and insect Navs are allosterically modulated by a variety of Nav lipophilic activators such as site-2, site-5, and site-7 toxins (e.g. veratridine, brevetoxin, and pyrethroids, respectively; Refs. 3, 7–10).
β-Toxins shift the voltage dependence of Nav activation to more negative membrane potentials upon binding to receptor site-4, assigned to extracellular loops in domains 2 and 3 (11–15). This class is divided into: (i) anti-mammalian β-toxins (e.g. Css4 from Centruroides suffusus suffusus; Ref. 4); (ii) β-toxins that affect both insect and mammalian Navs(e.g. Ts1 from Tityus serrulatus; Ref. 4); (iii) anti-insect selective depressant β-toxins (e.g. LqhIT2 from L. quinquestriatus hebraeus) typified by the flaccid paralysis they produce in blowfly larvae and depolarization followed by block of action potentials in an isolated cockroach axon (reviewed in Ref. 5); and (iv) anti-insect selective excitatory toxins (e.g. Bj-xtrIT from Hotentota judaica), which induce an immediate contraction paralysis upon injection of blowfly larvae, induce spontaneous repetitive firing in cockroach axon (16), and exhibit a unique structure (17, 18). Binding studies with scorpion α- and β-toxins have shown that receptor sites 3 and 4 of insect Navs interact allosterically, thus contributing to the synergistic effects in toxicity obtained upon their co-administration (19).
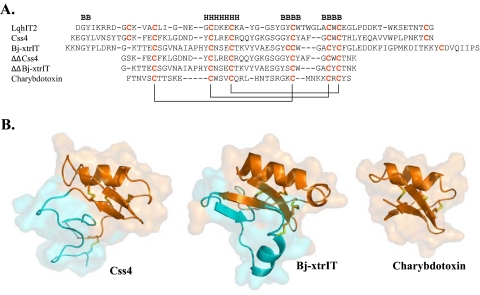
Short chain (30–50-residue-long) and long chain (60–76-residue-long) scorpion toxins that affect potassium and sodium channels, respectively, share a common βαββ scaffold with highly variable loops connecting the main secondary structure elements of the toxin core (see Fig. 1) (reviewed in Ref. 20). The structure of excitatory β-toxins, such as Bj-xtrIT (18), is unique. The core motif is very similar to those of other scorpion toxins (α-helix packed against a three-stranded β-sheet stabilized by three spatially conserved disulfide bonds: in Bj-xtrIT Cys-16–Cys-42, Cys-27–Cys-47, Cys-31–Cys-49; in Css4 Cys-16–Cys-41, Cys-25–Cys-46, Cys-29–Cys-48; see Fig. 1) but varies prominently in its C-terminal region and the spatial arrangement of its forth, non-conserved disulfide bond (in Bj-xtrIT, Cys-43–Cys-69; in Css4, Cys-12–Cys-65; Refs. 17, 18, 21). Moreover, the C-terminal region of Bj-xtrIT (residues 60–76) contains an additional α-helix, α2, which is unique for excitatory toxins (residues 63–69 in Bj-xtrIT; Refs. 18, 20). The differences in the C-tail arrangement, which stabilizes the conformation of the C-terminal region against the molecular core, were suggested to determine the functional divergence of scorpion toxins (17).
FIGURE 1.
Sequence alignment and three-dimensional structure of representative scorpion toxins. A, sequences were aligned according to the conserved cysteine residues, and the disulfide bonds formed between cysteine pairs are marked in solid lines. Dashes indicate gaps. Secondary structure motifs (B, β-strand; H, α-helix) in Css4 follow the published structure of Cn2 (Protein Data Bank accession 1Cn2). B, schematic diagrams of the Cα model structures of Css4, Bj-xtrIT, and charybdotoxin covered by semitransparent molecular surfaces. The structures of Bj-xtrIT and charybdotoxin are derived from the Protein Data Bank accession numbers 1BCG and 2CRD, respectively. The Css4 model is based on the NMR structure of Cn2 (25) and is spatially aligned with that of Bj-xtrIT and charybdotoxin. The moieties representing ΔΔBj-xtrIT and ΔΔCss4 are colored in orange on the ribbon structure of the parent toxins, and the cyan ribbons represent the deleted N- and C termini. The disulfide bonds are colored in yellow. Panel B was prepared using PyMOL.
A number of reports have described in detail the bioactive surfaces of a few scorpion β-toxins, the anti-insect excitatory and depressant toxins, Bj-xtrIT and LqhIT2 (22–24), and the anti-mammalian β-toxin, Css4 (25). These studies highlighted a conserved pharmacophore composed of a key negatively charged Glu at the α-helix motif, flanked by hydrophobic aromatic residues that may isolate the point of interaction from bulk solvent. The residues that comprise the pharmacophore of β-toxins belong to the protein core (Fig. 1), which is common to both long chain scorpion α and β-toxins and most short chain scorpion toxins affecting voltage-gated potassium channels (20, 26). An additional hydrophobic cluster of bioactive amino acids at the C-tail of Bj-xtrIT and on the loop connecting the second and the third β-strands of Css4 was suggested to be involved in determining toxin selectivity (5, 25).
To examine the commonality of the pharmacophore region in β-toxins and the extent of its contribution to recognition of receptor site-4, we deleted the N- and C-terminal regions of two β-toxins, Bj-xtrIT and Css4, and analyzed the binding properties of the resulting truncated derivatives (ΔΔβ-toxins). Unpredictably the ΔΔβ-toxins were non-toxic and did not bind at receptor site-4; nonetheless they were able to allosterically modulate α-toxin activity on insect and mammalian Navs and to antagonize the effects of brevetoxins. These results indicated that the seemingly inactive ΔΔβ-toxins were able to interact with the Nav.
EXPERIMENTAL PROCEDURES
Toxins and Mutagenesis—Bj-xtrIT, ΔΔBj-xtrIT, and LqhαIT were produced in a recombinant form as was described previously (16, 25), and Css4 and ΔΔCss4 were produced in fusion to a His tag extension at their N termini as described (25). PCR-driven mutagenesis, expression in Escherichia coli, in vitro folding, and purification of Css4 derivatives have been described in detail (25). Brevetoxins 2 and 3 (PbTx-2; PbTx-3) were purchased from Latoxan (Valance, France). Charybdotoxin was kindly provided by Prof. B. Attali, Tel Aviv University.
Binding Experiments—Locust neuronal membranes were prepared from dissected brains and ventral nerve cords of adult Locusta migratoria using an established method (7). Rat brain synaptosomes were prepared from adult (∼300 g) albino Wistar strain (laboratory-bred) as was described previously (27). Membrane protein concentration was determined by a Bio-Rad protein assay, using bovine serum albumin as standard. Css4 and Bj-xtrIT were radioiodinated by lactoperoxidase (catalog number L8257; 7 units/60 μl of reaction mix; Sigma) using 10 μg of toxin and 0.5 mCi of carrier-free Na125I (Amersham Biosciences,) and the monoiodotoxin was purified as was described previously (25).
Labeling of PbTx-3—Solution of PbTx-3 (1 mg/ml acetonitrile) was mixed with 0.1 Ci of NaBa3H4 (69 Ci/mmol) for 5 min reduction at room temperature. [3H]PbTx-3 was purified on a C18 high pressure liquid chromatography column using an isocratic gradient composed of 77% methanol and 23% water. The specific radioactivity of [3H]PbTx-3 was 17 Ci/mmol.
The composition of media used in binding assays and termination of the reactions was previously described (27, 28). Nonspecific binding was determined in the presence of 1–10 μm of the unlabeled toxin and typically consisted of 10–30% of total binding (for scorpion toxins). Equilibrium competition binding assays were performed and analyzed as was described previously (25). For [3H]PbTx-3, filters were dried 1 h at 80 °C, mixed with 5 ml of ULTIMUMA Gold (PerkinElmer Life Sciences), and the radioactivity was monitored. Nonspecific binding was determined in the presence of 2 μm PbTx-2. Each experiment was performed in duplicate and repeated at least three times as indicated (n). Data are presented as mean ± S.E. of the number of independent experiments.
Two-electrode Voltage Clamp Experiments—The genes encoding for the Drosophila melanogaster sodium channel α-subunit (DmNav1) and the auxiliary TipE subunit were kindly provided by J. Warmke, Merck, and M. S. Williamson, Division of Plant and Invertebrate Ecology-Rothamsted, UK, respectively. The genes encoding for the rat brain and skeletal muscle sodium channels (rNav1.2 and rNav1.4) were a gift from Dr. R. G. Kallen, University of Pennsylvania, Philadelphia, PA. These genes and that for the auxiliary subunit hβ1 were transcribed in vitro using T7 RNA-polymerase and the mMESSAGE mMACHINE™ system (Ambion, Austin, TX), and the cRNAs were injected into Xenopus laevis oocytes as was described previously (13). Currents were measured 1–2 days after injection using a two-electrode voltage clamp and a Gene Clamp 500 amplifier (Axon Instruments, Union City, CA). Data were sampled at 10 kHz and filtered at 5 kHz. Data acquisition was controlled by a Macintosh PPC 7100/80 computer, equipped with ITC-16 analog/digital converter (Instrutech Corp., Port Washington, NY), utilizing Synapse (Synergistic Systems, Sweden). Capacitance transients and leak currents were removed by subtracting a scaled control trace utilizing a P/6 protocol (29). Bath solution contained (in mm): 96 NaCl, 2 KCl, 1 MgCl2, 2 CaCl2, and 5 HEPES, pH 7.85. Oocytes were washed with the bath solution using a BPS-8 perfusion system (ALA Scientific Instruments, Westbury, NY) with a positive pressure of 4 p.s.i. Toxins were diluted with bath solution and applied directly to the bath to the final desired concentration.
GV Analysis—Mean conductance (G) was calculated from peak current/voltage relationship using the equation G = I/(V - Vrev), where I is the peak current, V is the membrane potential, and Vrev is the reversal potential. Normalized conductance voltage relations were fit with one Boltzman distribution according to the following equation G/Gmax = 1 + exp[(V½ - V)/k], where V½ is the respective membrane potential for which the mean conductance is half-maximal; k is the respective slope.
To obtain a dose-response curve for α-toxins, currents were elicited by depolarization to -10 mV from a holding potential of -80 mV in the presence of various toxin concentrations. At each toxin concentration, currents were allowed to reach a steady-state level prior to the final measurement, and the steady-state current at -10 mV was normalized relatively to the current in control.
RESULTS
The N and C termini of β-toxins vary in sequence, structure, and disulfide bond organization, but their core is conserved (Fig. 1) (Ref. 17). To examine the contribution of the pharmacophore of scorpion β-toxins (25) to their binding affinity, we deleted (Fig. 1) the first 10 N-terminal residues as well as the C-tail stretch beyond the penultimate Cys of Bj-xtrIT (anti-insect; 27 residues) and Css4 (anti mammalian; 18 residues). Since the ΔΔβ-toxins ended with a Cys residue, we added the triplet Thr-Asn-Lys, which appears at the C-tail of various potassium channel blockers (26). The Cys residues that remained unpaired in the two toxins (position 12 in Css4 and 43 in Bj-xtrIT) were substituted by Ser to prevent incorrect folding or dimerization. ΔΔCss4 and ΔΔBj-xtrIT were produced recombinantly using the E. coli expression system as was described for the unmodified parental toxins.
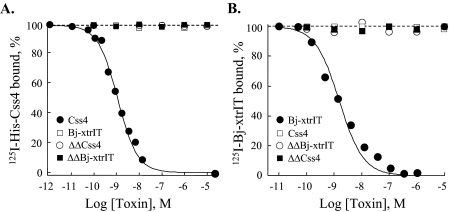
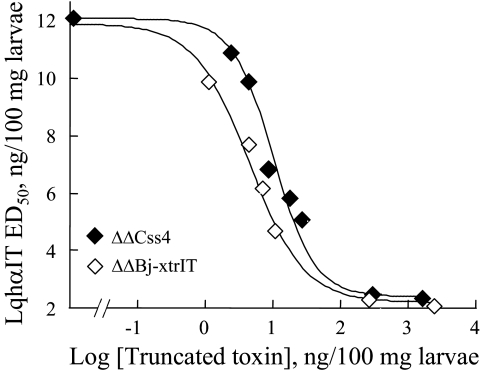
Functional Analysis of the ΔΔβ-Toxins—ΔΔBj-xtrIT and ΔΔCss4 were non-toxic to blowfly larvae and mice by injection even at very high concentrations (>20 nmol/g of animal) and were unable to displace 125I-Bj-xtrIT and 125I-Css4 at locust and rat brain neuronal membranes in binding competition assays (up to 20 μm; Fig. 2). These results indicated that the two ΔΔβ-toxins lost their ability to bind at receptor site-4 of Navs. Moreover, ΔΔBj-xtrIT and ΔΔCss4 (up to 20 μm) had no effect on the conductance and gating properties of the mammalian channels rNav1.2 (brain) and rNav1.4 (skeletal muscle), and the insect channel DmNav1, all expressed in X. laevis oocytes (Table 1). Thus, at first glance, these results have suggested that the truncated toxins lost any bioactive competence. At this stage, we used another sensitive measure to assess whether the truncated toxins could interact with the Nav. It has been shown that the interaction of scorpion α-toxins with receptor site-3 is modulated by a variety of ligands that bind at other receptor sites on the Nav, such as veratridine (site-2; Refs. 8,30), scorpion β-toxins (site-4; Ref. 19), brevetoxins (site-5; Refs. 7, 9), and pyrethroids (site-7; Ref. 10). We therefore tested whether ΔΔBj-xtrIT and ΔΔCss4 were capable of modulating the activity of the scorpion α-toxin LqhαIT on insects. Surprisingly, ΔΔBj-xtrIT and ΔΔCss4 increased 6-fold the toxicity of LqhαIT to blowfly larvae, with half-maximal doses (EC50) of 5.2 and 9.6 ng/100 mg, respectively (Fig. 3). This unexpected synergism prompted us to examine the binding capability of the ΔΔβ-toxins to Navs.
FIGURE 2.
The ΔΔβ-toxins do not compete with site-4 toxins. A, rat brain synaptosomes were incubated 60 min at 22 °C with 0.1 nm 125I-Css4 and increasing concentrations of Css4, Bj-xtrIT, ΔΔBj-xtrIT, and ΔΔCss4. Nonspecific binding, determined in the presence of 1 μm Css4 (comprising 10% of total binding), was subtracted. The calculated Ki values were: Css4, 0.98 ± 0.1 nm (n = 8); Bj-xtrIT, ΔΔBj-xtrIT, and ΔΔCss4, >>10,000 nm. B, locust neuronal membranes (160 μg) were incubated with 0.1 nm 125I-Bj-xtrIT 45 min at 22 °C. Nonspecific binding, measured in the presence of 1 μm unlabeled Bj-xtrIT (comprising 30% of total binding), was subtracted. The Ki value of Bj-xtrIT was 1.6 ± 0.3 nm (n = 3); Css4, ΔΔBj-xtrIT, and ΔΔCss4, >>10,000 nm.
TABLE 1.
Alterations of the mid-voltage of activation (V½) derived from conductance-voltage (G-V) curves of DmNav1/TipE, rNav1.2/hβ 1 and rNav1.4/hβ1 expressed in Xenopus oocytes and induced by PbTx-2 and the ΔΔβ-toxins The data represent the mean ± S.E. of at least six independent experiments. The oocytes were clamped at –100 mV and then 50-ms depolarization pulses from –90 to +50 mV were applied at 5-mV increments. The V½, was calculated as described under “Experimental Procedures.”
|
DmNav1/TipE |
rNav1.2/hβ1 |
rNav1.4/hβ1 |
||||
|---|---|---|---|---|---|---|
| μm | V½, mV | μm | V½, mV | μm | V½, mV | |
| Control | –15.9 ± 0.2 | –24.9 ± 0.3 | –24.9 ± 0.2 | |||
| PbTx-2 | 0.5 | –27.7 ± 0.3 | 1 | –33.4 ± 0.6 | 2 | –32.6 ± 0.5 |
| ΔΔBj-xtrIT | 20 | –17.0 ± 0.8 | 20 | –25.1 ± 0.4 | 20 | –25.0 ± 0.5 |
| ΔΔCss4 | 20 | –16.4 ± 0.2 | 20 | –24.9 ± 0.4 | 20 | –24.7 ± 0.4 |
| ΔΔBj-xtrIT + PbTx-2a | 1 + 2 | –16.4 ± 0.3 | 1 + 3 | –25.1 ± 0.3 | 2 + 5 | –25.1 ± 0.2 |
| ΔΔCss4+ PbTx-2a | 1 + 2 | –16.5 ± 0.3 | 1 + 3 | –25.0 ± 0.3 | 2 + 5 | –25.0 ± 0.2 |
The ΔΔβ-toxin was applied together with PbTx-2
FIGURE 3.
The toxicity of LqhαIT is enhanced by the ΔΔβ-toxins. Dose-response curves for toxicity of LqhαIT upon injection to blowfly larvae in the presence of increasing concentrations of ΔΔBj-xtrIT or ΔΔCss4 (in ng/100 mg of body weight). Forty larvae were used to generate each data point, which represents the ED50 equivalent of LqhαIT in the presence of the indicated dose of ΔΔBj-xtrIT or ΔΔCss4. The half-maximal doses of ΔΔBj-xtrIT or ΔΔCss4 (EC50) that increase the toxicity (decrease in ED50) of LqhαIT are 5.2 and 9.6 ng/100 mg, respectively. Maximal enhancement in LqhαIT toxicity (∼600%) is achieved in the presence of less than 1 μg of ΔΔBj-xtrIT or ΔΔCss4.
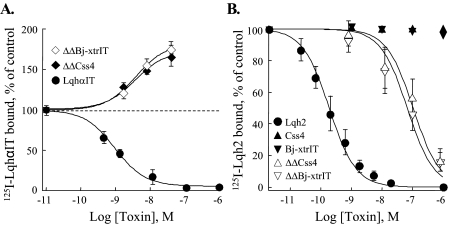
ΔΔBj-xtrIT and ΔΔCss4 were examined in binding studies using iodinated scorpion α-toxins that interact well with insect and rat brain neuronal membranes. In locust neuronal membranes, ΔΔBj-xtrIT and ΔΔCss4 increased 1.7-fold 125I-LqhαIT binding (EC50 2.9 ± 0.5 and 3.8 ± 1.4 nm, respectively; Fig. 4A). This positive cooperativity indicated that the ΔΔβ-toxins interact with the insect Nav at a site other than receptor site-3. Notably, in rat brain synaptosomes, ΔΔBj-xtrIT and ΔΔCss4 inhibited 125I-Lqh2 binding in a dose-dependent manner with an IC50 of 76 ± 18 and 105 ± 29 nm, respectively, whereas the parental β-toxins had no effect (Fig. 4B). It is noteworthy that 125I-ΔΔBj-xtrIT and 125I-ΔΔCss4 did not exhibit significant specific binding to insect or rat brain neuronal preparations, and therefore, direct assessment of their binding interaction could not be performed.
FIGURE 4.
Effects of the ΔΔβ-toxins on the binding of α-toxins. Rat brain and locust neuronal membranes were incubated with 0.1 nm 125I-toxin at room temperature for 20 or 45 min, respectively, in the presence of increasing concentrations of the indicated toxin derivatives. Nonspecific binding, measured in the presence of 1 μm unlabeled toxin, was subtracted. Data points represent mean ± S.E. of three independent experiments. A, enhancement of 125I-LqhαIT binding to locust neuronal membranes (160 μg of protein). The dashed line indicates maximal specific binding of 125I-LqhαIT under control conditions. The Ki value for LqhαIT was 1.1 ± 0.2 nm. Maximal increase in 125I-LqhαIT binding in the presence of ΔΔBj-xtrIT or ΔΔCss4 was 172 ± 11 and 163 ± 14% of the control with an EC50 of 2.9 ± 0.5 and 3.8 ± 1.4 nm, respectively. B, inhibition of 125I-Lqh2 binding to rat brain synaptosomes (41 μg of protein). Lqh2 displaces 125I-Lqh2 with a Ki of 0.2 ± 0.02 nm. ΔΔBj-xtrIT and ΔΔCss4 inhibit the binding of 125I-Lqh2 with an apparent Ki of 76 ± 18 and 105 ± 29 nm, respectively. Bj-xtrIT and Css4 (up to 20 μm) did not inhibit the binding of 125I-Lqh2.
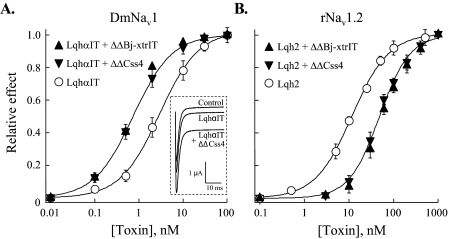
The allosteric effects of the ΔΔβ-toxins were analyzed on the activity of LqhαIT and Lqh2 on insect and rat brain Navs expressed in Xenopus oocytes. LqhαIT increases the sodium peak current and inhibits the fast inactivation of the insect sodium channel, DmNav1, with an EC50 of 2.6 ± 0.2 nm (Fig. 5A) (Ref. 3). When LqhαIT was applied together with either ΔΔBj-xtrIT or ΔΔCss4 in a 1:50 molar ratio, the EC50 of LqhαIT in the context of the mixture dropped to 0.6 ± 0.2 and 0.7 ± 0.2 nm, respectively (Fig. 5A), indicating enhancement in the α-toxin activity at the insect sodium channel. In contrast, joint application of either ΔΔBj-xtrIT or ΔΔCss4 in a 1:10 molar ratio with Lqh2, which inhibits the fast inactivation of rNav1.2 with an EC50 of 13.2 ± 0.3 nm (3), increased the EC50 of Lqh2 in the context of the mixture to 44.4 ± 1 and 42 ± 1.4 nm, respectively (Fig. 5B), indicating decrease in activity at this mammalian sodium channel. Once more, these opposite effects have indicated that the ΔΔβ-toxins, which by themselves had no effect on the sodium currents (Table 1), modulate the effect of site-3 α-toxins on mammalian and insect Navs in a different manner, further supporting their interaction with the sodium channels.
FIGURE 5.
Effects of the ΔΔβ-toxins on the activity of α-toxins. Oocytes expressing DmNav1/TipE and rNav1.2/hβ1 were clamped at -80 mV, and currents were elicited by step depolarization to -10 mV. Increasing concentrations of the toxins and toxin mixtures were applied, and their relative effect on the current inactivation (sustained current) at -10 mV was determined after 50 ms. A, activity of LqhαIT (EC50 = 2.6 ± 0.2 nm) and LqhαIT in a mixture with ΔΔBj-xtrIT or ΔΔCss4 (1:50 molar ratio) on DmNav1 provide the following EC50 values: 0.6 ± 0.2 and 0.7 ± 0.2 nm, respectively. Inset, representative current traces demonstrating the effect of 25 nm ΔΔCss4 on the level of the sustained current induced by 0.5 nm LqhαIT. B, activity of Lqh2 and Lqh2 in a mixture with ΔΔBj-xtrIT and ΔΔCss4 (1:10 molar ratio) on rNav1.2 provide the following EC50 values: 13.2 ± 0.3, 44.4 ± 1, and 42 ± 1.4 nm, respectively. The maximal inhibition of inactivation by LqhαIT or Lqh2 in the absence or presence of the ΔΔβ-toxins was identical.
Since the structure of the truncated toxins is composed of the βαββ fold as it appears in most scorpion toxins, we questioned the specificity of their interaction with the Nav by employing another short chain toxin of similar size and fold, charybdotoxin (from the scorpion L. quinquestriatus hebraeus; Fig. 1) (Ref. 26). Charybdotoxin, which is a blocker of voltage-gated potassium channels, had no effect on LqhαIT activity at DmNav1 and on Lqh2 activity at rNav1.2 (not shown), suggesting that the activity of the ΔΔβ-toxins is not derived only from their βαββ fold.
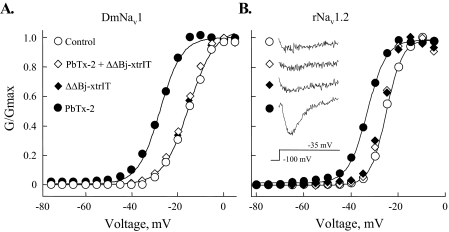
The ΔΔβ-Toxins Inhibit Brevetoxin Action on Navs—The ΔΔBj-xtrIT and ΔΔCss4 opposing modulations of the binding and activity of α-toxins at insect and mammalian Navs was reminiscent of that described for brevetoxins, which bind at receptor site-5 (7–9, 31). Therefore, we examined whether ΔΔBj-xtrIT had any effect on brevetoxin-2 (PbTx-2) toxicity in vivo. PbTx-2 is highly toxic to blowfly larvae (contraction and paralysis 60 s after injection; ED50 = 10 ng/100 mg of larvae), but when injected after or together with the ΔΔβ-toxins at 1:10 molar ratio, it was not toxic. Thus, the ΔΔβ-toxins act in vivo as antagonists of brevetoxin in insects. However, when the ΔΔβ-toxins were administered to blowfly larvae subsequent to PbTx-2 injection and after a paralytic effect already developed, even at a 1:100 molar ratio, the larvae were not resuscitated, indicating that the ΔΔβ-toxins were unable to abolish the brevetoxin effect. To further clarify this point, we examined the effects of PbTx-2 in the presence of the truncated toxins on rNav1.2, rNav1.4, and DmNav1 expressed in Xenopus oocytes. Although PbTx-2 in concentrations of 0.5–2 μm shifted the voltage dependence of activation of the three channels to more hyperpolarizing membrane potentials, pre- or co-application of ΔΔBj-xtrIT or ΔΔCss4 with PbTx-2 in a 1:2–3 molar ratio abolished these effects (Fig. 6 and Table 1). Once more, when the ΔΔβ-toxins were applied subsequent to PbTx-2, when its effect had already developed, they had no inhibitory effect (not shown).
FIGURE 6.
Inhibition of PbTx-2 effect by ΔΔBj-xtrIT. A and B, alteration in conductance-voltage relations of DmNav1/TipE (A) and rNav1.2/hβ1(B) by PbTx-2 in the presence or absence of ΔΔBj-xtrIT. Toxin concentrations and the activation parameters (V½) are as described in Table 1. Inset, rNav1.2/hβ1 currents induced by the indicated toxin in a test pulse to -35 mV. Similar results were obtained with ΔΔCss4 (Table 1).
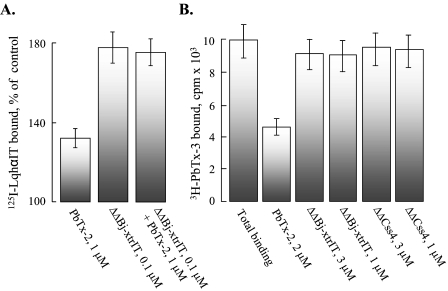
We further tested the effects of the ΔΔβ-toxins on the binding of α-toxins in the presence of PbTx-2. 1 μm PbTx-2 increased the binding of 125I-LqhαIT to locust neuronal membranes by 30% (19). 100 nm ΔΔBj-xtrIT increased the binding of 125I-LqhαIT to the same membranes by 70%. However, co-application of PbTx-2 and ΔΔBj-xtrIT had no additive effect on LqhαIT binding (Fig. 7A). These data suggest that the binding sites of brevetoxin and the ΔΔβ-toxins are not identical.
FIGURE 7.
The ΔΔβ-toxins do not compete with brevetoxin on binding to site-5. A, locust neuronal membranes were incubated with 0.1 nm 125I-LqhαIT as in Fig. 4. Saturating concentration of PbTx-2 (1 μm; Ref. 19) increased 125I-LqhαIT binding by 30 ± 8%. 0.1 μm ΔΔBj-xtrIT increased 125I-LqhαIT binding (maximal enhancement, Fig. 4) by 172 ± 11%, and in the presence of a mixture of both (1 μm PbTx-2 + 0.1 μm ΔΔBj-xtrIT), the increase in 125I-LqhαIT binding was comparable (168 ± 14%). B, [3H]PbTx-3 binding in the presence of the ΔΔβ-toxins. [3H]PbTx-3 (10 nm) was incubated with rat brain synaptosomes (70 μg) for 3 h at room temperature in the absence (Total binding) or presence of 2 μm PbTx-2 (nonspecific binding) or the indicated concentrations of ΔΔβ-toxins. Identical results were obtained when the ΔΔβ-toxins were preincubated with the membranes for 1 h prior to the addition of [3H]PbTx-3.
To examine whether the ΔΔβ-toxins were directly or indirectly involved with brevetoxin binding, we analyzed their effect on the binding of [3H]PbTx-3, a specific marker of receptor site-5 at rat brain synaptosomes (32). High concentrations (1–3 μm) of either ΔΔBj-xtrIT or ΔΔCss4 had no effect on [3H]PbTx-3 binding, not even when incubated for 1 h with the membranes prior to the addition of [3H]PbTx-3 (Fig. 7B). These results have indicated that the ΔΔβ-toxins do not compete for the brevetoxin binding site at rat brain Navs.
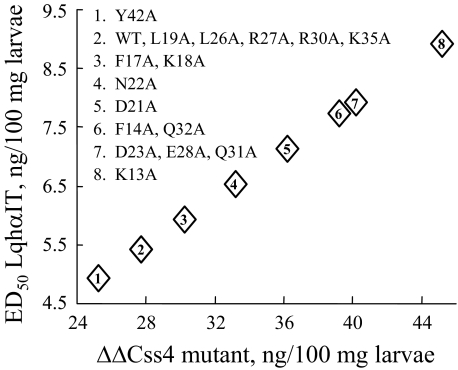
Residues Important for ΔΔCss4 Effect—The inability of ΔΔBj-xtrIT and ΔΔCss4 to compete on binding at receptor sites 3 (Figs. 4 and 5), 4 (Fig. 2), and 5 (Fig. 7B) have suggested that they recognize another receptor site on the sodium channel. Since the two ΔΔβ-toxins exhibited similar activities, we focused on ΔΔCss4 and substituted 16 putative solvent-exposed residues to Ala with the objective to identify those residues important for interaction with the channel. The ΔΔCss4 mutants were tested for their ability to influence LqhαIT toxicity by mixing each mutant in a 5:1 (w/w) ratio with LqhαIT, and the ED50 of the mixtures was determined on blowfly larvae (Fig. 8). A number of substitutions, which in the context of Css4 had no effect on the binding affinity (K13A, D21A, D23A, Q31A, and Q32A; Ref. 25), decreased the positive cooperative effect of ΔΔCss4 on LqhαIT, with the utmost effect achieved with K13A. In contrast, substitutions, which in the context of Css4 decreased substantially the binding affinity for rat brain synaptosomes, had no effect (L19A, R27A, and Y42A) or only a minor effect (F17A and N22A) on ΔΔCss4 ability to enhance LqhαIT toxicity (Fig. 8). Since Css4 and ΔΔCss4 do not bind at the same receptor site on the sodium channel, the difference of bioactive residues was not surprising. The only substitutions that affected both Css4 and ΔΔCss4 activity were E28A (hot spot) and F14A (numbering follows Css4).
FIGURE 8.
Analysis of ΔΔCss4 bioactive surface. ΔΔCss4 mutants mixed with LqhαIT in 5:1 (w/w) ratio were injected to blowfly larvae, and the ED50 of the mixture was determined. The y axis indicates the ED50 of LqhαIT in the presence of the indicated doses of ΔΔCss4 mutants (x axis). WT, unmodified ΔΔCss4. ED50 of LqhαIT is 12 ng/100 mg of larvae (see Fig. 3).
DISCUSSION
“Uncoating” the Exterior of Scorpion β-Toxins Exposes a Hidden Bioactive Surface—The C-tail, loops, turns, and unstructured stretches that connect the main secondary structure elements in long-chain scorpion toxins constitute a large portion of their exteriors and bear residues that participate in bioactivity (reviewed in Refs. 3 and 5). Here we show that removal of about 50% of the amino acid residues of Bj-xtrIT and Css4, which changed markedly their exteriors (Fig. 1), abolished any detectable activity and ability to bind at receptor site-4 on the Nav. However, the ability of ΔΔBj-xtrIT and ΔΔCss4 to reduce the activity of Lqh2 at the brain channel rNav1.2 and increase LqhαIT activity at receptor site-3 of insect channels (binding, effect on fast inactivation, and toxicity to blowfly larvae) indicated that they bound to the Nav. The bioactive residues of ΔΔCss4 are different from those of Css4 except for Glu-28, which was considered a hot spot in Css4 surface of interaction with the rNav1.2 (25). It is likely that in the context of the new functional surface of ΔΔCss4, Glu-28 is unable to interact with the site-4 residue as it did in the context of Css4.
From a structural viewpoint, the ability of ΔΔBj-xtrIT and ΔΔCss4 to bind to the Nav, as manifested in the modulation of receptor site-3, suggests that a masked functional surface was exposed by miniaturization of the two β-toxins. The probability that this hidden surface has been formed by coincidence is very low as it appears in two pharmacologically distinct toxins, Css4 and Bj-xtrIT. One way to rationalize such a finding is by assuming that scorpion toxins affecting sodium channels evolved from smaller peptides bearing the conserved βαββ core that were able to interact with sodium channels (26, 33). It is conceivable that along evolution, these peptides gained higher affinity and specificity for distinct Nav targets, whereas increasing in volume. Along this process, the bioactive surface of the ancestral toxin has been masked by new functional exteriors, such as those resolved nowadays by mutagenesis in various β-toxins (22, 23, 25). This hypothesis is in concert with the idea that diversification of long-chain scorpion toxins occurred mainly by mutations and slight structural alterations at the loops, turns, and C-tail rather than at theβαββ core (17). Thus, the truncation of these toxins may be considered an exercise in “reverse evolution” since it was opposite to the precedent natural path of evolution of these molecules.
The ΔΔβ-Toxins as Antagonists of Brevetoxins—The similar allosteric effects of the ΔΔβ-toxins and brevetoxins on the activity of α-toxins (Figs. 4, 5, 6, 7) (Refs. 7, 9, 31) have raised the possibility that they both interact with receptor site-5. However, because simultaneous application of ΔΔBj-xtrIT and PbTx-2 had no additive effect on LqhαIT binding at insect Navs and because ΔΔBj-xtrIT and ΔΔCss4 did not compete with brevetoxin on binding at receptor site-5 (Fig. 7), it was apparent that the truncated β-toxins bind at a different site. Interestingly, this binding induced allosterically a similar conformational alteration at site-3 to that induced by brevetoxins binding. This conclusion raises the question as to why the ΔΔβ-toxins are capable of inhibiting PbTx-2 effects on rNav1.2, rNav1.4, and DmNav1 activation only when pre- or co-applied (Fig. 6), whereas having no antagonistic effect when applied after PbTx-2. The 30-Å-long brevetoxin has been suggested to orient its “head” (Lacton ring A) down across the membrane, parallel to the transmembrane hydrophobic α helices S5 and S6 between domains 1 and 4 of the Nav, whereas its “tail” (“rigid region”; rings H-K) points outward (32, 34). Chemical modifications at the side chain of the K-ring of PbTx-3 (rigid region) hardly affected its binding but markedly influenced its activity (35, 36). In addition, brevetoxins that vary in their rigid region structure exhibit different effects on the binding of α-toxins at receptor site-3 (37). It is therefore likely that the rigid region of brevetoxins interacts with an external channel site whose conformational alteration allosterically affects the activity of α-toxins at receptor site-3. If the ΔΔβ-toxins interact at least partially with this external channel site, it may suggest that their surface of interaction with the channel, which is responsible for the allosteric effects, is involved in the inhibition of brevetoxin activity. This assumption is supported by previous observations that subtle modifications induced by tetrodotoxin binding to the Nav external pore are sufficient to prevent the allosteric inhibition caused by brevetoxin on the binding of a scorpion α-toxin to rat brain Navs (31, 37). Therefore, preapplication of the ΔΔβ-toxins may prevent brevetoxin activity without affecting its binding. As brevetoxins are responsible for massive fish kills and marine mammal mortalities, as well as illness in humans who ingest filter-feeding shellfish or inhale toxic aerosols (34), the ΔΔβ-toxins may serve as leads for development of brevetoxin antagonists.
General Implications—The continuous search for novel pharmacologically valuable compounds in arthropod venoms requires tedious fractionation and purification followed by biological assays to validate that a desired activity still persists at each purification step. Inactive fractions or substances are usually disregarded as they are not expected to yield a valuable end product. These basic considerations have led to the present situation where of 100–300 distinct polypeptides that can be monitored by mass spectroscopy in the venom of a single scorpion or a spider, the biological activity of only a small portion has been characterized (4, 38, 39). However, there are numerous reasons to believe that compounds that at first glance seem inactive should not be ignored. We have recently shown that scorpion β-toxins that bind to receptor site-4 at the extracellular region of Navs may influence allosterically the effects produced by α-toxins that bind at receptor site-3 (19). Moreover, even mere binding of a β-toxin, whose activity was abolished by mutagenesis, was shown to induce this allosteric effect at site-3 (19). We have also shown that the alleged anti-insect selective scorpion depressant toxins affect the mammalian skeletal muscle Nav provided that the channel was allosterically modulated by the binding of an α-toxin at receptor site-3 (40).
Here we demonstrate the use of the sensitive measure of allosteric interactions to evaluate the ability of substances that a priori were considered “biologically inactive” to bind and alter the Nav conformation. It is therefore rational to believe that when scorpion toxins that affect Navs degrade either in the venom glands (39) or in the stung animal (hemolymph or blood), their partially degraded derivatives may “boost” the impact of the entire venom. This phenomenon also provides a conceptual basis to future attempts to decrease doses of drugs by harmless, allosterically acting compounds.
This work was supported, in whole or in part, by National Institutes of Health Grant 1 U01 NS058039-01 (to M. G.). This work was also supported by the United States-Israel Binational Agricultural Research and Development Grants IS-3928-06 (to M. G. and D. G.) and IS-4066-07 (to D. G. and M. G.) and the Israeli Science Foundation Grants 1008/05 (to D. G.) and 909/04 (M. G.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article was selected as a Paper of the Week.
Footnotes
The abbreviation used is: Nav, voltage-gated sodium channel.
References
- 1.Catterall, W. A., Cestèle, S., Yarov-Yarovoy, V., Yu, F. H., Konoki, K., and Scheuer, T. (2007) Toxicon 49 124-141 [DOI] [PubMed] [Google Scholar]
- 2.Gordon, D. (1997) in Toxins and Signal Transduction (Lazarowici, P., and Gutman, Y., eds) pp. 119-149, Harwood Press, Amsterdam
- 3.Gordon, D., Karbat, I., Ilan, N., Cohen, L., Kahn, R., Gilles, N., Dong, K., Stühmer, W., Tytgat, J., and Gurevitz, M. (2007) Toxicon 49 452-472 [DOI] [PubMed] [Google Scholar]
- 4.Martin-Eauclaire, M. F., and Couraud, F. (1995) in Handk Neurotoxicology (Chang, L. W., and Dyer, R. S., eds) pp. 683-716, Marcel Dekker, New York
- 5.Gurevitz, M., Karbat, I., Cohen, L., Ilan, N., Kahn, R., Turkov, M., Stankiewicz, M., Stühmer, W., Dong, K., and Gordon, D. (2007) Toxicon 49 473-489 [DOI] [PubMed] [Google Scholar]
- 6.Catterall, W. A. (2000) Neuron 26 13-25 [DOI] [PubMed] [Google Scholar]
- 7.Gordon, D., Martin-Eauclaire, M. F., Cestèle, S., Kopeyan, C., Carlier, E., Ben Khalifa, R., Pelhate, M., and Rochat, H. (1996) J. Biol. Chem. 271 8034-8045 [DOI] [PubMed] [Google Scholar]
- 8.Catterall, W. A. (1992) Physiol. Rev. 72 S15-S48 [DOI] [PubMed] [Google Scholar]
- 9.Cestèle, S., Ben Khalifa, R., Pelhate, M., Rochat, H., and Gordon, D. (1995) J. Biol. Chem. 270 15153-1516 [DOI] [PubMed] [Google Scholar]
- 10.Gilles, N., Gurevitz, M., and Gordon, D. (2003) FEBS Lett. 540 81-85 [DOI] [PubMed] [Google Scholar]
- 11.Cestèle, S., Qu, Y., Rogers, J. C., Rochat, H., and Catterall, W. A. (1998) Neuron 21 919-931 [DOI] [PubMed] [Google Scholar]
- 12.Cestèle, S., Yarov-Yarovoy, V., Qu, Y., Sampieri, F., Scheuer, T., and Catterall, W. A. (2006) J. Biol. Chem. 281 21332-21344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shichor, I., Zlotkin, E., Ilan, N., Chikashvili, D., Stühmer, W., Gordon, D., and Lotan, I. (2002) J. Neurosci. 22 4364-4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leipold, E., Hansel, A., Borges, A., and Heinemann, S. H. (2006) Mol. Pharmacol. 70 340-347 [DOI] [PubMed] [Google Scholar]
- 15.Cohen, L., Ilan, N., Gur, M., Stuhmer, W., Gordon, D., and Gurevitz, M. (2007) J. Biol. Chem. 282 29424-29430 [DOI] [PubMed] [Google Scholar]
- 16.Froy, O., Zilberberg, N., Gordon, D., Turkov, D., Gilles, N., Stankiewicz, M., Pelhate, M., Loret, E., Oren, D., Shaanan, B., and Gurevitz, M. (1999) J. Biol. Chem. 274 5769-5776 [DOI] [PubMed] [Google Scholar]
- 17.Gurevitz, M., Gordon, D., Ben-Natan, S., Turkov, M., and Froy, O. (2001) FASEB J. 15 1201-1205 [DOI] [PubMed] [Google Scholar]
- 18.Oren, D. A., Froy, O., Amit, E., Kleinberger-Doron, N., Gurevitz, M., and Shaanan, B. (1998) Structure (Lond.) 6 1095-1103 [DOI] [PubMed] [Google Scholar]
- 19.Cohen, L., Lipstein, N., and Gordon, D. (2006) FASEB J. 20 1933-1935 [DOI] [PubMed] [Google Scholar]
- 20.Mouhat, S., Jouirou, B., Mosbah, A., De Waard, M., and Sabatier, J. M. (2004) Biochem. J. 378 717-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pintar, A., Possani, L. D., and Delepierre, M. (1999) J. Mol. Biol. 287 359-367 [DOI] [PubMed] [Google Scholar]
- 22.Cohen, L., Karbat, I., Gilles, N., Froy, O., Angelovici, R., Gordon, D., and Gurevitz, M. (2004) J. Biol. Chem. 279 8206-8211 [DOI] [PubMed] [Google Scholar]
- 23.Karbat, I., Turkov, M., Cohen, L., Kahn, R., Gordon, D., Gurevitz, M., and Frolow, F. (2007) J. Mol. Biol. 366 586-601 [DOI] [PubMed] [Google Scholar]
- 24.Karbat, I., Cohen, L., Gilles, N., Gordon, D., and Gurevitz, M. (2004) FASEB J. 18 683-689 [DOI] [PubMed] [Google Scholar]
- 25.Cohen, L., Karbat, I., Gilles, N., Ilan, N., Gordon, D., and Gurevitz, M. (2005) J. Biol. Chem. 280 5045-5053 [DOI] [PubMed] [Google Scholar]
- 26.Bontems, F., Roumestand, C., Gilquin, B., Menez, A., and Toma, F. (1991) Science 254 1521-1523 [DOI] [PubMed] [Google Scholar]
- 27.Gilles, N., Leipold, E., Chen, H., Heinemann, S. H., and Gordon, D. (2001) Biochemistry 40 14576-14584 [DOI] [PubMed] [Google Scholar]
- 28.Gilles, N., Krimm, I., Bouet, F., Froy, O., Gurevitz, M., Lancelin, J.-M., and Gordon, D. (2000) J. Neurochem. 75 1735-1745 [DOI] [PubMed] [Google Scholar]
- 29.Armstrong, C. M., and Bezanilla, F. (1974) J. Gen. Physiol. 63 533-552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon, D., and Zlotkin, E. (1993) FEBS Lett. 315 125-129 [DOI] [PubMed] [Google Scholar]
- 31.Cestèle, S., and Gordon, D. (1998) J. Neurochem. 70 1217-1226 [DOI] [PubMed] [Google Scholar]
- 32.Trainer, V. L., Baden, D. G., and Catterall, W. A. (1994) J. Biol. Chem. 269 19904-19909 [PubMed] [Google Scholar]
- 33.Froy, O., and Gurevitz, M. (1998) FASEB J. 12 1793-1796 [DOI] [PubMed] [Google Scholar]
- 34.Baden, D. G., Bourdelais, A. J., Jacocks, H., Michelliza, S., and Naar, J. (2005) Environ. Health Perspect. 113 621-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purkerson-Parker, S. L., Fieber, L. A., Rein, K. S., Podona, T., and Baden, D. G. (2000) Chem. Biol. 7 385-393 [DOI] [PubMed] [Google Scholar]
- 36.Bourdelais, A. J., Campbell, S., Jacocks, H., Naar, J., Wright, J. L., Carsi, J., and Baden, D. G. (2004) Cell. Mol. Neurobiol. 24 553-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cestèle, S., Sampieri, F., Rochat, H., and Gordon, D. (1996) J. Biol. Chem. 271 18329-18332 [DOI] [PubMed] [Google Scholar]
- 38.Tedford, H. W., Sollod, B. L., Maggio, F., and King, G. F. (2004) Toxicon 43 601-618 [DOI] [PubMed] [Google Scholar]
- 39.Pimenta, A. M., Stocklin, R., Favreau, P., Bougis, P. E., and Martin-Eauclaire, M. F. (2001) Rapid Commun. Mass Spectrom. 15 1562-1572 [DOI] [PubMed] [Google Scholar]
- 40.Cohen, L., Troub, Y., Turkov, M., Gilles, N., Ilan, N., Benveniste, M., Gordon, D., and Gurevitz, M. (2007) Mol. Pharmacol. 72 1220-1227 [DOI] [PubMed] [Google Scholar]