Abstract
Previous research suggests that the noradrenergic system modulates certain types of cognitive flexibility. This study compared various doses of beta-adrenergic antagonists for their effect on cognitive flexibility in problem solving, and how task difficulty interacts with this effect, as well as the effect of beta-adrenergic antagonists on other tasks. Anagram task performance was compared in 72 subjects using a within-subject design for propranolol at 20mg, 40mg, 60mg, and placebo in a double-blinded manner, and the effects of subject ability and task difficulty were examined. We also examined the effect of the 40mg propranolol dose on a range of other tasks. Overall, more anagram problems were solved while on propranolol 40mg than on placebo. Subjects least able to solve the problems benefited significantly from 40mg of propranolol. Also, for all subjects the most difficult problems were solved more quickly with propranolol 40mg than placebo. Benefits were also seen for word fluency and backward digit span. Therefore, noradrenergic modulation of cognitive flexibility is affected by how much difficulty the subject is encountering when searching for the solution, a pattern consistent with what might be expected in an effect on the search of the semantic and associative network.
Keywords: norepinephrine, propranolol, problem solving, executive function, semantic, language
Introduction
In our previous work, we had demonstrated that the noradrenergic system appears to exhibit a modulatory effect on performance of cognitive flexibility tasks requiring a broad search throughout the lexical/semantic network such as the anagram task (Beversdorf et al., 1999, 2002). For the purposes of this study, cognitive flexibility will, unless otherwise specified, refer to flexibility of access to the lexical-semantic and associative network in a verbal problem solving task (Beversdorf et al., 1999; Beversdorf et al., 2002; Kelley et al., 2005), in contrast to other forms of cognitive flexibility such as set-shifting. In these experiments, anagram performance after propranolol (a central and peripheral beta-adrenergic antagonist) was significantly better than after adrenergic agonists (Beversdorf et al., 1999) and than after peripheral-only beta-adrenergic antagonists (Beversdorf et al., 2002). This effect is proposed to result from a modulatory effect of norepinephrine on the signal-to-noise ratio in the cortex (Hasselmo et al., 1997; Heilman et al., 2003), whereby enhanced ‘signal’ may relate to superior performance on attention with increased noradrenergic activity and greater ‘noise’ with decreased noradrenergic activity may relate to increased intrinsic associative activity (Hasselmo et al., 1997; Alexander et al., 2007), and improved performance on those tasks requiring a high degree of flexibility of access to lexical-semantic and associative networks (Alexander et al., 2007). However, neither of these experiments revealed a significant difference between propranolol and placebo (Beversdorf et al., 1999; Beversdorf et al., 2002). Our subsequent research revealed that stress significantly impairs performance on these tasks, and propranolol does significantly improve performance while exposed to the stressor (Alexander et al., 2007). However, the question remained as to whether increased task difficulty in general could influence the effect of propranolol on task performance, which might interact with the aforementioned findings on stress. The question also remained as to the effects of varying doses of propranolol. Also, this effect may differ with other forms of cognitive flexibility, such as set shifting, which, in contrast, models predict may be enhanced by increased adrenergic activity (Aston-Jones and Cohen, 2005). Therefore, we also wished to examine the effect of propranolol on other forms of cognitive flexibility, as well as a range of other cognitive tasks.
Norepinephrine is important in arousal (Coull et al., 1997; Coull et al., 2004; Smith and Nutt, 1996). Furthermore, the prefrontal cortex, an area believed to be critical for various types of cognitive flexibility (Vikki et al., 1992; Karnath and Wallesch, 1992; Eslinger and Grattan, 1993; Duncan et al., 1995), has afferent projections to the locus coeruleus in the primate brain (Arnsten and Goldman-Rakic, 1984), which contains a majority of the noradrenergic neurons in the brain and sends extensive efferent fibers throughout the central nervous system (Barnes and Pompeiano, 1991). More recent findings have indicated that the noradrenergic system affects cognitive performance on specific tasks. Highly synchronized firing of noradrenergic neurons of the locus coeruleus in monkeys correlates with improvements in performance on a visual discrimination task, suggesting that this system is important in the regulation of goal-directed versus exploratory behaviors (Usher et al., 1999). A range of other cognitive effects have also been noted with noradrenergic agents, including effects on motor learning (Foster et al., 2006), response inhibition (Chamberlain et al., 2006b), working memory and emotional memory (Chamberlain et al., 2006a).
An increase in activity of the noradrenergic system occurs during stressful situations (Ward et al., 1983; Kvetnansky et al., 1998). Impairments in tasks involving flexibility of access to semantic/associative networks occurs with stress (Martindale and Greenough, 1973). Furthermore, studies with adolescents prone to test anxiety have shown that administration of a beta-adrenergic blocker (e.g. propranolol) improved test-taking performance (Faigel, 1991). Furthermore, as described above, stress also appears to impair flexibility of access to lexical/semantic and associative networks in subjects without any history of susceptibility to stress, an effect which is reversed by propranolol (Alexander et al., 2007). A recent study done to test the effects of anxiolytic medications on cognition found that lorazepam (an anxiolytic that acts on the GABAergic system rather than the noradrenergic system) did not significantly improve cognitive flexibility involving a search of the lexical/semantic network (Silver et al., 2004).
Previous research has further suggested that catecholaminergic agents have an effect on semantic networks independent of stressors. L-dopa was found to cause restriction of the semantic network in a semantic priming task (Kischka et al., 1996). Specifically, subjects were presented with a series of word pairs where the first word was paired with a closely related word, a distantly related word, a non-related word, or a non-word, and they were directed to indicate if the second series of letters was a word or a non-word. In the placebo condition, subjects recognized the closely and distantly related words more quickly than non-related words. L-Dopa, which is converted into dopamine and norepinephrine in the central nervous system, resulted in quicker recognition of the closely related words, but not the distantly related words, suggesting a catecholaminergic restriction of the semantic network. Such restriction of the semantic network may also apply to other neuronal networks, specifically those involved in searching for solutions to complex problems that would be implicated in the type of cognitive flexibility involved in our tasks.
In order to expand our previous findings on the anagram task (Beversdorf et al., 1999, 2002), and elucidate the most efficacious dose of beta-blocker, we compared performance on anagrams after administration of propranolol at 20, 40, and 60 milligrams to placebo. Performance was stratified according to task difficulty within subject, as well as between subjects according to ability to solve problems, in order to determine whether part of the effect of propranolol on performance under stress previously described (Alexander et al., 2007) could relate to effects of task difficulty. Performance on a number of other tasks was also assessed at our previously utilized 40mg dose to better determine the effect of noradrenergic modulation on other cognitive domains, including set shifting cognitive flexibility, word fluency (which may similarly involve access to lexical/semantic networks), spatial problem solving, and various aspects of memory.
Materials and Methods
Subjects
Seventy two normal healthy adults (36 males, 36 females) with a mean age of 22.75 ± 1.0 years, range 18–35 years, were recruited from the local university (including students and employees) and the surrounding area. All subjects were screened for exclusionary criteria. Subjects with a reported history of contraindications to beta-blockers or a reported history of learning disorders including dyslexia or attention deficit disorder were excluded, as were subjects with other psychiatric conditions such as depression. English was the primary language for all participants. The study protocol was approved by the Ohio State University Institutional Review Board and all subjects provided informed written consent before participation.
Procedures
Experiment 1
Each subject participated in four test sessions spaced four to seven days apart. Test version order and drug condition order were counterbalanced across equal numbers of male and female subjects for all sessions. Drug administration was conducted in a double-blind manner. Heart rate before medication administration was compared to heart rate at the time of testing (45 minutes after administration) for each session to determine the effectiveness of the drugs for autonomic changes.
To address dose effects and task difficulty effects of propranolol on anagrams, one of four sets of the anagram task was administered to assess cognitive flexibility for access to the lexical/semantic network at each test session (placebo, 20mg, 40mg, 60mg propranolol) 45 minutes after administration of the drug (as with our previous research (Beversdorf et al., 1999), and to allow sufficient time under peak for multiple tests in Experiment 2). This task required the subject to unscramble each of twenty words (anagrams) as quickly as possible with a maximum of two minutes allowed to solve them (Beversdorf et al., 1999, 2002; Silver et al., 2004). Failures were recorded as 120 seconds. Raw solution latencies were calculated, then, as with previous work (Beversdorf et al., 1999, 2002; Silver et al., 2004), the natural log of solution latency was then calculated. The logarithmic transformation was performed because the difference between a 5 second and 15 second solution latency is more meaningful for our purposes than the difference between a 105 and 115 second latency. Without this transformation, the results are largely driven by the results of the more difficult problems.
Experiment 2
For the other tasks, the effect or propranolol was assessed at doses previously studied on anagrams. Therefore, at the 40mg and placebo sessions, subjects completed a battery of neuropsychological tests assessing a number of cognitive domains subsequent to completing the anagrams as described above. The two sessions involving administration of the neuropsychological test battery included one of two versions of seven different tests and required approximately 1.5 hours to complete. For the other two sessions subjects received propranolol 20 mg or propranolol 60 mg and did not proceed to perform the subsequent neuropsychological testing after the anagrams.
For the neuropsychological battery during the placebo and propranolol 40 mg sessions, one of two sets of the Compound Remote Associates (CRA) test was given to assess cognitive flexibility of access to semantic/associative networks as is described for “insight-based” problem solving (Bowden and Beeman, 1998, 2003). In this task subjects were presented with a problem containing three words, each of which formed a compound word or phrase with the solution (e.g. problem: tooth/potato/heart – solution: SWEET). Subjects were given seven seconds to produce a response and solution latency was recorded, with failures were recorded as seven seconds. One of two versions of the Rey Complex Figure (RCF) memory task was given to assess visuospatial memory (Corwin and Bylsma, 1993; Osterrieth, 1944). Thirty minutes after copying the figure subjects were asked to recall the figure from memory. This task was scored for accuracy of recall of the figure based on a 36-point scale. In order to assess verbal fluency one of two versions of the Controlled Oral Word Association (COWA) test (letters and categories) was administered (Lezak, 1995). In this test subjects were given a letter of the alphabet (e.g. F, A, S) and a category (e.g. animals, clothes, vegetables) and asked to generate as many words as possible within one minute. To assess attention and working memory the digit span subtest derived from the Wechsler Adult Intelligence Scale (WAIS-III) was administered (Wechsler, 1981). One half of the problems from Raven Progressive Matrices (RPM) test (parts C, D, and E) were given to assess visuospatial problem solving skills (Raven, 1976; Raven et al., 2000). The number of problems solved correctly was recorded for this task. To assess verbal learning and memory one of two versions of the California Verbal Learning Test (CVLT) was given (Delis et al., 1987). The immediate recall score was used for comparison. Neuropsychological battery version order and drug condition order were counterbalanced across equal numbers of male and female subjects for all sessions, and drug administration remained double-blinded.
The Wisconsin Card Sorting Test (Heaton, 1981) was administered, in order to assess cognitive flexibility for set-shifting, during either the placebo session or propranolol 40 mg session once for each subject due to lack of reliability of repeat testing. The percent of total errors and total number of perseverative errors were used for comparison.
Analysis
Comparison was made between performance on each task after propranolol and placebo for each drug dosage. For t-tests, scores for all tasks were adjusted for test order and test version, utilizing the counterbalanced design in order to adjust for practice effects and possible variations in difficulty between different versions of the tests.
Experiment 1
As with our previous work, for each subject the sum of the natural logarithm of anagram completion times were calculated and compared between drug conditions (Beversdorf et al., 1999), in addition to the raw solution latencies. The overall solution latencies were compared using paired t-tests between each dose of propranolol and placebo to determine which drug condition revealed a significant effect. Similarly, the total number of anagrams solved correctly as well as the raw solution latencies (without logarithmic transformation) were compared using a paired t-test. To determine whether this drug proposed to aid the search through the network for remote solutions is be helpful for more difficult problems, subjects were also divided into three groups based on anagram solution latencies in the placebo condition –high, medium, and low. Repeated measures analysis of variance (ANOVA) was performed to determine whether an interaction effect was present suggesting that solution latency grouping affected the drug effects. If significant effects were revealed by ANOVA, the adjusted anagram solution times for those subject groups were compared using paired t-tests to clarify these effects. In order to determine if task difficulty had an effect on performance (independent of subject) individual anagram problems were ranked by difficulty based on mean subject latencies for the placebo condition. The five most difficult (longest average solution times) and the five most simple (shortest average solution times) problems were isolated from each list and adjusted solution times for these specified problems were then summed for each subject. Repeated measures ANOVA was performed to determine whether an interaction effect was present suggesting that task difficulty groupings affected the drug effects. If significant effects were revealed by ANOVA, performance on these sets of problems were then compared across drug conditions using paired t-tests to clarify these effects.
Experiment 2
Similar analyses were performed for the CRA task. Latency for each problem was summed for each list and compared across the two drug conditions using paired t-tests. The number of problems solved correctly per list across drug conditions was also compared in a similar manner. As with the anagrams, subjects were ranked and grouped in thirds (high, medium, and low latency) based on total solution times for the placebo condition. Solution times were then compared within these groups across drug conditions using ANOVA and subsequent paired t-tests, as with the anagrams. The CRA problems have been previously analyzed for difficulty (Bowden and Jung-Beeman, 2003). The five most difficult and five most simple problems were isolated and compared for each subject across drug conditions using ANOVA and subsequent paired t-tests, as with the anagrams.
The remaining tasks were analyzed to determine differences in scores between drug conditions (propranolol 40 mg versus placebo). Measures of the RCF, COWA, digit span, RPM, and CVLT (adjusted for test order and test version, as with the anagrams) were analyzed separately using paired t-tests. Scores on the Wisconsin Card Sorting Task were compared between subjects for the first test session due to lack of repeat testing reliability using between-subject t-tests.
For these multiple other tasks, where multiple t-tests were performed which were not driven by ANOVAs, Bonferroni corrections were performed.
Results
Heart rate decreased significantly from baseline for all doses of propranolol. Results of heart rate analysis are summarized in Table 1.
Table 1.
Summary of Heart Rate Results (HR = heart rate_
| HR baseline (mean ± std dev) | HR 45 minutes after medication administration (mean ± std dev) | Significance t(71) =, p = | HR on medication vs. placebo (after administration) Significance t(70) =, p = | |
|---|---|---|---|---|
| Placebo | 74.83 ± 9.87 | 76.58 ± 11.31 | 1.93, 0.06 | |
| Propranolol 20mg | 77.0 ± 8.52 | 72.88 ± 8.78 | 4.75, < 0.001 | 3.13, 0.002 |
| Propranolol 40mg | 75.59 ± 9.09 | 70.72 ± 8.55 | 4.92, < 0.001 | 5.29, < 0.001 |
| Propranolol 60mg | 74.68 ± 8.95 | 70.37 ± 9.17 | 4.82, < 0.001 | 5.47, < 0.001 |
Experiment 1
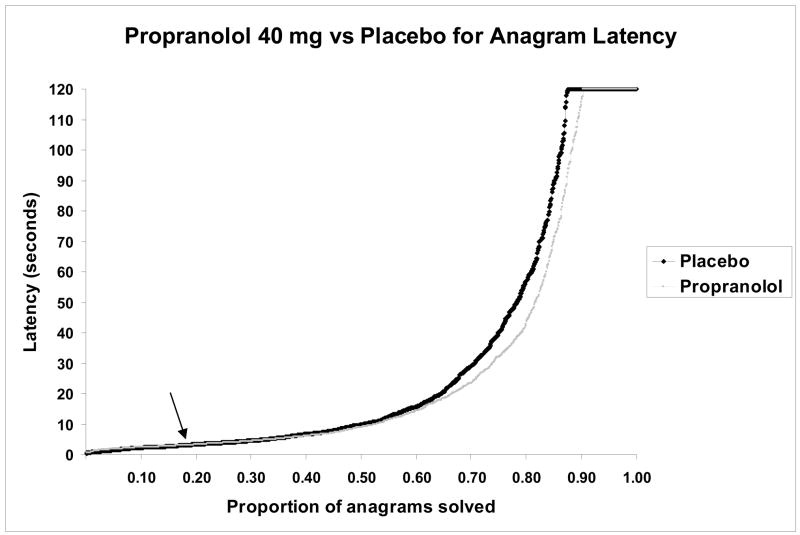
More anagrams were solved with the 40 mg dose of propranolol than with placebo (see Table 2). A significant improvement in raw latency scores to solve anagrams was observed in groups treated with 40 mg propranolol vs. placebo. However, as described above, the raw scores could be misleading as the result is driven mostly by the difficult problems. No dose of the propranolol produced a significant effect on the solution times (logarithmic transformed) for these tasks overall. To test whether a subject’s ability to solve problems affected the effect of drug on anagram problem solving, repeated measures ANOVA was performed examining whether individual variations in ability to solve problems (fastest 1/3 vs. middle 1/3 vs. slowest 1/3 of subjects in the placebo condition) interacted with the effect of administration of drug on performance, separately performed for each dose of propranolol. A significant drug by subject ability interaction effect was found for placebo vs. propranolol 20 mg (F(2,69) = 6.63, P = 0.002), for placebo vs. propranolol 40 mg (F(2,69) = 3.38, P = 0.040), and for placebo vs. propranolol 60 mg (F(2,69) = 4.26, P = 0.018). Subsequent between-subject t-tests demonstrated in the separate assessment of the performance of subjects with the most difficulty solving problems, that the group with the highest solution latency (the slowest 1/3) benefited significantly from 40 mg of propranolol (but not 20 mg or 60 mg) in ability to solve the anagrams as compared to placebo. Subjects that fell into the group with the lowest solution latency (fastest 1/3) were hindered by propranolol at 20 and 60 mg, but were not significantly impaired by 40 mg (Table 2). When the individual anagram problems (for all subjects) were stratified by difficulty, a significant drug by task difficulty interaction effect was also found for placebo vs. propranolol 20 mg (F(1,71) = 8.88, P = 0.004), for placebo vs. propranolol 40 mg (F(1,71) = 7.95, P = 0.006), and for placebo vs. propranolol 60 mg (F(1,71) = 7.94, P = 0.006). Subsequent within-subject t-tests demonstrated that problems that were the most difficult were solved significantly faster with propranolol 40 mg (but not 20 mg or 60 mg) than with placebo independent of subject. It was also found that the simple problems were solved significantly more slowly with propranolol at 20 and 60 mg as compared to placebo, but were not significantly impaired by 40 mg (Table 2). A plot of the proportion of problems solved vs. anagram solution times for all data points in the placebo and propranolol 40 mg conditions graphically illustrates the beneficial effect of 40mg propranolol at longer solution times (Figure 1), with no benefit at shorter solution times.
Table 2.
Summary of Anagrams Results (Ln = natural log)
| Index | n | Propranolol 40 mg | Propranolol 60 mg | Propranolol 20 mg | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± Std dev | t = | p = | Mean ± Std dev | t = | p = | Mean ± Std dev | t = | p = | Mean ± Std dev | ||
| Ln sum of solution times(total) | 72 | 49.89 ± 11.7 | 1.08 | 0.29 | 51.31 ± 11.6 | 0.58 | 0.57 | 51.40 ± 11.0 | 0.70 | 0.49 | 50.81 ± 11.5 |
| Number solved correctly | 72 | 17.92 ± 1.76 | 1.96 | 0.05* | 17.53 ± 1.94 | 0.50 | 0.62 | 17.64 ± 1.92 | 0.89 | 0.38 | 17.40 ± 2.36 |
| Raw sum of solution times(total) | 72 | 588.9 ± 247.6 | 2.36 | 0.02* | 638.4 ± 286.8 | 0.11 | 0.91 | 617.9 ± 238.5 | 0.98 | 0.33 | 641.2 ± 279.5 |
| Ln sum of solution times(most difficult problems) | 72 | 17.73 ± 3.28 | 2.67 | 0.01* | 18.25 ± 3.56 | 1.91 | 0.06 | 18.3 ± 3.07 | 1.30 | 0.20 | 18.92 ± 3.75 |
| Ln sum of solution times(simplest problems) | 72 | 8.91 ± 2.77 | 1.81 | 0.07 | 8.46 ± 3.25 | 2.13 | 0.04* | 8.91 ± 3.11 | 3.39 | 0.001* | 7.70 ± 3.13 |
| Ln sum of solution times(high latency subjects)† | 24 | 59.36 ± 7.17 | 3.84 | 0.04* | 61.03 ± 8.15 | 0.83 | 0.42 | 59.42 ± 7.39 | 1.90 | 0.07 | 62.45 ± 5.64 |
| Ln sum of solution times(medium latency subjects)† | 24 | 49.27 ± 8.81 | 0.58 | 0.57 | 50.20 ± 7.60 | 0.89 | 0.93 | 52.24 ± 6.93 | 1.24 | 0.23 | 50.33 ± 2.80 |
| Ln sum of solution times (low latency subjects)† | 24 | 40.67 ± 10.2 | 1.66 | 0.11 | 42.59 ± 9.58 | 2.85 | 0.009* | 42.24 ± 9.65 | 3.33 | 0.003* | 37.56 ± 7.05 |
= significant results, p < 0.05
= between subject comparison
Figure 1.
The proportion of problems solved vs. solution times of all of the anagram problems for all subjects for placebo and propranolol 40 mg. The arrow indicates the point where solution times for propranolol 40 mg become faster than solution times for placebo.
Experiment 2
Similarly, for the comparison of the sum of the solution times for CRA problems within subject between placebo and propranolol 40 mg, no significant differences were found. The total number of problems solved correctly also revealed no significant difference between drugs. However, a significant drug by subject ability interaction effect was found for the CRA (F(2,69) = 10.01, P < 0.0005). To further clarify this effect with subsequent t-tests, in assessment of performance of the subjects with the most difficulty solving problems, a significant decrease in CRA solution times for propranolol 40 mg was found for the high latency (slowest 1/3) group as compared to placebo. Furthermore, a significant increase in solution times was found for the low latency (fastest 1/3) group. No significant drug by task difficulty interaction effect was found for the CRA. Whereas it was not driven by a significant interaction on the ANOVA, further analysis was performed based on the hypothesis and the other previously described results. For the most difficult problems, independent of subject, t-tests revealed a trend towards better performance on propranolol 40 mg compared to placebo. No difference was found for the least difficult problems between drugs (Table 3).
Table 3.
Summary of other neuropsychological test results (CRA = Compound Remote Associates, COWA = Controlled Oral Word Association, CVLT = California Verbal Learning Test, RCF = Rey Complex Figure, RPM = Ravens Progressive Matrices, WCST = Wisconsin Card Sort Test)
| Index | n | Prop 40 mg mean ± std dev | Placebo mean ± std dev | t = | Significance p = |
|---|---|---|---|---|---|
| CRA Total sum of solution times | 72 | 162.29 ± 18.50 | 163.08 ± 17.25 | 0.37 | 0.72 |
| CRA Number solved correctly | 72 | 13.79 ± 3.99 | 12.94 ± 3.52 | 1.57 | 0.12 |
| CRA Difficult sum of solution times | 72 | 29.21 ± 4.84 | 30.54 ± 4.12 | 1.87 | 0.065 |
| CRA Simple sum of solution times | 72 | 21.22 ± 5.88 | 21.31 ± 6.00 | 0.08 | 0.93 |
| CRA High latency sum of solution times† | 24 | 170.13 ± 15.89 | 181.33 ± 8.67 | 3.88 | < 0.001* |
| CRA Medium latency sum of solution times† | 24 | 162.29 ± 15.19 | 163.27 ± 3.41 | 0.32 | 0.75 |
| CRA Low latency sum of solution times† | 24 | 154.44 ± 21.08 | 144.34 ± 10.68 | 2.51 | 0.02* |
| COWA total words by letter | 72 | 48.43 ± 9.61 | 44.84 ± 10.4 | 3.73 | 0.0004* |
| COWA total words by category | 72 | 54.1 ± 8.7 | 53.4 ± 9.0 | 0.72 | 0.47 |
| Digit span - total recall forward | 72 | 12.81 ± 2.01 | 12.24 ± 2.42 | 2.62 | 0.011* |
| Digit span - total recall backward | 72 | 9.63 ± 2.61 | 8.97 ± 2.77 | 2.81 | 0.006* |
| CVLT words on free recall trial 1 | 72 | 7.24 ± 1.93 | 7.39 ± 2.09 | 0.57 | 0.57 |
| RCF 30 minute recall score | 72 | 25.62 ± 5.21 | 25.48 ± 6.18 | 0.19 | 0.85 |
| RPM number correct | 72 | 17.2 ± 1.99 | 17.2 ± 1.87 | 0.002 | 0.998 |
| WCST percent of total errors† | 24 | 22.02 ± 9.98 | 18.74 ± 7.12 | 1.33 | 0.20 |
| WCST total number of perseverative errors† | 24 | 9.08 ± 6.86 | 7.71 ± 5.50 | 0.77 | 0.45 |
= significant results, p < 0.05
= between subject comparison
For the COWA test a significant increase in the number of words listed starting with a given letter by subjects was found while on propranolol 40 mg (Table 3). Analysis of the digit span task derived from the WAIS-III also revealed a significant increase in the number of digits repeated back while on propranolol 40 mg for the forward task as well as for the backward task. No other differences were found for COWA categories task, Raven Progressive Matrices, the California Verbal Learning task or the Rey Complex figure task (Table 3).
For the Wisconsin Card Sorting task, compared using between-subject ANOVA, no significant differences were found between propranolol 40mg and placebo in the percent of total errors, or the number of perseverative errors made (Table 3).
After Bonferroni correction for these multiple tasks not driven by ANOVA, the benefit for propranolol on the COWA letters task and a strong trend for benefit for digit span backwards remained.
Whereas this was not driven by the initial hypothesis, given the effects of subject ability on the anagram and CRA tasks, post hoc analysis was performed to examine for interaction effects between subject ability (top 1/3 vs. middle 1/3 vs. bottom 1/3 in the placebo condition) and effect of drug for the other tasks using a repeated measures ANOVA for those tasks not reaching significance above. Significant interaction effects were found for all such tasks (word fluency categories: (F(2,69) = 7.00, P = 0.002); Raven Progressive Matrices: (F(2,69) = 10.62, P < 0.0005); California Verbal Learning Test trial 1: (F(2,69) = 21.53, P < 0.0005); Rey complex figure recall: (F(2,69) = 23.92, P < 0.0005)). Performance was significantly better on propranolol 40 mg than on placebo for the bottom 1/3 on all of these tasks (word fluency categories: (t(23) = 3.18, p = 0.004); Raven Progressive Matrices: (t(23) = 2.55, p = 0.018); California Verbal Learning Test trial 1: (t(23) = 3.53, p = 0.001); Rey complex figure recall: t(23) = 5.87, p < 0.0005)). For the best 1/3, performance was significantly worse on propranolol 40 mg than on placebo for Raven Progressive Matrices, California Verbal Learning Test trial 1, and the Rey complex figure recall, with no significant difference between drugs for the word fluency categories task (word fluency categories: (t(23) = 1.17, p = 0.26); Raven Progressive Matrices: (t(23) = 4.02, p = 0.001); California Verbal Learning Test trial 1: (t(23) = 5.53, p < 0.0005); Rey complex figure recall: t(23) = 3.21, p = 0.004)).
Discussion
Previous research has demonstrated that performance on cognitive flexibility for tasks involving access to lexical/semantic networks is modulated by the noradrenergic system, but no significant difference was found when comparing propranolol and placebo (Beversdorf et al., 1999, 2002). Subsequent results showed a significant improvement on such tasks on propranolol in the setting of stressors (Alexander et al., 2007). Current results show that the benefit of beta-adrenergic blockade depends on the difficulty of the cognitive flexibility task as well as individual ability to solve these types of tasks in the unstressed condition. For subjects struggling with the task (subjects in the slowest 1/3) as well as for the most difficult tasks, 40 mg propranolol was beneficial. This would be consistent with what one might expect for a drug that might help in a search through the network for more remote solutions. Furthermore, 40 mg of propranolol was the only dose yielding significant benefit for difficult problems and for struggling subjects. The 20 mg and 60 mg doses did not yield significant benefit for these conditions, but revealed impaired performance for easy problems as well as for the subjects best able to solve problems. For the CRA (at 40 mg), benefit was also seen for struggling subjects (subjects in the slowest 1/3) as well as a beneficial trend for difficult problems, and impairment was also seen for the subjects best able to solve problems as was seen for the anagrams at the 20 mg and 60 mg doses.
Changes in noradrenergic activity elicit changes in the signal-to-noise ratio of cortical neurons. Specifically, increases in noradrenergic activity decrease background noise and may facilitate memory and learning specific to environmental cues, however increased “noise” with decreased noradrenergic activity may represent increased intrinsic associative activity (Hasselmo et al., 1997), which may aide in access to more remote nodes in the lexical/semantic and associative networks (Alexander et al., 2007). This need for greater access to more remote nodes in a network may be the mechanism by which difficult tasks and struggling subjects are influenced by noradrenergic modulation in problem solving. By using a beta-adrenergic antagonist (propranolol), responses to noradrenergic activity are decreased presumably by inhibition of neurotransmitter binding at the central target neuron from the locus coeruleus projection, thus decreasing the signal-to-noise ratio in cortical areas receiving noradrenergic projections. Based on the results of this study, individuals having the most difficulty solving cognitive flexibility tasks benefited from beta-adrenergic blockade whereas those individuals having the least amount of difficulty did not benefit from it. It appears that in conditions, such as problem solving involving access to lexical/semantic/associative networks, where effort is required to search for the correct solution, decreased noradrenergic activity may be beneficial. In conditions when the correct solution is immediately available, and may be the dominant response, noradrenergic antagonism is not beneficial. However, the magnitude of these effects is modest in unstressed normal subjects. Alternatively, the apparent specificity of these findings to one dose and certain levels of task difficulty could be related to optimization of arousal for performance on the inverted “U” curve (Cools and Robbins, 2004). Furthermore, it is also possible that the process of struggling with more difficult tasks could itself activate the noradrenergic system, affecting cognitive performance, and secondarily allowing an effect of propranolol, as appears to occur in rodent learning and memory experiments (Gold and van Buskirk, 1975, 1978a, 1978b), rather than simply resulting from modulation of signal-to-noise in a network search. Further investigation of this finding is warranted in other conditions where the noradrenergic system may be affected.
Improvements in the COWA letters task and the digit span tasks were also observed. However, the COWA letters task also involves a search of the lexical/semantic network to produce associated words, thus also possibly implicating the modulatory role of the noradrenergic system in the network search. This may explain the increase in words given by subjects while on the noradrenergic antagonist propranolol. Furthermore, the finding of the same task difficulty by drug interaction effect as with the cognitive flexibility tasks for the COWA categories (greater benefit for subjects in the bottom 1/3 of performance) seems to further support this hypothesis. The same interaction for the RPM my suggest that this effect extends to problem solving beyond the lexical/semantic network to other networks such as that of spatial representations, and the RCF and CVLT interaction findings suggest that this effect also may apply to accessing these networks for recall. However, these interaction findings would be equally supportive of the act of struggling activating the noradrenergic system and affecting performance, and secondarily allowing an effect of propranolol, as described above (Gold and van Buskirk, 1975, 1978a, 1978b). This will need further study.
The trend towards a digit span finding is less readily explained in this manner. The digit span task is considered to be a task of attention and working memory (Wechsler, 1981). While increased noradrenergic activity is required for attention related behavior, there may be an optimal level for best functioning on tasks such as these. As described above for anagrams, according to early theories on learning, a lower level of arousal is necessary for optimal performance on difficult cognitive tasks (Yerkes and Dodson, 1908). If noradrenergic activity is too high subjects may enter a state of hyper-arousal and be unable to focus attention on one specific task, while a very low level of noradrenergic activity may cause arousal to be too low to allow peak performance on any specific behavioral task. Perhaps this also relates to our finding of improved digit span on propranolol due to optimization of arousal on a challenging task. More research is needed to better understand the role of the noradrenergic system in this finding in order to disentangle the contributions of working memory and sustained attention by including more tasks focusing on each of these domains within this pharmacological setting, particularly as this contrasts with previous studies showing impaired working memory with propranolol (Chamberlain et al., 2006a).
The WCST did not reveal any differences in performance between drugs in the between-subject comparison. The WCST as a cognitive flexibility task involves set-shifting, rather than the search through a network for a remote solution as with the anagrams or CRA tasks. Usher et al (1999) suggested that increased norepinephrine might facilitate set shifting. Set shifting, though, usually involves shifting between a limited number of options (“constrained flexibility”), which would not be impaired by norepinephrine in our model, whereas tasks involving a search through the network for a remote solution among many options (“unconstrained flexibility”) for difficult tasks may be improved by norepinephrine. However, since repeat testing with the WCST would not be valid, comparison between drug conditions is only possible between subjects for the WCST. Thus, the weakness of a between-subjects analysis limits the conclusions that can be drawn from the results of this WCST aspect of the study.
More insight into the underlying mechanism of cognitive flexibility and noradrenergic modulation thereof may be relevant for treatment of conditions of dysregulated noradrenergic activity and otherwise impaired flexibility such as situational anxiety, cocaine withdrawal, and autism spectrum disorders (ASD). While literature supports the use of beta-adrenergic blockade in test taking situations and during public speaking for individuals susceptible to anxiety for these situations (Faigel, 1991; Drew et al., 1985; Lader, 1988; and Laverdue and Boulenger, 1991), more research is needed to find how this benefit extends to other situations involving anxiety. Furthermore, previous research has shown that the noradrenergic system is up-regulated during acute cocaine withdrawal (McDougle et al., 1994), and performance in acute withdrawal is impaired on tasks involving unconstrained flexibility (Kelley et al., 2005), suggesting that such patients may benefit from propranolol. Some benefit has been shown with propranolol among cocaine withdrawal subjects with greater withdrawal symptomatology in clinical studies (Kampman et al., 2001). Further research is needed to determine how propranolol or other noradrenergic agents affect this population. Patients with autism spectrum disorders have been found to have restricted semantic network flexibility (Beversdorf et al., 2000) and may also benefit from noradrenergic blockade. Some evidence suggests that there are social language benefits from propranolol in this population (Ratey et al., 1987). Further research is needed to determine how noradrenergic agents affect this population as well.
Acknowledgments
Special thanks to Jessica Alexander for help with data collection and Ryan M. Smith for protions of the analysis.
This research is supported by NIDA R21-DA015734-01 and NINDS K23-NS43222. Portions of this research were presented at the Cognitive Neuroscience Society, 2005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19:468–78. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Pompeiano M. Neurobiology of the locus coeruleus. Prog Brain Res. 1991;88:307–21. [Google Scholar]
- Beversdorf DQ, Hughes JD, Steinburg BA, Lewis LD, Heilman KM. Noradrenergic modulation of cognitive flexibility in problem solving. NeuroReport. 1999;10:2763–7. doi: 10.1097/00001756-199909090-00012. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Smith BW, Crucian GP, Anderson JM, Keillor JM, Barrett AM, et al. Increased discrimination of “false memories” in autism spectrum disorder. Proc Natl Acad Sci. 2000;97:8734–7. doi: 10.1073/pnas.97.15.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beversdorf DQ, White DM, Chever DC, Hughes JD, Bornstein RA. Central beta-adrenergic modulation of cognitive flexibility. NeuroReport. 2002;13:2505–7. doi: 10.1097/00001756-200212200-00025. [DOI] [PubMed] [Google Scholar]
- Bowden EM, Beeman MJ. Getting the right idea: semantic activation in the right hemisphere may help solve insight problems. Psychol Sci. 1998;9:435–40. [Google Scholar]
- Bowden EM, Jung-Beeman M. Normative data for 144 compound remote associate problems. Behav Res Meth, Instruments, and Computers. 2003;35:634–9. doi: 10.3758/bf03195543. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Müller U, Blackwell AD, Clark L, Robbins TW, Sahakian B. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–3. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Müller U, Blackwell AD, Robbins TW, Sahakian B. Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacol. 2006;188:397–407. doi: 10.1007/s00213-006-0391-6. [DOI] [PubMed] [Google Scholar]
- Corwin J, Bylsma FW. Translations of excerpts from Andre Rey’s psychological examination of traumatic encephalopathy and PA. Osterreith’s the Complex Figure Copy Test. Clin Neuropsychologist. 1993;7:3–15. [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Phil Trans R Soc Lond A. 2004;362:2871–88. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Dolan RJ, Frackowiak RSJ, Grasby PM. The neural correlates of the noradrenergic modulation of human attention, arousal and learning. Eur J Neurosci. 1997;9:589–98. doi: 10.1111/j.1460-9568.1997.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Coull JT, Jones MEP, Egan TD, Frith CD, Maze M. Attentional effects of noradrenaline vary with arousal level: selective activation of thalamic pulvinar in humans. NeuroImage. 2004;22:315–22. doi: 10.1016/j.neuroimage.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version. Psychological Corp; San Antonio, Texas: 1987. [Google Scholar]
- Drew PJ, Barnes JN, Evans SJ. The effect of acute beta-adrenoreceptor blockade on examination performance. Br J Clin Pharmacol. 1985;19:783–6. doi: 10.1111/j.1365-2125.1985.tb02714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–8. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM. Frontal lobe and frontal-striatal substrates for different forms of human cognitive flexibility. Neuropsychologia. 1993;31:17–28. doi: 10.1016/0028-3932(93)90077-d. [DOI] [PubMed] [Google Scholar]
- Faigel HC. The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin Pediatr. 1991;30:441–5. doi: 10.1177/000992289103000706. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Good DC, Fowlkes A, Sawaki L. Atomoxetine enhances a short-term model of plasticity in humans. Arch Phys Med Rehabil. 2006;87:216–21. doi: 10.1016/j.apmr.2005.08.131. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk Effects of alpha- and beta-adrenergic receptor antagonists on post-trial epinephrine modulation of memory: relationship to post-training brain norepinephrine. Behav Biol. 1975;13:145–53. doi: 10.1016/s0091-6773(78)93045-6. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk Facilitation of time-dependent memory processes with posttrial epinephrine. Behav Biol. 1978a;24:168–84. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk Posttraining brain norepinephrine concentration: correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behav Biol. 1978b;23:509–20. doi: 10.1016/s0091-6773(78)91614-0. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol. 1997;77:3326–39. doi: 10.1152/jn.1997.77.6.3326. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sort Test Manual. Psychological Assessment Resources; Odessa, FL: 1981. [Google Scholar]
- Heilman KM, Nadeau SE, Beversdorf DQ. Creative innovation: possible brain mechanisms. Neurocase. 2003;9:369–79. doi: 10.1076/neur.9.5.369.16553. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, et al. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Wallesch CW. Inflexibility of mental planning: a characteristic disorder with prefrontal lobe lesions. Neuropsychologia. 1992;30:1011–6. doi: 10.1016/0028-3932(92)90052-n. [DOI] [PubMed] [Google Scholar]
- Kelly BJ, Yeager KR, Pepper TH, Beversdorf DQ. Cognitive Impairment in Acute Cocaine Withdrawal. Cogn Behav Neurol. 2005;18:108–12. doi: 10.1097/01.wnn.0000160823.61201.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischka U, Kammer TH, Maier S, Weisbrod M, Thimm M, Spitzer M. Dopaminergic modulation of semantic network activation. Neuropsychologia. 1996;34:1107–13. doi: 10.1016/0028-3932(96)00024-3. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Sabban EL, Kopin IJ, Goldstein DS. Stressor specificity of peripheral catecholaminergic activation. Adv Pharmacol. 1998;42:556–60. doi: 10.1016/s1054-3589(08)60811-x. [DOI] [PubMed] [Google Scholar]
- Lader M. Beta-adrenergic antagonists in neuropsychiatry: an update. J Clin Psychiatr. 1988;49:213–23. [PubMed] [Google Scholar]
- Laverdue B, Boulenger JP. Medications beta-bloquantes et anxiete. Un interet therapeutique certain. [Beta-blocking drugs and anxiety. A proven therapeutic value ] L’Encephale. 1991;17:481–92. [PubMed] [Google Scholar]
- Lezak M. Neuropsychological Assessment. 3. Oxford University Press; Oxford New York: 1995. [Google Scholar]
- Martindale C, Greenough J. The differential effect of increased arousal on creative and intellectual performance. J Genet Psychol. 1973;123:329–35. doi: 10.1080/00221325.1973.10532692. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Black JE, Malison RT, Zimmerman RC, Kosten TR, Heninger GR, et al. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatr. 1994;51:713–9. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- Osterreith PA. Le test de copie d’une figure complexe; contribution a l’etude de la perception et de la memoire. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Ratey JJ, Bemporad J, Sorgi P, Bick P, Polakoff S, O’Driscoll G, et al. Brief Report: open trial effects of beta-blockers on speech and social behaviors in 8 autistic adults. J Autism Devel Disord. 1987;17:439–46. doi: 10.1007/BF01487073. [DOI] [PubMed] [Google Scholar]
- Raven JC. Standard Progressive Matrices Sets A,B,C,D &E. Oxford Psychologists Press; Oxford: 1976. [Google Scholar]
- Raven J, Raven JC, Court JH. Standard Progressive Matrices Manual. Oxford Psychologists Press Ltd; Oxford: 2000. [Google Scholar]
- Silver JA, Hughes JD, Bornstein RA, Beversdorf DQ. Effect of anxiolytics on cognitive flexibility in problem solving. Cogn Behav Neurol. 2004;17:93–7. doi: 10.1097/01.wnn.0000119240.65522.d9. [DOI] [PubMed] [Google Scholar]
- Smith A, Nutt D. Noradrenaline and attention lapses. Nature. 1996;380:291. doi: 10.1038/380291a0. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajowski J, Aston-Jones G. The role of the locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–54. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Vikki J. Cognitive flexibility and mental programming after closed head injuries and anterior and posterior cerebral excisions. Neuropsychologia. 1992;30:807–14. doi: 10.1016/0028-3932(92)90084-y. [DOI] [PubMed] [Google Scholar]
- Ward MM, Mefford IN, Parker SD, Chesney MA, Taylor CB, Keegan DL, et al. Epinephrine and norepinephrine responses to continuously collected human plasma to a series of stressors. Psychosom Med. 1983;45:471–86. doi: 10.1097/00006842-198312000-00002. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale – Revised. The Psychological Corporation; San Antonio, Texas: 1981. [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Compar Neurol Psychol. 1908;18:458–82. [Google Scholar]