Abstract
Objective
Preterm parturition has been associated with decidual vascular disorders and excessive thrombin generation. The objective of this study was to examine maternal plasma concentrations of protein Z in normal pregnancies, as well as in those presenting with spontaneous preterm labor (PTL) and intrauterine bleeding during pregnancy.
Study design
A cross-sectional study was designed to include patients with preterm labor and intact membranes and those with idiopathic intrauterine bleeding during pregnancy. Protein Z plasma concentrations were measured in the following groups: 1) normal pregnant women (n=71); 2) patients at term with (n=67) and without labor (n=88); 3) patients with spontaneous PTL before 34 weeks who were classified into: a) PTL with intra-amniotic infection/inflammation (IAI; n=35), b) PTL without IAI (n=54), and c) patients with PTL who delivered at term (n=49); and 4) patients with idiopathic intrauterine bleeding in the second and third trimester who were divided into: a) subsequent spontaneous PTL and delivery, and b) term delivery. Maternal plasma protein Z concentration was measured by a specific and sensitive immunoassay. Moreover, the amniotic fluid concentration of protein Z was determined in a subset of patients with preterm labor (n=30).
Results
1) There was no correlation between maternal plasma protein Z concentration and gestational age in normal pregnant women. 2) The mean maternal plasma concentration of protein Z was significantly lower in women during spontaneous labor at term than in those not in labor [mean: 2.15 μg/mL (95% CI: 2.01-2.29) vs. mean: 2.45 ± 0.52 μg/mL (95% CI: 2.34-2.56), respectively; p=0.001]; 3) Women with PTL without IAI who delivered preterm had a significantly lower mean protein Z concentration than normal pregnant women [mean: 2.12 μg/mL (95% CI: 1.98-2.26) vs. mean: 2.39 μg/mL (95% CI: 2.28-2.5); p=0.008); 4) Of interest, PTL with IAI was not associated with lower plasma concentrations of protein Z, nor were those with PTL who delivered at term (p>0.05 for each); 5) No differences were found in the maternal plasma concentrations of anti-protein Z antibodies between normal pregnancies and those with spontaneous PTL; 6) Patients with idiopathic intrauterine bleeding who had spontaneous PTL and delivery had a significantly lower mean plasma protein Z concentration than those who delivered at term [mean: 1.24 μg/mL (95% CI: 1.08-1.4) vs. mean: 1.49 ± 0.47 μg/mL (95% CI: 1.33-1.65), respectively; p=0.03]; and 7) Amniotic fluid was found to contain immunoreactive protein Z.
Conclusions
1) Patients with PTL leading to preterm delivery in the absence of IAI had a significantly lower plasma concentration of protein Z than those with normal pregnancies; 2) Patients with idiopathic intrauterine bleeding and subsequently spontaneous PTL and delivery had a significantly lower plasma concentration of protein Z than those with idiopathic intrauterine bleeding who delivered at term; and 3) Protein Z was present in the amniotic fluid of patients with PTL. Collectively, these observations suggest that a subgroup of patients with PTL have a haemostatic disorder which involves bleeding/thrombosis as a mechanism of disease.
Keywords: Coagulation, pregnancy, protein Z, amniotic fluid, parturition, thrombin, anti-protein Z antibodies, vaginal bleeding
INTRODUCTION
Spontaneous preterm labor and delivery is a syndrome caused by multiple pathologic processes that can activate the common terminal pathway of parturition [1]. A solid body of evidence supports the fact that systemic [2] and intrauterine infection/inflammation [3,4] are causally linked to preterm birth. The molecular mechanisms associated with this pathologic process have been defined [5,6] and involve pro-inflammatory cytokines [7-9], chemokines [10-12], prostaglandins [13,14], and pattern recognition receptors such as Toll-like receptors [15-18]. Infection accounts, however, for only a fraction of all cases of spontaneous preterm labor. Other pathological processes implicated in the preterm parturition syndrome include cervical insufficiency [19], uterine overdistension [20], abnormal allograft reaction [21], allergy [22,23], endocrine disorders [24-26], and vascular insults [27,28]. Indeed, Arias et al [27] reported an excess rate of placental vascular lesions in patients with spontaneous preterm labor. These observations have been confirmed by other investigators who found that failure of physiologic transformation of the myometrial segment of the spiral arteries and decidual vessel thrombosis are more frequent in patients with preterm labor with intact membranes than in normal pregnant women at term [28].
Normal pregnancy is a hypercoagulable state [29] characterized by increased generation of thrombin, as determined by increased concentrations of fibrinopeptide A [30,31], thrombin-antithrombin III (TAT) complex [32-34], and prothrombin fragments 1 and 2 [33,35,36]. Recent studies have implicated thrombin as an important enzyme in preterm parturition. In fact, there is evidence that increased thrombin generation is present in patients with preterm PROM [37,38] and spontaneous preterm labor with intact membranes [38-40]. Therefore, abnormalities of the hemostatic system may be involved in the pathophysiology of preterm parturition.
Protein Z is a vitamin K-dependent plasma glycoprotein with a molecular weight of 62 kDa and a plasma half-life of approximately 2.5 days [41,42]. This factor was originally identified in 1977 in bovine plasma by Prowse and Esnouf [43] and, in humans, by Broze and Miletich in 1984 [41] who reported that the mean plasma protein Z concentration has a considerably wider range than other vitamin K-dependent plasma proteins [42]. In vitro studies suggest that protein Z has an inhibitory effect on coagulation [44-46]. Indeed, protein Z binds to the protein Z-dependent protease inhibitor (ZPI) and enhances by 1000-fold the biological function of ZPI, which is to inhibit activated factor X (Xa) [44]. In the presence of phospholipids and calcium, protein Z and ZPI rapidly inactivate factor Xa [44,45]. Under normal conditions, factor Xa forms a complex with factor Va playing a central role in the conversion of prothrombin into thrombin [47,48]. Hence, protein Z is considered to regulate thrombin generation [45,46].
Because of its association with ischemic stroke [49] and acute coronary syndromes, protein Z deficiency has been proposed as a pro-coagulant state [50]. However, no consensus exists in the definition and the biological significance of protein Z deficiency, since protein Z deficiency as it has also been associated with bleeding tendency, even though the mechanism is poorly understood [51]. Recently, protein Z deficiency has been linked to early fetal loss [52] and adverse pregnancy outcomes such as preeclampsia, intrauterine growth restriction(IUGR), recurrent unexplained vaginal bleeding, and preterm parturition [53,54].
The objective of this study was to compare the maternal plasma concentration of protein Z in patients with: 1) spontaneous preterm labor and intact membranes, with and without intra-amniotic infection/inflammation (IAI), and those with normal pregnancies; 2) idiopathic intrauterine bleeding in the second and third trimester who subsequently had a spontaneous delivery in the index pregnancy, and those who delivered at term; and 3) in normal patients at term with and without spontaneous labor.
MATERIAL AND METHODS
Study population
A cross-sectional study was conducted by searching our clinical database and bank of biological samples, including patients in the following groups: 1) non-pregnant women in the secretory phase of their cycle not taking oral contraceptives (n=21); 2) normal pregnant women (n=71); 3) patients at term not in labor (n=88); 4) patients in spontaneous labor at term (n=67); and 5) patients with an episode of spontaneous preterm labor at <34 weeks of gestation (PTL). Women with PTL were classified into: a) PTL with IAI (n=35); b) PTL without IAI (n=54); and c) PTL who delivered at term (n=49). Women with preterm premature rupture of membranes, multiple pregnancies, fetal congenital malformations, and anticoagulant treatment were excluded.
A second population subject of this study consisted of a cohort of patients with vaginal bleeding (n=65) who met the following criteria: singleton gestation; vaginal bleeding of uterine origin; gestational age between 18 to 35 weeks; and amniocentesis with microbiological studies of amniotic fluid. The exclusion criteria for this part of the study were: preterm labor; preterm PROM; evidence of intra-amniotic infection; placenta previa; cervical bleeding (defined as bleeding seen on speculum exam originating from the ectocervix); clinical diagnosis of placental abruption; and evidence of a retroplacental clot by ultrasound at admission. This cohort of patients has been the subject of studies published by our group [55].
Definitions
Patients were considered to have a normal pregnancy outcome if they did not have any obstetrical, medical, or surgical complications of pregnancy, and delivered a term neonate of appropriate birth weight for gestational age [56] without congenital anomalies or complications. Preterm labor was diagnosed by the presence of at least two regular uterine contractions every 10 minutes associated with cervical changes, requiring admission to the hospital. Preterm delivery was defined as delivery before 37 weeks of gestation. Vaginal bleeding of uterine origin was diagnosed by sterile speculum examination confirming the presence of blood coming through the external os of the cervix. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. Intra-amniotic inflammation was diagnosed by amniotic fluid white blood cell count > 100 cells/mm3.
Sample collection
Samples of peripheral blood from pregnant and non-pregnant women were obtained by venipuncture and collected in tubes containing 0.109M trisodium citrate anticoagulant solution. The citrated plasma tubes were balanced and centrifuged at 1300 × g for 10 minutes at 4°C to separate cellular components from clear plasma, and the samples were stored at -70°C until assay. Transabdominal amniocentesis under ultrasonographic guidance was performed to assess the microbial state of the amniotic cavity, as well as determine fetal lung maturity in patients approaching term. Immediately upon retrieval, amniotic fluid was transported to the laboratory and cultured for aerobic and anaerobic bacteria as well as genital mycoplasmas. White blood cells count, glucose concentration and Gram-stain were also performed shortly after collection. Amniotic fluid not required for clinical purposes was centrifugated at 700 × g for 10 minutes at 4°C, and the supernatant was aliquoted and stored frozen at −70°C until analysis.
All women provided informed consent prior to the collection of maternal blood and amniotic fluid samples. The collection and utilization of samples for research purposes was approved by the institutional review boards of the Sotero del Rio Hospital (a major affiliate of the Catholic University), Wayne State University, and the National Institute of Child Health and Human Development (NICHD/NIH/DHHS).
Protein Z immunoassays in maternal plasma and amniotic fluid
Concentrations of protein Z in maternal plasma and amniotic fluid were determined by sensitive and specific immunoassays obtained from Diagnostica Stago (Asnieres-sur-Seine, France). This assay utilizes the quantitative sandwich enzyme immunoassay technique. Prior to assaying amniotic fluid samples, we conducted spike and recovery experiments, which produced parallel curves indicating that amniotic fluid constituents do not interfere with this assay system. Briefly, the standards and samples were incubated in duplicate wells of microtiter plate precoated with a monoclonal antibody specific for protein Z. Following incubation, repeated washings were performed to remove unbound materials and a second monoclonal antibody coupled with peroxidase directed against another epitope of protein Z was added to the assay plates. This step was followed by additional washes and an equal amount of stabilized chromogen; ortho-phenylenediamine (OPD) and urea peroxide substrate were added to each well. This initiates the development of color, which is halted at a set time by the addition of an acid solution. Optical densities were determined using a programmable micro plate reader (Bio-Tek Instruments, Winooski, Vermont, USA). Protein Z concentrations in the samples were determined by interpolation from individual standard curves. The calculated inter- and intra-assay coefficients of variation (CV) are 3.65% and 1.41% respectively. The calculated sensitivity of the protein Z immunoassay in our laboratory is 3.37ng/mLl.
Determination of IgM and IgG anti-protein Z antibodies concentrations in maternal plasma
Immunoassays to quantify anti-protein Z IgG and IgM isotypes were obtained from HYPHEN BioMed (Neuville-sur-Oise, France). Briefly, diluted citrated plasma samples were incubated in duplicate wells of the micro titer plates, which have been pre-coated with highly purified human protein Z. Repeated washing and aspiration removed all unbound materials from the assay plate. To detect bound antibodies of the IgG Isotype, further incubations with a peroxidase conjugated goat anti-human IgG which reacts specifically with the IgG Isotype were conducted. Similarly, to detect bound antibodies of the IgM isotype, further incubations with a peroxidase conjugated goat anti-human IgM were performed. Following repeated washings, a substrate solution, tetramethylbenzidine (TMB) in the presence of hydrogen peroxide was added and color developed in proportion to the amount of IgG or IgM bound in the initial step of the individual assays. The color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable micro titer plate spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentrations of anti-protein Z IgG or IgM in samples were determined by interpolation from individual standard curves composed of purified human anti-protein Z IgG or IgM (calibrators). The calculated inter- and intra-assay CV for anti-protein Z IgG isotype immunoassay in our laboratory were 6.03%, and 5.41%. The detection limit (sensitivity) for the anti-protein Z IgG isotype immunoassay was 1.11 AU/mL. The calculated inter- and intra-assay CV for anti-protein Z IgM isotype immunoassay were 7.04%, and 2.18% respectively. The detection limit for the anti-protein Z IgM isotype immunoassay was 2.02 AU/mLl.
Statistical analysis
Parametric (t-test and ANOVA) and non-parametric (Mann-Whitney and Kruskal-Wallis) tests were used for comparisons according to the data distribution. Post-hoc analysis was performed using Dunnett and Bonferroni tests to correct for multiple comparisons. The maternal plasma protein Z concentrations were expressed as mean and 95% confidence interval (CI). A p-value <0.05 was considered statistically significant. The statistical package used was SPSS v.12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics of the different study groups are displayed in Tables I and II. Women with a normal pregnancy had a significantly higher mean plasma concentration of protein Z than non-pregnant women [mean: 2.39 μg/mL (95% CI: 2.28-2.5) vs. mean: 1.18 μg/mL (95% CI: 0.96-1.4); p <0.001] (Figure 1). No correlation was found between gestational age and maternal plasma protein Z concentrations (r=0.06; p=0.6). Patients with spontaneous labor at term had a lower mean maternal plasma concentration of protein Z than patients at term not in labor [mean: 2.15 μg/mL (95% CI: 2.01-2.29) vs. mean: 2.45 ± 0.52 μg/mL (95% CI: 2.34-2.56), respectively; p=0.001] (Figure 2).
Table I.
Characteristics of the study population among patients presenting with preterm labor, and the normal pregnancy group
| Normal pregnancy (n=71) | Preterm labor with term delivery (n=49) | Preterm labor and delivery without IAI (n=54) | Preterm labor and delivery with IAI (n=35) | p* | |
|---|---|---|---|---|---|
| Maternal age
(years) |
24
(17-34) |
20
(15-39) |
22
(13-37) |
23
(16-33) |
NS |
| GA at blood draw
(weeks) |
31
(20-38) |
31
(25-34) |
30
(22-33) |
26
(20-33) |
<0.001 |
| GA at delivery
(weeks) |
40
(37-42) |
38
(37-41) |
32
(23-36) |
28
(21-36) |
<0.001 |
| Birthweight
(grams) |
3,320
(2,610-4,050) |
2,924
(2,320-4,170) |
1,680
(540-2,760) |
1,040
(400-2,950) |
<0.001 |
Values expressed as median (range)
IAI: intra-amniotic infection/inflammation, GA: gestational age, NS: not significant
Kruskall-Wallis test with Bonferroni correction.
Table II.
Clinical and demographic characteristics of the study population among patients presenting with idiopathic vaginal bleeding during pregnancy, according to the gestational age at delivery
| VB
Term delivery (n=36) |
VB
Preterm delivery (n=29) |
p* | |
|---|---|---|---|
| Maternal age
(years) |
26
(14-39) |
26
(15-44) |
NS |
| GA at blood draw
(weeks) |
29.4
(21.4-33.5) |
28.4
(21-34.4) |
NS |
| GA at delivery
(weeks) |
39.3
(37-42) |
32.4
(22.6-36.4) |
<0.001 |
| Birthweight
(grams) |
3,255
(2,410-4,880) |
2,205
(520-3210) |
<0.001 |
Values expressed as median (range)
VB: vaginal bleeding, GA: gestational age, NS: not significant
Mann-Whitney U test
Figure 1.
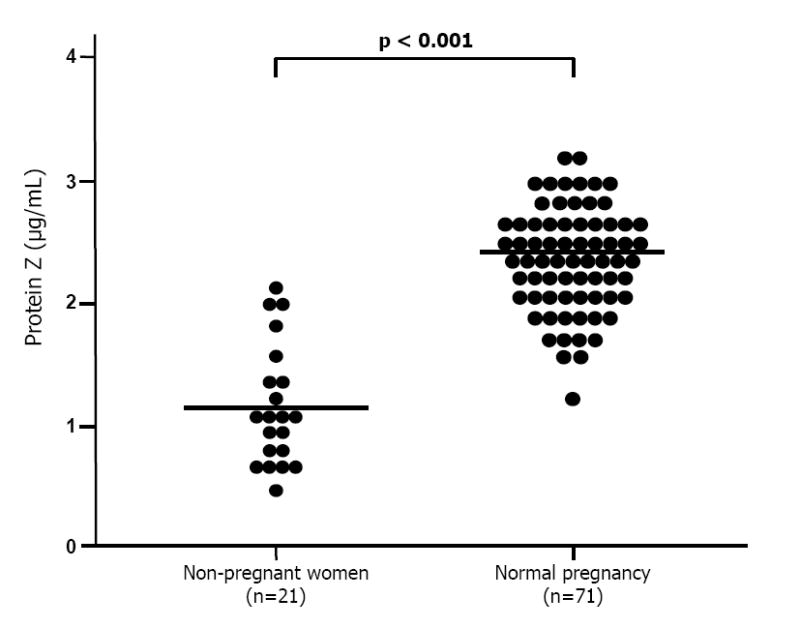
Maternal plasma concentrations of protein Z between non-pregnant (n=21) and normal pregnant women (n=71). The mean plasma concentration of protein Z in normal pregnant women was significantly higher than that of non-pregnant women (normal pregnant women, mean 2.39 μg/mL (95% CI: 2.28-2.5) vs. non-pregnant women, mean 1.18 μg/mL (95% CI: 0.96-1.4); p <0.001).
Figure 2.
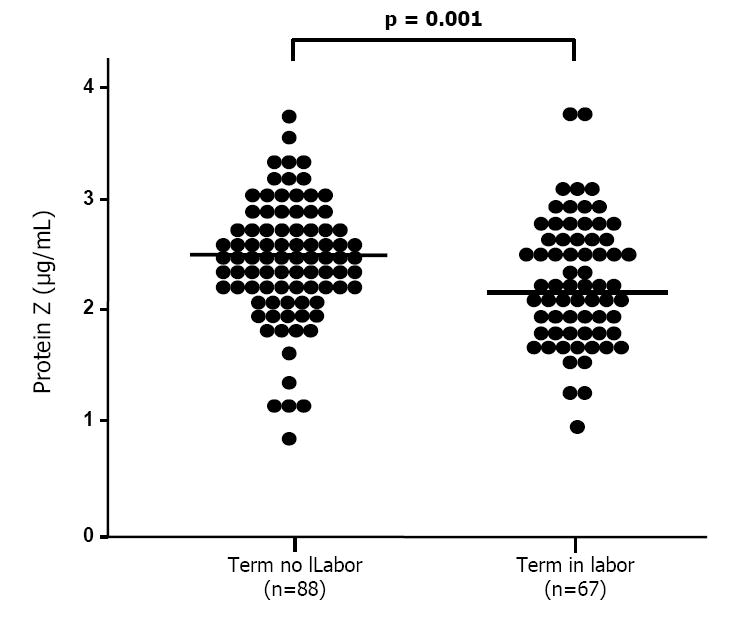
Maternal plasma concentrations of protein Z in pregnancies at term, among patients with (n=67) and without (n=88) labor. Patients with spontaneous labor at term have a significantly lower mean maternal plasma concentration of protein Z than that of patients at term not in labor (spontaneous labor at term, mean 2.15 μg/mL (95% CI: 2.01-2.29) vs. term not in labor, mean 2.45 μg/mL (95% CI: 2.34-2.56); p =0.001).
The mean plasma concentration of protein Z in women with spontaneous preterm labor who delivered preterm without IAI was significantly lower than that of normal pregnant women [mean: 2.12 μg/mL (95% CI: 1.98-2.26) vs. mean: 2.39 μg/mL (95% CI: 2.28-2.5), respectively; p=0.008] (Figure 3). In contrast, no significant differences were detected in the mean plasma concentrations of protein Z between patients with preterm labor and IAI who delivered preterm and those with normal pregnancies [mean: 2.18 μg/mL (95% CI: 2-2.36) vs. mean: 2.39 μg/mL (95% CI: 2.28-2.5), respectively; p=0.1]. Similarly, there were no major differences in the mean plasma concentration of this glycoprotein between patients with spontaneous preterm labor who delivered at term and normal pregnant women [mean: 2.28 ± 0.51 μg/mL (95% CI: 2.13-2.43) vs. mean: 2.39 μg/mL (95% CI: 2.28-2.5), respectively; p=0.46].
Figure 3.
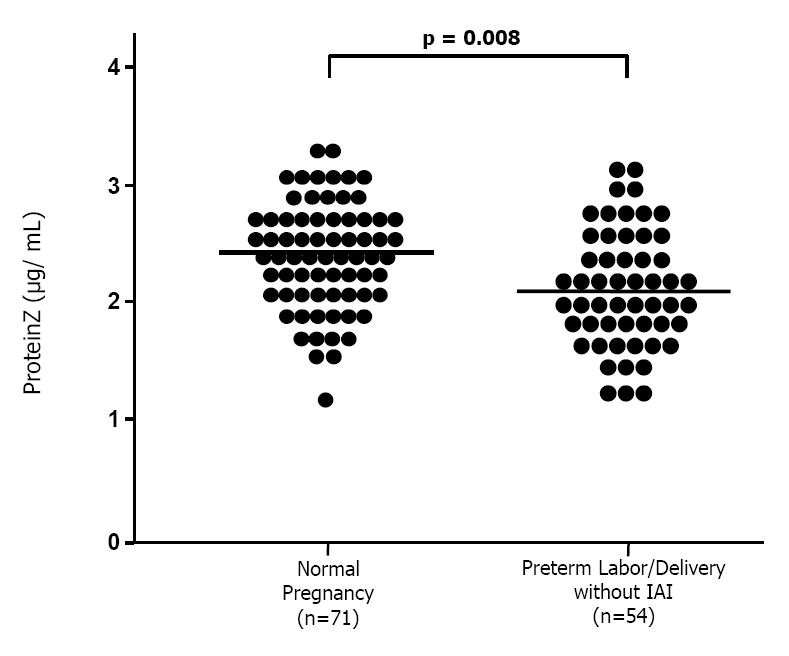
Maternal plasma concentrations of protein Z among patients with spontaneous preterm labor and delivery without intra-amniotic infection/inflammation (IAI; n=54) and those with normal pregnancies (n=71). Patients with spontaneous preterm labor and delivery without IAI had a significantly lower mean maternal plasma concentration of protein Z than that of normal pregnant women (spontaneous PTL and delivery without IAI, mean 2.12 μg/mL (95% CI: 1.98-2.26) vs. normal pregnant women, mean 2.39 μg/mL (95% CI: 2.28-2.5); p=0.008).
Maternal plasma concentrations of anti-protein Z IgG and IgM antibodies were not different among the study groups, indicating that the differences in protein Z plasma concentrations could not be explained on the basis of the anti-protein Z antibodies (Table III).
Table III.
Maternal plasma concentrations of anti-protein Z antibodies among patients presenting with preterm labor, and the normal pregnancy group
| Normal pregnancy (n=71) | Preterm labor with term delivery (n=49) | Preterm labor without IAI (n=54) | Preterm labor with IAI (n=35) | p* | |
|---|---|---|---|---|---|
| Anti-protein Z IgG antibody concentration (AU/mL) | 2.93
(0-500.5) |
2.61
(0-73.1) |
2.52
(0-9.7) |
2.88
(1.2-8.9) |
NS |
| Anti-protein Z IgM antibody concentration (AU/mL) | 12.3
(0-44) |
10.8
(0-47.4) |
11.9
(0-185.3) |
13.1
(3.5-68.7) |
NS |
Values expressed as median (range)
IAI: intra-amniotic infection/inflammation, GA: gestational age, NS: not significant
Kruskall-Wallis test with Bonferroni correction.
Among women presenting with idiopathic intrauterine bleeding during the second and third trimesters, those who subsequently had a spontaneous preterm labor and delivery without IAI had a lower mean plasma protein Z concentration than those with vaginal bleeding who delivered at term [mean: 1.24 μg/mL (95% CI: 1.08-1.4) vs. mean: 1.49 ± 0.47 μg/mLl (95% CI: 1.33-1.65), respectively; p=0.03] (Figure 4).
Figure 4.
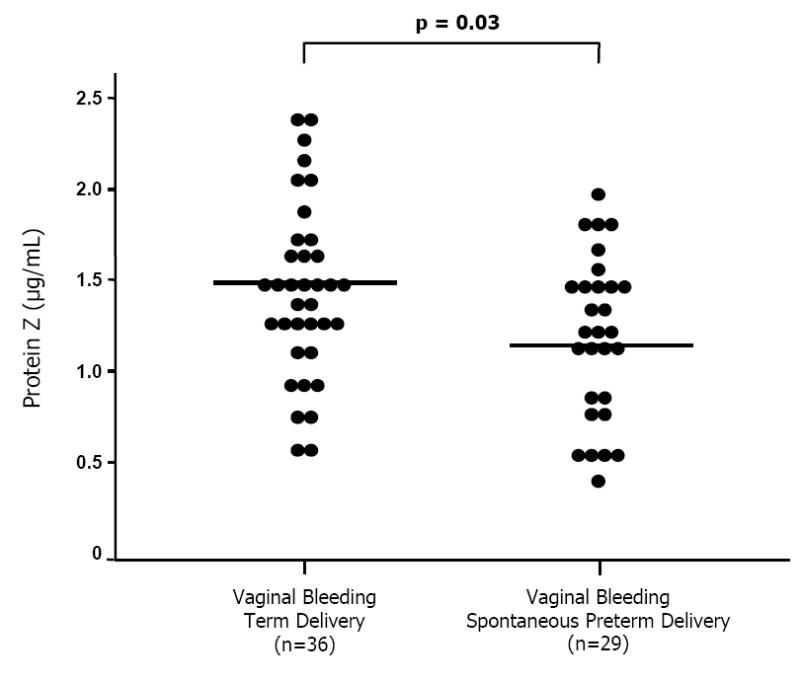
Maternal plasma protein Z concentrations among women presenting with idiopathic vaginal bleeding during pregnancy. Those who subsequently had a spontaneous preterm labor and delivery without intra-amniotic infection had a significantly lower mean plasma protein Z concentration than that of those with vaginal bleeding who delivered at term (spontaneous preterm labor and delivery, mean 1.24 μg/mL (95% CI: 1.08-1.4) vs. term delivery, mean 1.49 μg/mL (95% CI: 1.33-1.65); p=0.03).
Protein Z was measured in the amniotic fluid in a subset of patients with spontaneous PTL and intact membranes with (n=10) and without (n=10) IAI, and in those who delivered at term (n=10). The median amniotic fluid concentrations of protein Z were 28.6 ng/mL (11.2-133.2), 40.3 ng/mL (11.2-163.4) and 48.5 ng/mLl (15.6-169.9), respectively.
DISCUSSION
Principal findings of this study
1) Pregnancy is associated with a higher maternal plasma concentration of protein Z than the non-pregnant state. 2) Maternal plasma protein Z concentration did not change with advancing gestational age between 20-38 weeks of gestation. 3) Labor at term is associated with a lower mean maternal plasma concentration of protein Z than no labor. 4) Spontaneous preterm delivery in the absence of IAI is associated with a lower mean maternal plasma concentration of protein Z than normal pregnancies. 5) Similarly, patients with vaginal bleeding and negative amniotic fluid cultures, who subsequently delivered preterm, had a significantly lower plasma concentration of protein Z than those who delivered at term. 6) The maternal plasma concentration of anti-protein Z antibodies was not increased in women with preterm labor. 7) Protein Z is a normal constituent of amniotic fluid.
What is protein Z?
Human protein Z is a vitamin K-dependent protein that has been proposed to be synthesized by the liver [57]. The gene encoding protein Z is in chromosome 13q34 [58], and its structure is very similar to protein C and coagulation factors VII, IX and X [58-60]. In contrast to these serine proteases, this glycoprotein does not have proteolytic properties [61] due to the lack of histidine and serine residues necessary for serine protease activity [59,60].
Plasma protein Z concentration increases rapidly in the first months of life, is followed by slow increases during childhood, and reaches the concentration observed in adult life during puberty [42,62,63]. Miletich and Broze [42] reported that the mean plasma protein Z concentrations in 455 blood donors was 2.9 ± 1.0 μg/mL. Of note, the values extended between 0.6 to 5.7 μg/mL, a range considerably wider than that of other vitamin K-dependent plasma proteins [42].
When compared to normal controls [42,64], low plasma concentrations of protein Z have been reported in patients with liver disease [57], disseminated intravascular coagulation [64], amyloidosis [64], and in those taking oral anticoagulant treatment with warfarin. On the other hand, patients with idiopathic thrombocytopenic purpura [61] and chronic hemodialysis [65] have a higher plasma concentration of protein Z than normal controls.
An important role of protein Z in coagulation has been recently reported on the basis of in vitro experiments. Han et al [44] demonstrated that the procoagulant activity of factor Xa is reduced when incubated with protein Z in the presence of phospholipids and Ca++, suggesting an interaction between factor Xa and protein Z at the phospholipid surface. Moreover, Yin et al [46] reported that the presence of protein Z dramatically reduced factor Xa activity and thrombin generation.
Protein Z is a cofactor of ZPI, a 72 kDa glycoprotein present in human plasma [44]. In normal pooled plasma, which contains an excess of ZPI, all protein Z appears to be bound to ZPI [66]. The interaction of protein Z and ZPI retards and reduce thrombin generation in mixtures containing factor V, prothrombin, Ca++, and phospholipids. In similar mixtures containing factor Va, however, protein Z and ZPI do not inhibit thrombin generation [45]. Thus, it has been proposed that the anticoagulant effect of protein Z and ZPI may precede the activation of factor V and formation of protrombinase complex [61].
Two possible pathways for factor Xa inhibition by protein Z and ZPI have been suggested: 1) protein Z and factor Xa form a complex in the phospholipid surface, and this complex is then recognized by ZPI; 2) protein Z-ZPI complex is directed to the phospholipid surface and binds factor Xa [61]. Since it has been proposed that protein Z circulates bound to ZPI, the second mechanism may reflect the inhibition of factor Xa in human plasma.
Protein Z deficiency, thrombosis and hemorrhage
In vitro studies suggest that protein Z has an inhibitory effect on coagulation [44-46]. Thus, protein Z deficiency may predispose to a prothrombotic state. Evidence supporting this view includes the observation that protein Z deficiency (<1 μg/mL) is associated with prior ischemic stroke when compared to healthy individuals[49], and that patients with acute coronary syndrome have significantly lower plasma protein Z concentrations than comparable controls [50,67]. However, these results have not been confirmed by others [68-70].
Protein Z deficiency alone has been proposed to confer a modest risk for thrombosis [46,61]. This risk, however, is substantially increased when protein Z deficiency is associated with a thrombophilic state. Evidence in support of this includes: 1) knockout mice for protein Z have a normal phenotype in the absence of a thrombotic challenge, but when these mice are also homozygous for the factor V Leiden mutation, they have vascular thrombosis and excessive hepatic fibrin deposition [46]; 2) in humans, patients with factor V Leiden mutation and protein Z deficiency (<1 μg/mL) have a significantly higher incidence of deep venous thrombosis than normal patients [71]; 3) the odds ratio of venous thromboembolism in patients with factor V Leiden mutation is 5.2 (95% CI: 2.5-10.9), which is further increased to 14.6 (95% CI: 1.9-113) when their plasma concentrations of protein Z are below the 25th percentile (<1.34 μg/mL) [72]; and 4) among patients with anti-phospholipid antibodies and plasma protein Z below the 5th percentile, the odds ratio for thrombosis is 4.7 (95% CI: 1.1-19.4), as opposed to 7.5 (95% CI: 1.5-37.5) for arterial thrombosis [73].
An association between bleeding tendency and protein Z deficiency has been suggested by Kemkes-Matthes and Matthes [51], based on observations that patients with hemorrhagic disorders of unknown origin have significantly lower mean plasma concentrations of protein Z than healthy controls. In contrast, Miletich and Broze [42] have reported that 10% of blood donors have plasma protein Z concentrations below 50%, while patients with low protein Z concentrations due to oral anticoagulant therapy do not have abnormal bleeding times. Moreover, additional studies have found no association between protein Z deficiency and bleeding tendency [64,74,75].
Protein Z during pregnancy
Conflicting results have been reported for maternal plasma concentrations of protein Z in normal pregnancy. In this study, we found that pregnant women had a higher plasma concentration of protein Z than non-pregnant women. While this is consistent with a previous report [76], it is not with others [53,77]. Moreover, the observation that the maternal plasma concentration of protein Z does not change with advancing gestational age correlates the finding of a recent study [53]. In contrast, other studies have reported that the maternal plasma concentration of this glycoprotein increases [77] or decreases [54] with gestational age. The reasons for the discrepancies among studies are unclear. However, we wish to note that the current study focused on the second and third trimester, as opposed to the first trimester of pregnancy.
Protein Z deficiency has been associated with adverse pregnancy outcomes: 1) a higher proportion of patients with a previous fetal death (10 to 15 weeks of gestation) had protein Z deficiency (<1 mg/L) compared to the control group [52]; and 2) patients with adverse pregnancy outcome (defined as preeclampsia, IUGR, recurrent unexplained vaginal bleeding, and preterm parturition) had significantly lower mean plasma concentrations of protein Z than patients with normal pregnancy outcome. The authors proposed that changes in the maternal plasma protein Z concentration may have an important role in the regulation of thrombin generation during pregnancy [54]. This is relevant because excessive thrombin generation is associated with preterm parturition with intact or ruptured membranes [38], preeclampsia, and small for gestational age fetuses[78]. In contrast, a recent study [53] demonstrated that the median plasma concentration of protein Z in patients with preeclampsia, intrauterine growth restriction (IUGR), and late fetal demise intrauterine fetal demise (IUFD) was not significantly different than that of patients with normal pregnancy. However, the authors reported a higher frequency of protein Z deficiency (<1.2 mg/L) among patients with IUFD and IUGR compared to those with normal pregnancy [53].
Protein Z in spontaneous labor at term
The mean concentration of protein Z was significantly lower in women in spontaneous labor at term than in women not in labor at term, suggesting than physiologic parturition is associated with changes in the hemostatic system. This is consistent with the observation that labor is related to modifications in the coagulation and fibrinolytic system, including increased maternal plasma concentration of TAT complex, plasmin-alpha 2-plasmin inhibitor complex and D-dimer [79], as well as increased plasma concentration of the tissue-plasminogen activator[80].
Protein Z in preterm labor leading to preterm delivery
It is possible that changes in the maternal plasma concentration of protein Z may reflect activation of the coagulation cascade in patients with spontaneous preterm parturition without infection/inflammation, and in those with vaginal bleeding who subsequently deliver preterm. Thus, hemostatic disorders may constitute an additional mechanism of disease in the preterm parturition syndrome. It has been observed that patients with preterm labor and intact membranes who deliver preterm have a higher frequency of decidual vascular lesions than normal pregnant women at term [28]. In addition, vaginal bleeding [81,82] and retroplacental hematoma [83] are risk factors for spontaneous preterm delivery in the index pregnancy. It is likely that both decidual vessel thrombosis and vaginal bleeding of uterine origin may increase thrombin generation in the decidua. Therefore, thrombin may activate the common pathway of parturition. Evidence in support of this includes: 1) decidua is a rich source of tissue factor, the primary initiator of coagulation and thrombin activation [84]; 2) intrauterine administration of whole blood to pregnant rats stimulates myometrial contractility, while heparinized blood does not (heparin blocks the generation of thrombin )[85]; 3) thrombin stimulates myometrial contractility in a dose-dependent manner [85]; and 4) TAT complexes, a marker of in vivo generation of thrombin, are increased in plasma [38] and amniotic fluid [39] of women with preterm labor and preterm PROM.
Anti-protein Z antibodies during pregnancy and preterm parturition
An important observation in this study is that we found no significant differences in the mean plasma concentrations of anti-protein Z antibodies (IgG and IgM) between women with normal pregnancy and those with spontaneous preterm labor (with and without IAI who delivered preterm, and those who delivered at term). Thus, based on the findings of this study, the lower maternal plasma protein Z concentrations among these groups cannot be explained by the presence of these antibodies, as previously proposed in the case of other pregnancy complications [53].
A recent report has associated normal pregnancy with: 1) the development of anti-protein Z antibodies throughout gestation, independent of protein Z concentrations; and 2) a correlation between mean protein Z concentration and anti-protein Z IgM in the second and third trimester (r 0.26 and -0.28 respectively, p <0.05), but not for anti-protein Z IgG antibodies. The authors proposed that changes in anti-protein Z antibodies may contribute to the prothrombotic state of pregnancy [76]. Gris et al. [86] demonstrated that non-pregnant women with a history of adverse pregnancy outcomes [defined as history of unexplained primary recurrent embryo losses before the 8th week of gestation, one unexplained episode of fetal death from the 10th week of gestation, unexplained primary recurrent embryo losses associated with protein Z deficiency (<1 mg/L), and one episode of severe pre-eclampsia] had higher concentrations of anti IgG and anti-IgM antibodies. The authors reported that: 1) anti-protein Z IgG and IgM antibodies concentrations were not correlated with plasma protein Z concentrations in both cases and control groups; 2) the concentrations of anti-protein Z IgG and IgM antibodies were significantly higher in the group of patients with adverse pregnancy outcomes than in controls; 3) the global risk of adverse pregnancy outcome increased with higher concentrations of anti-protein Z antibodies; 4) IgG concentrations were significantly higher in women with fetal death and in women with recurrent embryo losses than in the controls; and 5) IgM concentrations were significantly higher in women with recurrent embryo losses, fetal death, severe pre-eclampsia, or recurrent embryo losses and protein Z deficiency than that of controls. Therefore, the study of anti-protein Z antibodies appears important, and for that reason it was the topic of the present article.
Protein Z in amniotic fluid
A novel observation made during the course of this study is that amniotic fluid contains immunoreactive protein Z. This finding was unexpected, since amniotic fluid does not contain all the components of the coagulation system. While further studies are required, our finding suggests that either protein Z may serve to inhibit the actions of thrombin in amniotic fluid [39] or have functions unrelated to the coagulation system.
Limitations of the study
An important limitation is the lack of information about the thrombophilic status of the patients included. However, the association between inherited thrombophilia and spontaneous preterm delivery is unclear at this time [54,87]. Indeed, screening for thrombophilia is not part of the standard of care in patients with spontaneous preterm labor [88].
Conclusions
1) Patients with spontaneous preterm labor without IAI and with idiopathic intrauterine bleeding in the absence of intra-amniotic infection have lower concentrations of protein Z than normal pregnant women. 2) The maternal plasma concentrations of anti-protein Z antibodies do not differ between women with normal pregnancies and those with spontaneous preterm labor (with IAI who delivered preterm, and those who delivered at term). 3) Protein Z is present in the amniotic fluid of patients with preterm labor.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. 2004:28–60. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madinger NE, Greenspoon JS, Ellrodt AG. Pneumonia during pregnancy: has modern technology improved maternal and fetal outcome? Am J Obstet Gynecol. 1989;161:657–62. doi: 10.1016/0002-9378(89)90373-6. [DOI] [PubMed] [Google Scholar]
- 3.Fidel PL, Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–75. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 4.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 5.Gomez R, Romero R, Mazor M, Ghezzi F, David C, Yoon BH. Role of infection in preterm labor and delivery. 1997:85–125. [Google Scholar]
- 6.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–55. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Mazor M, Wu YK, Avila C, Oyarzun E, Mitchell MD. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot Essent Fatty Acids. 1989;37:183–6. doi: 10.1016/0952-3278(89)90083-5. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 11.Kelly RW, Leask R, Calder AA. Choriodecidual production of interleukin-8 and mechanism of parturition. Lancet. 1992;339:776–7. doi: 10.1016/0140-6736(92)91896-g. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, Svinarich D, Cotton DB. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–13. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157:1461–7. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1988;34:141–5. doi: 10.1016/0952-3278(88)90137-8. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–63. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- 16.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–11. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–96. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 18.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams VM, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–55. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 20.Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol. 1990;10:347–50. [PubMed] [Google Scholar]
- 21.Romero R, Sepulveda W, Baumann P, Yoon BH, Brandt F, Gomez R, Mazor M, et al. The preterm labor syndrome: biochemical, cytologic, immunologic, pathologic, microbiologic, and clinical evidence that preterm labor is a heterogeneous disease. Am J Obstet Gynecol. 1993;168 [Google Scholar]
- 22.Romero R, Mazor M, Avila C, Quintero R, Munoz H. Uterine “allergy”: a novel mechanism for preterm labor. Am J Obstet Gynecol. 1991;164 [Google Scholar]
- 23.Bytautiene E, Romero R, Vedernikov YP, El Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol. 2004;191:1356–61. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 24.Csapo AI, Pohanka O, Kaihola HL. Progesterone deficiency and premature labour. Br Med J. 1974;1:137–40. doi: 10.1136/bmj.1.5899.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazor M, Hershkovitz R, Chaim W, Levy J, Sharony Y, Leiberman JR, Glezerman M. Human preterm birth is associated with systemic and local changes in progesterone/17 beta-estradiol ratios. Am J Obstet Gynecol. 1994;171:231–6. doi: 10.1016/0002-9378(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 26.Fidel PI, Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med. 1998;7:222–6. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<222::AID-MFM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 28.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 29.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52:176–82. [PubMed] [Google Scholar]
- 30.Romero R, Rickles FR, Matthews E, Scott D, Dinan C, Duffy T. Fibrinopeptide A during normal pregnancy. Am J Perinatol. 1988;5:70–3. doi: 10.1055/s-2007-999658. [DOI] [PubMed] [Google Scholar]
- 31.Pinto S, Abbate R, Rostagno C, Bruni V, Rosati D, Neri Serneri GG. Increased thrombin generation in normal pregnancy. Acta Eur Fertil. 1988;19:263–7. [PubMed] [Google Scholar]
- 32.de Boer K, ten Cate JW, Sturk A, Borm JJ, Treffers PE. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1989;160:95–100. doi: 10.1016/0002-9378(89)90096-3. [DOI] [PubMed] [Google Scholar]
- 33.Cadroy Y, Grandjean H, Pichon J, Desprats R, Berrebi A, Fournie A, Boneu B. Evaluation of six markers of haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. Br J Obstet Gynaecol. 1993;100:416–20. doi: 10.1111/j.1471-0528.1993.tb15264.x. [DOI] [PubMed] [Google Scholar]
- 34.Uszynski M. Generation of thrombin in blood plasma of non-pregnant and pregnant women studied through concentration of thrombin-antithrombin III complexes. Eur J Obstet Gynecol Reprod Biol. 1997;75:127–31. doi: 10.1016/s0301-2115(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 35.Comeglio P, Fedi S, Liotta AA, Cellai AP, Chiarantini E, Prisco D, Mecacci F, Parretti E, Mello G, Abbate R. Blood clotting activation during normal pregnancy. Thromb Res. 1996;84:199–202. doi: 10.1016/0049-3848(96)00176-4. [DOI] [PubMed] [Google Scholar]
- 36.Stone S, Hunt BJ, Seed PT, Parmar K, Khamashta MA, Poston L. Longitudinal evaluation of markers of endothelial cell dysfunction and hemostasis in treated antiphospholipid syndrome and in healthy pregnancy. Am J Obstet Gynecol. 2003;188:454–60. doi: 10.1067/mob.2003.14. [DOI] [PubMed] [Google Scholar]
- 37.Rosen T, Kuczynski E, O’Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med. 2001;10:297–300. doi: 10.1080/714904361. [DOI] [PubMed] [Google Scholar]
- 38.Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:368–73. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 39.Gomez R, Athayde N, Pacora P, Mazor M, Yoon BH, Romero R. Increased Thrombin in Intrauterine Inflammation. Am J Obstet Gynecol. 1998;178:S62. [Google Scholar]
- 40.Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol. 2001;185:1059–63. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- 41.Broze GJ, Jr, Miletich JP. Human Protein Z. J Clin Invest. 1984;73:933–8. doi: 10.1172/JCI111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miletich JP, Broze GJ., Jr Human plasma protein Z antigen: range in normal subjects and effect of warfarin therapy. Blood. 1987;69:1580–6. [PubMed] [Google Scholar]
- 43.Prowse CV, Esnouf MP. The isolation of a new warfarin-sensitive protein from bovine plasma. Biochem Soc Trans. 1977;5:255–6. doi: 10.1042/bst0050255. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Fiehler R, Broze GJ., Jr Isolation of a protein Z-dependent plasma protease inhibitor. Proc Natl Acad Sci U S A. 1998;95:9250–5. doi: 10.1073/pnas.95.16.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han X, Fiehler R, Broze GJ., Jr Characterization of the protein Z-dependent protease inhibitor. Blood. 2000;96:3049–55. [PubMed] [Google Scholar]
- 46.Yin ZF, Huang ZF, Cui J, Fiehler R, Lasky N, Ginsburg D, Broze GJ., Jr Prothrombotic phenotype of protein Z deficiency. Proc Natl Acad Sci U S A. 2000;97:6734–8. doi: 10.1073/pnas.120081897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53:505–18. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- 48.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–70. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 49.Vasse M, Guegan-Massardier E, Borg JY, Woimant F, Soria C. Frequency of protein Z deficiency in patients with ischaemic stroke. Lancet. 2001;357:933–4. doi: 10.1016/S0140-6736(00)04218-5. [DOI] [PubMed] [Google Scholar]
- 50.Fedi S, Sofi F, Brogi D, Tellini I, Cesari F, Sestini I, Gazzini A, Comeglio M, Abbate R, Gensini GF. Low protein Z plasma levels are independently associated with acute coronary syndromes. Thromb Haemost. 2003;90:1173–8. doi: 10.1160/TH03-04-0237. [DOI] [PubMed] [Google Scholar]
- 51.Kemkes-Matthes B, Matthes KJ. Protein Z deficiency: a new cause of bleeding tendency. Thromb Res. 1995;79:49–55. doi: 10.1016/0049-3848(95)00089-a. [DOI] [PubMed] [Google Scholar]
- 52.Gris JC, Quere I, Dechaud H, Mercier E, Pincon C, Hoffet M, Vasse M, Mares P. High frequency of protein Z deficiency in patients with unexplained early fetal loss. Blood. 2002;99:2606–8. doi: 10.1182/blood.v99.7.2606. [DOI] [PubMed] [Google Scholar]
- 53.Bretelle F, Arnoux D, Shojai R, D’Ercole C, Sampol J, Dignat F, Camoin-Jau L. Protein Z in patients with pregnancy complications. Am J Obstet Gynecol. 2005;193:1698–702. doi: 10.1016/j.ajog.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Paidas MJ, Ku DH, Lee MJ, Manish S, Thurston A, Lockwood CJ, Arkel YS. Protein Z, protein S levels are lower in patients with thrombophilia and subsequent pregnancy complications. J Thromb Haemost. 2005;3:497–501. doi: 10.1111/j.1538-7836.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- 55.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, Chaiworapongsa T, Espinoza J, Gonzalez R. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–7. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 56.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 57.Kemkes-Matthes B, Matthes KJ. Protein Z, a new haemostatic factor, in liver diseases. Haemostasis. 1995;25:312–6. doi: 10.1159/000217178. [DOI] [PubMed] [Google Scholar]
- 58.Fujimaki K, Yamazaki T, Taniwaki M, Ichinose A. The gene for human protein Z is localized to chromosome 13 at band q34 and is coded by eight regular exons and one alternative exon. Biochemistry. 1998;37:6838–46. doi: 10.1021/bi972002a. [DOI] [PubMed] [Google Scholar]
- 59.Ichinose A, Takeya H, Espling E, Iwanaga S, Kisiel W, Davie EW. Amino acid sequence of human protein Z, a vitamin K-dependent plasma glycoprotein. Biochem Biophys Res Commun. 1990;172:1139–44. doi: 10.1016/0006-291x(90)91566-b. [DOI] [PubMed] [Google Scholar]
- 60.Sejima H, Hayashi T, Deyashiki Y, Nishioka J, Suzuki K. Primary structure of vitamin K-dependent human protein Z. Biochem Biophys Res Commun. 1990;171:661–8. doi: 10.1016/0006-291x(90)91197-z. [DOI] [PubMed] [Google Scholar]
- 61.Broze GJ., Jr Protein Z-dependent regulation of coagulation. Thromb Haemost. 2001;86:8–13. [PubMed] [Google Scholar]
- 62.Kemkes-Matthes B, Matthes KJ. Protein Z. Semin Thromb Hemost. 2001;27:551–6. doi: 10.1055/s-2001-17966. [DOI] [PubMed] [Google Scholar]
- 63.Yurdakok M, Gurakan B, Ozbag E, Vigit S, Dundar S, Kirazli S. Plasma protein Z levels in healthy newborn infants. Am J Hematol. 1995;48:206–7. doi: 10.1002/ajh.2830480315. [DOI] [PubMed] [Google Scholar]
- 64.Bertolino G, Montani N, Lorenzutti F, Balduini CL, Gamba G. Behaviour of plasma protein Z levels in patients with abnormal haemostasis: Correlation with coagulation screening times. Thromb Haemost. 1997;78 [Google Scholar]
- 65.Usalan C, Erdem Y, Altun B, Arici M, Haznedaroglu IC, Yasavul U, Turgan C, Caglar S. Protein Z levels in haemodialysis patients. Int Urol Nephrol. 1999;31:541–5. doi: 10.1023/a:1007179631603. [DOI] [PubMed] [Google Scholar]
- 66.Tabatabai A, Fiehler R, Broze GJ., Jr Protein Z circulates in plasma in a complex with protein Z-dependent protease inhibitor. Thromb Haemost. 2001;85:655–60. [PubMed] [Google Scholar]
- 67.Sofi F, Cesari F, Vigiani S, Fatini C, Marcucci R, Giglioli C, Valente S, Abbate R, Gensini GF, Fedi S. Protein Z plasma levels in different phases of activity of coronary atherosclerosis. J Thromb Haemost. 2005;3:2254–8. doi: 10.1111/j.1538-7836.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 68.Kobelt K, Biasiutti FD, Mattle HP, Lammle B, Wuillemin WA. Protein Z in ischaemic stroke. Br J Haematol. 2001;114:169–73. doi: 10.1046/j.1365-2141.2001.02913.x. [DOI] [PubMed] [Google Scholar]
- 69.Lopaciuk S, Bykowska K, Kwiecinski H, Czlonkowska A, Kuczynska-Zardzewialy A. Protein Z in young survivors of ischemic stroke. Thromb Haemost. 2002;88:536. [PubMed] [Google Scholar]
- 70.Morange PE, Juhan-Vague I. Protein Z plasma levels are not associated with the risk of coronary heart disease: the PRIME Study. J Thromb Haemost. 2004;2:2050–1. doi: 10.1111/j.1538-7836.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- 71.Kemkes-Matthes B, Nees M, Kuhnel G, Matzdorff A, Matthes KJ. Protein Z influences the prothrombotic phenotype in Factor V Leiden patients. Thromb Res. 2002;106:183–5. doi: 10.1016/s0049-3848(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 72.Martinelli I, Razzari C, Biguzzi E, Bucciarelli P, Mannucci PM. Low levels of protein Z and the risk of venous thromboembolism. J Thromb Haemost. 2005;3:2817–9. doi: 10.1111/j.1538-7836.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- 73.Forastiero RR, Martinuzzo ME, Lu L, Broze GJ. Autoimmune antiphospholipid antibodies impair the inhibition of activated factor X by protein Z/protein Z-dependent protease inhibitor. J Thromb Haemost. 2003;1:1764–70. doi: 10.1046/j.1538-7836.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 74.Gamba G, Bertolino G, Montani N, Spedini P, Balduini CL. Bleeding tendency of unknown origin and protein Z levels. Thromb Res. 1998;90:291–5. doi: 10.1016/s0049-3848(98)00036-x. [DOI] [PubMed] [Google Scholar]
- 75.Ravi S, Mauron T, Lammle B, Wuillemin WA. Protein Z in healthy human individuals and in patients with a bleeding tendency. Br J Haematol. 1998;102:1219–23. doi: 10.1046/j.1365-2141.1998.00908.x. [DOI] [PubMed] [Google Scholar]
- 76.Paidas M, Ku D, Arkel Y, Triche E, Fortunato C, Hamar B, Ku E, et al. Normal pregnancy is associated with the development of protein S and protein Z antibodies, independent of PS and PZ levels. Am J Obstet Gynecol. 2004;191 [Google Scholar]
- 77.Quack Loetscher KC, Stiller R, Roos M, Zimmermann R. Protein Z in normal pregnancy. Thromb Haemost. 2005;93:706–9. doi: 10.1160/TH04-08-0532. [DOI] [PubMed] [Google Scholar]
- 78.Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim YM, Berman S, Edwin S, Yoon BH, Romero R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;11:362–7. doi: 10.1080/jmf.11.6.362.367. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe T, Minakami H, Sakata Y, Matsubara S, Tamura N, Obara H, Wada T, Onagawa T, Sato I. Effect of labor on maternal dehydration, starvation, coagulation, and fibrinolysis. J Perinat Med. 2001;29:528–34. doi: 10.1515/JPM.2001.073. [DOI] [PubMed] [Google Scholar]
- 80.Yoshimura T, Ito M, Nakamura T, Okamura H. The influence of labor on thrombotic and fibrinolytic systems. Eur J Obstet Gynecol Reprod Biol. 1992;44:195–9. doi: 10.1016/0028-2243(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 81.Williams MA, Mittendorf R, Lieberman E, Monson RR. Adverse infant outcomes associated with first-trimester vaginal bleeding. Obstet Gynecol. 1991;78:14–8. [PubMed] [Google Scholar]
- 82.Signore CC, Sood AK, Richards DS. Second-trimester vaginal bleeding: correlation of ultrasonographic findings with perinatal outcome. Am J Obstet Gynecol. 1998;178:336–40. doi: 10.1016/s0002-9378(98)80022-7. [DOI] [PubMed] [Google Scholar]
- 83.Nagy S, Bush M, Stone J, Lapinski RH, Gardo S. Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet Gynecol. 2003;102:94–100. doi: 10.1016/s0029-7844(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 84.Lockwood CJ, Krikun G, Papp C, Toth-Pal E, Markiewicz L, Wang EY, Kerenyi T, Zhou X, Hausknecht V, Papp Z, et al. The role of progestationally regulated stromal cell tissue factor and type-1 plasminogen activator inhibitor (PAI-1) in endometrial hemostasis and menstruation. Ann N Y Acad Sci. 1994;734:57–79. doi: 10.1111/j.1749-6632.1994.tb21736.x. [DOI] [PubMed] [Google Scholar]
- 85.Elovitz MA, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J Obstet Gynecol. 2000;183:799–804. doi: 10.1067/mob.2000.108897. [DOI] [PubMed] [Google Scholar]
- 86.Gris JC, Amadio C, Mercier E, Lavigne-Lissalde G, Dechaud H, Hoffet M, Quere I, Amiral J, Dauzat M, Mares P. Anti-protein Z antibodies in women with pathologic pregnancies. Blood. 2003;101:4850–2. doi: 10.1182/blood-2002-12-3802. [DOI] [PubMed] [Google Scholar]
- 87.Resch B, Gallistl S, Kutschera J, Mannhalter C, Muntean W, Mueller WD. Thrombophilic polymorphisms--factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations--and preterm birth. Wien Klin Wochenschr. 2004;116:622–6. doi: 10.1007/s00508-004-0223-9. [DOI] [PubMed] [Google Scholar]
- 88.Paidas MJ, Ku DH, Langhoff-Roos J, Arkel YS. Inherited thrombophilias and adverse pregnancy outcome: screening and management. Semin Perinatol. 2005;29:150–63. doi: 10.1053/j.semperi.2005.05.008. [DOI] [PubMed] [Google Scholar]


