Abstract
Plasma retinol-binding protein (RBP), the principal carrier of vitamin A in the blood, delivers vitamin A from liver, the site of storage, to distant organs that need vitamin A, such as the eye, brain, placenta, and testis. STRA6 is a high-affinity membrane receptor for RBP and mediates vitamin A uptake in these target organs. STRA6 is a 74-kDa multi-transmembrane domain protein that represents a new class of membrane transport protein. In this study, we used an unbiased strategy by analyzing >900 random mutants of STRA6 to study its structure and function, and we identified an essential RBP-binding domain in STRA6. Mutations in any of the three essential residues in this domain can almost completely abolish binding of STRA6 to RBP and its vitamin A uptake activity from holo-RBP without affecting its cell surface expression. We have also functionally characterized the mutations in human STRA6 that cause severe birth defects as well as several human polymorphisms. All STRA6 mutants associated with severe birth defects have largely abolished vitamin A uptake activity, consistent with the severe clinical phenotypes. In addition, we have identified a human polymorphism that significantly reduces the vitamin A uptake activity of STRA6. Interestingly, the residue affected by this polymorphism is located in the RBP-binding domain we identified, and the polymorphism causes decreased vitamin A uptake by reducing RBP binding. This study identifies an essential functional domain in STRA6 and a human polymorphism in this domain that leads to reduced vitamin A uptake activity.
Plasma retinol-binding protein (RBP)2 is the principal carrier of vitamin A in the blood (1–4). It was first proposed in the 1970s that there exists a cell surface receptor that mediates vitamin A uptake from RBP on the retinal pigment epithelium and small intestine cells (5–10). During the past 3 decades, there has been mounting evidence for the existence of RBP receptors on diverse types of tissues, including the placenta (11–13), choroid plexus (12, 14), Sertoli cells and peritubular cells of the testis (12, 15–18), macrophages (19), and skin (12, 20). There are also indirect pieces of evidence for the existence of an RBP receptor. For example, in an unbiased search for a serum factor that stimulates the growth of B cells, it was found that the vitamin A-RBP complex (holo-RBP) is this factor (21). Using an unbiased strategy combining photo-cross-linking, high-affinity purification, and mass spectrometry, the RBP receptor was identified as STRA6, a multi-transmembrane protein of previously unknown function (22). STRA6 binds to RBP with high affinity and specificity and facilitates the transport of vitamin A into the cell. STRA6 was originally identified as a retinoic acid-stimulated gene in cancer cell lines (23, 24).
STRA6 has not been systematically characterized at the structural and functional level and is not homologous to any protein with known function. In this study, we used two complementary approaches to study the structure and function of STRA6. The first approach was to create a large number of random mutants of STRA6 and systematically analyze their properties. An analogous large-scale mutagenesis strategy has been used successfully to study potassium channels (25–27). The second approach was to analyze naturally occurring human STRA6 mutants and polymorphisms. The advantage of the first approach is that it is unbiased and is especially suitable for analyzing proteins that have no obvious functional domains and that are not homologous to any protein of known function like STRA6. The advantage of the second approach is that it can provide the biochemical basis for the human genetics of STRA6. Recent human genetic studies found that mutations in STRA6 are associated with severe pathological phenotypes such as mental retardation, anophthalmia, congenital heart defects, lung hyperplasia, duodenal stenosis, pancreatic malformations, and intrauterine growth retardation (28, 29). In this study, we identified the functional defects in these naturally occurring human STRA6 mutants and polymorphisms.
EXPERIMENTAL PROCEDURES
Production of Random Mutants and Site-directed Mutagenesis of STRA6—Random mutagenesis of bovine STRA6 cDNA was performed using the GeneMorph II random mutagenesis system (Stratagene) between the HindIII and XbaI sites (from residues 232 to 362). The mutagenesis protocol was optimized so that each mutant contains one to four mutations; this is an acceptable rate because not all mutations cause an amino acid change. To facilitate the examination of the cell surface expression of each mutant, mutagenesis was performed on STRA6 tagged with a high-affinity binding site for α-bungarotoxin as described below. Site-directed mutagenesis of human STRA6 or bovine STRA6 was performed by PCR.
Examination of Cell Surface Expression of STRA6 Mutants Using α-Bungarotoxin—α-Bungarotoxin and its high-affinity binding site (BBS) provide an efficient tagging system to study cell surface proteins (30). An amino acid sequence containing BBS was inserted into an extracellular loop of bovine STRA6 between residues 133 and 134 (STRA6-BBS) (22). Because STRA6-BBS behaves similarly to wild-type STRA6 (22) and BBS is located extracellularly, live cells transfected with the STRA6-BBS construct can be stained with Alexa Fluor 594-conjugated α-bungarotoxin (Molecular Probes) to examine STRA6 cell surface expression. Briefly, STRA6 mutants with a BBS tag were transfected into HEK293 cells growing on gelatin-coated coverslips. Twenty-four h after transfection, Alexa Fluor 594-conjugated α-bungarotoxin was added to the medium at 5 μg/ml. After a 1-h incubation at 37 °C, the cells were washed with Hanks' buffered salt solution (HBSS) three times and fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. After three more washes with HBSS, the coverslips containing cells were mounted onto slides using Vectashield mounting medium (Vector Laboratories). The cell surface expression of each mutant was individually examined by fluorescence microscopy.
Assay for Vitamin A Uptake Activity Using [3H]-Retinol/RBP—The production of [3H]-retinol/RBP was performed similarly as described (22). All STRA6 variants were cotransfected with lecithin:retinol acyltransferase for the vitamin A uptake assay. To determine cellular [3H]-retinol uptake from [3H]-retinol/RBP, cells were washed with HBSS before incubation with [3H]-retinol/RBP diluted in serum-free medium for 1 h at 37 °C. The reactions were stopped by removing the medium, washing the cells with HBSS, and solubilizing the cells in 1% Triton X-100 in PBS. Radioactivity was measured with a scintillation counter.
Quantitation of RBP Binding Activity—Quantitation of RBP binding to STRA6 using alkaline phosphatase-RBP fusion protein was performed similarly as described (31). Briefly, transfected cells grown on 12-well cell culture plates were washed once with HBSS and incubated with alkaline phosphatase-RBP fusion protein diluted in serum-free medium at 37 °C for 1 h. The reaction was stopped by washing cells twice with HBSS. The cells were then lysed in 150 μl of cold PBS containing 1% Triton X-100 and protease inhibitors per well. The cell lysates were centrifuged at 10,000 × g at 4 °C for 5 min. The supernatants were removed and heated at 65 °C for 1 h to inactivate endogenous alkaline phosphatase activity. Fifty μl of the heated lysate were mixed with 200 μl of p-nitrophenyl phosphate (Sigma) and incubated at 37 °C for 1 h for the alkaline phosphatase color reaction. The reactions were stopped by adding 50 μl of 3 m NaOH, and then the lysate was transferred to a 96-well plate for reading in a microplate reader at 405 nm.
Quantitation of Cell Surface Expression of STRA6 Mutants—We introduced a Myc tag at the position where BBS is inserted in STRA6-BBS (STRA6-Myc) to quantify its cell surface expression using anti-Myc antibody. Briefly, 24 h after transfection of COS-1 cells with STRA6 variants with the Myc tag, anti-Myc antibody was added to the medium for 1 h. After three washes with HBSS, the cells were incubated in 5% normal rabbit serum in serum-free medium for 15 min. Rabbit anti-mouse antibody conjugated to alkaline phosphatase (Pierce) diluted in 5% normal rabbit serum in serum-free medium was then added to the cells and incubated for 1 h at 37 °C. After four washes with HBSS, cells were lysed in 1% Triton X-100 in PBS with protease inhibitors. Insoluble materials were removed by centrifugation at 16,000 × g for 5 min at 4 °C. Alkaline phosphatase activity in the supernatant was measured using p-nitrophenyl phosphate as described above.
Live Cell Staining and Permeabilized Cell Staining—Briefly, STRA6-Myc constructs were transfected into HEK293 cells growing on gelatin-coated coverslips. At 24 h after transfection, purified anti-Myc monoclonal antibody was added to the medium. After a 1-h incubation at 37 °C, the cells were washed with HBSS three times and fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. After three washes with PBS, the fixed cells were incubated with blocking buffer (5% normal goat serum and 0.3% Triton X-100 in PBS) for 1 h at room temperature. The cells were then incubated with Alexa Fluor 488-conjugated goat anti-mouse antibody (Molecular Probes) diluted in blocking buffer for another hour at room temperature. After three washes with PBS, the coverslips containing cells were mounted onto slides using VectorShield mounting medium. The cell surface expression of STRA6-Myc proteins was examined by fluorescence microscopy. Permeabilized staining was performed similarly to live cell staining procedures except that cells were incubated with the primary antibody after they were fixed.
RESULTS
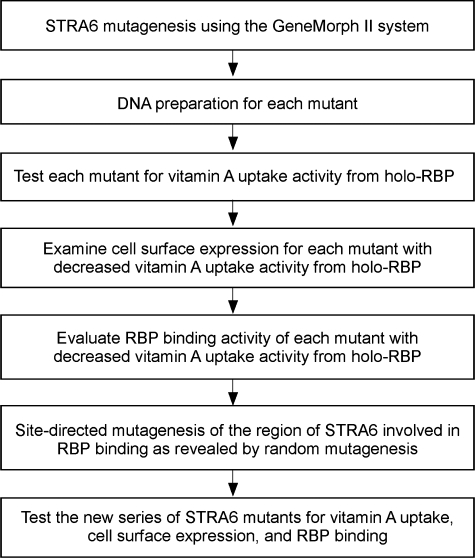
An Unbiased Strategy to Study the Structure and Function of STRA6—Because STRA6 has not been systematically characterized at the structural level and little is known about its structure, we designed an unbiased strategy to study its structure and function (Fig. 1). Our previous small-scale analysis identified a bovine STRA6 mutant (Q310H/L315P) with normal surface expression but impaired RBP binding (22). By analyzing the individual mutations (Q310H and L315P), we found that L315P is responsible for the loss of RBP binding activity (data not shown). However, residue 315 is located in a region of STRA6 predicted to be a transmembrane domain (completely devoid of hydrophilic residues). Given the large impact of proline on protein structure beyond the mutated residue, residue 315 may not be directly involved in RBP binding. Instead, this mutant implicated a larger region of STRA6 around residue 315 in RBP interaction. Therefore, we performed an unbiased large-scale mutational analysis of the region around this residue (residues 232–362). This strategy is more practical than mutating the whole gene because of its large size and the lack of prior knowledge available about its structure.
FIGURE 1.
Outline of experimental procedures for an unbiased approach to study the structure and function of STRA6.
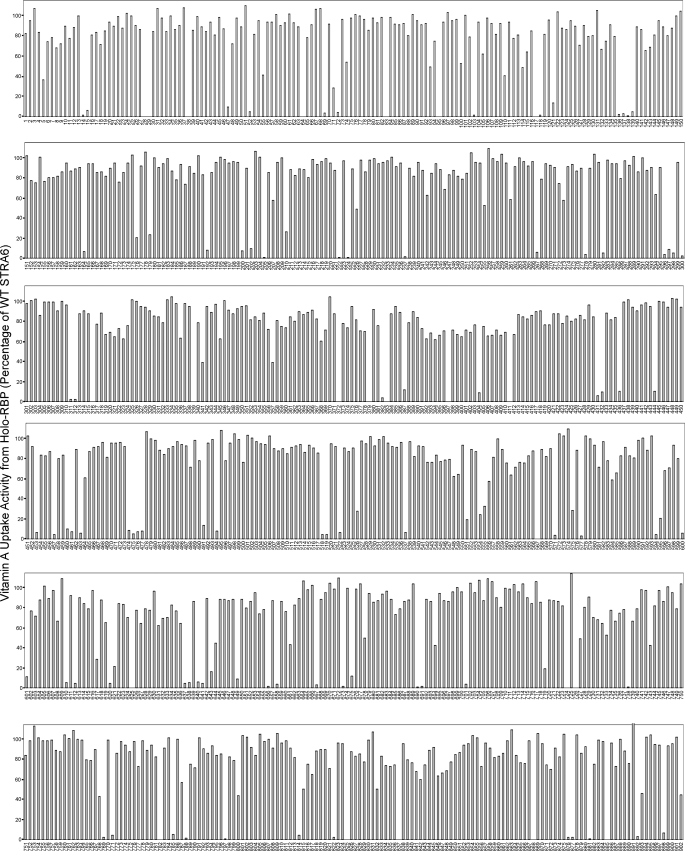
Using random mutagenesis, we produced 902 random mutants of STRA6. The mutagenized protein is a tagged version of STRA6, which made it possible to efficiently examine STRA6 cell surface expression, as described below. We optimized the mutagenesis protocol so that each mutant contained one to four mutations. This was verified by sequencing. We found that no identical mutation occurred in more than one clone. These mutants were then individually transfected into COS-1 cells together with lecithin:retinol acyltransferase for vitamin A uptake assay from [3H]-retinol/RBP. As shown in Fig. 2, a large number of mutants had significantly reduced vitamin A uptake activity. Among them, 141 mutants had <50% of the wild-type STRA6 activity in vitamin A uptake from holo-RBP, and 102 mutants had <10% of the wild-type activity. Sequence analysis revealed mutations scattered throughout the mutagenized region of STRA6. Most of the severely defective mutants are nonsense or frameshift mutations that introduce premature stop codons.
FIGURE 2.
Vitamin A uptake activities from holo-RBP of 902 random bovine STRA6 mutants. Wild-type (WT) STRA6 activity is defined as 100%. Mutant names are shown below the x axis.
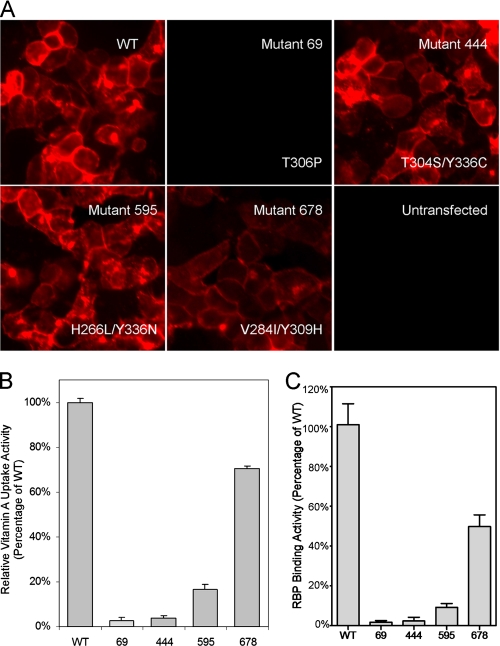
Cell Surface Expression and RBP Binding Activity of STRA6 Mutants—α-Bungarotoxin and BBS provide an efficient tagging system to study membrane proteins (30, 32). We found previously that insertion of BBS into an extracellular loop of STRA6 does not interfere with either cell surface expression or vitamin A uptake activity (22). This STRA6-BBS construct is an ideal template for mutagenesis because mutants can be efficiently screened for cell surface expression. We examined all defective mutants for their cell surface expression. We found that most defective mutants were absent or expressed poorly on the cell surface, which is sufficient to account for their loss of retinol uptake activity. These results demonstrate the dependence of vitamin A uptake activity on the cell surface expression of STRA6. Examples of two STRA6 mutants with complete (mutant 69, T306P) or partial (mutant 678, V284I/Y309H) loss of surface expression are shown in Fig. 3. Strikingly, only two of the 141 defective STRA6 mutants had a level of cell surface expression similar to the wild-type protein (Fig. 3). These two mutants (mutant 444, T304S/Y336C, and mutant 595, H266L/Y336N) had largely abolished vitamin A uptake activity from holo-RBP (Figs. 2 and 3). We further tested RBP binding for these two mutants and found that their loss of RBP binding was largely responsible for their loss of vitamin A uptake activity from holo-RBP (Fig. 3).
FIGURE 3.
Characterization of STRA6 mutants generated by random mutagenesis. A, cell surface expression of STRA6 mutants: two defective mutants with normal cell surface expression (mutants 444 and 595), one mutant with weak cell surface expression (mutant 678), and one mutant with complete loss of cell surface expression (mutant 69). The specific mutations are indicated. B, vitamin A uptake activity of STRA6 mutants 69, 444, 595, and 678. C, RBP binding activity of STRA6 mutants 69, 444, 595, and 678. WT, wild type.
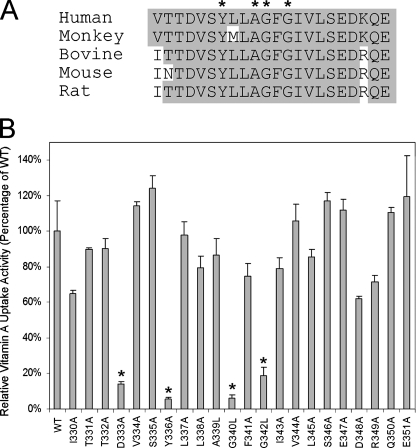
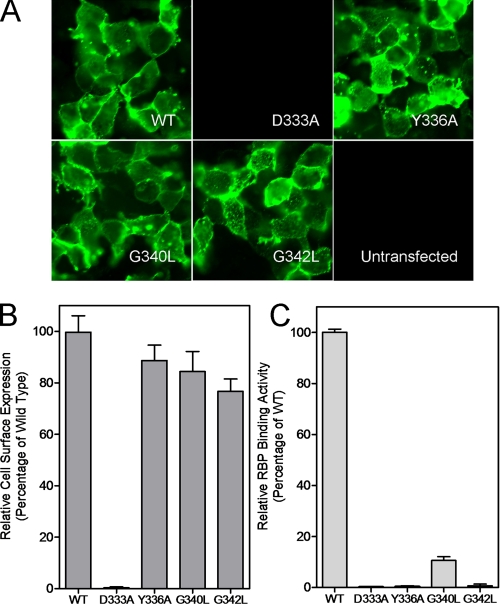
Identification and Characterization of an RBP-binding Domain in STRA6—Both of the severe STRA6 mutants with normal cell surface expression have an alteration of Tyr336. Several other mutants with defective vitamin A uptake activity (with partial or complete loss of surface expression) also point to the region around Tyr336 as potentially important in STRA6 function (data not shown). Therefore, we decided to perform systematic alanine-scanning mutagenesis of the region of STRA6 around Tyr336 by changing glycine and alanine to leucine and all other residues to alanine. We performed this site-directed mutagenesis using a Myc-tagged STRA6 (the Myc is located in the same extracellular loop where the BBS is located), which makes it possible to examine cell surface expression by immunostaining and quantify cell surface expression. Of the 22 mutants in this series, four showed significantly reduced vitamin A uptake activity from holo-RBP (D333A, Y336A, G340L, and G342L) (Fig. 4). These four residues are highly conserved in evolution (Fig. 4). Further characterization of the cell surface expression of these mutant proteins showed that one mutant, D333A, lost its cell surface expression, but the other three had normal cell surface expression, as assessed by both live cell staining and Myc quantitation (Fig. 5). The normal cell surface expression but loss of vitamin A uptake activity of the Y336A mutant is consistent with the phenotypes of the two mutants that affect this residue identified through random mutagenesis (Fig. 3). All three mutants with normal surface expression lost RBP binding activity (Fig. 5), demonstrating that their loss of vitamin A uptake activities is due to defective RBP binding.
FIGURE 4.
Alanine-scanning mutagenesis of the putative RBP-binding region in STRA6 that was identified by random mutagenesis. Glycine and alanine residues were mutated to leucine. A, sequence alignment of the region from different species. Residues where mutations lead to significant loss of vitamin A uptake are indicated by asterisks. B, vitamin A uptake activity from holo-RBP for this series of STRA6 mutants. Mutants with significant loss of vitamin A uptake are indicated by asterisks (D333A, Y336A, G340L, and G342L). WT, wild type.
FIGURE 5.
Characterization of four defective STRA6 mutants identified through alanine-scanning mutagenesis. A, cell surface expression of STRA6 mutants D333A, Y336A, G340L, and G342L. All mutants maintained cell surface expression except D333A. B, quantitation of cell surface expression of the four STRA6 mutants. C, RBP binding activity of the four STRA6 mutants. WT, wild type.
Characterization of Human STRA6 Mutations Associated with Severe Birth Defects and Human STRA6 Polymorphisms—In an unbiased genetic search for the gene responsible for a recessive human disease causing birth defects, the responsible gene was identified as STRA6 (28), the RBP receptor, consistent with the essential roles of vitamin A in human embryonic development. This finding was further confirmed by another human genetic study identifying mutations in STRA6 responsible for Matthew-Wood syndrome (29). Mutations identified in these studies include five point mutations, a frameshift mutation leading to a premature stop codon (28), and insertion/deletion mutations leading to premature stop codons (29). The five point mutations (P90L/T321P, P293L, R644M, and R655C) occur in conserved residues (from fish to human) (28). Because the biochemical defects caused by point mutations are not known, we decided to study the detailed mechanistic defects in STRA6 caused by these five point mutations. For the two mutations found in one patient (P90L/T321P), the mutations were analyzed singly and combined. There are also four non-synonymous human STRA6 polymorphisms in the GenBank™ Data Bank: D26N, G339S, L517F, and I527M. We analyzed the effect of these polymorphisms on STRA6 functions. For all human variants, we produced them in an untagged and Myc-tagged human STRA6 background, which allows examination of cell surface expression. We systematically analyzed their vitamin A uptake activity from holo-RBP, their cell surface expression, and their RBP binding activity.
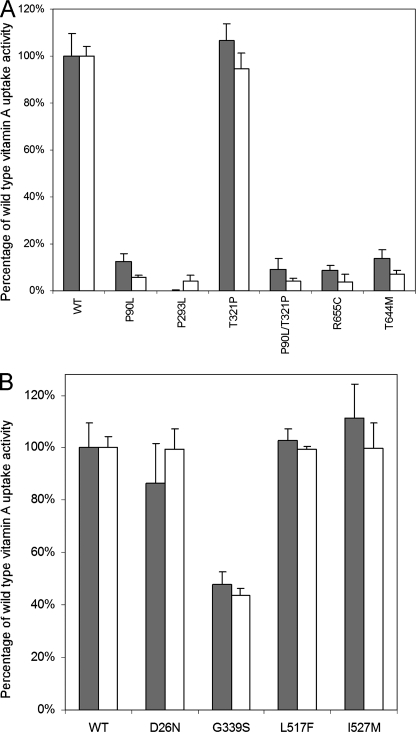
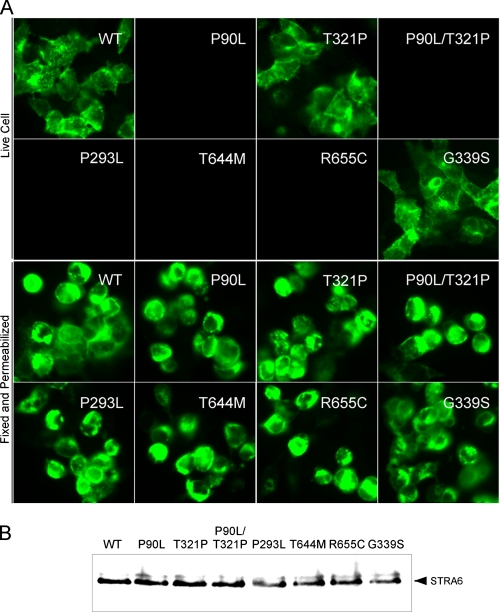
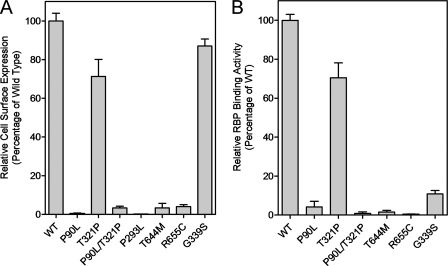
All human STRA6 mutations associated with birth defects except T321P almost completely abolished vitamin A uptake activity of STRA6 from holo-RBP (Fig. 6). This suggests that for the patient carrying the P90L/T321P double mutations, P90L is the causal mutation. All mutations except T321P led to the loss of cell surface expression of STRA6 (Fig. 7). Unlike the mutant that loses expression because of a frameshift mutation (28), permeabilized immunostaining and Western blot analysis showed that all five point mutants were still expressed (Fig. 7). Therefore, these mutations either cause misfolding of STRA6 or interfere with its targeting mechanism to prevent cell surface expression, which can account for their loss of vitamin A uptake activity from holo-RBP. To distinguish between these two possibilities, we performed a cell-free vitamin A uptake assay using membrane prepared from transfected cells as we have performed previously for wild-type STRA6 (22). Again, the mutants also lacked vitamin A uptake activity in the cell-free system (data not shown). This experiment suggests that the mutations affect the proper folding of STRA6 instead of its cell surface targeting. They are mechanistically similar to most defective STRA6 mutants generated by random mutagenesis in that they fail to be expressed on the cell surface.
FIGURE 6.
Vitamin A uptake activity from holo-RBP for human STRA6 variants. Human STRA6 variants were produced in both the untagged (gray bars) and Myc-tagged (white bars) human STRA6 background. A, vitamin A uptake activity from holo-RBP of human STRA6 missense mutants associated with birth defects (P90L, P293L, T321P, P90L/T321P, R655C, and T644M). B, vitamin A uptake activity of human STRA6 polymorphisms (D26N, G339S, L517F, and I527M). The activity of wild-type (WT) STRA6 (tagged or untagged) is defined as 100%.
FIGURE 7.
Analysis of protein expression of human STRA6 variants (P90L, T321P, P90L/T321P, P293L, T644M, R655C, and G339S). A, live cell staining of human STRA6 variants to examine cell surface expression (upper panels) and fixed and permeabilized cell staining of human STRA6 variants (lower panels). B, Western blot analysis of the expression of human STRA6 variants. WT, wild type.
For the four human STRA6 polymorphisms, three (D26N, L517F, and I527M) did not have a significant effect on vitamin A uptake activity from holo-RBP, but the G339S polymorphism led to significantly reduced vitamin A uptake activity. We found by both live cell staining (Fig. 7) and cell surface expression quantitation (Fig. 8) that this polymorphism did not affect the cell surface expression of STRA6. It caused decreased vitamin A uptake activity by reducing STRA6 RBP binding activity (Fig. 8).
FIGURE 8.
Quantitation of cell surface expression and RBP binding activities of human STRA6 variants (P90L, T321P, P90L/T321P, P293L, T644M, R655C, and G339S). A, quantitation of cell surface expression. The expression level of wild-type (WT) STRA6 is defined as 100%. B, RBP binding activities. The activity of wild-type STRA6 is defined as 100%.
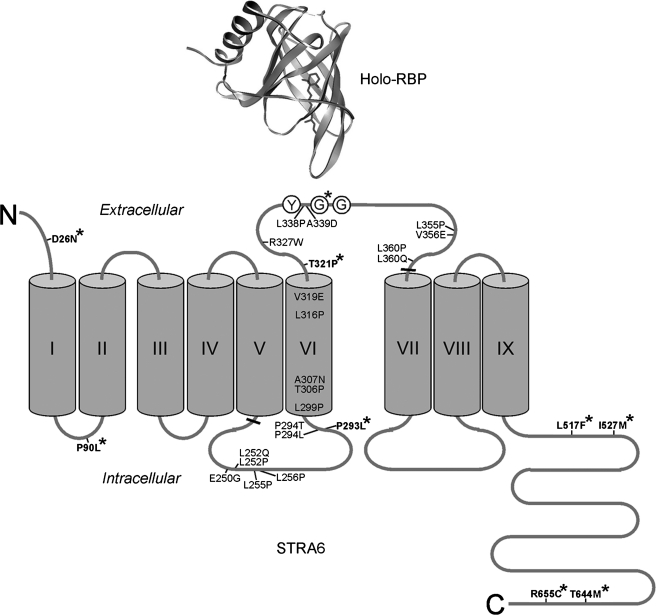
Key STRA6 Mutations and Polymorphisms in the Transmembrane Topology Model of STRA6—In an independent study, we experimentally determined the transmembrane topology of STRA6 (33). The locations of the key STRA6 mutations and polymorphisms in this topology model are summarized in Fig. 9. Mutations identified by random mutagenesis that cause severe loss of STRA6 function are located throughout the region of STRA6 subjected to the mutagenesis. The region where the three essential residues involved in RBP binding (Tyr336, Gly340, and Gly342 in bovine STRA6) are located was predicted to be a transmembrane domain in bovine STRA6 (but not in mouse STRA6) by two computer programs (SOSUI and TMPRED). This contrasts with the fact that it contains several hydrophilic residues, including arginine, aspartate, and glutamate. The identification of key residues involved in RBP interaction in this region demonstrates the advantage of using an unbiased experimental approach to study STRA6 instead of relying on targeted mutagenesis based on predicted models. The human polymorphism that causes reduced RBP binding affects residue 339. Interestingly, human STRA6 Gly339 is equivalent to bovine STRA6 Gly340, which we identified as a critical residue in the interaction of STRA6 with RBP through random mutagenesis and alanine scanning (Fig. 9).
FIGURE 9.
Locations of key STRA6 mutations and polymorphisms analyzed in this study in the transmembrane topology model of STRA6. Transmembrane domains are indicated with Roman numerals. Thickened lines mark the region of STRA6 subjected to random mutagenesis. Single bovine STRA6 mutants with severe functional defects (defined as vitamin A uptake activity <10% of the wild type) are shown. The three essential STRA6 residues involved in RBP interaction (Tyr336, Gly340, and Gly342 in bovine STRA6) are drawn as circled Y, G, and G, respectively. Natural human mutations and polymorphisms are shown in boldface and marked with asterisks (residue numbers according to human STRA6). The residue affected by human polymorphism G339S is equivalent to bovine Gly340 (marked with an asterisk). The diagram of holo-RBP is based on code 1HBP in the Protein Data Bank.
DISCUSSION
The power of mutagenesis to study protein function has been illustrated by the excellent mechanistic studies of rhodopsin. A large number of natural and artificial rhodopsin mutants have revealed deep mechanistic insights into rhodopsin activation, phosphorylation, inactivation by arrestin, interaction with transducin, interaction with its chromophore, and rhodopsin trafficking. This study represents the first systematic analysis of the structure and function of STRA6, the high-affinity membrane receptor for RBP. Although STRA6 is not homologous to any protein of known function (and therefore has no obvious functional domains), its multi-transmembrane domains and its function as the RBP receptor in mediating vitamin A uptake imply that it has extracellular domains that participate in RBP interaction. Because ligand binding may involve multiple interacting sites, the large size of STRA6 and its absence of obvious functional domains make it impractical to perform site-directed mutagenesis as the initial approach to identify the RBP-binding domain(s). Indeed, most defective mutants generated through random mutagenesis consisted of mutants that failed to be expressed on the cell surface. Because the loss of cell surface expression (by either misfolding or loss of proper targeting) is sufficient to account for the loss of vitamin A uptake activity, these mutants do not reveal the functional domains in STRA6. Interestingly, most of the severe missense mutations involve proline residues (either change to or from proline) (Fig. 9), consistent with the unique role of proline in protein structure. These mutations likely cause large structural changes and misfolding of STRA6, which can prevent cell surface expression. Several natural human mutations also involve proline, similar to most of the severe mutations identified in our large-scale mutagenesis (Fig. 9).
By focusing on mutants with normal cell surface expression, our large-scale mutagenesis study identified an essential RBP-binding domain in STRA6. Because mutation in any one of three key residues in this domain is sufficient to decimate the RBP binding and vitamin A uptake activities of STRA6, these three residues are likely located in the contact site for the STRA6-RBP interaction. This finding also further demonstrated the specific interaction between RBP and STRA6. Coincidentally, one of the key residues for RBP binding identified by alanine scanning in bovine STRA6 is also the residue affected by a polymorphism in human STRA6 that leads to partial loss of RBP binding. This study does not imply that these key residues are the only residues in this region involved in STRA6 interaction with RBP. It is possible that there are other key residues in this region, but their mutations cause misfolding of the protein, or that there are other residues involved in RBP binding, but mutating a single residue does not cause as profound an effect on RBP binding as Tyr336, Gly340, and Gly342.
The function of the RBP receptor in human development has been demonstrated by two human genetic studies. Mutations in human STRA6 cause either embryonic lethality or severe birth defects in many organ systems (28, 29). These phenotypes are consistent with the vital functions of vitamin A in embryonic development. In this study, we showed that the point mutations in human STRA6 associated with birth defects cause severe or complete loss of vitamin A uptake activity from holo-RBP due to their absence of cell surface expression. These biochemical defects are consistent with the severe clinical phenotypes associated with these mutations (28). These mutations are mechanistically similar to the common mutation in the cystic fibrosis transmembrane conductance regulator (Δ508) that causes cystic fibrosis in that they have defective cell surface expression (34).
Only two natural RBP mutations have been identified in humans, and they cause vision defects such as the dystrophy of retinal pigment epithelium, an essential cell type in vision (35). This phenotype is consistent with the high expression levels of STRA6 in retinal pigment epithelium cells, which need to absorb large amount of vitamin A for proper visual functions. How can we reconcile the relatively restricted phenotypes caused by the two RBP mutations in humans (35) and the more severe and systemic phenotypes caused by STRA6 mutations in humans (28, 29)? First, biochemical analysis showed that the only two natural mutations found in human RBP cause only a partial loss of RBP function (36). This is in sharp contrast to the complete or nearly complete loss of STRA6 function described in this work. The human RBP mutants can still bind retinol (36). Second, holo-RBP formed by the mutant RBP can still bind transthyretin like wild-type RBP. The major defect identified is the relatively faster release of retinol in the presence of lipid membranes. The vision defect associated with human RBP mutations suggests that vision is most sensitive to the partial loss of RBP function. RBP is highly conserved in evolution. The fact that human RBP mutations are so rarely identified (only two so far) is consistent with its essential function. Moreover, the detection limit of serum retinol/RBP in patients with RBP mutations in the previous study was 200 nm (35), which is still much higher than the Kd (50 nm) of the STRA6-RBP interaction (22). One potential function of the high-affinity interaction between RBP and its receptor is to help vertebrates survive vitamin A-deficient conditions, which can lower serum RBP levels. Vitamin A-deficient conditions are common for most, if not all, vertebrates living in natural environments. Furthermore, even for complete nulls, the loss of RBP and RBP receptor function does not necessarily generate the same phenotypes in the same species. For example, placental delivery of vitamin A from maternal RBP to the human embryo is very different between RBP null embryos and RBP receptor null embryos. A human embryo without the RBP receptor can be directly impacted by its loss of function in the placental absorption of vitamin A from maternal RBP. In contrast, a human embryo without RBP still has its functional RBP receptor and functional maternal RBP to ensure sufficient vitamin A delivery to the embryo through the placenta.
During human evolution (including certain populations in the modern world), different human populations have dramatic differences in diet. The diet of certain human populations consists almost entirely of animal products rich in vitamin A. Because vitamin A is toxic at high concentrations, it may be advantageous to have reduced vitamin A uptake as an adaptation to this unique diet. The selective pressure on this polymorphism is likely exerted during pregnancy because too much vitamin A (as well as too little) can cause birth defects (37). In this study, we identified a human polymorphism in STRA6 with reduced vitamin A uptake activity. Future systematic human genetic study is necessary to determine the distribution of this polymorphism in present day human populations.
This work was supported, in whole or in part, by National Institutes of Health Grant 1R01EY018144 (to H. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: RBP, retinol-binding protein; BBS, α-bungarotoxin-binding site; HBSS, Hanks' buffered salt solution; PBS, phosphate-buffered saline.
References
- 1.Goodman, D. S. (1984) in The Retinoids (Sporn, M. B., Boberts, A. B., and Goodman, D. S., eds) pp. 41-88, Academic Press, Orlando, FL
- 2.Newcomer, M. E., and Ong, D. E. (2000) Biochim. Biophys. Acta 1482 57-64 [DOI] [PubMed] [Google Scholar]
- 3.Blaner, W. S. (1989) Endocr. Rev. 10 308-316 [DOI] [PubMed] [Google Scholar]
- 4.Zanotti, G., and Berni, R. (2004) Vitam. Horm. 69 271-295 [DOI] [PubMed] [Google Scholar]
- 5.Bok, D., and Heller, J. (1976) Exp. Eye Res. 22 395-402 [DOI] [PubMed] [Google Scholar]
- 6.Heller, J. (1975) J. Biol. Chem. 250 3613-3619 [PubMed] [Google Scholar]
- 7.Heller, J., and Bok, D. (1976) Exp. Eye Res. 22 403-410 [DOI] [PubMed] [Google Scholar]
- 8.Rask, L., and Peterson, P. A. (1976) J. Biol. Chem. 251 6360-6366 [PubMed] [Google Scholar]
- 9.Maraini, G., and Gozzoli, F. (1975) Investig. Ophthalmol. 14 785-787 [PubMed] [Google Scholar]
- 10.Chen, C. C., and Heller, J. (1977) J. Biol. Chem. 252 5216-5221 [PubMed] [Google Scholar]
- 11.Sivaprasadarao, A., and Findlay, J. B. (1988) Biochem. J. 255 561-569 [PMC free article] [PubMed] [Google Scholar]
- 12.Smeland, S., Bjerknes, T., Malaba, L., Eskild, W., Norum, K. R., and Blomhoff, R. (1995) Biochem. J. 305 419-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivaprasadarao, A., Boudjelal, M., and Findlay, J. B. (1994) Biochem. J. 302 245-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald, P. N., Bok, D., and Ong, D. E. (1990) Proc. Natl. Acad. Sci. U. S. A. 87 4265-4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shingleton, J. L., Skinner, M. K., and Ong, D. E. (1989) Biochemistry 28 9641-9647 [DOI] [PubMed] [Google Scholar]
- 16.Bishop, P. D., and Griswold, M. D. (1987) Biochemistry 26 7511-7518 [DOI] [PubMed] [Google Scholar]
- 17.Bhat, M. K., and Cama, H. R. (1979) Biochim. Biophys. Acta 587 273-281 [DOI] [PubMed] [Google Scholar]
- 18.Davis, J. T., and Ong, D. E. (1995) Biol. Reprod. 52 356-364 [DOI] [PubMed] [Google Scholar]
- 19.Hagen, E., Myhre, A. M., Smeland, S., Halvorsen, B., Norum, K. R., and Blomhoff, R. (1999) J. Nutr. Biochem. 10 345-352 [DOI] [PubMed] [Google Scholar]
- 20.Torma, H., and Vahlquist, A. (1984) Arch. Dermatol. Res. 276 390-395 [DOI] [PubMed] [Google Scholar]
- 21.Buck, J., Ritter, G., Dannecker, L., Katta, V., Cohen, S. L., Chait, B. T., and Hammerling, U. (1990) J. Exp. Med. 171 1613-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi, R., Yu, J., Honda, J., Hu, J., Whitelegge, J., Ping, P., Wiita, P., Bok, D., and Sun, H. (2007) Science 315 820-825 [DOI] [PubMed] [Google Scholar]
- 23.Bouillet, P., Sapin, V., Chazaud, C., Messaddeq, N., Decimo, D., Dolle, P., and Chambon, P. (1997) Mech. Dev. 63 173-186 [DOI] [PubMed] [Google Scholar]
- 24.Szeto, W., Jiang, W., Tice, D. A., Rubinfeld, B., Hollingshead, P. G., Fong, S. E., Dugger, D. L., Pham, T., Yansura, D. G., Wong, T. A., Grimaldi, J. C., Corpuz, R. T., Singh, J. S., Frantz, G. D., Devaux, B., Crowley, C. W., Schwall, R. H., Eberhard, D. A., Rastelli, L., Polakis, P., and Pennica, D. (2001) Cancer Res. 61 4197-4205 [PubMed] [Google Scholar]
- 25.Bichet, D., Lin, Y. F., Ibarra, C. A., Huang, C. S., Yi, B. A., Jan, Y. N., and Jan, L. Y. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 4441-4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi, B. A., Lin, Y. F., Jan, Y. N., and Jan, L. Y. (2001) Neuron 29 657-667 [DOI] [PubMed] [Google Scholar]
- 27.Minor, D. L., Jr., Masseling, S. J., Jan, Y. N., and Jan, L. Y. (1999) Cell 96 879-891 [DOI] [PubMed] [Google Scholar]
- 28.Pasutto, F., Sticht, H., Hammersen, G., Gillessen-Kaesbach, G., Fitzpatrick, D. R., Nurnberg, G., Brasch, F., Schirmer-Zimmermann, H., Tolmie, J. L., Chitayat, D., Houge, G., Fernandez-Martinez, L., Keating, S., Mortier, G., Hennekam, R. C., von der Wense, A., Slavotinek, A., Meinecke, P., Bitoun, P., Becker, C., Nurnberg, P., Reis, A., and Rauch, A. (2007) Am. J. Hum. Genet. 80 550-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golzio, C., Martinovic-Bouriel, J., Thomas, S., Mougou-Zrelli, S., Grattagliano-Bessieres, B., Bonniere, M., Delahaye, S., Munnich, A., Encha-Razavi, F., Lyonnet, S., Vekemans, M., Attie-Bitach, T., and Etchevers, H. C. (2007) Am. J. Hum. Genet. 80 1179-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekine-Aizawa, Y., and Huganir, R. L. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 17114-17119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh, J. C., Rattner, A., Smallwood, P. M., and Nathans, J. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 3546-3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dellis, O., Dedos, S. G., Tovey, S. C., Taufiq Ur, R., Dubel, S. J., and Taylor, C. W. (2006) Science 313 229-233 [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi, R., Yu, J., Wiita, P., Ter-Stepanian, M., and Sun, H. (2008) Biochemistry, in press [DOI] [PMC free article] [PubMed]
- 34.Denning, G. M., Anderson, M. P., Amara, J. F., Marshall, J., Smith, A. E., and Welsh, M. J. (1992) Nature 358 761-764 [DOI] [PubMed] [Google Scholar]
- 35.Seeliger, M. W., Biesalski, H. K., Wissinger, B., Gollnick, H., Gielen, S., Frank, J., Beck, S., and Zrenner, E. (1999) Investig. Ophthalmol. Vis. Sci. 40 3-11 [PubMed] [Google Scholar]
- 36.Folli, C., Viglione, S., Busconi, M., and Berni, R. (2005) Biochem. Biophys. Res. Commun. 336 1017-1022 [DOI] [PubMed] [Google Scholar]
- 37.Collins, M. D., and Mao, G. E. (1999) Annu. Rev. Pharmacol. Toxicol. 39 399-430 [DOI] [PubMed] [Google Scholar]