Abstract
CRP2 (cysteine-rich protein) is a vascular smooth muscle cell (VSMC)-expressed LIM-only protein. CRP2 associates with the actin cytoskeleton and interacts with transcription factors in the nucleus to mediate smooth muscle cell gene expression. Using Csrp2 (gene symbol of the mouse CRP2 gene)-deficient mice, we previously demonstrated that an absence of CRP2 enhances VSMC migration and increases neointima formation following arterial injury. Despite its importance in vascular injury, the molecular mechanisms controlling CRP2 expression in VSMC are largely unknown. Transforming growth factor β (TGFβ), a key factor present in the vessel wall in the early phases of arterial response to injury, plays an important role in modulating lesion formation. Because both CRP2 and TGFβ are mediators of VSMC responses, we examined the possibility that TGFβ might regulate CRP2 expression. TGFβ significantly induced CRP2 mRNA and protein expression in VSMCs. Promoter analysis identified a conserved cAMP-responsive element (CRE)-like site (TAACGTCA) in the Csrp2 promoter that was critical for basal promoter activity and response to TGFβ. Gel mobility shift assays revealed that mainly ATF2 bound to this CRE-like element, and mutation of the CRE sequences abolished binding. TGFβ enhanced the activation of ATF2, leading to increased phospho-ATF2 levels within the DNA-protein complexes. Furthermore, ATF2-transactivated Csrp2 promoter activity and TGFβ enhanced this activation. In addition, a phosphorylation-negative ATF2 mutant construct decreased basal and TGFβ-mediated Csrp2 promoter activity. Our results show for the first time in VSMC that TGFβ activates ATF2 phosphorylation and Csrp2 gene expression via a CRE promoter element.
Vascular smooth muscle cells (VSMCs)2 of the arterial wall play a critical role in the development of occlusive vascular lesions. In normal vessels, VSMCs are quiescent, differentiated, and contractile and regulate vascular tone and blood pressure. In response to arterial injury, VSMCs undergo a phenotypic transition whereby they migrate and proliferate from the media into the intima, contributing to vascular lesion formation and arteriosclerosis (1).
The LIM domain is a double zinc finger structure that functions as a modular protein-binding interface that mediates protein-protein interactions (2–6). By binding to target proteins and serving as an adaptor molecule in the assembly of multiprotein complexes, LIM proteins participate in diverse biological processes (6, 7). The LIM-only cysteine-rich protein (CRP) family, which includes CRP1 (8, 9), CRP2/SmLIM (10), and CRP3/MLP (muscle LIM protein) (11), is characterized by two tandem LIM domains, each followed by a short glycine-rich repeat (12, 13). CRP1 is expressed in several cell types, including vascular and visceral smooth muscle cells (SMCs) (8, 14), whereas CRP2 is mainly expressed in VSMCs, particularly arterial SMC (10, 15, 16). CRP3 is specifically expressed in cardiac and skeletal muscle (11, 17). CRPs are involved in promoting protein assembly along the actin-based cytoskeleton (7, 13, 18). CRPs associate with the actin cytoskeleton via interacting with the actin-cross-linking protein α-actinin and adhesion plaque protein zyxin (4, 13, 19). In addition, CRPs also interact with transcription factors to activate gene transcription in the nucleus (20, 21). Given their prominent cytoskeletal association and presence in the nucleus, CRPs have both a major cytoskeletal function and a role controlling gene expression (6). A cytoskeletal function of CRP3 was demonstrated in mice lacking CRP3; those mice developed dilated cardiomyopathy with hypertrophy and heart failure after birth (17). Ultrastructural analysis revealed dramatic disruption of cardiomyocyte cytoarchitecture (17). Further supporting a function of CRP3 in maintaining the cytoarchitecture of striated muscle cells, the Drosophila homolog of muscle LIM protein, Mlp84B, was recently demonstrated to cooperate with d-titin to maintain muscle structural integrity (22).
We recently identified a key role for the LIM domain-containing protein, CRP2, in the development of arteriosclerosis (16). Following femoral artery wire injury, CRP2 expression persisted in the first few days and then decreased but was not abolished in the vessel wall by 14 days (16), implicating a role for CRP2 in vascular injury. By gene deletion experiments, we demonstrated that a lack of CRP2 increased neointima formation following arterial injury in mice (16). Importantly, the increased intimal thickening in Csrp2 (gene symbol of the mouse CRP2 gene)-deficient mice correlated with enhanced VSMC migration into the intima (16). This migratory role of CRP2 in arterial SMC emphasizes a cytoskeleton-associated function of CRP2 protein.
In response to injury, many cytokines and growth factors are released within injured blood vessels (1, 23, 24). These mediators in turn alter gene expression patterns of vascular cells, including SMC marker genes, leading to phenotypic modulation of VSMC and neointima formation. Despite its important function in the pathogenesis of arteriosclerosis, the molecular mechanisms controlling CRP2 expression in the context of vascular injury remain largely unknown. We showed previously that one of these factors, PDGF-BB, down-regulates CRP2 mRNA expression (10). Given that an absence of CRP2 promotes VSMC migration both in vitro and in vivo (16), induction of CRP2 by factors present in the injured vessels might serve as a protective mechanism and reduces VSMC migration and subsequent neointima formation.
In the current study, we sought to identify factors, present in the context of vascular injury, that up-regulated CRP2. CRP2 regulation by these factors might ultimately protect against intimal thickening. TGFβ, a key factor present in the vessel wall in the early phases of the arterial response to injury plays an important role in modulating lesion formation. We identified that TGFβ induces CRP2 protein and mRNA expression. Analysis of the Csrp2 promoter by reporter gene transfection experiments revealed that a conserved CRE-like motif located at bp –461 to –454 of the Csrp2 gene was required for both basal and TGFβ-induced activation of the Csrp2 promoter. We further demonstrated that TGFβ activates ATF2 phosphorylation and Csrp2 gene expression via a CRE promoter element.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents—Primary VSMCs were isolated from mouse or rat aortas and cultured in Dulbecco's modified Eagle's medium as described previously (16, 25). Cells were passaged every 3–5 days, and experiments were performed on cells 6–8 passages from primary culture. Actinomycin D and cycloheximide were purchased from Sigma.
Western Blot Analysis—Approximately 1 × 106 VSMCs were plated on 100-mm cell culture dishes and incubated overnight. Cells were treated with human recombinant TGFβ (10 ng/ml; PeproTech, Rocky Hill, NJ) for various times and lysed in extraction buffer (25 mm Tris, pH 7.4, 150 mm NaCl, 0.5% sodium deoxycholate, 2% Nonidet P-40, and 0.2% SDS) containing protease inhibitors (Complete™; Roche Applied Science). Cell extracts were cleared by centrifugation and subjected to SDS-PAGE using Novex gels (Invitrogen). Protein concentrations were determined by BCA protein assay (Pierce), and 20 μg of protein were loaded per lane. Separated proteins were then transferred to Protran nitrocellulose membranes (Whatman Schleicher & Schuell), followed by immunoblotting (26). Membranes were incubated with CRP2-(91–98) antiserum (16) and monoclonal α-tubulin antibody (Sigma) to verify equivalent loading. To assess phosphorylation of ATF2 and CREB, cells were plated, incubated overnight, serum-starved (0.5% fetal bovine serum in Dulbecco's modified Eagle's medium) for 24 h, and then stimulated with TGFβ. Protein extracts were prepared in extraction buffer containing protease inhibitor Complete™ and phosphatase inhibitor mixture 1 and 2 (Sigma) at the indicated time points. Membranes were incubated with antibodies for phospho-ATF2 (Thr71) (phosphorylated Thr71 of human ATF2; Cell Signaling Technology, Danvers, MA) that recognizes both Thr51/Thr53 dually phosphorylated and Thr51 singly phosphorylated mouse ATF2, total ATF2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), phospho-CREB that recognizes phosphorylated Ser133 of CREB (Upstate Cell Signaling Solutions, Charlottesville, VA), or total CREB (Santa Cruz Biotechnology).
Northern Blot Analysis and Real Time Quantitative Polymerase Chain Reaction—Total RNA was isolated from VSMCs using the RNeasy Mini RNA isolation kit (Qiagen, Valencia, CA), and Northern blot analysis was performed as described (26). The filters were hybridized with a random primed 32P-labeled mouse CRP2 cDNA open reading frame probe (15). To correct for loading differences, the filters were hybridized to a 32P-labeled oligonucleotide complementary to 18 S ribosomal RNA. The signal intensity was measured on a PhosphorImager using ImageQuant software (GE Healthcare). In cyclohexamide experiments, 1 μg of RNA was reverse transcribed to cDNA using the SuperScript III first strand synthesis system (Invitrogen). Real time quantitative PCR was performed with the 7500 real time PCR system (ABI) using 2× SYBR Green PCR master mix (ABI) and 1% of the starting 1 μg of RNA with the following primers for CRP2: forward, 5′-CTGACTGAGAAAGAAGGCGAAATC-3′; reverse, 5′-TGCTGGCTGTTTCACAGTAGTGA-3′. β-Actin was used as the internal control with the following primers: forward, 5′-GAGAGGTATCCTGACCCTGAAG-3′; reverse, 5′-TGATCTGGGTCATCTTTTCACGG-3′. Quantification was performed by the comparative CT method.
Migration Assays—To assess migration, wild type and Csrp2 null (Csrp2–/–) VSMCs (16) were serum-starved (0.5% fetal bovine serum in Dulbecco's modified Eagle's medium) for 24 h and treated with or without TGFβ (10 ng/ml) for 12 h, and then migration assays were performed as described (16). Cells were placed in the upper chamber of 6-well transwell plates (8-μm pore size; Costar) in triplicate (300,000 cells/well). The bottom chambers were filled with 0.5% fetal bovine serum medium containing PDGF-BB (10 ng/ml) (Peprotech) as a chemoattractant. Cells migrating through the filters after 2 h were quantified and normalized to the cell number of wild type without TGFβ treatment.
Luciferase Reporter and Expression Plasmid Constructs—The mouse Csrp2 luciferase reporter plasmid –795Csrp2-luc was described previously (27). To generate a series of 5′-deletion constructs, Csrp2 promoter deletion fragments were generated by PCR with the use of Pfu polymerase (Stratagene, La Jolla, CA) and cloned into the luciferase reporter pGL2-Basic (Promega, Madison, WI). These constructs share a common 3′-end at bp +40 but differ at the 5′-end at bp –573, –549, –480, and –438. With the –795Csrp2-luc construct as a template, site-directed mutagenesis was performed using Pfu polymerase to create an internal deletion of bp –480 to –438 to generate the –795Δ(480/438)Csrp2-luc construct. Site-directed mutagenesis was performed to mutate the CRE site from –461TAACGTCA to CACCGTAA (mutated bases are underlined) to generate the –795CREmutCsrp2-luc construct. All constructs were confirmed by DNA sequencing. The mouse ATF2 expression plasmid pCMV·SPORT6-ATF2 was purchased from Invitrogen. The open reading frame of ATF2 was amplified with Pfu polymerase using pCMV·SPORT6-ATF2 as a template and cloned into pFLAG-CMV5 vector (Sigma) to generate pFLAG-CMV5-ATF2. Site-directed mutagenesis was then performed using Pfu polymerase to mutate the three potential phosphorylation sites (Thr51, Thr53, and Ser72) to alanines (T51A, T53A, and S72A) (28) to generate phosphorylation-negative mutant pFLAG-CMV5-ATF2-AAA, which also functions as a dominant negative mutant (28).
Transient Transfection Assays—Approximately 1.6 × 105 VSMCs were plated onto each well of 6-well plates and allowed to attach overnight. Cells were then transfected using FuGENE 6 reagent (Roche Applied Science) (21). To correct for differences in transfection efficiency, 500 ng each of the luciferase plasmids and pCMVβ (Clontech) were cotransfected into cells. Two hours following transfection, cells were treated with or without TGFβ (10 ng/ml). Luciferase and β-galactosidase activities were measured 24 h later; luciferase activity was normalized to β-galactosidase activity. For cotransfection experiments, –795Csrp2-luc (0.33 μg/well) and expression plasmid (0.67 μg/well) pCMV-CREB (Clontech), pCMV·SPORT6-ATF2, or empty vector were cotransfected into VSMCs. Similarly, for phosphorylation-negative ATF2 experiments, –795Csrp2-luc and pFLAG-CMV5-ATF2-AAA or empty vector pFLAG-CMV5 were cotransfected into VSMCs. For dominant negative Smad3 experiments, –795Csrp2-luc or 3TP-Lux (29) was cotransfected with pRK5-Smad3ΔC, which lacks C-terminal phosphorylation and activation sites (30) or empty pRK5 vector into VSMCs. Two hours following transfection, the indicated wells were treated with or without TGFβ. Because pCMVβ contains a consensus CRE site for potential ATF2 binding (data not shown), we omitted pCMVβ for transfection efficiency control and normalized luciferase activity to total protein content as described by Owens and co-workers (31). Each construct was transfected at least three times, and each transfection was performed in triplicate.
Electrophoretic Mobility Shift Assays—Nuclear extracts were prepared from VSMCs, and DNA-binding assays were performed as described (27). Complementary oligonucleotides from the Csrp2 promoter bp –467 to –448 containing the CRE-like sequence (in boldface type), 5′-TTTTCATAACGTCATTTGTT-3′ or three bases mutated (underlined) in the core sequence 5′-TTTTCACACCGTAATTTGTT-3′, and the CRE consensus sequence 5′-ATTGCCTGACGTCAGAGAGC-3′ (32) were synthesized. Additional mutant oligonucleotides with one or two bases mutated (underlined) in the core sequence (in boldface type) were also synthesized: mut1, 5′-TTTTCACAACGTCATTTGTT-3′ and mut2 5′-TTTTCACAACGCCATTTGTT-3′. Annealed complementary oligonucleotides were end-labeled with [γ-32P]ATP and T4 polynucleotide kinase (New England Biolabs). To determine the specificity of the DNA-protein complexes, competition assays were performed with a 200-fold molar excess of unlabeled double-stranded oligonucleotides. To characterize the specific DNA-binding proteins, nuclear proteins were incubated with antibodies to ATF-2, CREB, or c-Jun (Santa Cruz Biotechnology). To detect phosphorylated proteins in the DNA-protein complexes, nuclear proteins were incubated with antibodies to phospho-ATF2 (Thr71) (Cell Signaling Technology) or phospho-CREB (Ser133) (Upstate Cell Signaling Solutions).
Chromatin Immunoprecipitation Assays—Mouse VSMCs were cultured in 150-mm dishes and fixed with formaldehyde as described in the instructions of the EZ ChIP chromatin immunoprecipitation kit (Upstate Cell Signaling Solutions). The fixed cells were harvested and prepared for chromatin immunoprecipitation according to the manufacturer's instructions. Following DNA fragmentation by sonication, aliquots of the samples equivalent to 2 × 106 cells were used in each reaction and subsequently incubated with 5 μg of CREB antibody (Santa Cruz Biotechnology), phospho-CREB (Ser133) antibody (Upstate Cell Signaling Solutions), or normal rabbit IgG (Upstate Cell Signaling Solutions). Quantitative PCR (as described under “Northern Blot Analysis and Real Time Quantitative Polymerase Chain Reaction”) was subsequently performed using immunoprecipitated DNA. Aliquots of samples equivalent to 1% of initial cell lysate for each reaction were processed, and DNA was purified to use as input DNA control. The primers used to amplify a 165-bp fragment of the mouse Csrp2-CRE were 5′-GTGGGTTTCTTCCCCCCTCAG-3′ (forward) and 5′-CAGGATAGTCTCTGGTCAGAATC-3′ (reverse), and the primers used to amplify a 92-bp fragment of the mouse cyclin D1-CRE were 5′-CTGCCCGGCTTTGATCTCT-3′ (forward) and 5′-CTCTGGAGGCTGCAGGAC-3′ (reverse). As an additional negative control, a 164-bp fragment within intron 1 of the Csrp2 gene was also amplified by quantitative PCR with the following primers: forward, 5′-TTCCCTTACTGCGGTGTCTCTC-3′; reverse, 5′-ACACATATCCTGGGGGCTGAAG-3′.
RESULTS
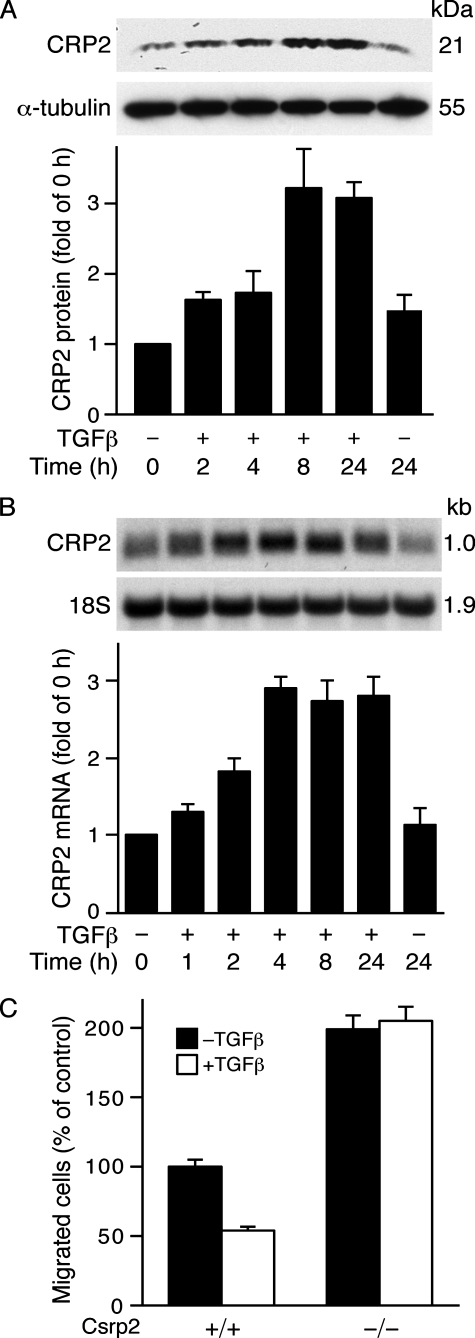
TGFβ Induces CRP2 Expression in Vascular Smooth Muscle Cells—TGFβ, a key factor present in the vessel wall in the early phases of arterial response to injury, plays important roles in modulating lesion formation (33). Because CRP2 also has a significant role in modulating this response, we examined the possibility that TGFβ might regulate CRP2 expression. We treated VSMCs with TGFβ and evaluated CRP2 protein levels by Western blot analysis. The physiological concentrations of TGFβ have been reported to be ∼2–3 ng/ml in plasma and cells (34, 35). However, a higher concentration of 10 ng/ml has been routinely used in cultured cells (34, 36, 37). To mimic the likely higher concentrations of cytokines released at the injured arterial microenvironment we used 10 ng/ml TGFβ for our studies. TGFβ induced CRP2 protein expression as early as 2 h, and the induction continued at the 4 h time point (Fig. 1A). A 3-fold induction was observed at 8 h, and this level of induction was maintained at 24 h when compared with untreated controls (Fig. 1A). Similar to the protein results, Northern blot analysis revealed that TGFβ induced CRP2 mRNA expression in a time-dependent manner (Fig. 1B). A maximal 3-fold induction was observed at 4 h, which preceded maximal protein induction at 8 h (Fig. 1A), and was maintained at 24 h when compared with untreated controls (Fig. 1B). To determine the significance of CRP2 induction, we treated wild type and Csrp2–/– VSMC with or without TGFβ for 12 h to obtain maximal induction of CRP2 in wild type cells and then performed migration assays. Interestingly, compared with untreated controls, TGFβ treatment reduced wild type VSMC migration by ∼50%, whereas Csrp2–/– VSMC migration was not affected (Fig. 1C), indicating that up-regulation of CRP2 correlated with a blunted VSMC motility.
FIGURE 1.
TGFβ increases CRP2 expression in VSMCs. A, TGFβ induces CRP2 protein expression. VSMCs were exposed to TGFβ (10 ng/ml), and protein extracts were harvested at the indicated time points. Total protein was also extracted from unstimulated control cells. Western blot analyses were performed using 20 μg of total protein/lane. After electrophoresis, proteins were transferred to nitrocellulose membranes and incubated with a polyclonal primary antiserum specific for CRP2-(91–98) and a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody. The blot was visualized with enhanced chemiluminescence and exposed to film. Blots were subsequently probed for α-tubulin for normalization. CRP2 protein induction is expressed relative to control without TGFβ stimulation at 0 h. Values are mean ± S.E. of three experiments. B, up-regulation of CRP2 mRNA by TGFβ. VSMCs were treated with TGFβ (10 ng/ml) for the indicated times. Northern blot analysis was performed with 10 μg of total RNA/lane. After electrophoresis, RNA was transferred to nitrocellulose filters and hybridized with a random primed 32P-labeled mouse CRP2 cDNA probe that hybridized to a 1.0-kb CRP2 message. The blots were subsequently hybridized with a 32P-labeled 18 S oligonucleotide to verify loading. The signal intensity of each RNA sample hybridized to the CRP2 probe was divided by that hybridized to the 18 S probe. The normalized intensities were expressed relative to control without TGFβ treatment at 0 h. Values are mean ± S.E. of 3–5 experiments. C, TGFβ treatment reduces wild type but not Csrp2–/– VSMC migration in response to PDGF-BB. Wild type (+/+) and Csrp2–/– (–/–) VSMCs were serum-starved for 24 h, treated without or with TGFβ (10 ng/ml) for 12 h and then plated in triplicate in 6-well transwell plates for migration assays using PDGF-BB (10 ng/ml) as a chemoattractant. Cells migrating through the filters after 2 h were quantified and normalized to the cell number of wild type without TGFβ treatment. Values are mean ± S.D. of two experiments.
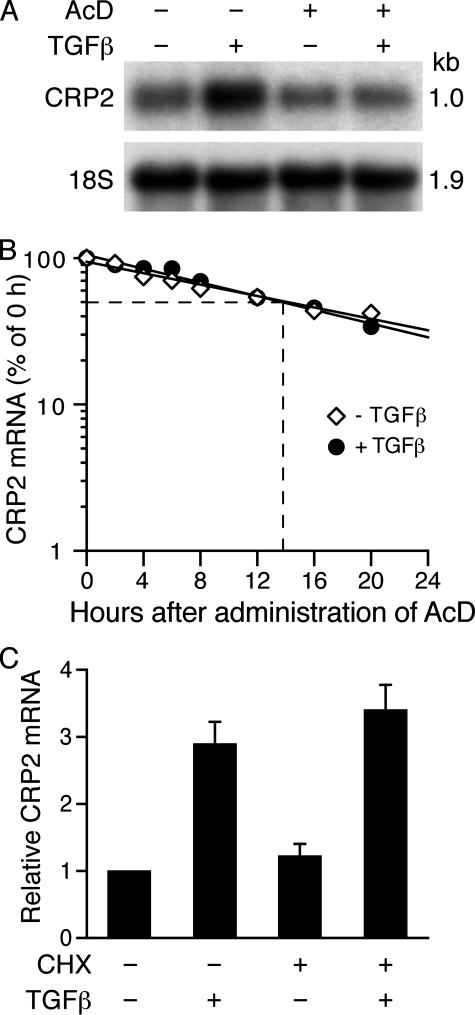
TGFβ Does Not Alter the Half-life of CRP2 mRNA—To begin to elucidate the mechanisms underlying CRP2 mRNA induction, we performed experiments with the transcriptional inhibitor actinomycin D. VSMCs were preincubated with vehicle or actinomycin D for 30 min and then treated for 4 h with or without TGFβ. Northern analysis revealed that in the absence of actinomycin D, TGFβ substantially induced CRP2 mRNA expression, whereas actinomycin D blocked induction of CRP2 mRNA (Fig. 2A). Given that TGFβ can regulate mRNA levels of many target genes by altering mRNA stability and half-life (38–40), we measured the half-life of CRP2 mRNA in VSMCs stimulated with or without TGFβ. In the absence of TGFβ, the half-life of CRP2 mRNA was ∼14 h (Fig. 2B). The half-life of CRP2 mRNA in TGFβ-treated cells was also ∼14 h, indicating that TGFβ has no effect on CRP2 mRNA half-life (Fig. 2B).
FIGURE 2.
TGFβ induction of CRP2 mRNA does not alter CRP2 mRNA half-life or require new protein synthesis. A, TGFβ does not alter CRP2 mRNA half-life. VSMCs were pretreated with vehicle (95% ethanol) or transcriptional inhibitor actinomycin D (AcD) (10 μg/ml) for 30 min and then stimulated without or with TGFβ (10 ng/ml) for 4 h and analyzed as in Fig. 1B. TGFβ induced CRP2 mRNA expression in the absence of AcD. In comparison, AcD blocked the CRP2 mRNA induction by TGFβ. B, TGFβ does not alter CRP2 mRNA half-life. VSMCs were stimulated with or without TGFβ (10 ng/ml) for 4 h, and then AcD (10 μg/ml) was administered to the cells. Total RNA was extracted at the indicated times after administration of AcD, and Northern blot analyses were performed as in Fig. 1B. The normalized intensity was then plotted as a percentage of the 0 h value (in log scale) against time. C, CRP2 induction by TGFβ does not require new protein synthesis. VSMCs were pretreated with vehicle (DMSO) or protein synthesis inhibitor cyclohexamide (CHX) (10 μg/ml) for 30 min and then stimulated without or with TGFβ (10 ng/ml) for 4 h. RNA was harvested, reverse transcribed, and analyzed by real time quantitative PCR assays. Cyclohexamide did not prevent the induction of CRP2 mRNA by TGFβ. Values are mean ± S.E. of three experiments.
CRP2 Induction by TGFβ Does Not Require New Protein Synthesis—To further examine whether CRP2 mRNA induction by TGFβ required protein synthesis, we preincubated VSMCs with protein synthesis inhibitor cyclohexamide for 30 min and then treated for 4 h with or without TGFβ. Real time quantitative PCR analysis showed that cyclohexamide did not prevent CRP2 mRNA induction by TGFβ (Fig. 2C), demonstrating that transcriptional activation of Csrp2 by TGFβ does not require new protein synthesis.
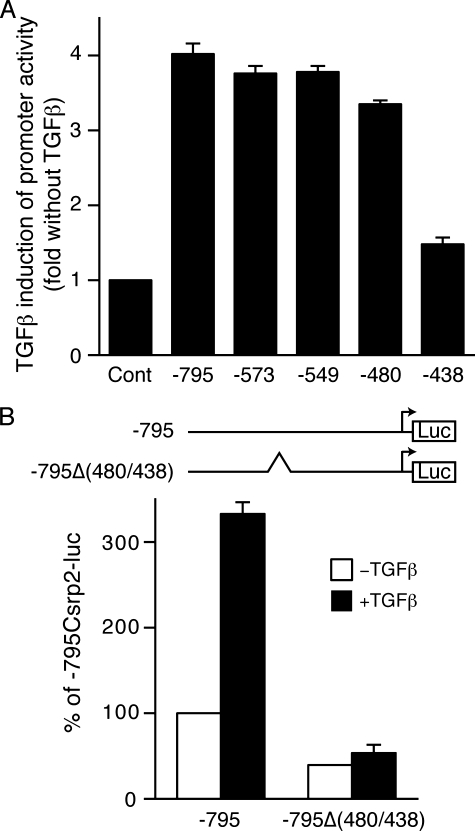
The Csrp2 Promoter Region bp –480 to –438 Is Important for TGFβ Induction—To determine whether elements responsible for TGFβ induction were present in the Csrp2 promoter, we transiently transfected VSMCs with luciferase plasmid –795Csrp2-luc containing –795 bp of the Csrp2 promoter. We demonstrated previously that this region is sufficient to drive lacZ reporter gene expression in VSMCs of blood vessels in transgenic mice (21). TGFβ increased –795Csrp2 promoter activity by ∼4-fold (Fig. 3A), indicating that the –795 proximal promoter contained TGFβ-responsive elements for the induction of CRP2. To identify the elements, a series of Csrp2 5′-deletion promoter-luciferase constructs were generated and transiently transfected into VSMCs. Similar to the –795 construct, the –573 construct showed a 3.8-fold induction by TGFβ (Fig. 3A). Deletion of the 5′-sequences to bp –549 retained TGFβ inducibility (Fig. 3A). Additional deletion to bp –480 only slightly diminished the responsiveness to TGFβ. Significantly, further deletion to bp –438 abolished the promoter induction by TGFβ (Fig. 3A), indicating that the region between bp –480 and –438 was required for the TGFβ stimulation of Csrp2 promoter activity. To determine the functional importance of this region in the Csrp2 promoter, we generated a –795Δ(480/438)Csrp2-luc construct that had a 43-bp internal deletion from bp –480 to –438 within the context of –795Csrp2-luc construct. Transient transfection experiments revealed that deletion of this region reduced basal promoter activity by >50% (Fig. 3B, white bars). Furthermore, internal deletion of this region also abolished the TGFβ induction (Fig. 3B, filled bars), consistent with the 5′-deletion studies (Fig. 3A).
FIGURE 3.
The Csrp2 promoter region bp –480 to –438 is important for TGFβ induction. VSMCs were transiently transfected with Csrp2 promoter-luciferase reporter plasmids (500 ng/well) in triplicate using FuGENE 6. All wells received 500 ng of pCMVβ to normalize for transfection efficiency. Two hours after transfection, cells were treated with or without TGFβ (10 ng/ml) for 24 h and harvested for luciferase and β-galactosidase activity assays. A, 5′ deletion constructs of Csrp2 promoter-luciferase reporter plasmids. TGFβ induction is expressed relative to control without TGFβ of each construct. Values are mean ± S.E. of three experiments. B, the –795 Csrp2 wild type –795 and mutant –795Δ(480/438) (an internal deletion of bp –480 to –438) promoter constructs are schematically depicted in the top panel. Luciferase activity is expressed relative to –795 without TGFβ treatment. Values are mean ± S.E. of three experiments.
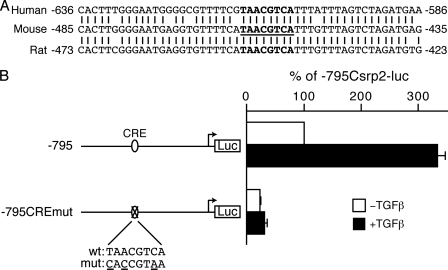
The CRE-like Element Is Critical for Basal and Inducible Activity of the Csrp2 Promoter—Comparison of the sequences from the mouse promoter bp –485 to –435 with human and rat promoters (GenBank™ accession numbers U95017 and NW04774, respectively) revealed that this region is highly conserved across species (Fig. 4A). Through transcription factor data bases (BCM Search Launcher; TRANSFAC) searches and closer examination of the sequences, we identified a putative CRE-like element (TAACGTCA) (Fig. 4A, boldface type). To test the potential function of this conserved CRE-like site, which is one base divergent from the consensus CRE site (TGACGTCA), we generated a luciferase construct, –795CREmutCsrp2-luc, with three bases mutated (underlined) within the putative CRE site (CACCGTAA). Compared with the control, the –795CREmutCsrp2-luc construct substantially reduced basal promoter activity, similar to that of the internal deletion construct, –795Δ(480/438)Csrp2-luc (Fig. 4B, white bars). Most importantly, mutation of this site abrogated TGFβ-mediated induction of the Csrp2 promoter (Fig. 4B, black bars).
FIGURE 4.
The CRE-like site is functionally important for basal and TGFβ induction of Csrp2 promoter activity. A, conservation of the putative CRE site among species. Sequence alignment of corresponding regions of human and rat promoter sequences to the mouse promoter. A putative CRE site (TAACGTCA) is in boldface type and underlined in the mouse sequence. B, the putative CRE site is important for Csrp2 promoter activity. The –795 Csrp2 wild type (wt) and CRE mutant (mut) promoter constructs are schematically depicted in the left panel. VSMCs were transiently transfected with Csrp2 luciferase reporter constructs (500 ng/well) containing –795 or –795CREmut with putative CRE site mutated and pCMVβ (500 ng/well) to normalize for transfection efficiency in triplicate using FuGENE 6 transfection reagent. Two hours after transfection, cells were treated with or without TGFβ (10 ng/ml) for 24 h. Cells were then harvested for luciferase and β-galactosidase activity assays. Luciferase activity is expressed relative to –795 without TGFβ treatment.
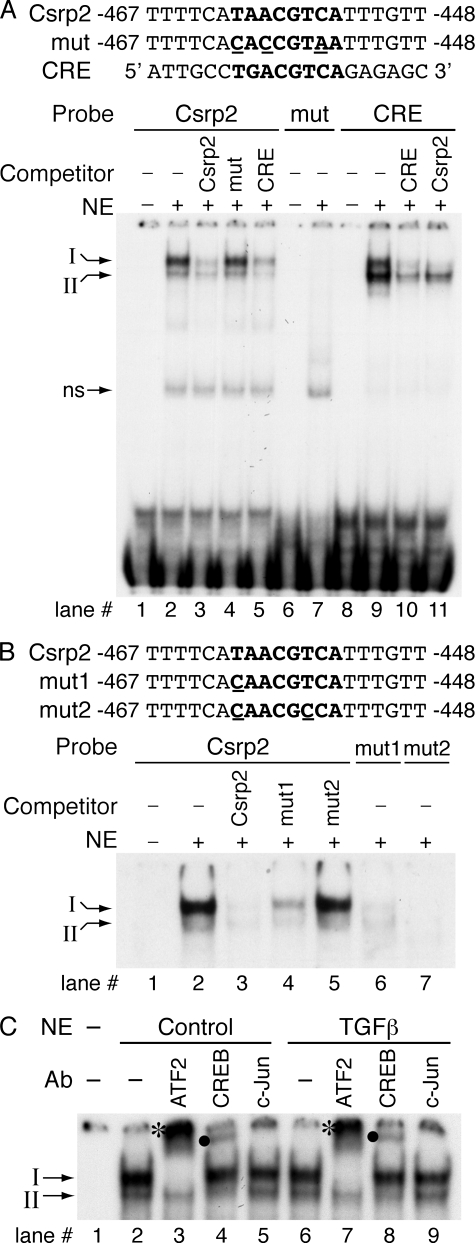
Nuclear Proteins Binding to the CRE-like Site of the Csrp2 Promoter—Given the functional importance of the CRE-like site in regulating Csrp2 promoter activity, we were interested in characterizing the transcription factors that bound to this site. We first tested whether nuclear proteins from control VSMCs (i.e. not treated with TGFβ) bound to the Csrp2-CRE site by gel mobility shift assays. Oligonucleotide sequences used in the gel shift assays are indicated (Fig. 5A). Incubation of VSMC nuclear extracts with an oligonucleotide probe encoding bp –467 to –448 of the Csrp2 promoter resulted in two prominent DNA-protein complexes, a dominant upper complex I and a minor lower complex II (Fig. 5A, lane 2). These two complexes were specific, because they were competed by excess unlabeled identical probe (Fig. 5A, lane 3) but not by an unrelated probe (data not shown). The mutant oligonucleotides (Fig. 5A, mut) with three bases mutated (CACCGTAA) failed to compete away the binding complexes (Fig. 5A, lane 4). Conversely, no specific complex formation was observed when the mutant was used as a probe (Fig. 5A, lane 7), further indicating that nuclear factors bound specifically to this CRE-like site. The consensus CRE oligonucleotides also competed away the complexes (Fig. 5A, lane 5), indicating that this is a bona fide CRE site. When 32P-labeled consensus CRE was used as a probe, the intensity of complex I relative to complex II was reduced compared with the Csrp2 probe (Fig. 5A, lane 9 versus lane 2). The identical unlabeled CRE competitor abolished the complexes, confirming the specificity of the two complexes (Fig. 5A, lane 10). Intriguingly, the Csrp2 competitor primarily abolished binding of complex I and to a lesser degree with complex II (Fig. 5A, lane 11). Taken together, these results suggest that nuclear factors that bound to Csrp2-CRE differ from that bound to consensus CRE. To examine whether less severe mutations are sufficient to disrupt nuclear protein binding, we performed additional gel shift assays using oligonucleotides with one or two bases mutated in the Csrp2-CRE core sequence (Fig. 5B). Consistently, incubation of VSMC nuclear extracts with Csrp2 oligonucleotide probe resulted in complex I and II formation (Fig. 5B, lane 2). Complexes I and II were abolished by the addition of unlabeled identical (Fig. 5B, lane 3) oligonucleotides as competitors. mut1 with one base mutated at the core partially competed away complexes (Fig. 5B, lane 4), whereas mut2 with two bases mutated did not compete away the complexes (Fig. 5B, lane 5). These data suggested that mutation at the first base of the CRE core partially retained the ability to bind to nuclear proteins. In comparison, a two-base mutation rendered the oligonucleotides unable to bind to the cognate nuclear proteins. In agreement with these results, gel shift assays using 32P-labeled mut1 oligonucleotides resulted in minimal complex formation (lane 6), whereas 32P-labeled mut2 oligonucleotides did not result in any specific complex formation (lane 7), demonstrating the importance of Csrp2-CRE core sequences for nuclear protein binding.
FIGURE 5.
Nuclear proteins binding to the CRE-like element of Csrp2 promoter. A, oligonucleotide sequences used in the electrophoretic mobility shift assays (EMSAs) are shown. The core sequence of CRE-like and consensus CRE sites are in boldface type, and mutated sequence is underlined. EMSAs were performed with double-stranded oligonucleotides corresponding to bp –467 to –448 of the Csrp2 promoter. The addition of nuclear extracts (10 μg) from VSMCs to the 32P-labeled Csrp2 probe resulted in two major retarded DNA-protein complexes, designated I and II (arrows on left)(lane 2). A nonspecific band (ns) is indicated. Complexes I and II were abolished by the addition of unlabeled identical (Csrp2, lane 3) or consensus CRE (lane 5) oligonucleotides as competitors but not by the addition of three bases mutated (mut) oligonucleotides (lane 4). Conversely, EMSAs using 32P-labeled mutated oligonucleotides did not result in specific complex formation (lane 7). As a comparison, EMSAs using 32P-labeled CRE oligonucleotides were performed. The addition of nuclear extracts to the CRE probe resulted in the formation of complexes I and II (lane 9), which were competed away by identical unlabeled oligonucleotides (CRE; lane 10). The addition of unlabeled Csrp2 mainly abolished complex I and to a lesser degree complex II (lane 11). B, oligonucleotides used in the EMSAs are indicated. Csrp2 oligonucleotides contain the core sequence of CRE-like site (in boldface type), whereas mut1 has a one-base mutation (underlined) in the core and mut2 has two bases mutated (underlined). As in A, complex I and II were abolished by the addition of unlabeled identical (Csrp2, lane 3) oligonucleotides as competitors. mut1 partially competed away the complexes (lane 4), whereas mut2 did not compete away the complexes (lane 5). EMSAs using 32P-labeled mut1 oligonucleotides resulted in low intensity complex I and II formation (lane 6), whereas 32P-labeled mut2 oligonucleotides did not result in specific complex formation (lane 7). C, nuclear extracts from control (lanes 2–5) or TGFβ treated for 15 min (lanes 6–9) VSMCs were incubated with 32P-labeled Csrp2 probe without the addition of antibodies (lanes 2 and 6) or antibodies specific to ATF2 (lanes 3 and 7), CREB (lanes 4 and 8), or c-Jun (lanes 5 and 9). ATF2 antibody completely supershifted complex I to an upper band (*, lanes 3 and 7), whereas CREB antibody supershifted complex II to an upper complex (•, lanes 4 and 8). Incubation with c-Jun antibody did not produce supershifted bands (lanes 5 and 9).
To identify the composition of these complexes, we screened several known CRE-binding proteins for their ability to supershift the Csrp2-CRE-protein complex. Antibodies specific for ATF2, CREB, or c-Jun were added to the binding reactions and incubated with VSMC nuclear extracts (Fig. 5C). ATF2 antibodies completely supershifted complex I, as indicated by the disappearance of complex I, and the appearance of a larger complex near the top of the gel (Fig. 5C, lane 3, asterisk). The lower complex II was not supershifted by ATF2 antibodies. These data suggested that ATF2 was the main factor present in complex I. CREB antibodies supershifted complex II to a larger complex (Fig. 5C, lane 4, dot) but not complex I, indicating the complex II contained CREB. In comparison, c-Jun was not present in either complex, since c-Jun antibodies did not supershift complex I or II (Fig. 5C, lane 5), although this antibody has been previously shown to supershift c-Jun complexes (41).
We next determined whether TGFβ affected levels of ATF2/CREB binding to the CRE site. Since CRP2 mRNA induction by TGFβ was evident at 1 h (Fig. 1B), and DNA-protein complex formation would precede mRNA induction, we used nuclear extracts from VSMCs treated with TGFβ for 15 and 30 min. Interestingly, gel mobility shift assays using nuclear extracts from VSMCs treated with TGFβ for 15 min revealed similar intensity of complexes I and II (Fig. 5C, lanes 6–9) with control nuclear extracts (Fig. 5C, lanes 2–5). Using nuclear extracts from VSMCs treated with TGFβ for 30 min also showed similar DNA-protein complex patterns as controls (data not shown).
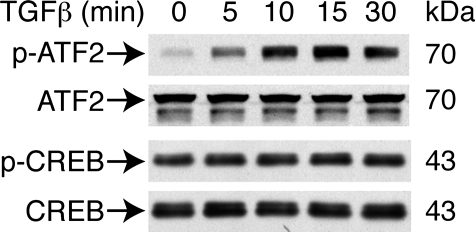
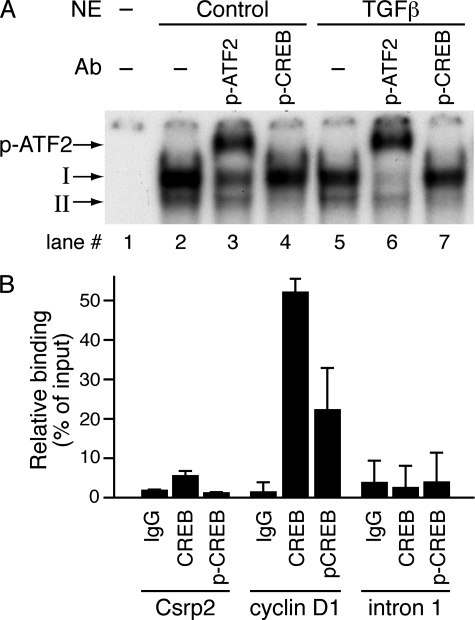
TGFβ Increases Phosphorylation Levels of ATF-2 But Not CREB—CREB/ATF2 can bind to CRE in an unphosphorylated and transcriptionally inactive form (42–44). Phosphorylation of ATF2 or CREB within the activation domain leads to their activation of gene expression (45, 46). Thus, we hypothesized that TGFβ might increase the phosphorylation of ATF2 and CREB in VSMCs. To investigate this possibility, VSMCs were treated with TGFβ and protein extracts harvested 5–30 min after treatment. Western blot analysis revealed that TGFβ increased ATF2 phosphorylation as early as 5 min after stimulation but did not alter total ATF2 levels (Fig. 6). In contrast, TGFβ did not affect either phosphorylated CREB levels, which were present at baseline, or total amounts of CREB (Fig. 6). To test the hypothesis that ATF2 and its phosphorylation levels might be responsible for TGFβ induction of CRP2, we performed supershift assays using antibodies specific for phospho-ATF2 or phospho-CREB. Phospho-ATF2 antibodies partially supershifted complex I (Fig. 7A, lane 3) when control nuclear extracts were used. When nuclear extracts from VSMCs treated with TGFβ were used in the reactions, most of complex I was supershifted (Fig. 7A, lane 6). TGFβ increased the ratio of phospho-ATF2 to unphosphorylated ATF2 within complex I from 1.35 ± 0.06 of controls to 3.09 ± 0.29 (n = 3), indicating that TGFβ enhanced phosphorylation of ATF2 within the DNA-protein complex. Phospho-CREB antibodies appeared to disrupt complex II from both the control and TGFβ-treated nuclear extracts (Fig. 7A, lanes 4 and 7); however, the results were not conclusive. To investigate further whether CREB and p-CREB bound to Csrp2-CRE in the intact native chromatin, we performed quantitative ChIP assays. The CRE of the cyclin D1 promoter with the core sequence identical to that of Csrp2-CRE has been shown mainly to bind CREB (46); thus, mouse cyclin D1-CRE was used as a positive control. CREB binding to the cyclin D1 promoter was substantially higher than to the Csrp2 promoter (52 and 6%, respectively; Fig. 7B), indicating much lower levels of CREB bound to Csrp2-CRE than cyclin D1-CRE. ChIP assays using p-CREB antibodies revealed that, compared with 22% of p-CREB bound to cyclin D1-CRE, p-CREB binding to Csrp2 was barely detectable (Fig. 7B). Taken together, the results of gel shifts and ChIP assays suggested that low levels of CREB bound to Csrp2-CRE; however, the bound CREB were probably not phosphorylated.
FIGURE 6.
TGFβ increases the phosphorylation of ATF2 but not CREB. VSMCs were stimulated with TGFβ (10 ng/ml) and activation of ATF2 and CREB was determined using cell lysates harvested at the indicated time points by Western blot analysis. Phosphorylation of ATF2 and CREB was detected by using phospho-ATF2 (p-ATF2) and p-CREB antibodies, respectively. To verify equal loading, the blots were probed with total ATF2 and CREB antibodies. A representative of three independent experiments is shown.
FIGURE 7.
TGFβ enhances phosphorylation of ATF2 within the DNA-protein complex. A, EMSAs were performed with double-stranded oligonucleotides corresponding to bp –467 to –448 of the Csrp2 promoter. The addition of nuclear extracts (NE; 10 μg) from control or TGFβ-treated for 15 min VSMCs to the 32P-labeled Csrp2 probe resulted in retarded DNA-protein complex I and II (lanes 2 and 5). To detect phosphorylation of ATF2 and CREB within the complexes, nuclear extracts were incubated with antibodies (Ab) specific to phospho-ATF2 or phospho-CREB before the reactions. Phospho-ATF2 (p-ATF2) antibody supershifted complex I to a larger complex (p-ATF2→, lanes 3 and 6), whereas phospho-CREB antibody disrupted complex II (lanes 4 and 7). B, quantitative ChIP assays. VSMCs were cross-linked with formaldehyde and DNA fragmented by sonication. Chromatin was immunoprecipitated with normal rabbit IgG, CREB, or p-CREB antibodies. Aliquots of samples equivalent to 1% of initial cell lysate for each reaction were processed, and DNA was purified to use as input DNA control. Quantitative PCR was performed to amplify a 165-bp fragment flanking the mouse Csrp2-CRE (Csrp2) and a 92-bp fragment flanking the mouse cylin D1-CRE (cyclin D1). As an additional negative control, a 164-bp fragment within intron 1 of the Csrp2 gene (intron 1) was also amplified by quantitative PCR. Binding activity is expressed as percentage relative to input DNA. Values are mean ± S.D. of two or three experiments.
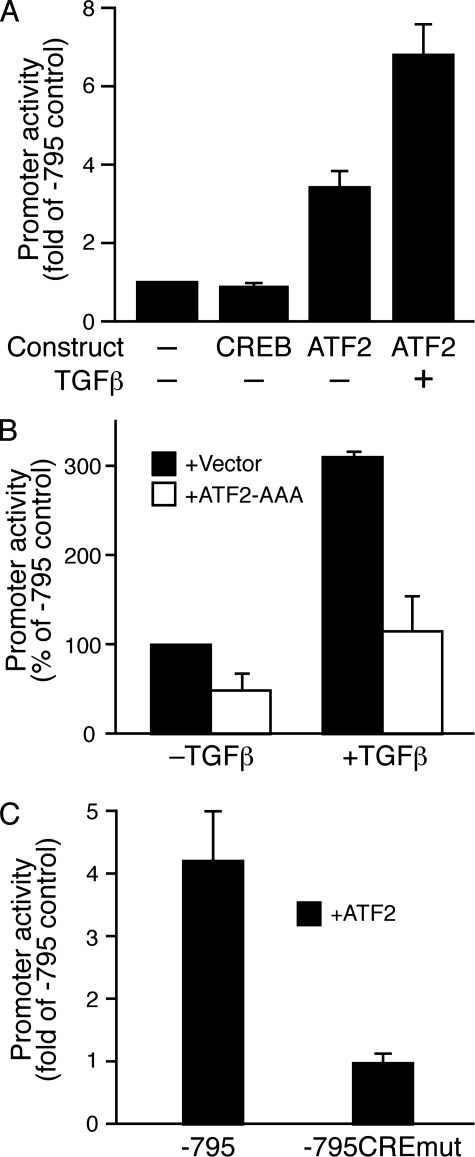
Transcriptional Activation of the Csrp2 Promoter by ATF2 via the CRE Site—To evaluate the functional role of CREB and ATF2 in the induction of CRP2, we cotransfected –795Csrp2-luc construct with expression plasmids in transient transfection assays. CREB did not increase Csrp2 promoter activity (Fig. 8A). In contrast, ATF2 expression increased Csrp2 promoter activity by ≥3-fold (Fig. 8A), and TGFβ further enhanced the transcriptional activation by ATF2 7-fold (Fig. 8A). To further confirm the function of ATF2 and the importance of its phosphorylation, we cotransfected –795Csrp2-luc with a phosphorylation-negative ATF2 mutant (ATF2-AAA), in which the three important phosphorylation residues Thr51, Thr53, and Ser72 were mutated to alanines; thus, it also functions as a dominant negative construct (28). In the absence of TGFβ, ATF2-AAA decreased Csrp2 promoter activity by 50% (Fig. 8B), suggesting that endogenous ATF2 phosphorylation contributed to Csrp2 promoter activity. TGFβ consistently increased promoter activity by ≥3-fold; interestingly, ATF2AAA reduced the TGFβ induction to baseline (Fig. 8B), demonstrating the critical importance of ATF2 phosphorylation in the transcriptional regulation of Csrp2 by TGFβ. We then tested whether ATF2 activated the Csrp2 promoter through the identified CRE site. Transfection experiments revealed that mutation of the CRE sequences reduced ATF2 transactivation of Csrp2 promoter by 77% (Fig. 8C). Taken together, our data demonstrate that ATF2 regulated Csrp2 gene expression via a novel CRE element and that TGFβ signaling via ATF2 induced CRP2 expression in VSMCs.
FIGURE 8.
ATF2 up-regulates Csrp2 promoter activity via the CRE site in VSMCs. A, VSMCs were transiently co-transfected with luciferase reporter plasmid –795Csrp2-luc and expression plasmid pCMV-CREB or pCMV· SPORT6-ATF2 in triplicate using FuGENE 6 transfection reagent. Empty pCMV vector was added to keep constant the total content of DNA. Two hours following transfection, cells were treated with or without TGFβ (10 ng/ml) for 24 h. Cells were then harvested for luciferase activity and total protein assays. Csrp2 promoter activity was plotted as -fold induction compared with activity of –795 with empty vector. Values are mean ± S.E. of three experiments. B, the reporter plasmid –795Csrp2-luc and phosphorylation-negative ATF2 mutant ATF2-AAA or the empty vector pFLAG-CMV5 were cotransfected into VSMCs and similarly treated as in A. Csrp2 promoter activity was plotted as percentage activity of –795 cotransfected with vector but without TGFβ. Values are mean ± S.D. C, VSMCs were transiently co-transfected with Csrp2 luciferase reporter plasmid –795 or –795CREmut and expression plasmid pCMV·SPORT6-ATF2 or empty vector in triplicate using FuGENE 6 transfection reagent. Cells were harvested 24 h later for luciferase activity and total protein assays. Csrp2 promoter activity was plotted as -fold induction compared with activity of –795 without ATF2. Values are mean ± S.E.
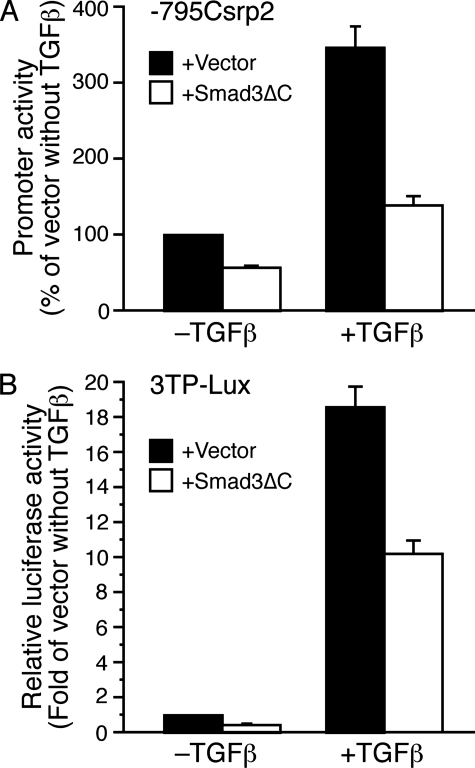
Smad3 Contributes to the Transcriptional Regulation of Csrp2 by TGFβ—Given the role of Smad3 in mediating numerous TGFβ signaling pathways (30, 47, 48), we examined the significance of Smad3 in the regulation of Csrp2 by TGFβ. We cotransfected –795Csrp2-luc with Smad3ΔC, a dominant negative Smad3 expression plasmid that lacks C-terminal phosphorylation and activation sites (30). In transient transfection assays, Smad3ΔC decreased Csrp2 promoter activity by 43% in the absence of TGFβ (Fig. 9A). Furthermore, Smad3ΔC reduced TGFβ induction from 350 to 139% (Fig. 9A). As a positive control, we used TGFβ/Smad3-inducible 3TP-Lux reporter construct, which contains three copies of the TRE and a TGFβ-responsive PAI-1 promoter (29) in transfections. Smad3ΔC reduced 3TP-Lux activity to a similar degree as the Csrp2 promoter in the absence or presence of TGFβ (Fig. 9B). Together, these data indicate that Smad3 also contributed to the transcriptional regulation of Csrp2 by TGFβ.
FIGURE 9.
Dominant negative Smad3 decreases Csrp2 promoter activity in the absence and presence of TGFβ. A, VSMCs were transiently cotransfected with luciferase reporter plasmid –795Csrp2-luc and empty vector pRK5 or expression plasmid pRK5-Smad3ΔC. Two hours following transfection, cells were treated with or without TGFβ (10 ng/ml) for 24 h. Cells were then harvested for luciferase activity and total protein assays. Csrp2 promoter activity was plotted as percentage of activity of –795 with empty vector without TGFβ. Values are mean ± S.E. of three experiments. B, VSMCs were transiently cotransfected with 3TP-Lux reporter plasmid and empty vector pRK5 or expression plasmid pRK5-Smad3ΔCasin A. 3TP-Lux luciferase activity was plotted as -fold induction compared with activity of vector without TGFβ. Values are mean ± S.E.
DISCUSSION
We recently demonstrated a key role for CRP2 in the development of arteriosclerosis (16). An absence of CRP2 enhances intimal thickening following arterial injury, implicating a protective function of CRP2 against neointima formation. In line with a vascular protective role of CRP2, by microarray analysis Watanabe et al. (49) showed that estrogen, which has been proposed to have atheroprotective effects, up-regulates CRP2/SmLIM in the vascular wall. In the current study, we sought to identify factor(s) that could potentially up-regulate CRP2 expression in the context of vascular injury, which might serve as a protective mechanism against intimal thickening. We demonstrated that TGFβ up-regulated CRP2 protein and mRNA expression. Furthermore, we identified a conserved CRE-like element that was critical for basal and TGFβ-induced activation of the Csrp2 promoter. In addition, TGFβ increased phospho-ATF2 binding to the Csrp2-CRE element. More importantly, TGFβ, via ATF2, transactivated the Csrp2 promoter via this CRE element in VSMCs.
The CRP2 induction by TGFβ in primary cultures of VSMC is consistent with a recent report in A10 cell line (50); however, the transcriptional mechanisms were not investigated in this prior study. TGFβ, a multifunctional cytokine, is a key factor present in the blood vessel wall in the early phases of the arterial response to injury and plays a significant role in modulating lesion formation (33, 51). TGFβ regulates many SMC marker genes and can enhance SMC differentiation (48, 52). Despite numerous studies, the precise role of TGFβ in vascular injury remains controversial (53, 54). Although TGFβ has been suggested to promote lesion formation (33, 51); interestingly, disruption of a TGFβ signaling pathway in Smad3 null mice enhances neointimal hyperplasia in response to injury. This latter study indicates an inhibitory and protective role for TGFβ in vascular lesion formation (53).
By 5′-promoter deletion experiments, we identified a region at bp –480 to –438 important for TGFβ induction of the Csrp2 promoter (Fig. 3). The sequence conservation of this region across mouse, human, and rat promoters further indicated a functional importance (Fig. 4A). Indeed, internal deletion of this region in the context of the larger –795 promoter construct abolished TGFβ induction (Fig. 3B). Surprisingly, it also reduced the basal promoter activity by >50% (Fig. 3B). Mutation of a conserved CRE-like motif (TAACGTCA), which has one base divergent from the consensus CRE motif (TGACGTCA), within this region at bp –461 to –454 of the mouse Csrp2 promoter also abolished TGFβ induction and reduced basal promoter activity (Fig. 4B). These complementary experiments demonstrated an essential role of the CRE motif in conferring Csrp2 basal and TGFβ-inducible promoter activity in VSMCs. Supporting a critical role of CRE in regulating VSMC gene expression, the homeobox transcription factor Hex modulates CRE-dependent transcription of SMemb/non-muscle myosin heavy chain-B gene in VSMCs (55). Additionally, a consensus CRE located in the intronic enhancer of the Csrp1 gene, a member of the CRP family, binds CREB and was important for CRP1 expression in VSMCs (56). Taken together, it appears that CRE elements might regulate several functionally significant genes in VSMCs.
Members of the ATF/CREB family of transcription factors form homodimers or heterodimers with the Fos/Jun family member, leading to transcriptional activation of target genes via CRE elements (TGACGTCA) (42, 57). The heterogeneity in dimer composition contributes to the functional diversification of ATF/CREB complexes (57). In addition, variations within the CRE core sequences affect binding affinities of these complexes (58). Interestingly, our results showed that predominantly ATF2 bound to the Csrp2-CRE site (Fig. 5C). Although ATF2 homodimers and ATF2/c-Jun heterodimers have identical affinities for the CRE (59), ATF2 probably bound to the Csrp2-CRE as a homodimer, because an ATF2-specific antibody completely supershifted complex I, but c-Jun antibody did not (Fig. 5C). This binding pattern differs from the consensus CRE (Fig. 5A) or Csrp1-CRE complexes (56), where CREB was the predominant binding factor. The differential transcription factor binding might be due to one base divergence of the core sequence (from G to A in the second base). However, the CRE of the cyclin D1 promoter with the identical core sequence with Csrp2-CRE has been shown to mainly bind CREB and activate cyclin D1 promoter in rat insulinoma INS-1 cells (46). ChIP assays using VSMCs demonstrated that in contrast to cyclin D1-CRE, much lower levels of CREB bound to Csrp2-CRE (Fig. 7B), suggesting that flanking sequences might confer the specificity for transcription factor binding.
The functional role of ATF2 in activating the Csrp2 promoter was confirmed by transiently overexpressing ATF2 in transfection experiments (Fig. 8A). Although ATF2 can bind to CREs in the unphosphorylated state (60), TGFβ enhanced ATF2 phosphorylation (Fig. 6) and increased phospho-ATF2 levels binding to Csrp2-CRE (Fig. 7A), implicating the importance of ATF2 phosphorylation in mediating TGFβ induction of CRP2 expression. The significance of post-translational modification rather than new protein synthesis in TGFβ-mediated transcriptional activation of Csrp2 was supported by the findings that protein synthesis inhibitor cyclohexamide did not prevent CRP2 mRNA induction by TGFβ (Fig. 2C). A phosphorylation-negative mutant, which also functions as a dominant negative mutant, reduced basal and TGFβ-mediated Csrp2 promoter activity (Fig. 8B), further demonstrating the critical importance of ATF2 phosphorylation in regulating CRP2 expression. In contrast to ATF2, CREB probably played a minimal role in regulating Csrp2 gene expression, given its very low levels of binding (particularly p-CREB) to Csrp2-CRE both in vitro and in vivo and that cotransfection of CREB did not increase Csrp2 promoter activity (Fig. 8A). Interestingly, TGFβ signaling molecule Smad3 also contributed to the transcriptional regulation of Csrp2 (Fig. 9). However, further studies are needed to investigate the underlying mechanisms.
In conclusion, we identified a conserved CRE element (TAACGTCA) in the Csrp2 promoter that was critical for basal promoter activity and responsiveness to TGFβ. Our results show for the first time in VSMC that TGFβ activates ATF2 phosphorylation and Csrp2 gene expression via a CRE promoter element, which may represent a previously unrecognized mechanism of VSMC gene expression. Understanding the transcriptional activation of CRP2 may help to elucidate the molecular mechanisms that control VSMC gene expression in vascular disease.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-057977 (to S.-F. Y.) and HL-078869 (to M. D. L.). This work was also supported by National Health Research Institutes (Taiwan) Grant 97A1-CVPP02-014 (to S.-F. Y.) and National Science Council (Taiwan) Grant 96-2321-B-400-004-MY2 (to S.-F. Y.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: VSMC, vascular smooth muscle cell; CRP, cysteine-rich protein; SMC, smooth muscle cell; TGFβ, transforming growth factor β; CRE, cAMP-responsive element; CREB, cAMP-responsive element-binding protein; p-CREB, phosphorylated CREB; PDGF, platelet-derived growth factor; AcD, actinomycin D; EMSA, electrophoretic mobility shift assay; ChIP, chromatin immunoprecipitation.
References
- 1.Owens, G. K., Kumar, M. S., and Wamhoff, B. R. (2004) Physiol. Rev. 84 767–801 [DOI] [PubMed] [Google Scholar]
- 2.Schmeichel, K. L., and Beckerle, M. C. (1994) Cell 79 211–219 [DOI] [PubMed] [Google Scholar]
- 3.Feuerstein, R., Wang, X., Song, D., Cooke, N. E., and Liebhaber, S. A. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 10655–10659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arber, S., and Caroni, P. (1996) Genes Dev. 10 289–300 [DOI] [PubMed] [Google Scholar]
- 5.Dawid, I. B., Breen, J. J., and Toyama, R. (1998) Trends Genet. 14 156–162 [DOI] [PubMed] [Google Scholar]
- 6.Kadrmas, J. L., and Beckerle, M. C. (2004) Nat. Rev. Mol. Cell. Biol. 5 920–931 [DOI] [PubMed] [Google Scholar]
- 7.Weiskirchen, R., and Gunther, K. (2003) BioEssays 25 152–162 [DOI] [PubMed] [Google Scholar]
- 8.Weiskirchen, R., and Bister, K. (1993) Oncogene 8 2317–2324 [PubMed] [Google Scholar]
- 9.Crawford, A. W., Pino, J. D., and Beckerle, M. C. (1994) J. Cell Biol. 124 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain, M. K., Fujita, K. P., Hsieh, C.-M., Endege, W. O., Sibinga, N. E., Yet, S.-F., Kashiki, S., Lee, W.-S., Perrella, M. A., Haber, E., and Lee, M.-E. (1996) J. Biol. Chem. 271 10194–10199 [DOI] [PubMed] [Google Scholar]
- 11.Arber, S., Halder, G., and Caroni, P. (1994) Cell 79 221–231 [DOI] [PubMed] [Google Scholar]
- 12.Weiskirchen, R., Pino, J. D., Macalma, T., Bister, K., and Beckerle, M. C. (1995) J. Biol. Chem. 270 28946–28954 [DOI] [PubMed] [Google Scholar]
- 13.Louis, H. A., Pino, J. D., Schmeichel, K. L., Pomies, P., and Beckerle, M. C. (1997) J. Biol. Chem. 272 27484–27491 [DOI] [PubMed] [Google Scholar]
- 14.Henderson, J. R., Brown, D., Richardson, J. A., Olson, E. N., and Beckerle, M. C. (2002) J. Histochem. Cytochem. 50 107–111 [DOI] [PubMed] [Google Scholar]
- 15.Yet, S.-F., Folta, S. C., Jain, M. K., Hsieh, C.-M., Maemura, K., Layne, M. D., Zhang, D., Marria, P. B., Yoshizumi, M., Chin, M. T., Perrella, M. A., and Lee, M.-E. (1998) J. Biol. Chem. 273 10530–10537 [DOI] [PubMed] [Google Scholar]
- 16.Wei, J., Gorman, T. E., Liu, X., Ith, B., Tseng, A., Chen, Z., Simon, D. I., Layne, M. D., and Yet, S.-F. (2005) Circ. Res. 97 1323–1331 [DOI] [PubMed] [Google Scholar]
- 17.Arber, S., Hunter, J. J., Ross, J., Jr., Hongo, M., Sansig, G., Borg, J., Perriard, J. C., Chien, K. R., and Caroni, P. (1997) Cell 88 393–403 [DOI] [PubMed] [Google Scholar]
- 18.Pomies, P., Louis, H. A., and Beckerle, M. C. (1997) J. Cell Biol. 139 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmeichel, K. L., and Beckerle, M. C. (1998) Biochem. J. 331 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong, Y., Flick, M. J., Kudla, A. J., and Konieczny, S. F. (1997) Mol. Cell. Biol. 17 4750–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang, D. F., Belaguli, N. S., Iyer, D., Roberts, W. B., Wu, S. P., Dong, X. R., Marx, J. G., Moore, M. S., Beckerle, M. C., Majesky, M. W., and Schwartz, R. J. (2003) Dev. Cell 4 107–118 [DOI] [PubMed] [Google Scholar]
- 22.Clark, K. A., Bland, J. M., and Beckerle, M. C. (2007) J. Cell Sci. 120 2066–2077 [DOI] [PubMed] [Google Scholar]
- 23.Ross, R. (1993) Nature 362 801–809 [DOI] [PubMed] [Google Scholar]
- 24.Tedgui, A., and Mallat, Z. (2006) Physiol. Rev. 86 515–581 [DOI] [PubMed] [Google Scholar]
- 25.Günther, S., Alexander, R. W., Atkinson, W. J., and Gimbrone, M. A. (1982) J. Cell Biol. 92 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yet, S.-F., Perrella, M. A., Layne, M. D., Hsieh, C.-M., Maemura, K., Kobzik, L., Wiesel, P., Christou, H., Kourembanas, S., and Lee, M.-E. (1999) J. Clin. Invest. 103 R23–R29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang, Y.-F., Wei, J., Liu, X., Chen, Y.-H., Layne, M. D., and Yet, S.-F. (2003) Am. J. Physiol. 285 H1675–1683 [DOI] [PubMed] [Google Scholar]
- 28.Gupta, S., Campbell, D., Derijard, B., and Davis, R. J. (1995) Science 267 389–393 [DOI] [PubMed] [Google Scholar]
- 29.Wrana, J. L., Attisano, L., Carcamo, J., Zentella, A., Doody, J., Laiho, M., Wang, X. F., and Massague, J. (1992) Cell 71 1003–1014 [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Y., Feng, X., We, R., and Derynck, R. (1996) Nature 383 168–172 [DOI] [PubMed] [Google Scholar]
- 31.Yoshida, T., Sinha, S., Dandre, F., Wamhoff, B. R., Hoofnagle, M. H., Kremer, B. E., Wang, D. Z., Olson, E. N., and Owens, G. K. (2003) Circ. Res. 92 856–864 [DOI] [PubMed] [Google Scholar]
- 32.Ishida, A., Fujita, N., Kitazawa, R., and Tsuruo, T. (2002) J. Biol. Chem. 277 26217–26224 [DOI] [PubMed] [Google Scholar]
- 33.Majesky, M. W., Lindner, V., Twardzik, D. R., Schwartz, S. M., and Reidy, M. A. (1991) J. Clin. Invest. 88 904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng, J., Thongngarm, T., Nakajima, M., Yamashita, N., Ohta, K., Bates, C. A., Grunwald, G. K., and Rosenwasser, L. J. (2005) Int. Arch. Allergy Immunol. 138 151–160 [DOI] [PubMed] [Google Scholar]
- 35.Fogel-Petrovic, M., Long, J. A., Misso, N. L., Foster, P. S., Bhoola, K. D., and Thompson, P. J. (2007) Int. Immunopharmacol. 7 1924–1933 [DOI] [PubMed] [Google Scholar]
- 36.Feinberg, M. W., Jain, M. K., Werner, F., Sibinga, N. E., Wiesel, P., Wang, H., Topper, J. N., Perrella, M. A., and Lee, M. E. (2000) J. Biol. Chem. 275 25766–25773 [DOI] [PubMed] [Google Scholar]
- 37.Liu, G., Ding, W., Neiman, J., and Mulder, K. M. (2006) J. Biol. Chem. 281 29479–29490 [DOI] [PubMed] [Google Scholar]
- 38.Tsukada, S., Westwick, J. K., Ikejima, K., Sato, N., and Rippe, R. A. (2005) J. Biol. Chem. 280 10055–10064 [DOI] [PubMed] [Google Scholar]
- 39.DiCamillo, S. J., Yang, S., Panchenko, M. V., Toselli, P. A., Naggar, E. F., Rich, C. B., Stone, P. J., Nugent, M. A., and Panchenko, M. P. (2006) Am. J. Physiol. 291 L232–L243 [DOI] [PubMed] [Google Scholar]
- 40.Dibrov, A., Kashour, T., and Amara, F. M. (2006) Growth Factors 24 1–11 [DOI] [PubMed] [Google Scholar]
- 41.Sreeramaneni, R., Chaudhry, A., McMahon, M., Sherr, C. J., and Inoue, K. (2005) Mol. Cell. Biol. 25 220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hai, T., and Curran, T. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 3720–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herr, I., van Dam, H., and Angel, P. (1994) Carcinogenesis 15 1105–1113 [DOI] [PubMed] [Google Scholar]
- 44.Li, X. Y., and Green, M. R. (1996) Genes Dev. 10 517–527 [DOI] [PubMed] [Google Scholar]
- 45.Livingstone, C., Patel, G., and Jones, N. (1995) EMBO J. 14 1785–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim, M. J., Kang, J. H., Park, Y. G., Ryu, G. R., Ko, S. H., Jeong, I. K., Koh, K. H., Rhie, D. J., Yoon, S. H., Hahn, S. J., Kim, M. S., and Jo, Y. H. (2006) J Endocrinol 188 623–633 [DOI] [PubMed] [Google Scholar]
- 47.Qing, J., Liu, C., Choy, L., Wu, R. Y., Pagano, J. S., and Derynck, R. (2004) Mol. Cell. Biol. 24 1411–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinha, S., Hoofnagle, M. H., Kingston, P. A., McCanna, M. E., and Owens, G. K. (2004) Am. J. Physiol. 287 C1560–C1568 [DOI] [PubMed] [Google Scholar]
- 49.Watanabe, T., Akishita, M., Nakaoka, T., He, H., Miyahara, Y., Yamashita, N., Wada, Y., Aburatani, H., Yoshizumi, M., Kozaki, K., and Ouchi, Y. (2004) Life Sci. 75 1219–1229 [DOI] [PubMed] [Google Scholar]
- 50.Herrmann, J., Borkham-Kamphorst, E., Haas, U., Van de Leur, E., Fraga, M. F., Esteller, M., Gressner, A. M., and Weiskirchen, R. (2006) Biochem. Biophys. Res. Commun. 345 1526–1535 [DOI] [PubMed] [Google Scholar]
- 51.Nikol, S., Isner, J. M., Pickering, J. G., Kearney, M., Leclerc, G., and Weir, L. (1992) J. Clin. Invest. 90 1582–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah, N. M., Groves, A. K., and Anderson, D. J. (1996) Cell 85 331–343 [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi, K., Yokote, K., Fujimoto, M., Yamashita, K., Sakamoto, A., Kitahara, M., Kawamura, H., Maezawa, Y., Asaumi, S., Tokuhisa, T., Mori, S., and Saito, Y. (2005) Circ. Res. 96 904–912 [DOI] [PubMed] [Google Scholar]
- 54.Yokote, K., Kobayashi, K., and Saito, Y. (2006) Trends Cardiovasc. Med. 16 240–245 [DOI] [PubMed] [Google Scholar]
- 55.Sekiguchi, K., Kurabayashi, M., Oyama, Y., Aihara, Y., Tanaka, T., Sakamoto, H., Hoshino, Y., Kanda, T., Yokoyama, T., Shimomura, Y., Iijima, H., Ohyama, Y., and Nagai, R. (2001) Circ. Res. 88 52–58 [DOI] [PubMed] [Google Scholar]
- 56.Najwer, I., and Lilly, B. (2005) Am. J. Physiol. 289 C785–C793 [DOI] [PubMed] [Google Scholar]
- 57.van Dam, H., and Castellazzi, M. (2001) Oncogene 20 2453–2464 [DOI] [PubMed] [Google Scholar]
- 58.Benbrook, D. M., and Jones, N. C. (1994) Nucleic Acids Res. 22 1463–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benbrook, D. M., and Jones, N. C. (1990) Oncogene 5 295–302 [PubMed] [Google Scholar]
- 60.Maekawa, T., Sakura, H., Kanei-Ishii, C., Sudo, T., Yoshimura, T., Fujisawa, J., Yoshida, M., and Ishii, S. (1989) EMBO J. 8 2023–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]