Abstract
Ocular dominance column formation in visual cortex depends on both the presence of subplate neurons and the endogenous expression of neurotrophins. Here we show that deletion of subplate neurons, which supply glutamatergic inputs to visual cortex, leads to a paradoxical increase in brain-derived neurotrophic factor mRNA in the same region of visual cortex in which ocular dominance columns are absent. Subplate neuron ablation also increases glutamic acid decarboxylase-67 levels, indicating an alteration in cortical inhibition. These observations imply a role for this special class of neurons in modulating activity-dependent competition by regulating levels of neurotrophins and excitability within a developing cortical circuit.
Ocular dominance columns in the visual cortex of higher mammals form as axons from the lateral geniculate nucleus (LGN), representing the two eyes gradually remodel their terminal arbors into a series of alternating eye-specific patches within layer 4 (1, 2). The final pattern of ocular dominance columns can be altered by imbalance of activity between the two eyes (3, 4), implying a mechanism of activity-dependent synaptic competition. Because column formation is prevented by blockade of retinal activity (5) or changes in the availability of neurotrophins (6, 7)—in particular, brain-derived neurotrophic factor (BDNF) or neurotrophin-4, ligands of the high-affinity receptor for BDNF, TrkB—it is possible that LGN neurons compete for cortically derived neurotrophins released in an activity-dependent manner (reviewed in refs. 8–11). Because BDNF mRNA is both present in visual cortex and regulated by retinal activity (refs. 12, 13; E.S.L. and C.J.S., unpublished work), the role of activity in ocular dominance column formation may be to drive the production of cortical BDNF.
The segregation of LGN axons into ocular dominance columns is also prevented by the selective ablation of subplate neurons at the appropriate developmental time (14, 15). A subset of these neurons sends axonal projections into the developing cortical plate, where they form glutamatergic connections with cortical neurons, including those of layer 4 (16, 17). Because subplate neurons provide excitatory inputs to cortex, and because neurotrophin gene expression is regulated by neural activity, subplate neurons may influence ocular dominance column formation by regulating levels of neurotrophins. Here we have tested this hypothesis by ablating subplate neurons in the cat visual cortex and by examining the expression of the neurotrophin BDNF.
Materials and Methods
All procedures were performed according to University of California Berkeley Animal Care and Use Committee approved protocols.
Subplate Ablations.
Kainic acid injections (0.5 μl each of a 10-mg/ml solution in sterile 0.9% saline; Sigma) were made into the subplate/white matter underlying visual cortex in the posterior pole of the lateral gyrus in anesthetized cats (n = 26) between postnatal day 2 (P2) and P8, as described previously (14, 15). Fluorescent latex microspheres (Lumafluor, Naples, FL, in a ratio of 9:1 kainic acid/microspheres) were coinjected for visualization of the injection sites and to limit spread of the kainic acid (14, 15). Each animal received two injections, spaced approximately 1 mm apart anteroposteriorly, producing a zone of ablation up to several millimeters in extent (see refs. 14, 15 for complete details). Control animals were either identically injected with saline plus microspheres (9:1; n = three animals) into the visual subplate or were unmanipulated (n = 2). In three additional control animals, 0.5-μl injections of kainic acid plus microspheres were injected into layer 4 of visual cortex rather than into the subplate.
In Situ Hybridization.
In situ hybridization was performed as previously described (18), by using probes specific for cat BDNF or neurotrophin-3 (NT-3). Cat clones corresponding to unprocessed BDNF and NT-3 were cloned by PCR by using primers derived from homologous sequences from pig (BDNF) (19) and mouse (NT-3) (20). The PCR primers used for amplification of cat sequences were as follows: for BDNF, 5′-GAGAATCGATGACCATCCTTTTCCTT-3′ and 5′-ATATGGATCCCTATCTTCCCCTCTTAAT-3′, and for NT-3, 5′- ATAAGGATCCATGTCCATCTTGTTTTATG-3′ and 5′-ATATGGATCCTCATGTTCTTCCAATTTTTC-3′.
Transneuronal Transport of 3H-proline.
Transneuronal transport via intraocular injections of 3H-proline was performed (1, 14, 15) on four subplate neuron-ablated cats. Anesthetized animals received a monocular injection of 2 mCi 3H-proline (102 Ci/mmol; Amersham Pharmacia). After 7 to 10 days, tissue was prepared for in situ hybridization. Adjacent sections were either dipped for autoradiography and exposed for 6 wk to visualize ocular dominance columns (1) or had in situ hybridization performed on them before processing for autoradiography; 3-wk exposure of this tissue allows the visualization of both columns and mRNA localization on the same section.
Glutamic Acid Decarboxylase (GAD) Immunohistochemistry.
Eight subplate neuron-ablated and four unmanipulated control animals were examined for GAD immunohistochemistry (17) by using a rabbit anti-GAD-67 antibody (Chemicon) and Vectastain Elite ABC kit (Vector Laboratories) with diaminobenzidine (DAB; Sigma). To allow for comparisons and to ensure consistency across sections and animals, all sections were reacted for the same amount of time (20 min) in DAB solution, and control and experimental hemispheres from each animal were reacted simultaneously.
Results
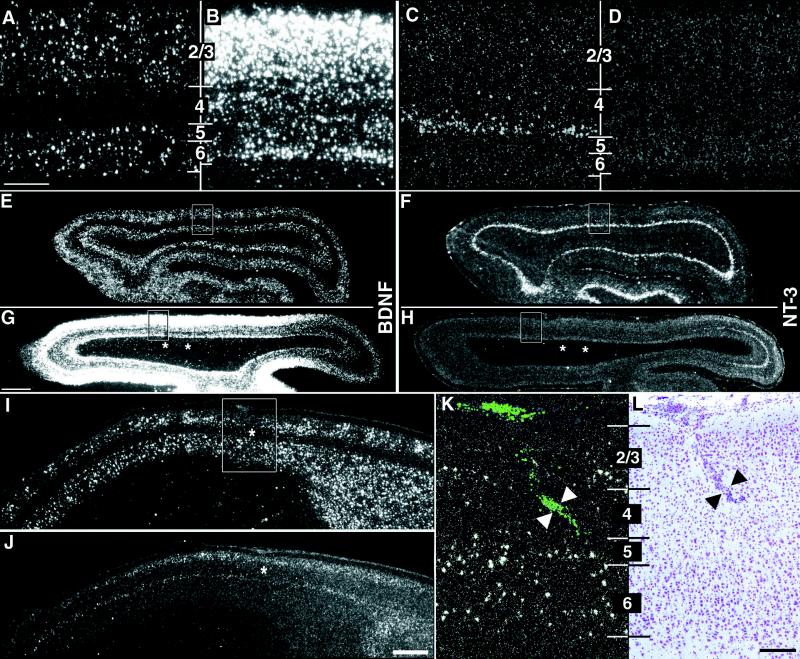
To ablate subplate neurons, the excitotoxin kainic acid was injected locally into visual subplate during the first postnatal week in cats (14, 15), about 10 days before LGN axons can be detected segregating into ocular dominance columns (1). At this time, subplate neurons, which are generated before other cortical neurons and hence mature earlier, are selectively vulnerable to kainate-induced excitotoxicity [refs. 14, 15, 21; also see supplemental data (www.pnas.org)]. The consequence of subplate neuron ablation on BDNF mRNA expression was examined by using in situ hybridization. As a control, we also investigated the expression of NT-3, which is not thought to be involved in ocular dominance column formation (6, 7) or visual system plasticity (22). At P28, about a week after the onset of column formation and at the height of the critical period for ocular dominance column plasticity (24, 25), BDNF and NT-3 mRNAs are expressed in distinct laminar patterns within visual cortex of normal animals (Fig. 1 A and E, C and F) (23). BDNF mRNA is detectable in layers 2/3, 5, and 6, but only in a few layer 4 neurons (Fig. 1A). NT-3 mRNA, on the other hand, is expressed primarily in the neurons at the base of layer 4 (layer 4c) (Fig. 1C), although expression is occasionally also detectable in layer 2. However, at P28 after subplate neuron ablation 3 wk earlier, there are profound changes in the pattern and levels of BDNF and NT-3 mRNA surrounding the injection site (Fig. 1 B and G, D and H). BDNF mRNA is dramatically up-regulated throughout the cortical plate, including in layer 4, the target layer for thalamocortical axons. Unexpectedly, NT-3 mRNA is down-regulated in layer 4c to such an extent that no NT-3 mRNA is detectable near the injection sites. BDNF and NT-3 mRNA levels are often altered for several millimeters (Fig. 1 G and H), as expected because of the spacing of the injection sites and the very broad tangential extent of the terminal arbors of subplate neurons (26).
Figure 1.
Alteration of neurotrophin gene expression after kainic acid injections into subplate but not cortical plate. (A–H) Horizontal sections through primary visual cortex at P28 showing in situ hybridization for BDNF and NT-3 mRNA in an unmanipulated case (A and E, C and F) and in a P28 animal that received injections of kainic acid into the subplate at P7 (B and G, D and H). High-magnification photomicrographs in A–D correspond to boxed regions in E–H. BDNF mRNA levels surrounding the injection sites increase throughout the cortical plate after subplate ablation (B); NT-3 mRNA levels in layer 4c decrease below the level of detectability (D). Alterations in mRNA levels for BDNF and NT-3 are present for many millimeters surrounding the two injection sites (asterisks in G and H), whereas the hybridization pattern appears normal for both neurotrophins far posteriorly (right in G and H). (I–L) Neurotrophin mRNA expression does not change after injections of kainic acid directly into layer 4. Sagittal sections through primary visual cortex showing in situ hybridization for BDNF (I and K) or NT-3 (J) mRNA at P28 after an injection of kainic acid into layer 4 of primary visual cortex on P7. The patterns and levels of expression are indistinguishable from unmanipulated or saline-injected animals at the same age. High-magnification photomicrograph in K corresponds to the boxed region in I. Fluorescent beads coinjected with the kainic acid are visible in layer 4 of visual cortex (arrowheads in K), and a scar along the injection track is visible in an adjacent section counterstained with cresyl violet (arrowheads in L). Asterisks (I and J) denote injection sites. Numbers denote cortical layers. Anterior is A–H Left and in I–L Right. Scale bars (A–D, K–L), 200 μm; (E–H, I and L), 2 mm.
Changes in the laminar pattern and in levels of expression of these genes occur in all animals examined at either 4 days (n = 2, data not shown) or 3 wk (n = 11 animals) postablation. As reported previously (14, 15), the extent of subplate neuron loss varies from animal to animal, ranging from several hundred microns to several millimeters away from the kainate injection sites. Similarly, changes in BDNF and NT-3 mRNA levels are centered on the kainate injection sites and vary in extent (compare Fig. 1 G and H, showing a large effect, with Fig. 2B showing a particularly small effect). Gene expression changes persist for at least 6–7 wk (n = nine animals, Fig. 2B), although at these later timepoints the elevated BDNF mRNA levels are more variable and not always as robust as those present at 3 wk. Of note, changes in the expression patterns of mRNA for BDNF or NT-3 are not detectable at 24 hr postkainate injection (n = 2; data not shown); furthermore, these changes in neurotrophin mRNA occur in the cortex in the vicinity of the injection sites and are not observed either in the thalamus or in the contralateral visual cortex of 3-wk (n = 4) and 6-wk (n = 4) postkainate animals (data not shown). Thus, ablation of subplate neurons leads to delayed but enduring changes in the expression of BDNF and NT-3 mRNAs.
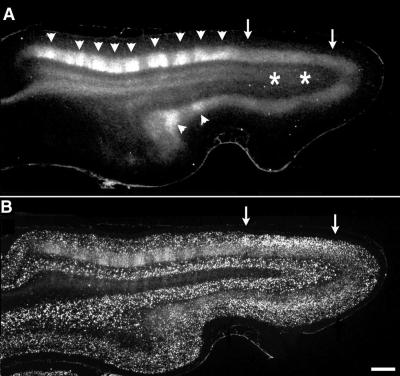
Figure 2.
Correlation of area of disrupted ocular dominance columns with region of BDNF mRNA up-regulation. (A) Horizontal section through primary visual cortex in a P56 animal after kainic acid injections into visual subplate at P8 and a monocular injection of 3H-proline at P46. The patchy appearance of label in layer 4 (arrowheads) anterior to the kainic acid injection sites (asterisks in white matter) indicates the presence of normal ocular dominance columns. However, the uniform band of label in layer 4 (region between arrows) overlying the injection sites indicates that ocular dominance columns have failed to form there. (B) Section adjacent to A, double labeled for transneuronally transported radioactive label, and in situ hybridization for BDNF mRNA. The region lacking ocular dominance columns corresponds to the region in which BDNF mRNA levels are persistently increased even at this older age (arrows). Anterior Left, medial Upper. Bar = 1 mm.
An essential control is to demonstrate that the changes in neurotrophin gene expression are not caused by a direct effect of kainic acid on neurons of the cortical plate, particularly those of layer 4. This is a reasonable supposition because previous work has shown that injections of kainic acid into superficial layers of the cortex do not affect cortical cytoarchitecture or ocular dominance column formation (14, 15). To further confirm this conclusion, kainic acid was injected directly into layer 4 rather than subplate at P7. Such cortical injections do not alter the normal pattern of BDNF and NT-3 mRNA expression within visual cortex when examined at P28 (Fig. 1 I–L, n = 3). Thus, changes in gene expression are specific to the ablation of subplate neurons and are not caused by direct effects of kainic acid on cortical neurons.
Because infusion of exogenous BDNF is known to prevent ocular dominance column formation (6, 7), the up-regulation of BDNF mRNA after subplate neuron ablation could explain why columns fail to form in subplate-ablated animals. If so, the region lacking ocular dominance columns after subplate neuron ablation should coincide with the region of elevated BDNF mRNA expression. We examined this prediction in animals (n = 4) at 7 wk of age, when ocular dominance columns in layer 4 have normally almost completely formed (1). To reveal ocular dominance columns, transneuronal transport of 3H-proline injected into one eye was used to label LGN axon terminals within layer 4. As expected after subplate ablation at P7 (14, 15), there is a uniform band of transneuronally transported label in layer 4 overlying the ablated region, indicating lack of ocular dominance columns; the patchy appearance of label further away denotes the presence of normal columns (Fig. 2A). The lower intensity of transneuronal labeling in the region of disrupted columns has been observed previously (14, 15) and is expected, because the same number of LGN axons representing the 3H-proline-injected eye now must project to twice the normal area of layer 4. [Note that previous studies have demonstrated that the subplate ablations do not affect LGN neurons, many of which are retrogradely labeled with fluorescent microspheres coinjected with the kainic acid many weeks earlier (15).] In situ hybridization for BDNF mRNA on an adjacent section (Fig. 2B) demonstrates a clear correlation: BDNF mRNA is increased in the region of cortex lacking ocular dominance columns (Fig. 2, arrows) and is at normal low levels where columns are present (Fig. 2A, arrowheads). In all four animals examined, the region of altered BDNF mRNA expression correlates closely with the area lacking ocular dominance segregation. (Note that at these older ages, necessary to determine whether ocular dominance columns had formed, the elevation of BDNF mRNA is not as marked as that observed at 3 wk postablation; shown in Fig. 1). These observations are entirely consistent with the suggestion that subplate neuron ablation prevents ocular dominance column formation by elevating BDNF expression.
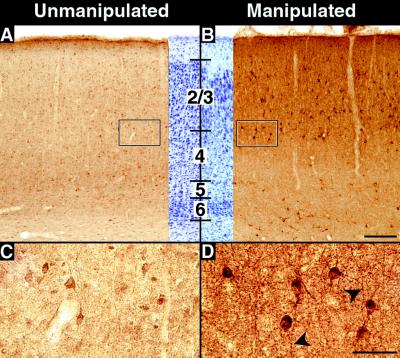
Recent evidence has suggested that BDNF can regulate the phenotype and function of inhibitory γ-amino-butyric-acid (GABA)-positive interneurons in the cerebral cortex (for review, see ref. 27). Thus, we examined the expression of the major enzyme that synthesizes GABA, GAD-67 (28), in kainate-injected animals. GAD-67 protein levels in subplate-ablated cortex are substantially higher in cell bodies in cortical layers 2–4 (Fig. 3B) near the injection sites (n = six animals at P28), as compared with the unmanipulated hemispheres of the same animals or of normal controls (n = two animals) (Fig. 3A). Immunoreactive neurons in these layers are larger and more intensely stained than labeled neurons in the uninjected hemisphere (Fig. 3 C and D). Whereas cell-body staining increases primarily in the superficial layers, neuropil staining increases throughout all cortical layers near the injection sites, including in the white matter. Elevations in GAD-67 immunostaining persist for at least 6 wk after subplate ablation (experimental animals: n = 2; control animals: n = 2; data not shown).
Figure 3.
Effects of subplate ablation on GAD expression. (A–D) GAD-67 immunostaining in primary visual cortex at P28 after injection of kainic acid into the subplate on P7. (A) GAD-67 immunostaining in the hemisphere contralateral to the kainic acid injection, showing levels of staining comparable to that of unmanipulated control animals. (B) GAD-67 immunostaining in visual cortex overlying injection sites in the subplate-ablated hemisphere from the same animal shown in A. Neuropil immunostaining is significantly increased throughout the cortical plate and in the underlying white matter. (C and D) High-magnification photomicrographs of the boxed areas at the border of layers 3 and 4 in A and B. Immunopositive cells are larger and more intensely labeled in the manipulated D than the unmanipulated C hemisphere. The middle portions of A and B represent cresyl violet counterstained sections adjacent to stained sections. Heavily labeled processes are visible (arrowheads) in D. Numbers denote cortical layers. Bars = (A and B), 200 μm; (C and D), 50 μm.
Expression levels of the genes examined in this study are known to be activity dependent in other paradigms. For example, BDNF and GAD are known to increase and NT-3 to decrease after seizure-induced increases in neural activity in adult animals (29–31). In fact, in 17/27 kainate-treated animals studied here, careful daily observation revealed seizures that were delayed in onset after kainic acid injection (average onset 2–3 days postinjection; range 1–7 days) and were also transient in duration (2–10 days). This behavioral correlate argues that subsequent to the loss of subplate neurons, profound changes in neural activity within cortical circuits must occur. [See also supplemental data (www.pnas.org.).]
Discussion
The tight correlation observed here between the region of visual cortex in which ocular dominance columns are disrupted and the extent of increased levels of BDNF mRNA suggests that altered levels of BDNF are responsible for the failure of thalamocortical axons representing the two eyes to segregate after subplate neuron ablation. Previous work involving infusions of BDNF or neurotrophin-4 or antagonists of trkB signaling (6, 7) have demonstrated that either too much or too little signaling through trkB can prevent the formation of ocular dominance columns. One current model of ocular dominance column formation is that LGN axons corresponding to the two eyes compete with one another for limiting amounts of cortically derived neurotrophins (8–11, 32). In support of this model, LGN axons are known to express trkB during the critical period for ocular dominance column formation (33, 34), implying a direct action of BDNF on LGN axons. Raising levels of BDNF, as occurs after subplate neuron ablation, could simply remove the competition between LGN axons for this neurotrophin before segregation can occur. Because BDNF mRNA levels increase throughout the cortex after subplate neuron ablation, including in layer 4, it is highly likely that these axons have easy access to this neurotrophin, which could affect their patterns of branching and synaptic stability.
A second way in which increased BDNF could be influencing ocular dominance column formation is indirectly, by altering the excitability of neurons in cortical circuits. BDNF is known to affect phenotypic markers and quantal amplitudes of local circuit inhibitory neurons (27, 35, 41). Possibly as a result of increased BDNF levels, subplate neuron ablation increases the immunostaining of inhibitory (GAD-67-positive) cortical neurons. Because alterations in inhibitory transmission are known to affect synaptic plasticity, including cortical LTP and ocular dominance column plasticity (36–39), enhancement of GABAergic transmission could alter or even prevent the synaptic remodeling required for ocular dominance column formation. In this context, it is interesting to note that monocular deprivation in conjunction with infusion of either GABA agonists (38, 39) or BDNF (42) causes a paradoxical shift in favor of the nondeprived eye, suggesting a common underlying mechanism.
These results provide a unifying link between ocular dominance column formation, neurotrophin gene expression, and inhibitory transmission in the developing visual system: subplate neurons. Because subplate neurons send a glutamatergic projection into the cortex, we expected that removal of subplate neurons might prevent column formation by depriving the cortex of excitation necessary for the production of neurotrophins. Paradoxically, rather than a decrease in BDNF mRNA, we found an increase in expression of this gene. Furthermore, we saw unexpected changes in two other genes, NT-3 (decrease) and GAD-67 (increase). One explanation that could account for changes in all of these genes is that subplate neuron ablation leads to a transient increase in cortical excitability (as supported by the development of transient seizures in some animals), implying that subplate neurons normally supply significant synaptic drive to inhibitory cortical neurons. The requirement for subplate neurons in the formation of ocular dominance columns can be understood in the context of a role in modulating activity-dependent competition by regulating levels of neurotrophins and cortical inhibition. These findings may also have clinical significance, because some instances of perinatal brain injury and epilepsy are known to be associated with distinct abnormalities of the subplate/white matter (40), which could lead to similar alterations in connectivity, neurotrophin gene expression, and GABAergic phenotype.
Supplementary Material
Acknowledgments
We thank Denise Escontrias, Cynthia Cowdrey, and Sandra Wiese for expert technical assistance. We are also grateful to Andreas Hohn for assistance in cloning the cat neurotrophin homologues and to Michael DeFreitas for surgical assistance. This work was supported by National Institutes of Health Grant EY 02858 and an Alcon Research Institute Award to C.J.S., a National Science Foundation predoctoral fellowship to E.S.L., a Howard Hughes Medical Institute predoctoral fellowship to E.M.F., and University of California at San Francisco Child Health Research Center National Institute of Child Health and Human Development Grant no. HD28825-07 to P.S.M. C.J.S. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- GAD
glutamic acid decarboxylase
- LGN
lateral geniculate nucleus
- NT-3
neurotrophin-3
- Pn
postnatal day n
- trkB
high-affinity Trk receptors for BDNF and neurotrophin-4
References
- 1.LeVay S, Stryker M P, Shatz C J. J Comp Neurol. 1978;179:223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- 2.Katz L C, Shatz C J. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 3.Wiesel T N, Hubel D H. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 4.Hubel D H, Wiesel T N, LeVay S. Philos Trans R Soc Lond B. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 5.Stryker M P, Harris W A. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 7.Cabelli R J, Shelton D L, Segal R A, Shatz C J. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 8.Shatz C J. In: Molecular and Cellular Approaches to Neural Development. Cowan W M, Jessell T M, Zipursky S L, editors. Oxford: Oxford University Press; 1997. pp. 509–524. [Google Scholar]
- 9.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 10.Bonhoeffer T. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 11.McAllister A K, Katz L C, Lo D C. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 12.Castren E, Zafra F, Thoenen H, Lindholm D. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozzi Y, Pizzorusso T, Cremisi F, Rossi F M, Barsacchi G, Maffei L. Neuroscience. 1995;69:1133–1144. doi: 10.1016/0306-4522(95)00321-9. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh A, Shatz C J. Science. 1992;255:1441–1443. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh A, Shatz C J. J Neurosci. 1994;14:3862–3880. doi: 10.1523/JNEUROSCI.14-06-03862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friauf E, Shatz C J. J Neurophysiol. 1991;66:2059–2071. doi: 10.1152/jn.1991.66.6.2059. [DOI] [PubMed] [Google Scholar]
- 17.Finney E M, Stone J R, Shatz C J. J Comp Neurol. 1998;398:105–118. [PubMed] [Google Scholar]
- 18.Corriveau R A, Huh G S, Shatz C J. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 19.Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde Y A. Nature (London) 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 20.Hohn A, Leibrock J, Bailey K, Barde Y A. Nature (London) 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- 21.Coyle J T, Bird S J, Evans R H, Gulley R L, Nadler J V, Nicklas W J, Olney J W. Neurosci Res Bull. 1981;19:331–427. [PubMed] [Google Scholar]
- 22.Riddle D R, Lo D C, Katz L C. Nature (London) 1995;378:189–191. doi: 10.1038/378189a0. [DOI] [PubMed] [Google Scholar]
- 23.Lein, E. S., Hohn, A. & Shatz, C. J. (1999) J. Comp. Neurol., in press. [DOI] [PubMed]
- 24.Hubel D H, Wiesel T N. J Physiol (London) 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blakemore C, Van Sluyters R C. J Physiol (London) 1974;237:195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friauf E, McConnell S K, Shatz C J. J Neurosci. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marty S, Berzaghi Mda P, Berninger B. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- 28.Hendrickson A E, Tillakaratne N J, Mehra R D, Esclapez M, Erickson A, Vician L, Tobin A J. J Comp Neurol. 1994;343:566–581. doi: 10.1002/cne.903430407. [DOI] [PubMed] [Google Scholar]
- 29.Garcia M L, Garcia V B, Isackson P J, Windebank A J. NeuroReport. 1997;8:1445–1449. doi: 10.1097/00001756-199704140-00024. [DOI] [PubMed] [Google Scholar]
- 30.Kornblum H I, Sankar R, Shin D H, Wasterlain C G, Gall C M. Mol Brain Res. 1997;44:219–228. doi: 10.1016/s0169-328x(96)00224-0. [DOI] [PubMed] [Google Scholar]
- 31.Dugich-Djordjevic M M, Tocco G, Lapchak P A, Pasinetti G M, Najm I, Baudry M, Hefti F. Neuroscience. 1992;47:303–315. doi: 10.1016/0306-4522(92)90246-x. [DOI] [PubMed] [Google Scholar]
- 32.Maffei L, Berardi N, Domenici L, Parisi V, Pizzorusso T. J Neurosci. 1992;12:4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allendoerfer K L, Cabelli R J, Escandon E, Kaplan D R, Nikolics K, Shatz C J. J Neurosci. 1994;14:1795–1811. doi: 10.1523/JNEUROSCI.14-03-01795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabelli R J, Allendoerfer K L, Radeke M J, Welcher A A, Feinstein S C, Shatz C J. J Neurosci. 1996;16:7965–7980. doi: 10.1523/JNEUROSCI.16-24-07965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutherford L C, DeWan A, Lauer H M, Turrigiano G G. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkwood A, Bear M F. Neuroscience. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensch T K, Fagiolini M, Mataga N, Stryker M P, Baekkeskov S, Kash S F. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiter H O, Stryker M P. Proc Natl Acad Sci USA. 1988;85:3623–3627. doi: 10.1073/pnas.85.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hata Y, Stryker M P. Science. 1994;265:1732–1735. doi: 10.1126/science.8085163. [DOI] [PubMed] [Google Scholar]
- 40.Volpe J J. Neurology of the Newborn. Philadelphia: Saunders; 1995. , Chap. 2 and 5. [Google Scholar]
- 41.Huang Z J, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear M F, Maffei L, Tonegawa S. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 42.Galuske R A W, Kim D S, Castren E, Thoenen H, Singer W. Eur J Neurosci. 1996;8:1554–1559. doi: 10.1111/j.1460-9568.1996.tb01618.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.