Short abstract
Differential Hox gene expression make the thumb special
Abstract
Asymmetric regulation of Hox gene expression pre-dates the appearance of tetrapod digits, and was co-opted in the development of 'thumbness'. This asymmetric expression correlates with independent morphological evolutionary variation of digit 1.
Each finger on your hand is uniquely identified by its relative size, position and shape. But on closer inspection, only one digit is really different from the rest - the thumb (digit 1). Digit 1 has two bones (phalanges) whereas all the other fingers have three. Only the thumb can be moved away from the other digits, a phenomenon called opposability, and on a more subtle anatomical level, growth of the corresponding metacarpal bone in the hand is proximal for the thumb but distal for all the other digits. In every respect the thumb stands out (no pun intended) as qualitatively different. With the appearance of a paper in Genes and Development by Montavon et al. [1], we now have a detailed understanding of some of the molecular mechanisms that make this anterior-most digit special.
How is the thumb different?
The earliest trace of a difference between the future thumb and the other digits is the expression of a subfamily of Hox genes, the AbdB-related group of Hox genes in the D cluster. Hox genes code for transcription factors, and most jawed vertebrates (with the exception of teleost (bony) fishes) have the Hox genes in four tightly linked clusters, called A, B, C and D [2]. Each gene cluster has the same 3' to 5' arrangement of corresponding genes, with those determining digit identity located at the 5' end of the cluster. In the embryo, the future hand or foot expresses four of these genes - HoxD-13, HoxD-12, HoxD-11 and HoxD-10 [3]. The embryonic territories in which digits 2 to 5 develop express all four genes, whereas the thumb develops from a territory where only HoxD-13 is expressed. There is much evidence that this association between the absence of HoxD-12 to HoxD-10 expression and thumb identity is causally important. Misexpression of HoxD-12 [4] or HoxD-11 [5] in the digit 1 territory often leads to a digit 2-like morphology in the first digit. The thumb's characteristic gene-expression pattern even follows it when, during evolution, the digits have shifted their embryological position, as is the case in a bird's wing [6]. The most anterior digit in the avian wing has the morphology of a thumb but develops at a position that would normally give rise to digit 2. The expression of HoxD genes is like that of a thumb, however, confirming that digit 1 has indeed changed places [7]. The differential expression of HoxD genes is obviously important for 'thumbness'. So how is this differential expression achieved?
Montavon et al. [1] developed a quantitative model of the regulatory program leading to the differential expression of HoxD genes in the developing mouse front paw, using experimental data from a set of deletion and duplication mutations of the HoxD cluster and quantifying the levels of HoxD mRNA with reverse transcription PCR (RT-PCR). Their story is one of two mechanisms - topological proximity of genes and enhancers, and differential affinity of HoxD gene promoters for enhancer regions. Two enhancers located 5' of the HoxD cluster influence the transcription of the 5' HoxD genes. One is called GCR, located some 180 kb upstream of HoxD-13, and the other is called Prox, located between the GCR and the 5' end of the HoxD cluster. Montavon et al. [1] measured how genes closer to the enhancers GCR and Prox are more strongly transcribed than genes located more 3' - the effect of topological proximity.
Furthermore, there is evidence that different HoxD genes have differential affinity for the enhancer complex, thus generating a differential rate of transcription. Mechanically, the process seems to consist of three steps (Figure 1). First the enhancer complexes forming at GCR and Prox associate, and then together they attach to a region between HoxD-13 and Evx2, which is a gene upstream of HoxD-13. From there, the enhancer complex starts to engage with promotor regions in the neighborhood of this point on the chromosome and initiates transcription at a rate related to the affinity between the promoter and the enhancer.
Figure 1.
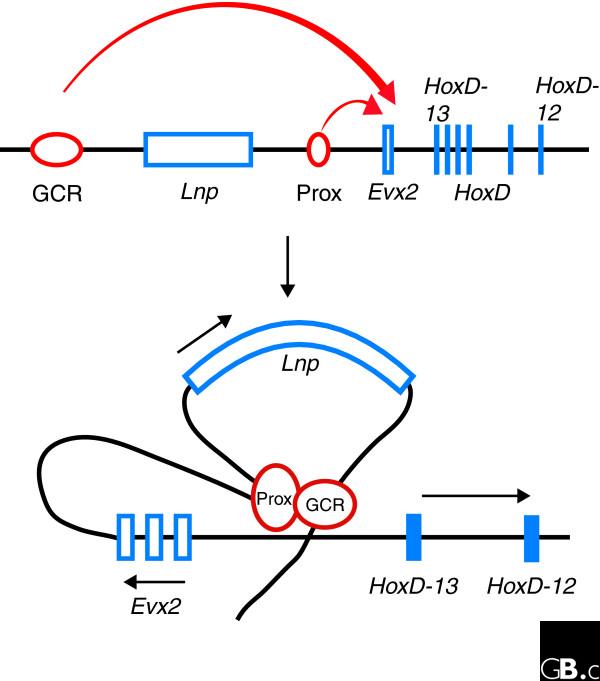
Regulation of 5' HoxD genes through interactions with two upstream enhancers. Two enhancers, GCR and Prox, are situated upstream of the HoxD genes, separated from each other by a gene called 'Luna Park' (Lnp). Both enhancers attach together to a region between HoxD-13 and the next upstream gene, Evx2, and from there interact with the genes in the HoxD cluster. The intensity of expression is then determined by two factors. First is the proximity of a gene to the enhancers, such that HoxD-13 is expressed at a much higher level than HoxD-10. Second, the differential affinity of the promoters of the HoxD genes for the enhancer complex also modulates their expression (not shown here). Reproduced with permission from [1].
The study by Montavon et al. [1] is one of the first quantitative models of transcriptional regulation in limb development that is strongly supported by quantitative experimental evidence. It teaches us a number of important lessons about the evolution of gene regulation. One is that, at least in the case of Hox genes, the inherent asymmetry in the physical arrangement of the genes leads to an intrinsic non-equivalence in transcription levels. This is an inherent constraint, in the sense that no adaptive reason is needed to explain why HoxD-13 is more strongly expressed than HoxD-10. Montavon et al. suggest that a reduction of global levels of HoxD products in digit 1 can explain why HoxD-10, HoxD-11 and HoxD-12 are not detectable there. These intrinsic asymmetries lead to differential gene expression that can become the template for the evolution of asymmetries in morphological characters, as is the case for the thumb relative to all other digits.
The asymmetry of HoxD transcription associated with digit 1 could be reflected in the independence of evolutionary variation in digit 1 with respect to the other digits: digit 1 is the digit most frequently reduced or lost during evolution ('Morse's law' [8]). In a remarkable recent paper in the Journal of Experimental Zoology, Reno et al. [9] show a pattern of correlated morphological variation in the evolution of elements of the forelimbs of the Anthropoidea (the monkeys, a group showing plenty of evolution in digit morphology). This pattern coincides with the late HoxD gene expression domains the authors observed in mouse forelimbs. For instance, Reno et al. show that changes in length of digits 2-5 in monkeys usually correspond to each other and to change in length of the distal forearm (Figure 2a), whereas changes in digit 1 are independent. Accordingly, in late stages of mouse development, the expression domain of HoxD-11 comprises digits 2-5 as well as the distal-most part of the forearm (Figure 2b), but is absent in digit 1. Reno et al. [9] suggest that up- or downregulation of the growth effects of HoxD-11 within this conserved expression domain can explain the observed phylogenetic pattern. It is notable that in this case, the correspondence was found for late expression patterns; indeed, the late-phase expression domain of a HoxD gene can be considerably different from its earlier expression (see discussion of HoxD-13 in [10]). Digital condensations remain undetermined until late stages [11], against the common intuition that all patterning must occur in early limb buds. The evolutionary pattern in monkeys seems to reflect the importance of this fact.
Figure 2.
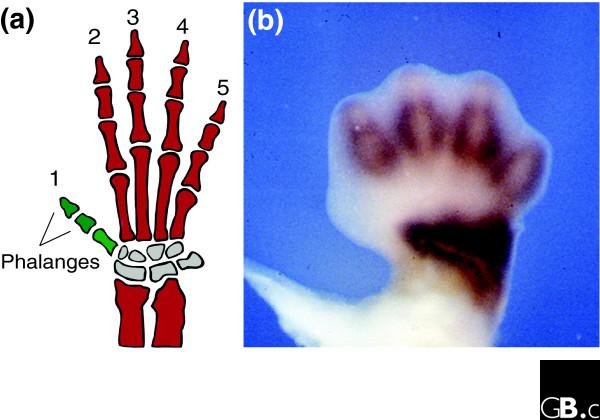
Digit evolution and HoxD expression. (a) In the evolution of the forelimb in different monkey species, morphological variation in digits 2-5 and the distal forearm (dark red) is correlated, whereas variation in digit 1 (green) corresponds to other independent regions. This phylogenetic pattern can be explained by variations in the late expression of HoxD-11 in the distal forearm and digits 2-5. (b) Domains of late HoxD-11 expression in an embryonic mouse paw. HoxD-11 expression is indicated by brown staining. Digits are numbered in the same order as in (a). The position where the thumb will develop is on the left. Modified from [9].
Building on the past
The Hox gene expression patterns observed in developing mouse paws by Montavon et al. [1] and others are already present in basal bony fish such as paddle fish [12] and lungfish [13], and even in cartilaginous sharks [14], as an anterior region where developing fin rays express neither HoxD-11 nor HoxD-12. Hence, the Hox expression pattern necessary for thumb/digit 1 development did not evolve with the origin of digits or the thumb. The phenotypic differences between the thumb and the other digits evolved by taking advantage of an ancient asymmetry in the expression of transcription-factor genes that is at least as old as the jawed vertebrates. This asymmetry in gene expression in turn seems to have arisen not by adaptive pressure but because of a genetic constraint. A similar scenario has been demonstrated for the evolution of pigment patterns in the fly wing, where the expression domains of transcription factors leading to the development of a feature are phylogenetically older than the pigment spot itself [15]. Expression domains or 'regulatory regions' appear to constrain evolution, such that despite changes in the associated morphological outcome, each region remains independent. Biology is most exciting when explanatory narratives reach from the depths of molecular mechanisms to the broad patterns of macro-evolutionary diversification. We are most fortunate to live at a time when this conceptual reach is being achieved.
References
- Montavon T, Garrec J-FL, Kerzberg M, Duboule D. Modelling HOX genes regulation in digits: reverse collinearity and the molecular origin of thumbness. Genes Dev. 2008;22:236–259. doi: 10.1101/gad.1631708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle FH, Bartels JL, Bentley KL, Kappen C, Murta MT, Pendelton JW. Evolution of Hox genes. Annu Rev Genet. 1994;28:423–442. doi: 10.1146/annurev.ge.28.120194.002231. [DOI] [PubMed] [Google Scholar]
- Nelson CE, Morgan BA, Burke AC, Laufer E, DiMambro E, Murtaugh LC, Gonzales E, Tessasollo L, Parada L, Tabin C. Analysis of Hox gene expression in the chick limb bud. Development. 1996;122:1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- Knezevic V, DeSanto R, Schugart K, Huffstadt U, Chiang C, Mahon KA, Mackem S. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Izpisúa-Belmonte JC, Duboule D, Tabin CJ. Targeted mis-expression of Hox-4.6 in the avian limb bud causes apparent homeotic transformations. Nature. 1992;358:236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Gauthier JA. 1,2,3=2,3,4: A solution to the problem of the homology of the digits in the avian hand. Proc Natl Acad Sci USA. 1999;96:5111–5116. doi: 10.1073/pnas.96.9.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas A, Fallon JF. Birds have dinosaur wings: the molecular evidence. J Exp Zoolog B Mol Dev Evol. 2005;304:86–90. doi: 10.1002/jez.b.21023. [DOI] [PubMed] [Google Scholar]
- Shapiro MD. Developmental morphology of limb reduction in Hemiergis (Squamata: Scincidae): chondrogenesis. osteogenesis, and heterochrony. J Morphol. 2002;254:211–231. doi: 10.1002/jmor.10027. [DOI] [PubMed] [Google Scholar]
- Reno PL, McCollum MA, Cohn MJ, Meindl RS, Hamrick M, Lovejoy CO. Patterns of correlation and covariation of anthropoid distal forelimb segments correspond to Hoxd expression territories. J Exp Zoolog B Mol Dev Evol. 2007. doi:10.1002/jez.b.21207. [DOI] [PubMed]
- Vargas AO, Fallon JF. The digits of the wing of birds are 1, 2, and 3. A review. J Exp Zoolog B Mol Dev Evol. 2005;304:206–219. doi: 10.1002/jez.b.21051. [DOI] [PubMed] [Google Scholar]
- Dahn RD, Fallon JF. Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science. 2000;289:438–441. doi: 10.1126/science.289.5478.438. [DOI] [PubMed] [Google Scholar]
- Davis MC, Dahn RD, Shubin NH. An autopodial-like pattern of Hox expression in fins of a basal actinopterygian fish. Nature. 2007;447:473–476. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- Johanson Z, Joss J, Boisvert CA, Ericsson R, Sutija M, Ahlberg PE. Fish fingers: digit homology in sarcopterygian fish fins. J Exp Zoolog B Mol Dev Evol. 2007;308:757–768. doi: 10.1002/jez.b.21197. [DOI] [PubMed] [Google Scholar]
- Freitas R, Zhang G, Cohn MJ. Biphasic Hoxd gene expression in shark paired fins reveals an ancient origin of distal limb domain. PLoS ONE. 2007;2:e754. doi: 10.1371/journal.pone.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme B, Gompel N, Rokas A, Kassner VA, Williams TM, Yeh SD, True JR, Carroll SB. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature. 2006;440:1050–1053. doi: 10.1038/nature04597. [DOI] [PubMed] [Google Scholar]


